Abstract
Endogenous cardiotonic glycosides bind to the inhibitory binding site of the plasma membrane sodium pump (Na+/K+-ATPase). Plasma levels of endogenous cardiotonic glycosides increase in several disease states, such as essential hypertension and uremia. Low concentrations of ouabain, which do not inhibit Na+/K+-ATPase, induce cell proliferation. The mechanisms of ouabain-mediated response remain unclear. Recently, we demonstrated that in opossum kidney (OK) proximal tubular cells, low concentrations of ouabain induce cell proliferation through phosphorylation of protein kinase B (Akt) in a calcium-dependent manner. In the present study, we identified ERK as an upstream kinase regulating Akt activation in ouabain-stimulated cells. Furthermore, we provide evidence that low concentrations of ouabain stimulate Na+/K+-ATPase-mediated 86Rb uptake in an Akt-, ERK-, and Src kinase-dependent manner. Ouabain-mediated ERK phosphorylation was inhibited by blockade of intracellular calcium release, calcium entry, tyrosine kinases, and phospholipase C. Pharmacological inhibition of phosphoinositide-3 kinase and Akt failed to inhibit ouabain-stimulated ERK phosphorylation. Ouabain-mediated Akt phosphorylation was inhibited by U0126, a MEK/ERK inhibitor, suggesting that ouabain-mediated Akt phosphorylation is dependent on ERK. In an in vitro kinase assay, active recombinant ERK phosphorylated recombinant Akt on Ser473. Moreover, transient transfection with constitutively active MEK1, an upstream regulator of ERK, increased Akt phosphorylation and activation, whereas overexpression of constitutively active Akt failed to stimulate ERK phosphorylation. Ouabain at low concentrations also promoted cell proliferation in an ERK-dependent manner. These findings suggest that ouabain-stimulated ERK phosphorylation is required for Akt phosphorylation on Ser473, cell proliferation, and stimulation of Na+/K+-ATPase-mediated 86Rb uptake in OK cells.
Keywords: opossum kidney cells, sodium/potassium adenosine triphosphatase, extracellular signal-regulated kinase, cell proliferation
Na+/K+-ATPase, or the Na+ pump, is a member of the P-type ATPases. The primary function of Na+/K+-ATPase is to establish and maintain normal Na+ and K+ gradients across the plasma membrane (18). In kidney proximal tubules, the activity of Na+/K+-ATPase localized to the basolateral membrane establishes ion gradients that provide driving force for vectorial transport of various solutes and ions from the tubular lumen to the renal vasculature. Na+/K+-ATPase-mediated regulation of proximal renal tubule sodium reabsorption is a major determinant of total body sodium homeostasis, extracellular fluid volume status, and blood pressure control (46).
Cardiac glycosides such as ouabain are potent inhibitors of Na+/K+-ATPase-mediated ion transport (3). In some cells however, ouabain at low concentrations appears to act like a steroid hormone and not an ion transport inhibitor. Ouabain activates several signaling proteins including phosphoinositide-3 kinase (PI-3K), tyrosine kinases, the Ras-Raf MEK pathway, and protein kinase C (PKC) in cardiac myocytes. This occurs through the activation of epidermal growth factor receptor (EGFR) and appears to cause hypertrophy (23, 24, 27, 30, 31, 43–45). In proximal tubule cells, a 1 nM concentration of ouabain is sufficient to activate extracellular-regulated kinase (ERK) and stimulate cell proliferation (6). Ouabain is also known to trigger calcium oscillations in kidney proximal tubular cells (1). Significantly, plasma levels of endogenous cardiotonic glycosides (ouabain and marinobufagenin), normal products of mammalian adrenal glands, are increased in various disease states such as essential hypertension (29) and chronic renal failure (19), experimental uremia (14), and high salt intake-induced hypertension (28).
We recently demonstrated that 10 nM ouabain induced Akt-Ser473 phosphorylation and promoted cell proliferation (22). However, results from other laboratories point to a role for ERK in ouabain response that leads to cell proliferation (6). Thus several questions remain for better understanding of how these two proliferative pathways regulate cell proliferation. Akt and ERK pathways could independently promote cell proliferation. Alternatively, cross talk or interdependence between ERK and Akt signaling pathways could exist. The goal of the present study was to decipher the relationship, if any, between ERK and Akt pathways from the context of Na+/K+-ATPase-mediated ion transport. Akt is present in the cytosol of unstimulated cells. Phospholipids generated by activation of PI-3K bind to the pleckstrin homology (PH) domain of Akt, recruiting it to the plasma membrane. Once at the plasma membrane, Akt is activated by phosphorylation on two sites, namely, Thr308 and Ser473 (36). Although Thr308 is known to be phosphorylated by 3′-phosphoinositide-dependent kinases-1 (PDK1), the identity of PDK2, which phosphorylates Ser473, has been debated for several years (7). Several candidate kinases have been reported to function as PDK2, including integrin-linked kinase (4, 40), Akt itself (39), MAP kinase-activated protein kinase-2 (MK2; Ref. 35), double-stranded DNA-dependent protein kinase (8, 15), ataxia telangiectasia mutated kinase (42), and, most recently, mammalian target of rapamycin (mTOR; Refs. 17, 38). In this study we present data suggesting that in ouabain-stimulated cells, ERK is required for Akt phosphorylation at Ser473. Consistent with this idea, we have demonstrated that ouabain-stimulated Akt phosphorylation in opossum kidney (OK) proximal tubular cells is dependent on ERK phosphorylation. Interestingly, the response was associated with stimulation of Na+/K+ transport in a manner dependent on Src kinase.
EXPERIMENTAL PROCEDURES
Materials
Ouabain, 8-(diethylamino)octyl-3,4,5-trimethoxybenzoate (TMB-8), and SKF-96365 were purchased from Sigma (St. Louis, MO). Akt inhibitor [1L-6-hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate], genistein, LY-294002, and edelfosine (ET18-OCH3, an inhibitor of phosphatidylinositol-dependent phospholipase C) were purchased from Calbiochem-EMD Biosciences (San Diego, CA). Constitutively active, dominant negative, and wild-type MEK1 and Akt cDNAs in pUSE vector, empty vector (pUSE), recombinant constitutively active and inactive ERK2, Akt1, the NH2-terminal pleckstrin homology domain of Akt (PH domain, amino acids 1–144), control small interference RNA (siRNA) plasmid (pKD, catalog no. 62-001), and siRNA plasmid pKD-MEK1-v2 (catalog no. 62–152) were purchased from Upstate Biotechnologies (Charlottesville, VA). GenePorter transfection reagent was purchased from Genlantis (Gene Therapy Systems, San Diego, CA). Cell proliferation kit and U0126 were purchased from Promega. Antibodies against phospho-ERK1/2, total ERK1 and ERK2, and horseradish peroxidaselinked secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phospho-Ser473-Akt (antibody no. 9271), phospho-Thr308-Akt (antibody no. 9275), phospho-Ser9-glycogen synthase kinase-3β (GSK3β) (antibody no. 9336), phospho-Ser2448-mTOR (antibody no. 2971), total Akt (antibody no. 9272), total GSK (antibody no. 9315), total mTOR (antibody no. 2972), Akt2 (antibody no. 7503), and Akt3 (antibody no. 7506) were purchased from Cell Signaling Technology (Danvers, MA). All other chemicals were purchased from Sigma unless otherwise specified.
