Abstract
Background and Purpose
Using a model of embolic stroke, the present study tested the hypothesis that blockage of endothelin-1 with S-0139, a specific endothelin type A receptor (ETA) antagonist, enhances the neuroprotective effect of recombinant tissue plasminogen activator (rtPA) by suppressing molecules that mediate thrombosis and blood brain barrier (BBB) disruption induced by ischemia and rtPA.
Methods
Rats (n=104) subjected to embolic middle cerebral artery (MCA) occlusion were randomly divided into 1 of 4 infusion groups with 26 rats per group: (1) the control group in which rats were administered saline, (2) the monotherapy rtPA group in which rtPA was intravenously administered at a dose of 10 mg/kg 4 hours after MCA occlusion, (3) the monotherapy S-0139 group in which S-0139 was intravenously given 2 hours after MCA occlusion, and (4) the combination of rtPA +S-0139 group in which S-0139 and rtPA were given 2 and 4 hours after MCA occlusion, respectively. Measurements of infarct volume and parenchymal hemorrhage, behavioral outcome, and immunostaining were performed on rats euthanized 1 and 7 days after stroke.
Results
The combination therapy of S-0139 and rtPA significantly (P<0.01) reduced infarct volume (24.8±0.9% versus 33.8±1.5% in control) and hemorrhagic area (7.1±6.1 µm2 versus 36.5±19.2 µm2 in control) and improved functional recovery compared with control saline-treated animals. Immunostaining analysis revealed that the combination therapy had the synergistically suppressed ischemia- and rtPA-induced ICAM-1, protease-activated receptor 1 (PAR-1), as well as accumulation of platelets in cerebral microvessels. Furthermore, the combination treatment synergistically reduced loss of laminin, ZO1, and occludin in cerebral vessels.
Conclusions
These data suggest that S-0139 provides the neuroprotection by suppressing ischemia- and rtPA-triggered molecules that evoke thrombosis and BBB disruption.
Keywords: antagonist, embolic, integrity, patency, rat, stroke
The endothelins (ETs) are potent vasoactive peptides and ET-1 to ET-3 induce vasoconstriction through the ET receptor subtype, ETA.1 ETA is expressed in cerebral endothelial cells and regulates blood brain barrier (BBB) function.1–4 Acute stroke patients exhibit significantly elevated ET-1 levels in plasma and cerebrospinal fluid.5,6 In experimental stroke, increased ETs exacerbate BBB leakage, whereas blockage of ETA with S-0139, a specific ETA antagonist, suppresses disruption of BBB permeability and brain edema.3,4,7
Although the neuroprotective effects of S-0139 have been demonstrated,3,4 it has not been examined whether blockage of ETA with S-0139 enhances thrombolysis. As disruption of the BBB and an increase risk of hemorrhage limit the therapeutic window for thrombolysis of stroke with tissue plasminogen activator (tPA) to 3 hours,8,9 there remains a need for agents that reduce the incidence and severity of bleeding and are effective in extending the therapeutic window for rht-PA. Using a model of embolic stroke, the present study tested the hypothesis that a combination treatment of S-0139 with tPA significantly reduce infarct volume when tPA is given 4 hours after stroke. We further hypothesized that the combination treatment reduces expression of molecules that mediate thrombosis, leukocyte adhesion, and BBB disruption, and thereby reduces ischemic cell damage.
Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
General Procedures
Male Wistar rats were anesthetized with isoflorane (1% to 2.5% in a mixture of 70% N2O and 30% O2) with the use of a face mask. The rectal temperature was maintained at 37±0.5°C throughout the surgical procedure by means of a feedback-regulated water heating system.