Cell culture
Wild-type OK cells were a generous gift from Dr. Steven Scheinman (Health Sciences Center, Syracuse, NY). Cells were maintained in minimal essential medium with Earl’s salts supplemented with 10% fetal calf serum, and 1% penicillin/streptomycin. Cell culture and all other studies were carried out at 37°C in a humidified atmosphere of 95% air-5% CO2. Cells were fed twice a week and split once a week at a 1:4 ratio. All experiments were carried out using cells at 90–95% confluence. Cells grown on six-well culture plates were washed with serum-free medium for 24 h before use.
Western blot analysis
OK cells were treated with 10 nM ouabain for 15 min. The cells were lysed in lysis buffer containing 20 mM Tris, pH 7.4, 150 mM NaCl, 20 mM NaF, 1 mM EGTA, 1 mM EDTA, 5 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 1 µg/ml aprotinin, 1 µg/ml leupeptin, 10 µl/ml phosphatase inhibitor cocktail 1, 0.5% Nonidet P-40, and 1% Triton X-100. The cell lysate was homogenized by passing through a 27.5-gauge needle and centrifuged at 20,000 g at 4°C. The supernatant proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. The nitrocellulose membrane was incubated in 5% nonfat dry milk in 20 mM Tris, 150 mM NaCl, and 0.05% Tween 20 (TTBS) at room temperature for 1 h to inhibit nonspecific binding, followed by overnight incubation at 4°C with anti-phospho-ERK1/2, phospho-Ser473, phospho-Thr308-Akt, phospho- Ser2448-mTOR, or phospho-Ser9-GSK3 antibodies in 5% milk in TTBS. Location of specific antibodies was detected by incubation with peroxidase-labeled secondary antibodies at 1:2,000 dilution in 5% milk in TTBS, followed by development with enhanced chemiluminescence (New England Biolabs). The bands imaged by chemiluminescence were analyzed by densitometry. The films were scanned using a Personal Densitometer SI (Molecular Dynamics).
Transfection of MEK1, Akt, or MEK1 siRNA plasmid cDNA and Akt activity
Constitutively active (CA-MEK), dominant negative (D-MEK), or wild-type MEK1 (WT-MEK1), constitutively active (CA-Akt), dominant negative (D-Akt), or wild-type Akt (WT-Akt) in mammalian expression vector pUSE, or empty pUSE vector was transiently transfected in OK cells using GenePorter transfection reagent according to the manufacturer’s protocol and as described previously (21). Akt activity was determined using Western blot analysis with phospho-Ser9-GSK3 antibodies. Plasmid siRNA for MEK1 (pKD-MEK1) or pKD negative control siRNA plasmid was transfected as described above.
In vitro phosphorylation of recombinant inactive Akt by recombinant active ERK2
In vitro kinase assay for Akt phosphorylation by recombinant active ERK2 was carried at 30°C for 1 h by adding 1 µl of active recombinant ERK2 (0.1 µg/µl) to 29 µl of kinase buffer containing 20 mM HEPES, 10 mM MgCl2, 10 mM MnCl2, 1 mM dithiothreitol, either 1 µM ATP (cold) or 1 µl of [γ-32P]ATP, and 1 µl of recombinant inactive Akt1 (0.1 µg/µl) or the pleckstrin homology (Akt-PH, amino acids 1–144) domain of Akt. Active recombinant Akt or MAP kinase-activated protein kinase-2 (MK2; a kinase known to phosphorylate Akt at Ser473; Ref. 35) was used as positive control to identify the phosphorylated Akt band. The reaction was terminated by the addition of 6 µl of 6× Laemmli buffer. The samples were boiled for 3 min, the products were resolved by 4–12% gradient SDS-PAGE, and Akt phosphorylation was detected using either autoradiography or Western blot analysis with phospho-Ser473-Akt antibodies.
Ouabain-sensitive 86Rb uptake
Ouabain-sensitive 86Rb uptake was measured as described previously (21, 22) as an index of Na+/K+-ATPase-mediated ion transport. OK cells were pretreated with 5 µM monensin for 30 min. The cells were exposed to 10 nM ouabain for 5 min before a trace amount of 86Rb (~1 µCi/ml 86RbCl) was added in DMEM without serum. Uptake was carried out for 10 min such that total ouabain treatment time was 15 min, after which the cells were washed five to six times with ice-cold PBS. One-half of the cells received ouabain (final concentration 1 mM) added 15 min before the start of 86Rb uptake. The cells were lysed overnight in 0.5 N NaOH containing 0.1% Triton X-100 at 37°C. An aliquot (100 µl) of the lysate was used to measure radioactivity. The difference between 86Rb uptake measured in the presence of 1 mM and 10 nM of ouabain was used as a measure of Na+/K+-ATPase-mediated transport activity. Uptake data are expressed as nanomoles of 86Rb accumulated per milligram of protein per minute.
Measurement of cell proliferation
The number of living cells was determined using the CellTiter 96 AQueous One solution reagent (catalog no. G3580; Promega) according to the manufacturer’s recommendations as reported previously (22). Briefly, cells were cultured in a 96-well plate (100 µl of culture medium per well) for 6 h and then exposed to 10 nM or 10 µM ouabain for 24 h in the continued presence or absence of U0126. The growth of cells as reflected by metabolism of the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was determined using CellTiter 96 AQueous One solution reagent. Titer reagent (20 µl) was added directly to culture wells and incubated for 3 h, and the absorbance at 490 nm was measured with a 96-well plate reader to quantify the amount of formazan product. Cells in one lane of the plate (6 wells) were washed twice with 1× PBS and lysed in 0.5% Triton X-100 to determine the amount of protein. The data are expressed as optical density (OD at 490 nm) per milligram of protein.
Protein determination
Protein concentration was determined using a bicinchoninic acid protein kit (Sigma) with BSA as standard.
Statistics
Data are means ± SE. The n values represent the number of separate experiments. Each experiment was done in triplicate. P value was calculated using SigmaStat software utilizing Student’s t-test or one-way ANOVA followed by Bonferroni’s analysis. A P value <0.05 was a priori considered statistically significant.
RESULTS
Effect of ERK on ouabain-mediated Akt phosphorylation
We demonstrated previously that 10 nM ouabain increases Akt phosphorylation (22). To determine whether ERK plays a role in ouabain-mediated Akt phosphorylation, cells were treated for 15 min with 10 nM ouabain in the presence or absence of 10 µM U0126, an inhibitor of upstream activator of ERK (MEK1), or 50 µ MAkt inhibitor, an Akt inhibitor that interacts in a complementary manner with the positively charged pocket formed by β1–β2 and β3–β4 loops of the PH domain of Akt, thereby preventing the binding of inositol 1,4,5-trisphosphate and translocation of Akt to membrane (17). As shown in Fig. 1A, ouabain-mediated Akt phosphorylation at both Ser473 (left) and Thr308 (right) was prevented by inhibition of both Akt and MEK1. Ouabain-mediated Akt phosphorylation resulted in phosphorylation of Akt substrate GSK3 at Ser9, which was also prevented by inhibition of Akt and ERK (Fig. 1B, left). Ouabain also promoted phosphorylation of mTOR at Ser2448 (Fig. 1B, right). However, mTOR phosphorylation was independent of both Akt and ERK. To confirm that inhibition of ERK prevents ouabain-stimulated Akt phosphorylation, cells were transfected with either control siRNA plasmid (pKD-negative control) or pKD-MEK1-siRNA plasmid 48 h before treatment with 10 nM ouabain. As shown in Fig. 1C, transfection with MEK1 siRNA plasmid abolished ouabain-stimulated Akt and ERK phosphorylation. In cells transfected with control siRNA plasmid, ouabain stimulated both Akt and ERK phosphorylation.
Fig. 1.
Effect of ERK on ouabain-mediated Akt phosphorylation. Opossum kidney (OK) cells were treated for 15 min with 10 nM ouabain in the presence or absence of Akt inhibitor or U0126. Cells were lysed, and the cell lysates were subjected to 10% SDSPAGE, transferred, and probed with phospho (p)-Ser473 antibodies (A, top left) or p-Thr308-Akt antibodies (A, top right), p-Ser9-glycogen synthase kinase-3β (GSK3β) antibodies (B, top left), or p-Ser2448-mammalian target of rapamycin (mTOR) antibodies (B, top right). The nitrocellulose membranes were stripped and reprobed for total proteins (A and B, bottom). Representative Western blots are shown. Phosphorylated (phospho) and total band densities were analyzed by densitometry, and the results (means ± SE from 3 independent experiments) are presented as the ratio of phosphorylated to total protein band density. *P < 0.05 by Student’s t-test. C: OK cells were transfected with either negative control small interference RNA (siRNA; pKD-negative control siRNA) or pKD-MEK1 siRNA plasmid. Cells were treated for 15 min with 10 nM ouabain 48 h after transfection, and the lysates were subjected to 10% SDS-PAGE, transferred, and probed with p-Ser473-Akt (top left) or p-ERK1/2 antibodies (top right). The nitrocellulose membranes were stripped and reprobed with total Akt or total ERK antibodies (bottom). Representative Western blots are shown. Phosphorylated and total protein band densities were analyzed by densitometry, and the results (means ± SE from 3 independent experiments) are presented as the ratio of phosphorylated to total protein band density. *P < 0.05 by Student’s t-test.
To confirm the dependence of Akt phosphorylation on ERK, OK cells were transiently transfected with cDNA for constitutively active (CA) or dominant negative (D) MEK1 or Akt. As shown in Fig. 2A, transfection with CA-MEK1 increased ERK phosphorylation. Transfection with D-MEK1, CA-Akt, or D-Akt had no discernible effect on ERK phosphorylation. Akt phosphorylation increased in cells transfected with CA-MEK1 compared with D-MEK1, CA-Akt, D-Akt, or empty vector (pUSE; Fig. 2B). Next we determined whether Akt is activated upon phosphorylation by ERK. Akt activity was assessed by measuring phosphorylation of the Akt substrate GSK3 at Ser9. As shown in Fig. 2C, left, transfection with CA-MEK1 and CA-Akt increased GSK3 phosphorylation compared with untransfected control cells or cells transfected with D-MEK1, D-Akt, or empty vector. To confirm that the effects of transfection with CA-MEK1 or CA-Akt were specific, in a separate experiment cells were transfected with vector (pUSE), WT-MEK1, or WT-Akt. Cells were lysed 24 h after transfection and analyzed using Western blot analysis with phospho-ERK1/2 or phospho-Ser473-Akt antibodies. As shown in Fig. 2C, right, transfection with WT-MEK1 or WT-Akt did not induce phosphorylation of ERK or Akt. To confirm the ability of ERK to directly phosphorylate Akt, an in vitro Akt kinase assay was performed. As shown in Fig. 2D, ERK caused marked increase in Akt phosphorylation as determined by 32P autoradiography (left) and Western blotting using phospho-Ser473-Akt antibodies (right).
Fig. 2.
Effect of constitutively active MEK1 transfection on Akt phosphorylation. OK cells were transiently transfected with catalytically active (CA-MEK1) or dominant negative MEK1 (D-MEK1), CA- or D-Akt, or empty pUSE vector cDNA (V). Cells were lysed 6 h after transfection, and the lysates were subjected to 10% SDS-PAGE, transferred, and probed with p-ERK1/2 antibodies (A, top), p-Ser473-Akt (B, top), or p-Ser9-GSK3β antibodies (C, top left). The nitrocellulose membranes were stripped and reprobed with respective total proteins (A–C, bottom). Representative Western blots are shown. Phosphorylated and total protein band densities were analyzed by densitometry, and the results (means ± SE from 3 independent experiments) are presented as the ratio of phosphorylated to total protein band density. *P< 0.05 by Student’s t-test. Akt-T, transfected Akt; C, control. C, right: OK cells were transiently transfected with vector, wild-type (WT)-Akt, or WT-MEK1 and lysed 24 h after transfection. The lysates were subjected to 10% SDS-PAGE, transferred, and probed with p-Ser473-Akt or p-ERK1/2 antibodies. Representative Western blots from 2 independent experiments are shown. D: recombinant inactive Akt1 was in vitro phosphorylated by catalytically active recombinant ERK2 in the presence of radioactive (left) or nonlabeled ATP (right) as described in EXPERIMENTAL PROCEDURES. A representative autoradiogram of phosphorylated proteins (left) is shown. Solid arrows indicate the phosphorylated Akt band, whereas broken arrow shows autophosphorylation of the respective kinase. The gels were exposed for 1 h to detect autophosphorylation of active recombinant Akt (positive control, last lane). Samples that underwent phosphorylation in presence of nonlabeled ATP were transferred to nitrocellulose membranes and probed with p-Ser473-Akt antibodies. The blots were stripped and probed with total Akt antibodies. The blots were again stripped and reprobed with ERK2 antibodies. A representative Western blot from 2 independent experiments is shown.
To determine whether dependence of Akt on ERK in ouabain-mediated signaling cascade is specific to ouabain, cells were treated for 15 min with 10 µM forskolin (PKA activator) or 1 µM PMA (PKC activator). As shown in Fig. 3, both forskolin and PMA increased ERK phosphorylation. However, forskolin and PMA had no effect on Akt phosphorylation.
Fig. 3.