Animal Models
Rats weighing 375 to 400 g were subjected to embolic MCA occlusion.10 Briefly, a single intact, fibrin-rich, 24-hour-old homologous clot was placed at the origin of the MCA via a 15-mm length of modified polyethylene catheter (PE-50).10
Experimental Protocols
To examine the effect of S-0139 on extending the therapeutic window of recombinant tPA (rtPA), rats were subjected to the embolic MCA occlusion and randomized into 1 of 4 infusion groups with 26 rats per group: (1) the control group, in which rats were administered saline, (2) the monotherapy rtPA (Genetics) group, in which rtPA was infused intravenously at a dose of 10 mg/kg 4 hours after MCA occlusion (10% bolus and the remainder at a continuous infusion over a 30 minutes interval via a syringe infusion pump, Harvard Apparatus), (3) the monotherapy S-0139 group, in which S-0139 was infused intravenously at a dose of 3 mg/kg/h for 22 hours (n=6) or 72 hours (n=20) infusion starting 2 hours after MCA occlusion, and (4) the combination of rtPA + S-0139 group in which S-0139 and rtPA (10 mg/kg) were given 2 hours and 4 hours after MCA occlusion, respectively. S-0139 was infused intravenously at a dose of 3 mg/kg/h for 22 hours (n=6) or 72 hours (n=20). Six rats per group were euthanized 24 hours after MCA occlusion for immunostaining analysis, whereas 20 rats from each group were euthanized 7 days after MCA occlusion for measurements of infarct volume and the incidence of hemorrhage.
Neurological and Behavioral Assessment
To detect sensorimotor impairments, an array of behavioral tests including foot-fault, modified neurological severity score (mNSS), and adhesive-removal test were performed before MCA occlusion and at 1 and 7 days after MCA occlusion by an investigator blinded to the experimental groups. These tests are sensitive and reliable indices of sensorimotor impairments after ischemic stroke and have been applied extensively in our laboratory to assess neurological outcome after MCA occlusion in rats.11–16
Measurements of Infarct Volume and Hemorrhage
Infarct volume and petechial hemorrhage were measured on hematoxylin and eosin (H&E) stained 7 coronal sections from rats euthanized 7 days after MCA occlusion via MicroComputer imaging device (MCID) system (Imaging Research) according to our published protocols.10 Petechial hemorrhage, defined as a cluster of red blood cells outside of the lumen of blood vessels, is presented as total area of hemorrhage (µm2) which is a summation of the area of hemorrhage from each section.10
Immunohistochemistry
Immunostaining was performed on frozen brain coronal sections from rats euthanized 24 hours after MCA occlusion, according to our published methods.8,13,16,17 To detect adhesive molecule expression in the cerebral microvessels, a monoclonal anti-rat intercellular adhesion molecule-1 (ICAM-1) antibody (BD Bioscience) was used at a titer of 1:50. To examine the integrity of cerebral microvessels, a rabbit polyclonal antitype IV collagen antibody (Abcam) and a rabbit polyclonal antilaminin antibody (Sigma) were used at a titer of 1:500 and 1:200, respectively. To detect protease-activated receptor 1 (PAR-1) in the brain, a mouse monoclonal antibody against human PAR-1 (Santa Cruz Biotechnology) was used at a titer of 1:500. To detect platelets, a rabbit polyclonal antithrombocytes antibody (Inter-Cell Technologies Inc) was used at a tier of 1:1000. To examine vascular tight junctions, a monoclonal antioccludin (Zymed) and a rabbit polyclonal anti-ZO1 antibody (Zymed) were used at a titer of 1:200 and 1:100, respectively.
Quantification
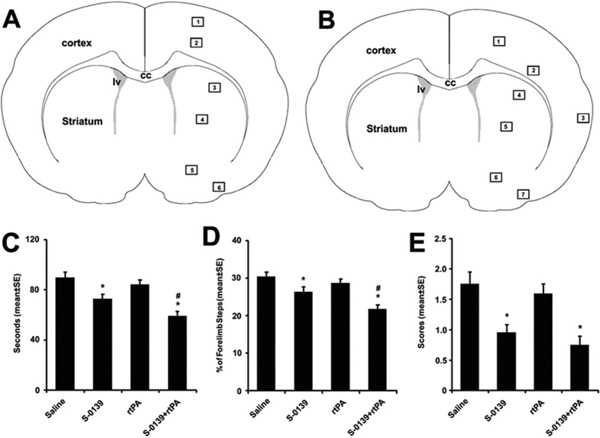
Frozen coronal sections used in the present study were located at the center of the territory supplied by the MCA (between anterior-posterior [AP] + 10.6 mm-genu corpus callosum and AP + 8.74 mm-anterior commissure crossing).18 A total of 4 sections (each one at 400 µm apart) per rat were used for immunostaining. The numbers of ICAM, and PAR-1 immunoreactive vessels were counted throughout the entire ipsilateral hemisphere because normal cerebral vessels do not express these genes. The numbers of thrombocyte immunoreactive vessels were measured from 6 0.55-mm2 fields located in the ischemic boundary (Figure 1A). The numbers of immunoreactive vessels in the 4 sections per rat were averaged to obtain a mean number for each brain according to published methods.19 Data are presented as the number of vessels.
Figure 1.
A schematic of coronal sections shows 6 (A) and 7 (B) areas where images were acquired for analysis of thrombocytes and vascular integrity, respectively. The effects of S-0139 on behavioral tests (C to E). Monotherapy of S-0139 given 2 hours after stroke and combination therapy of S-0139 given 2 hours and rtPA administered 4 hours after stroke improve neurological function measured by adhesive removal test (C), foot-fault test (D), and mNSS (E) 7 days after MCA occlusion. *P<0.05 vs the saline group and #P<0.05 vs monotherapy of S-0139 group. N=20 rats/group. CC indicates corpus callosum; lv, lateral ventricle.
Measurements of occludin, ZO-1, collagen type IV, and laminin positive vessels were performed in the ischemic boundary (Figure 1B, 0.55 mm2×7 fields) and homologous areas of the contralateral hemisphere. Briefly, occludin, ZO-1, collagen type IV, and laminin immunoreactive vessels were digitized under a 40× objective (Olympus) via the MCID system. Density of vessels in each field of view was determined by the MCID system. Data are presented as a percent of vessels in the ipsilateral hemisphere versus the contralateral hemisphere.
Statistical Analysis
Analysis of variance (ANOVA) for repeated measures, with fixed factors rtPA and S-0139 and repeated factor sample, was used to test the synergy of combination treatments rt-PA and S-0139. Analysis began with testing for rtPA by S-0139 interaction, followed by a subgroup analysis. A significant treatment interaction between rtPA and S0139 at the critical level of 0.05 was further evaluated for superadditive effect (synergy) or subadditive effect. All data are presented as mean±SE, and a P<0.05 was considered as statistically significant.
Results
The Combination Therapy Is Neuroprotective
In rats subjected to embolic MCAo, monotherapy of S-0139 2 hours after MCA occlusion significantly reduced ischemic lesion volume (27.9±1.4% of the contralateral hemisphere, n=20) compared with the controls (34.3±2.8%, n=20), which was associated with significant improvement of functional outcome 7 days after MCA occlusion (Figure 1C to 1E). In contrast, monotherapy of rtPA 4 hours after MCA occlusion neither reduced infarct volume (34.9±1.4%, n=20 versus 33.8±1.5%, n=20 in control), nor improved functional recovery (Figure 1C to 1E), but significantly (P<0.05) increased hemorrhagic area (116.8±33.8 µm2 versus 36.5±19.2 µm2 in control). Combination of S-0139 at 2 hours and rtPA at 4 hours after MCA occlusion significantly (P<0.01) reduced infract volume (24.8±0.9%, n=20 versus 33.8±1.5%, n=20 in control) and hemorrhagic area (7.1±6.1 µm2 versus 36.5±19.2 µm2 in control) and improved functional recovery compared with the rats treated with saline (Figure 1C to 1E).