Effect of forskolin and PMA on Akt and ERK phosphorylation. Cells were treated for 15 min with either 10 µM forskolin or 1 µM PMA. Cell lysates were subjected to 10% SDS-PAGE, transferred, and probed with p-Ser473-Akt or total Akt (top) or p-ERK1/2 or total ERK antibodies (bottom). Representative Western blots from 2 independent experiments are shown.
Effect of ouabain on ERK phosphorylation
OK cells were treated for 15 min with 10 nM ouabain, a concentration of ouabain previously found to phosphorylate Akt and stimulate 86Rb uptake and cell proliferation (22). ERK phosphorylation in ouabain-treated cells was examined using Western blot analysis. As shown in Fig. 4, ouabain markedly increased ERK phosphorylation.
Fig. 4.
Effect of ouabain on ERK phosphorylation. OK cells were treated for 15 min with ouabain. The cell lysates were subjected to 10% SDS-PAGE, transferred, and probed for p-ERK1/2. The nitrocellulose membranes were stripped and reprobed for total ERK. Phosphorylated and total protein band densities were analyzed by densitometry, and the results (means ± SE from 3 independent experiments) are presented as the ratio of phosphorylated to total protein band density. *P < 0.05 by Student’s t-test.
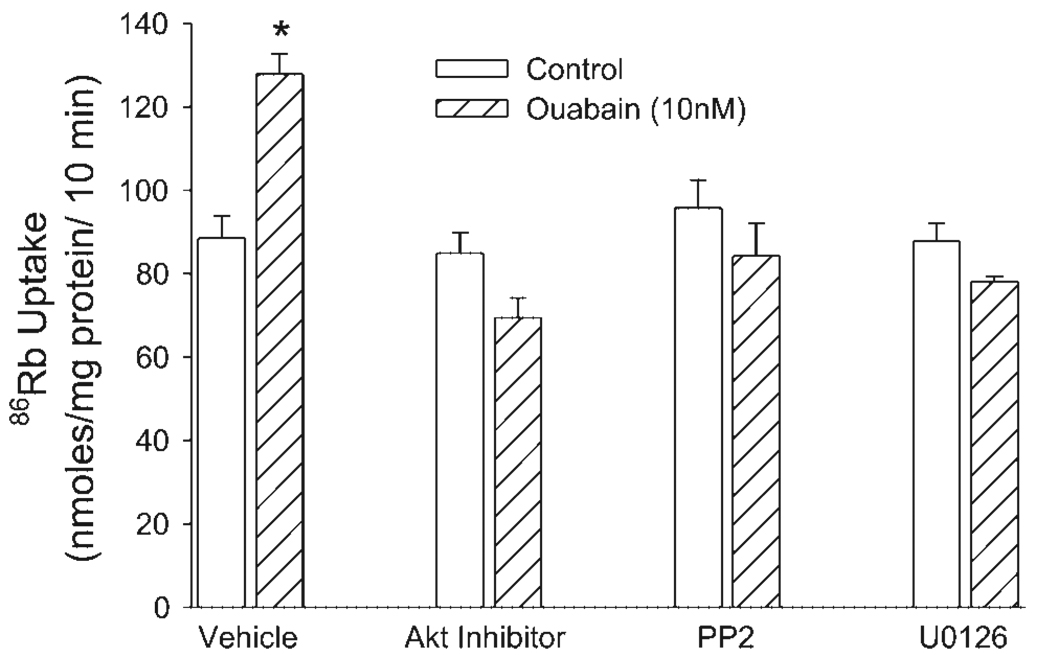
Influence of Akt, ERK, and Src kinase on ouabain-mediated stimulation of 86Rb uptake
We have previously demonstrated that 10 nM ouabain increases 86Rb uptake in OK cells (22). To determine the influence of Akt, ERK, and Src kinase on stimulation of 86Rb uptake, cells were treated for 15 min in the continued presence and absence of 50 µM Akt inhibitor, 10 µM U0126, or 100 nM PP2 (a Src kinase inhibitor). Ouabain-sensitive 86Rb uptake was measured as described in EXPERIMENTAL PROCEDURES. As shown in Fig. 5, 10 nM ouabain increased 86Rb uptake. The ouabain-mediated increase in 86Rb uptake was prevented by inhibitors of Akt, ERK, and Src kinase.
Fig. 5.
Role of Akt, Src kinase, and ERK on ouabain-stimulated 86Rb uptake. Intact cells were pretreated for 30 min with Akt inhibitor, PP2, or U0126, followed by incubation with 10 nM ouabain for 5 min at 25°C. Ouabain-sensitive 86Rb uptake was measured over a 10-min period such that the total time of ouabain treatment was 15 min. Each bar represents mean ± SE (nmol 86Rb uptake ·mg protein−1 ·10 min−1) from 6 independent experiments performed in triplicate. *P < 0.05 by Student’s t-test.
Influence of tyrosine kinase and PI-3K on ouabain-mediated ERK phosphorylation
Askari and colleagues (8) have demonstrated that ouabain at low concentration activates ERK through a mechanism that involves the EGFR and Src tyrosine kinase (43). To examine the role of tyrosine kinase and PI-3K in ouabain-mediated ERK phosphorylation, OK cells were treated for 15 min with 10 nM ouabain in the continued presence or absence of 1 µM genistein (tyrosine kinase inhibitor), 100 nM PP2 (Src kinase inhibitor), 5 µM LY-294002 (PI-3K inhibitor), 50 µM Akt inhibitor, or 10 µM U0126 (MEK-1 inhibitor). As shown in Fig. 6, ouabain-mediated ERK phosphorylation was inhibited by genistein, PP2 (A), and U0126 (B). In contrast, Akt inhibitor and LY-294002 had no effect on ouabain-mediated ERK phosphorylation (Fig. 6B).
Fig. 6.
Role of Akt, phosphoinositide-3 kinase (PI-3K), and Src kinase on ouabain-mediated ERK phosphorylation. OK cells were treated for 15 min with 10 nM ouabain in the presence of PP2 (an inhibitor of Src kinase) or genistein (a tyrosine kinase inhibitor) (A) or Akt inhibitor, LY-294002 (LY; an inhibitor of PI-3K), or U0196 (an inhibitor of MEK1) (B). The cell lysates were subjected to 10% SDS-PAGE, transferred, and probed for p-ERK1/2. The blots were stripped and reprobed for total ERK. Representative Western blots from 3 independent experiments are shown. Phosphorylated and total protein band densities were analyzed by densitometry, and the results (means ± SE from 3 independent experiments) are presented as the ratio of phosphorylated to total protein band density. *P < 0.05 by Student’s t-test. G, genistein; Ou, ouabain.