The Combination Therapy of rtPA and S-0139 Reduces ICAM-1 Expression
Upregulation of ICAM-1 facilitates adhesion of neutrophils to endothelial cells, which contributes to secondary thrombosis in cerebral microvessels.16,20 Endothelin-1 increases ICAM-1 expression on cultured rat aortic endothelial cells.21 To examine whether ETA antagonist, S-0139, blocks ICAM-1 expression on cerebral endothelial cells, immune-staining for ICAM-1 was performed. Occlusion of the MCA resulted in induction of ICAM-1 in cerebral vessels (Figure 2G), which is consistent with our previous findings.17 The monotherapy of S-0139 given 2 hours after MCA occlusion significantly reduced the number of ICAM-1–positive vessels compared with the control group (Figure 2G), whereas the monotherapy of rtPA given 4 hours after MCA occlusion further increased (P<0.05) the number of ICAM-1–positive vessels (Figure 2A to 2C and 2G). However, the combination therapy of rtPA (4 hours) and S-0139 (2 hours) had the synergistic effect of suppressing ICAM-1 in microvessels (Figure 2D to 2F and 2G), suggesting that S-0139 suppresses ischemia- and rtPA-upregulated ICAM-1 expression in cerebral endothelial cells.
Figure 2.
Immunostaining shows ICAM-1 immunoreactive vessels in the ischemic hemisphere from representative rats treated with rtPA alone (A through C) and S-0139 in combination with rtPA (D through F). C and F indicate the cortex. B and E represent the striatum. G, Quantitative data of ICAM-1 immunoreactive vessels (n=6 per group, # vs saline and * vs rtPA). Bar=25 µm (B to F). Monotherapy of rtPA was administered 4 hours after stroke, whereas combination therapy of S-0139 and rtPA was given 2 and 4 hours after stroke, respectively.
The Combination Therapy of rtPA and S-0139 Reduces PAR-1 and Thrombosis
PAR-1, a receptor for thrombin, induces thrombosis.16 To examine the effect of S-0139 on formation of secondary thrombosis, we measured PAR-1 and thrombocyte immune-reactive vessels. The monotherapy of S-0139 administered 2 hours after stroke significantly reduced the number of PAR-1 (Figure 3E) and thrombocyte (Figure 3F) positive vessels, whereas the monotherapy of rtPA given 4 hours after stroke significantly augmented the number of PAR-1 (Figure 3A and 3E) and thrombocyte (Figure 3C and 3F) immunoreactive vessels compared to saline-treated animals. The combination therapy of rtPA and S-0139 substantially decreased the number of PAR-1 (Figure 3B and 3E) and thrombocyte (Figure 3D and 3F) positive vessels compared to saline control, and statistical analysis revealed that the combination therapy has a synergistic effect, indicating that blockage of ETA by S-0139 abolishes stroke- and rtPA-induced secondary thrombosis.
Figure 3.
Photomicrographs show PAR-1 (A and B) and platelet (C and D) immunoreactive vessels in the ischemic boundary of the cortex (area 2 of Figure 1A) from representative rats treated with rtPA alone (A and C) or S-0139 in combination with rtPA (B and D). E and F, Quantitative data of PAR-1 and thrombocyte immunoreactive vessels (n=6 per group), respectively. Please see methods for details of quantification of PAR-1 and thrombocyte immunoreactive vessels. Bar=25 µm (A to D). Monotherapy of rtPA was administered 4 hours after stroke, whereas combination therapy of S-0139 and rtPA was given 2 and 4 hours after stroke, respectively.
The Combination Therapy of rtPA and S-0139 Augments Vascular Integrity
To examine the effects of S-0139 on cerebral microvascular integrity, we measured collagen type IV and laminin immunereactivity, 2 of the major basal lamina components of cerebral microvessels,9 and the tight junction proteins, occluding and ZO1. Consistent with previous findings,16 embolic stroke dramatically reduced collagen type IV (Figure 4D), laminin (Figure 4H), ZO1 (Figure 5B and 5E), and occludin (Figure 5G and 5J) immunoreactive vessels in the ipsilateral hemispheres compared with the homologous area of the contralateral hemisphere. Treatment with S-0139 2 hours after stroke significantly blocked loss of laminin (Figure 4H), ZO1 (Figure 5C and 5E), and occludin (Figure 5G and 5J), but not collagen IV immunoreactive vessels (Figure 4H). However, the monotherapy of rtPA administered 4 hours after stroke exacerbated reduction of collagen type IV (Figure 4B and 4D), laminin (Figure 4F and 4H), ZO1 (Figure 5D and 5E), and occludin (Figure 5H and 5J) immunoreactive vessels. The combination therapy of rtPA and S-0139 abolished exacerbation of these immunoreactive vessels triggered by ischemia and rtPA (Figure 4C, 4D, Figure 5D, 5E, 5I, and 5J).
Figure 4.
Photomicrographs show collagen IV (A to C) and laminin (E to G) immunoreactive vessels in the cortex of homologous areas of the contralateral hemisphere (A and E) and in the ischemic boundary of the cortex (areas 1 and 2 of Figure 1B) from representative rats treated with rtPA alone (B and F) or S-0139 in combination with rtPA (C and G). D and H, Quantitative data of percentage of collagen IV and laminin, respectively, positive vessels in the ipsilateral hemisphere vs the contralateral hemisphere (n=6 per group). Bar=20 µm (A to G). Monotherapy of rtPA was administered 4 hours after stroke, whereas combination therapy of S-0139 and rtPA was given 2 and 4 hours after stroke, respectively.
Figure 5.
Photomicrographs show ZO1 (A to D) and occludin (F to I) immunoreactive vessels in the cortex of homologous areas of the contralateral hemisphere (A and F) and in the ischemic boundary of the cortex (areas 1 and 2 of Figure 1B) from representative rats treated with saline (B and G), rtPA alone (C and H), or S-0139 in combination with rtPA (D and I). E and J, Quantitative data of percentage of ZO1 and occludin, respectively, positive vessels in the ipsilateral hemisphere vs the contralateral hemisphere. (n=6 per group). Bar=20 µm (A to I). Monotherapy of rtPA was administered 4 hours after stroke, whereas combination therapy of S-0139 and rtPA was given 2 and 4 hours after stroke, respectively.
Discussion
The present study demonstrates that combination treatment of rtPA (4 hours) and S-0139 (2 hours) significantly reduced infarct volume and improved neurological outcome without increasing the incidence of hemorrhagic transformation compared with animals treated with saline and rtPA alone (4 hours). Immunostaining analysis revealed that the combination therapy synergistically blocked ischemia- and rtPA-induced ICAM-1 and PAR-1 expression as well as accumulation of platelets in cerebral microvessels. Furthermore, the combination treatment synergistically reduced loss of laminin, ZO1, and occludin in cerebral vessels. These data indicate that S-0139, an ETA antagonist, in combination with rtPA at 4 hours after stroke has a neuroprotective effect, whereas S-0139 suppresses ischemia- and rtPA-triggered molecules that evoke thrombosis and BBB disruption and likely contributes to the beneficial effects of the combination therapy.
S-0139 is a potent ETA antagonist and has neuroprotective effects in experimental models of stroke and BBB disruption.3,4 In a filament model of transient MCA occlusion, S-0139 given 1 hour after transient MCA occlusion has been shown to reduce infarct volume, which was associated with decreased BBB leakage and neutrophil infiltration.4 However, S-0139 does not have a neuroprotective effect when it is given to the rat subjected to permanent MCA occlusion.22 Administration of rtPA 2 hours after embolic MCA occlusion reduces infarct volume and improves neurological outcome.23 The present study shows that monotherapy of rtPA given 4 hours after MCA occlusion did not have the neuroprotective effects and exacerbated hemorrhage, which is consistent with previous findings in this model of embolic stroke.16,24,25 However, blockage of ET-1 with S-0139 substantially reduced the infarct volume and hemorrhage induced by rtPA. Moreover, the combination therapy 4 hours after stroke has a synergistic effect on improvement of functional outcome. These data suggest that S-0139 is effective to reduce the adverse side effects of thrombolysis with rtPA.