Role of calcium
We have recently demonstrated that Akt phosphorylation by 10 nM ouabain is dependent on calcium (22). To examine the role of calcium on ouabain-mediated ERK phosphorylation, OK cells were treated for 15 min with 10 nM ouabain in the continued presence or absence of 100 µM TMB-8 (an inhibitor of calcium store release). Ouabain-mediated ERK phosphorylation was suppressed by TMB-8 (Fig. 7A). To determine whether extracellular calcium plays a role in ouabain-mediated ERK phosphorylation, cells were treated for 15 min with 10 nM ouabain in the presence or absence of 1 mM EGTA. As shown in Fig. 7B, EGTA prevented ouabain-mediated ERK phosphorylation. To examine the requirement for calcium entry via store-operated calcium channels, cells were exposed for 15 min to 10 nM ouabain in the presence or absence of 25 µM SKF-96365. SKF-96365 prevented ouabain-mediated ERK phosphorylation (Fig. 7B). To further confirm the requirement for intracellular calcium in ERK phosphorylation, cells were treated for 15 min with 10 nM ouabain in the presence of BAPTA-AM (20 µM). BAPTA-AM also prevented ouabain-mediated ERK phosphorylation (Fig. 7B).
Fig. 7.
Role of calcium on ouabain-mediated ERK phosphorylation. OK cells were treated for 15 min with 10 nM ouabain in the presence of 8-(diethylamino)-octyl-3,4,5-trimethoxybenzoate (TMB-8; A) or EGTA, BAPTA-AM, or SKF- 96365 (B). The cell lysates were subjected to 10% SDS-PAGE, transferred, and probed for p-ERK1/2. The blots were stripped and reprobed for total ERK. Representative Western blots from 3 independent experiments are shown. Phosphorylated and total protein band densities were analyzed by densitometry, and the results (means ± SE from 3 independent experiments) are presented as the ratio of phosphorylated to total protein band density. *P < 0.05 by Student’s t-test. B, BAPTA; SKF, SKF-96365; E, EGTA.
Role of phospholipase C
To determine whether phospholipase C (PLC) plays a role in ouabain-mediated ERK phosphorylation, cells were treated for 15 min with 10 nM ouabain in the continued presence or absence of 30 µM edelfosine, an inhibitor of phosphatidylinositol-dependent PLC. As shown in Fig. 8, edelfosine suppressed ouabain-mediated ERK phosphorylation. The data suggest that ERK phosphorylation by ouabain is dependent on PLC.
Fig. 8.
Role of PLC on ouabain-mediated ERK phosphorylation. OK cells were treated for 15 min with 10 nM ouabain in presence of edelfosine (a PLC inhibitor). The cell lysates were subjected to 10% SDS-PAGE, transferred, and probed for p-ERK1/2. The blots were stripped and reprobed for total ERK. A representative Western blot from three independent experiments is shown. Phosphorylated and total protein band densities were analyzed by densitometry, and the results (means ± SE from 3 independent experiments) are presented as the ratio of phospho- to total protein band density. *P < 0.05 by Student’s t-test.
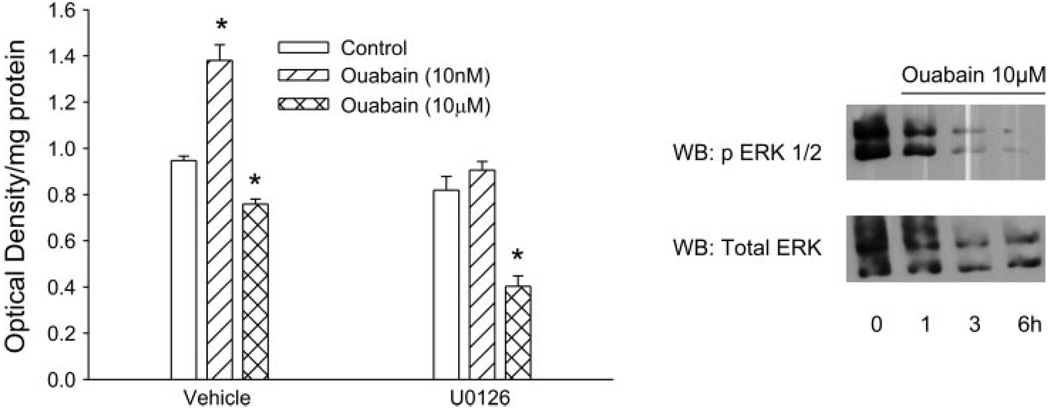
Effect of ERK on ouabain-stimulated cell proliferation
We and others have shown that ouabain at low concentration increases the rate of cell proliferation in an Akt-dependent (22) and calcium-dependent manner (22, 25). To examine the influence of ERK on ouabain-induced cell proliferation, cells were treated with 10 nM or 10 µM ouabain for 24 h in the presence or absence of U0126. As shown in Fig. 9, 10 nM ouabain increased proliferation by ~30%. U0126 abolished the increase in proliferation. It is noteworthy that treatment with 10 µM ouabain decreased cell number, an effect that was enhanced by treatment with U0126 together with 10 µM ouabain. Added alone, U0126 did not significantly alter cell proliferation. At a 10 µM concentration, ouabain decreased ERK phosphorylation (Fig. 9, right). This effect appears to be due to decreased ERK expression.
Fig. 9.
Effect of ERK on ouabain-stimulated cell proliferation. OK cells were treated with 10 nM or 10 µM ouabain after the cells were attached to the 96-well plate for 24 h. Cell proliferation was measured as described in EXPERIMENTAL PROCEDURES. Each data point represents the mean ± SE from 24 independent experiments. *P < 0.05 compared with control by 1-way ANOVA followed by Bonferroni’s analysis. Some cells were lysed after 1, 3, or 6 h after ouabain (10 µM) treatment. The cell lysates were subjected to 10% SDS-PAGE, transferred, and probed for p-ERK1/2. The blots were stripped and reprobed for total ERK. A representative Western blot from 2 independent experiments is shown.
DISCUSSION
Previously, we identified a role for Akt phosphorylation in cell proliferation response to nanomolar concentrations of ouabain (22). Studies by Askari and coworkers (23, 26, 27, 30, 31, 43–45) suggest that Na+/K+-ATPase in cardiac myocytes acts as a signal transducer in the sense that interaction of Na+/K+-ATPase with extracellular ouabain leads to activation of the ERK/MAP kinase pathway. In the present study we report evidence that ouabain causes phosphorylation of Akt through an ERK-dependent pathway. Akt phosphorylation in ouabain-treated cells was prevented by inhibition of ERK. Transient transfection of constitutively active MEK1 (an upstream regulator of ERK) mimicked the action of ouabain on Akt phosphorylation. Furthermore, we confirmed that ERK-mediated phosphorylation increases Akt activity as evidenced by increased phosphorylation of an Akt substrate, GSK3. A 10 nM concentration of ouabain also stimulated OK cell growth. Importantly, inhibition of upstream activator of ERK (MEK) by U0126 abolished the effect of 10 nM ouabain on cell proliferation. An in vitro kinase assay with active recombinant ERK and recombinant Akt demonstrated the ability of ERK to directly phosphorylate Akt on Ser473. Western blot analysis suggests that OK cells express Akt1 and Akt2 but not Akt3 (data not shown). Whether ERK phosphorylates Akt1 or Akt2 cannot be determined from the data presented, because the phospho-Akt Ser473 antibodies we used were not isoform specific. This effect of ERK on Akt phosphorylation appears to be specific for ouabain-induced signaling cascade, since both forskolin and PMA stimulated ERK phosphorylation without any effect on Akt phosphorylation. The results presented do not allow us to speculate on the mechanism of forskolin- or PMA-induced ERK phosphorylation.