Molecular mechanisms underlying the neuroprotective effects of S-0139 on stroke and on thrombolysis have not been investigated. Using immunohistochemistry, the present study shows that S-0139 significantly reduced ICAM-1 and PAR-1 immunoreactive vessels in ischemic boundary regions, when S-0139 was administered 2 hours after embolic stroke. More importantly, we found that S-0139 (2 hours) in combination with rtPA (4 hours) has a synergistic effect on decreasing these immunoreactive vessels. Stroke induces adhesion molecule expression including ICAM-1 in cerebral vessels, which facilitates leukocyte adhesion.26 When neutrophils adhere to injured endothelium, they interact with platelets and form clots that contribute to secondary thrombosis in cerebral microvessels and disruption of the BBB.26 Delayed fibrinolysis with rht-PA further increased in ICAM-1 expression and neutrophil accumulation in ipsilateral cerebral vessels.27 The present study suggests that endothelin-1 could mediate stroke- and thrombolysis-upregulated ICAM-1 expression, which is consistent with previous findings showing that endothelin-1 induces ICAM-1 expression on cultured endothelial cells.21 Moreover, our data suggest that ETA may be involved in PAR-1 expression. PAR-1 is the prototype thrombin receptor, and plasmin, which is generated from the cleavage of plasminogen by rht-PA, activates PAR-1 that also promotes thrombosis formation.28 Blockage of ETA with BQ123 inhibits the effect of PAR-1 activated by thrombin on NMDA-mediated nociceptive activity.29 Collectively, these data suggest that suppressing ICAM-1 and PAR-1 expression by S-0139 likely contributes to the beneficial effect of this ETA antagonist on cerebral vascular patency. Indeed, the present study demonstrates that monotherapy of S-0139 and combination therapy of S-1039 with rtPA significantly reduced platelet aggregation in cerebral vessels.
The present study shows that either monotherapy of S-0139 or combination therapy of S-0139 with rtPA significantly reduced degradation of collagen IV and laminin immunoreactivity, two of the major basal lamina components of cerebral microvessels.9 Concurrently, both therapies significantly reduced loss of endothelial tight junction proteins ZO1 and occludin. These data suggest that S-0139 could benefit cerebral vascular integrity after stroke and thrombolysis with rtPA.
In the present study, we used a model of embolic MCAo that mimics the malignant MCA infarction of human stroke.10,30,31 In this model, we demonstrate that development of secondary thrombosis contributes to impairment of cerebral microvascular patency and integerity.9 Stroke elevates endothelin-1 levels in cerebral endothelium and astrocytes and astrocytes are integral to composite the BBB.32,33 ETA is expressed in cerebral endothelial cells.1–4 Consistently, the present study suggests that S-0139 has multi-targeted effects on cerebral microvascular thrombosis and microvascular integrity. However, future studies are warranted to delineate signaling pathways that mediate interaction between rtPA and ET-1 receptor activation, which extend the therapeutic window of rtPA to 4 hours after stroke.
In summary, our data indicate that the combination therapy of S-0139 and rtPA 4 hours after stroke is neuroprotective and that S-0139 augments cerebral vascular patency and integrity by suppressing ischemia- and rtPA-triggered molecules that mediate thrombosis and BBB disruption.
Acknowledgments
We appreciate Qing-e Lu and Yuping Yang for technical assistance.
Sources of Funding
This work was supported by an unrestricted research grant from Pharmaceutical Research Division, Shionogi & Co Ltd, Osaka, Japan, and CNS Program & Clinical Development, Shionogi USA Inc, Florham Park, NJ.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: Three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanimirovic DB, Yamamoto T, Uematsu S, Spatz M. Endothelin-1 receptor binding and cellular signal transduction in cultured human brain endothelial cells. J Neurochem. 1994;62:592–601. doi: 10.1046/j.1471-4159.1994.62020592.x. [DOI] [PubMed] [Google Scholar]
- 3.Narushima I, Kita T, Kubo K, Yonetani Y, Momochi C, Yoshikawa I, Ohno N, Nakashima T. Highly enhanced permeability of blood-brain barrier induced by repeated administration of endothelin-1 in dogs and rats. Pharmacol Toxicol. 2003;92:21–26. doi: 10.1034/j.1600-0773.2003.920104.x. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo Y, Mihara S, Ninomiya M, Fujimoto M. Protective effect of endothelin type a receptor antagonist on brain edema and injury after transient middle cerebral artery occlusion in rats. Stroke. 2001;32:2143–2148. doi: 10.1161/hs0901.94259. [DOI] [PubMed] [Google Scholar]
- 5.Volpe M, Cosentino F. Abnormalities of endothelial function in the pathogenesis of stroke: The importance of endothelin. J Cardiovasc Pharmacol. 2000;35:S45–S48. doi: 10.1097/00005344-200000002-00011. [DOI] [PubMed] [Google Scholar]
- 6.Franceschini R, Gandolfo C, Cataldi A, Del Sette M, Rolandi A, Corsini G, Rolandi E, Barreca T. Twenty-four-hour endothelin-1 secretory pattern in stroke patients. Biomed Pharmacother. 2001;55:272–276. doi: 10.1016/s0753-3322(01)00059-2. [DOI] [PubMed] [Google Scholar]
- 7.Mihara S, Nakajima S, Matumura S, Kohnoike T, Fujimoto M. Pharmacological characterization of a potent nonpeptide endothelin receptor antagonist 97–139. J Pharmacol Exp Ther. 1994;268:1122–1128. [PubMed] [Google Scholar]
- 8.Zhang ZG, Chopp M, Goussev A, Lu D, Morris D, Tsang W, Powers C, Ho KL. Cerebral microvascular obstruction by fibrin is associated with upregulation of pai-1 acutely after onset of focal embolic ischemia in rats. J Neurosci. 1999;19:10898–10907. doi: 10.1523/JNEUROSCI.19-24-10898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang ZG, Zhang L, Tsang W, Goussev A, Powers C, Ho K, Morris D, Smyth SS, Coller BS, Chopp M. Dynamic platelet accumulation at the site of the occluded middle cerebral artery and in downstream microvessels is associated with loss of microvascular integrity after embolic middle cerebral artery occlusion. Brain Res. 2001;912:181–194. doi: 10.1016/s0006-8993(01)02735-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of embolic focal cerebral ischemia. Brain Research. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, Zhang Z. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Zhang ZG, Zhang R, Morris D, Lu M, Coller BS, Chopp M. Adjuvant treatment with a glycoprotein iib/iiia receptor inhibitor increases the therapeutic window for low-dose tissue plasminogen activator administration in a rat model of embolic stroke. Circulation. 2003;107:2837–2843. doi: 10.1161/01.CIR.0000068374.57764.EB. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Zhang ZG, Zhang C, Zhang RL, Chopp M. Intravenous administration a gpiib/iiia receptor antagonist extends the therapeutic window of intra-arterial tenecteplase-tissue plasminogen activator in a rat stroke model. Stroke. 2004;35:2890–2895. doi: 10.1161/01.STR.0000147963.68238.da. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Zhang ZG, Ding GL, Jiang Q, Liu X, Meng H, Hozeska A, Zhang C, Li L, Morris D, Zhang RL, Lu M, Chopp M. Multitargeted effects of statin-enhanced thrombolytic therapy for stroke with recombinant human tissue-type plasminogen activator in the rat. Circulation. 2005;112:3486–3494. doi: 10.1161/CIRCULATIONAHA.104.516757. [DOI] [PubMed] [Google Scholar]