Several kinases have been proposed as a hydrophobic motif kinase that phosphorylates Akt on Ser473. These include integrin-linked kinase (4, 40), PKCα (33), PKCβII (20), double-stranded DNA-dependent protein kinase (8, 15), ataxia telangiectasia mutated kinase (41), and mTOR (16, 37). Akt has also been shown to autophosphorylate at Ser473 (39). The data presented suggest that in the ouabain-stimulated signaling cascade, ERK is required for Akt phosphorylation at Ser473. In this study, using RNA interference-mediated knockdown and pharmacological inhibitors, we have shown that inhibition of ERK phosphorylation prevents ouabain-mediated Akt phosphorylation at Ser473. Furthermore, we have demonstrated that overexpression of catalytically active MEK1, an upstream activator of ERK1/2, induces Akt phosphorylation. Our results are in agreement with studies by Delehedde et al. (5), who showed that hepatocyte growth factor/scatter factor (HGF/SF) activates Akt phosphorylation in an ERK-dependent manner, leading to cell migration in rat mammary fibroblasts. They demonstrated that HGF/SF-mediated Akt phosphorylation was prevented by PD-98059. Kotova et al. (24) demonstrated that in human skeletal muscle cells, ouabain induces glycogen synthesis through a pathway involving Src kinase, ERK1/2, and p90rsk1. They further showed that activation of ERK1/2 leads to activation of p90rsk1. The data presented do not allow us to speculate whether ERK directly or indirectly through p90rsk1 phosphorylates Akt at Ser473 in the ouabain-stimulated signaling cascade. However, studies on the in vitro kinase reaction suggest the ability of ERK to directly phosphorylate Akt. The data presented also rule out the dependence of Akt phosphorylation at Ser473 on mTOR in cells stimulated with ouabain, since both U0126 and Akt inhibitor had no effect on ouabain-induced mTOR phosphorylation.
We and others have demonstrated that low doses of ouabain increase Na+/K+-ATPase-mediated 86Rb uptake (9–11, 22). However, the mechanism underlying stimulation of 86Rb uptake is not well understood. Our data suggest that the observed 86Rb uptake stimulation is dependent on a signaling cascade initiated by 10 nM ouabain. The response was prevented by inhibitors of Akt, ERK, and Src kinase. This fits in with a report by Al-Khalili et al. (2), who showed that ERK, Akt, and tyrosine kinases contribute to insulin-dependent increase of 86Rb uptake in human skeletal muscle cells. In that study, the stimulation of 86Rb uptake was due to increased expression of Na+/K+-ATPase protein in the plasma membrane. Kotova et al. (24) recently demonstrated that cardiotonic glycosides increase glycogen synthesis through an ERK- and Src-dependent pathway in human skeletal muscle cells. Oweis et al. (32) demonstrated that ouabain at a concentration of 100 nM inhibits Na+/K+ exchanger (NHE3) activity, reduces NHE3 mRNA, and suppresses NHE3 promoter activity in LLC-PK1 cells. Furthermore, they showed that the decrease in NHE3 activity was mediated through Src kinase and tyrosine kinase-dependent mechanisms. However, the authors did not measure Na+/K+-ATPase activity at a 100 nM concentration. Whether ouabain-stimulated increase in 86Rb uptake in our study is due to an increase in phosphorylation of Na+/K+-ATPase α-subunit, increased abundance of active Na+/K+-ATPase molecules at the plasma membrane, or indirectly due to stimulation of NHE3 activity remains to be determined.
Several investigators have demonstrated that ouabain mediates ERK phosphorylation through Src kinase and PLC activation (38, 45). Importantly, it has been demonstrated that Src kinase associates with Na+/K+-ATPase when LLC-PK1 cells are exposed to low concentrations of ouabain (38). We have recently demonstrated that Akt phosphorylation is dependent on PLC (22). Based on the observed ability of PP2 (Src kinase inhibitor), genistein (tyrosine kinase inhibitor), and edelfosine (PLC inhibitor) to suppress ERK phosphorylation by ouabain, the results from the present study suggest that ERK phosphorylation in OK cells is dependent on both Src kinase and PLC. Studies from the laboratory of Askari and colleagues (23, 30, 31) suggest that ERK phosphorylation by ouabain may occur through two independent pathways. one through Src-mediated activation of Ras-Raf MEK pathway and the other through PLC-dependent, PKC-mediated direct phosphorylation of ERK. However, from the data presented in this report, we cannot determine whether Src and PLC are linked or are two independent pathways.
Unlike Akt phosphorylation, ouabain-mediated ERK phosphorylation was not prevented by inhibitors of PI-3K, suggesting that ERK phosphorylation is independent of PI-3K. This observation may be explained by the fact that Akt requires binding of phosphoinositides to the NH2-terminal PH domain for its translocation to the membrane, where Akt then undergoes phosphorylation at Thr308 and Ser473 to become fully active (36). Generation of phosphoinositides by PI-3K is thus an important step in regulation of Akt activity. In contrast, ERK phosphorylation may not require PI-3K as was suggested by Kotova et al. (24).
Ouabain is known to alter cytoplasmic calcium dynamics even at ouabain concentrations too low to inhibit Na+/K+-ATPase pump activity. For example, Aizman et al. (1) demonstrated that ouabain elicits calcium oscillations in proximal tubule cells. Findings in the present study suggest that phosphorylation of ERK is dependent on both extracellular and intracellular calcium. The ouabain-mediated ERK phosphorylation was suppressed under conditions where the concentration of extracellular calcium was reduced and by TMB-8, a nonspecific intracellular calcium inhibitor that can prevent release of calcium from cytoplasmic stores. Chelation of intracellular calcium by BAPTA-AM prevented ouabain-mediated ERK phosphorylation, confirming the requirement of intracellular calcium in the ouabain-initiated signaling cascade. Importantly, the ERK response was sensitive to SKF-96365, an inhibitor of calcium entry via store-operated calcium channels. In the presence of SKF-96365, ouabain did not phosphorylate ERK. It is noteworthy that ouabain is reported to elicit activation of store-operated calcium channels in astrocytes (13), and there is evidence pointing toward disturbance of calcium dynamics following interaction of ouabain with the α2-isoform of Na+/K+-ATPase (12, 13).
The role of ouabain in cell proliferation, growth, and apoptosis has been difficult to define. Some studies suggest that ouabain causes cell proliferation and hypertrophy (6, 30), whereas others have shown that ouabain treatment leads to apoptosis (34, 42). Different response patterns may depend on the concentration of ouabain. We and others have shown that 10 nM ouabain significantly stimulates cell growth. The effect of ouabain on cell proliferation can be prevented by inhibition of calcium mobilization (22, 25) or by Akt inhibition (22). In the present study we confirmed that the effect of ouabain on cell proliferation requires ERK phosphorylation and does not require inhibition of Na+/K+-ATPase-mediated ion transport. Indeed, a reduction in cell number and decreased ERK expression was seen following exposure of OK cells to ouabain at a concentration of 10 µM, which is sufficient to cause ~40% inhibition in Na+/K+-ATPase-mediated ion transport.
In summary, the findings demonstrate that ERK phosphorylation by ouabain is required for Akt phosphorylation and cell proliferation in OK cells. The findings point to a link between Akt and ERK phosphorylation and the effect of 10 nM ouabain on Na+/K+-ATPase-mediated 86Rb uptake and cell proliferation. The findings support the notion that ouabain is able to change the pattern of cell growth at concentrations significantly lower than the concentration required to inhibit Na+/K+-ATPase-mediated ion transport.
ACKNOWLEDGMENTS
We thank Dr. Eleanor Lederer, Professor of Medicine, University of Louisville, for critical reading of the manuscript. We also thank Nina Lesousky and Sudarshana Sengupta for expert technical assistance.
GRANTS
The work was supported by a Fellowship Grant from The American Heart Association, Ohio Valley Affiliate (to S. J. Khundmiri) and a Scientist Development Grant (to S. J. Khundmiri and M. J. Rane), National Institutes of Health Grants EY040414 (to N. A. Delamere) and R56 AI059165 (to M. J. Rane), and a Project Grant from the J. Graham Brown Cancer Center, University of Louisville (to N. A. Delamere).
REFERENCES
- 1.Aizman O, Uhlen P, Lal M, Brismar H, Aperia A. Ouabain, a steroid hormone that signals with slow calcium oscillations. Proc Natl Acad Sci USA. 2001;98:13420–13424. doi: 10.1073/pnas.221315298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Khalili L, Kotova O, Tsuchida H, Ehren I, Feraille E, Krook A, Chibalin AV. ERK1/2 mediates insulin stimulation of Na+, K+-ATPase by phosphorylation of the α-subunit in human skeletal muscles. J Biol Chem. 2004;279:25211–25218. doi: 10.1074/jbc.M402152200. [DOI] [PubMed] [Google Scholar]
- 3.Askari A, Kakar SS, Huang W. Ligand binding sites of the ouabain-complexed (Na+ + K+)-ATPase. J Biol Chem. 1988;263:235–242. [PubMed] [Google Scholar]
- 4.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/Akt by integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delehedde M, Sergeant N, Lyon M, Rudland PS, Fernig DG. Hepatocyte growth factor/scatter factor stimulates migration of rat mammary fibroblasts through both mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt pathways. Eur J Biochem. 2001;269:4423–4429. doi: 10.1046/j.1432-1327.2001.02363.x. [DOI] [PubMed] [Google Scholar]
- 6.Dmitrieva RI, Doris PA. Ouabain is a potent promoter of growth and activator of ERK1/2 in ouabain-resistant rat renal epithelial cells. J Biol Chem. 2003;278:28160–28166. doi: 10.1074/jbc.M303768200. [DOI] [PubMed] [Google Scholar]
- 7.Dong LQ, Liu F. PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am J Physiol Endocrinol Metab. 2005;289:E187–E196. doi: 10.1152/ajpendo.00011.2005. [DOI] [PubMed] [Google Scholar]
- 8.Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004;279:41189–41196. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- 9.Ferrai P, Ferrandi M, Valentini G, Bianchi G. Rostafuroxin: an ouabain antagonist that corrects renal and vascular Na+-K+-ATPase alterations in ouabain and adducin-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290:R529–R539. doi: 10.1152/ajpregu.00518.2005. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari P, Torielli L, Ferrandi M, Padoani G, Duzzi L, Florio M, Conti F, Melloni P, Vesci L, Corsico N, Bianchi G. PST2238: a new antihypertensive compound that antagonizes the long-term pressor effect of ouabain. J Pharmacol Exp Ther. 1998;285:83–94. [PubMed] [Google Scholar]
- 11.Gao J, Wymore RS, Wang Y, Gaudette GR, Krukenkamp IB, Cohen IS, Mathias RT. Isoform-specific stimulation of cardiac Na/K pumps by nanomolar concentrations of glycosides. J Gen Physiol. 2002;119:297–312. doi: 10.1085/jgp.20028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golovina VA, Song H, James PF, Lingrel JB, Blaustein MP. Na+ pump α-2 subunit expression modulates Ca2+ signaling. Am J Physiol Cell Physiol. 2003;284:C475–C486. doi: 10.1152/ajpcell.00383.2002. [DOI] [PubMed] [Google Scholar]
- 13.Hartford AK, Messer ML, Moseley AE, Lingrel JB, Delamere NA. Na-K ATPase α2 inhibition alters calcium responses in optic nerve astrocytes. Glia. 2004;45:229–237. doi: 10.1002/glia.10328. [DOI] [PubMed] [Google Scholar]
- 14.Harwood S, Mullen AM, McMohan AC, Dawnay A. Plasma OLC is elevated in mild experimental uremia but is not associated with hypertension. Am J Hypertens. 2001;14:1112–1115. doi: 10.1016/s0895-7061(01)02219-1. [DOI] [PubMed] [Google Scholar]
- 15.Hill MM, Feng J, Hemmings BA. Identification of a plasma membrane Raft-associated PKB Ser473 kinase activity that is distinct from ILK and PDK1. Curr Biol. 2002;12:1251–1255. doi: 10.1016/s0960-9822(02)00973-9. [DOI] [PubMed] [Google Scholar]
- 16.Hresko RC, Mueckler mTOR-rictor is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Qiao L, Wang S, Rong S, Meuillet EJ, Berggren M, Gallegos A, Powis G, Kozikowski AP. 3-(Hydroxymethyl)-bearing phosphatidylinositol ether lipid analogues and carbonate surrogates block PI3-K, Akt, and cancer cell growth. J Med Chem. 2000;43:3045–3051. doi: 10.1021/jm000117y. [DOI] [PubMed] [Google Scholar]
- 18.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 2003;228:134–142. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 19.Kariya K, Sano H, Yamanishi J, Saito K, Furuta Y, Fukuzaki H. A circulating Na+-K+ ATPase inhibitor, erythrocyte sodium transport and hypertension in patients with chronic renal failure. Clin Exp Hypertens A. 1986;8:167–183. doi: 10.3109/10641968609074770. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami Y, Nishimoto H, Kitaura J, Maeda-Yamamoto M, Kato RM, Littman DR, Rawlings DJ, Kawakami T. Protein kinase CβII regulates Akt phosphorylation on Ser-473 in a cell type- and stimulus-specific fashion. J Biol Chem. 2004;279:47720–47725. doi: 10.1074/jbc.M408797200. [DOI] [PubMed] [Google Scholar]