- 17.Zhang RL, Chopp M, Zaloga C, Zhang ZG, Jiang N, Gautam SC, Tang WX, Tsang W, Anderson DC, Manning AM. The temporal profiles of ICAM-1 protein and mRNA expression after transient MCA occlusion in the rat. Brain Res. 1995;682:182–188. doi: 10.1016/0006-8993(95)00346-r. [DOI] [PubMed] [Google Scholar]
- 18.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. II ed. VIII. New York, NY: Academic Press Inc.; 1986. [Google Scholar]
- 19.Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhang RL, Chopp M, Li Y, Zaloga C, Jiang N, Jones ML, Miyasaka M, Ward PA. Anti-ICAM-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurology. 1994;44:1747–1751. doi: 10.1212/wnl.44.9.1747. [DOI] [PubMed] [Google Scholar]
- 21.Hayasaki Y, Nakajima M, Kitano Y, Iwasaki T, Shimamura T, Iwaki K. ICAM-1 expression on cardiac myocytes and aortic endothelial cells via their specific endothelin receptor subtype. Biochem Biophys Res Commun. 1996;229:817–824. doi: 10.1006/bbrc.1996.1886. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Zhang L, Zhang Z, Lu M, Chopp M. Neuroprotective effects of s-0139, an endothelin type a receptor antagonist, on transient ischemic stroke and therapeutic window of rtPA in the rat. Stroke. 2008;39:671. [Google Scholar]
- 23.Zhang RL, Zhang ZG, Chopp M. Increased therapeutic efficacy with rt-PA and anti-cd18 antibody treatment of stroke in the rat. Neurology. 1999;52:273–279. doi: 10.1212/wnl.52.2.273. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Q, Zhang ZG, Zhang L, Ding GL, Li L, Ewing JR, Lu M, Whitton P, Hu J, Li QJ, Zhang RL, Chopp M. MRI evaluation of treatment of embolic stroke in rat with intra-arterial and intravenous rt-PA. J Neurol Sci. 2004;224:57–67. doi: 10.1016/j.jns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Ding G, Jiang Q, Zhang L, Zhang Z, Knight RA, Soltanian-Zadeh H, Lu M, Ewing JR, Li Q, Whitton PA, Chopp M. Multiparametric isodata analysis of embolic stroke and rt-PA intervention in rat. J Neurol Sci. 2004;223:135–143. doi: 10.1016/j.jns.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Zhang RL, Chopp M, Jiang N, Tang WX, Prostak J, Manning AM, Anderson DC. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke. 1995;26:1438–1442. doi: 10.1161/01.str.26.8.1438. discussion 1443. [DOI] [PubMed] [Google Scholar]
- 27.Zhang RL, Zhang ZG, Chopp M, Zivin JA. Thrombolysis with tissue plasminogen activator alters adhesion molecule expression in the ischemic rat brain. Stroke. 1999;30:624–629. doi: 10.1161/01.str.30.3.624. [DOI] [PubMed] [Google Scholar]
- 28.Ishihara H, Connolly AJ, Zeng D, Kahn ML, Zheng YW, Timmons C, Tram T, Coughlin SR. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- 29.Fang M, Kovacs KJ, Fisher LL, Larson AA. Thrombin inhibits NMDA-mediated nociceptive activity in the mouse: Possible mediation by endothelin. J Physiol. 2003;549:903–917. doi: 10.1113/jphysiol.2002.036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 31.Carmichael ST. Rodent models of focal stroke: Size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jesmin S, Maeda S, Mowa CN, Zaedi S, Togashi H, Prodhan SH, Yamaguchi T, Yoshioka M, Sakuma I, Miyauchi T, Kato N. Antagonism of endothelin action normalizes altered levels of VEGF and its signaling in the brain of stroke-prone spontaneously hypertensive rat. Eur J Pharmacol. 2007 doi: 10.1016/j.ejphar.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Lo AC, Chen AY, Hung VK, Yaw LP, Fung MK, Ho MC, Tsang MC, Chung SS, Chung SK. Endothelin-1 overexpression leads to further water accumulation and brain edema after middle cerebral artery occlusion via aquaporin 4 expression in astrocytic end-feet. J Cereb Blood Flow Metab. 2005;25:998–1011. doi: 10.1038/sj.jcbfm.9600108. [DOI] [PubMed] [Google Scholar]