- 21.Khundmiri SJ, Dean WL, McLeish KR, Lederer ED. Parathyroid hormone-mediated regulation of Na+-K+-ATPase requires ERK-dependent translocation of protein kinase Cα. J Biol Chem. 2005;280:8705–8713. doi: 10.1074/jbc.M408606200. [DOI] [PubMed] [Google Scholar]
- 22.Khundmiri SJ, Metzler MA, Ameen M, Amin V, Rane MJ, Delamere NA. Ouabain induces cell proliferation through calcium-dependent phosphorylation of Akt (protein kinase B) in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol. 2006;291:C1247–C1257. doi: 10.1152/ajpcell.00593.2005. [DOI] [PubMed] [Google Scholar]
- 23.Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie Z. Multiple signal transduction pathways link Na+/K+ -ATPase to growth-related genes in cardiac myocytes: the role of Ras and mitogen-activated protein kinases. J Biol Chem. 1998;273:15249–15256. doi: 10.1074/jbc.273.24.15249. [DOI] [PubMed] [Google Scholar]
- 24.Kotova O, Al-Khalili A, Talia S, Hooke C, Fedorova OV, Bagrov AY, Chibalin AV. Cardiotonic steroids stimulate glycogen synthesis in human skeletal muscle cells via Src- and ERK1/2-dependent mechanism. J Biol Chem. 2006;281:20085–20094. doi: 10.1074/jbc.M601577200. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Zelenin S, Aperia A, Aizman O. Low doses of ouabain protect from serum deprivation-triggered apoptosis and stimulate kidney cell proliferation via activation of NF-κB. J Am Soc Nephrol. 2006;17:1848–1857. doi: 10.1681/ASN.2005080894. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Tian J, Haas M, Shapiro JI, Askari A, Xie Z. Ouabain interaction with cardiac Na+/K+ -ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem. 2000;275:27838–27844. doi: 10.1074/jbc.M002950200. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Mohammadi K, Aynafshar B, Wang H, Li D, Liu J, Ivanov AV, Xie Z, Askari A. Role of caveolae in signal-transducing function of cardiac Na+/K+ -ATPase. Am J Physiol Cell Physiol. 2003;284:C1550–C1560. doi: 10.1152/ajpcell.00555.2002. [DOI] [PubMed] [Google Scholar]
- 28.Manunta P, Hamilton BP, Hamlyn JM. Salt intake and depletion increase circulating levels of endogenous ouabain in normal men. Am J Physiol Regul Integr Comp Physiol. 2006;290:R553–R559. doi: 10.1152/ajpregu.00648.2005. [DOI] [PubMed] [Google Scholar]
- 29.Manunta P, Messaggio E, Ballabeni C, Sciarrone MT, Lanzani C, Ferrandi M, Hamlyn JM, Cusi D, Galletti F, Bianchi G. Salt Sensitivity Study Group of the Italian Society of Hypertension. Plasma ouabain-like factor during acute and chronic changes in sodium balance in essential hypertension. Hypertension. 2001;38:198–203. doi: 10.1161/01.hyp.38.2.198. [DOI] [PubMed] [Google Scholar]
- 30.Mohammadi K, Kometiani P, Xie Z, Askari A. Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem. 2001;276:42050–42056. doi: 10.1074/jbc.M107892200. [DOI] [PubMed] [Google Scholar]
- 31.Mohammadi K, Liu L, Tian J, Kometiani P, Xie Z, Askari A. Positive inotropic effect of ouabain on isolated heart is accompanied by activation of signal pathways that link Na+/K+-ATPase to ERK1/2. J Cardiovasc Pharmacol. 2003;41:609–614. doi: 10.1097/00005344-200304000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Oweis S, Wu L, Kiela PR, Zhao H, Malhotra D, Ghishan FK, Xie Z, Shapiro JI, Liu J. Cardiac glycoside downregulates NHE3 activity and expression in LLC-PK1 cells. Am J Physiol Renal Physiol. 2006;290:F997–F1008. doi: 10.1152/ajprenal.00322.2005. [DOI] [PubMed] [Google Scholar]
- 33.Partovian C, Simons M. Regulation of protein kinase B/Akt activity and Ser473 phosphorylation by protein kinase Cα in endothelial cells. Cell Signal. 2004;16:951–957. doi: 10.1016/j.cellsig.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Pchejetski D, Taurin S, Sarkissian SD, Lopina OD, Pshezhestsky AV, Tremblay J, deBlois D, Hamet P, Orlov SN. Inhibition of Na+, K+-ATPase by ouabain triggers epithelial cell death independently of inversion of [Na+]i/[K+]i ratio. Biochem Biophys Res Commun. 2003;301:735–744. doi: 10.1016/s0006-291x(02)03002-4. [DOI] [PubMed] [Google Scholar]
- 35.Rane MJ, Coxon PY, Powell DW, Webster R, Klein JB, Pierce W, Ping P, McLeish KR. p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J Biol Chem. 2001;276:3517–3523. doi: 10.1074/jbc.M005953200. [DOI] [PubMed] [Google Scholar]
- 36.Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem. 2003;278:27828–27835. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
- 37.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 38.Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell. 2006;17:317–326. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toker A, Newton AC. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J Biol Chem. 2000;275:8271–8274. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- 40.Troussard AA, Mawji NM, Ong C, Mui A, St-Arnaud R, Dedhar S. Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J Biol Chem. 2003;278:22374–22378. doi: 10.1074/jbc.M303083200. [DOI] [PubMed] [Google Scholar]
- 41.Viniegra JG, Martinez N, Modirassari P, Hernandez Losa J, Parada Cobo C, Sanchez-Arevalo Lobo VJ, Avceves-Luquero CI, Alavrez-Vallina L, Ramon YCS, Rojas JM, Sanchez-Prieto R. Full activation of PKB/Akt in response to insulin or ionizing radiation is mediated through ATM. J Biol Chem. 2005;280:4029–4036. doi: 10.1074/jbc.M410344200. [DOI] [PubMed] [Google Scholar]
- 42.Xiao AY, Wei L, Xia S, Rothman S, Yu SP. Ionic mechanism of ouabain-induced concurrent apoptosis and necrosis in individual cultured cortical neurons. J Neurosci. 2002;22:1350–1362. doi: 10.1523/JNEUROSCI.22-04-01350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie Z, Askari A. Na+/K+-ATPase as a signal transducer. Eur J Biochem. 2002;269:2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- 44.Xie Z, Cai T. Na+-K+-ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003;3:157–168. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- 45.Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell. 2005;16:4034–4045. doi: 10.1091/mbc.E05-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Norian JM, Magyar CE, Holstein-Rathlou NH, Mircheff AK, McDonough AA. In vivo PTH provokes apical NHE3 and NaPi2 redistribution and Na-K-ATPase inhibition. Am J Physiol Renal Physiol. 1999;276:F711–F719. doi: 10.1152/ajprenal.1999.276.5.F711. [DOI] [PubMed] [Google Scholar]