Short abstract
A new classification system for adverse drug reactions based on time course and susceptibility as well as dose responsiveness should improve drug development and management of adverse reactions
The pharmacological classification of adverse drug reactions whose causality has been established currently rests on the perceived dose dependence and predictability of the adverse reaction. It is based on a proposal of Rawlins and Thompson, prefigured by others (see table A on bmj.com), to classify adverse drug reactions into two types1: type A reactions, dose dependent and predictable from the known pharmacology of the drug; and type B reactions, not dose dependent and unpredictable.2 This classification is simple; it helps drug regulation because prelicensing studies can reveal type A reactions,3 and it predicts that dose titration will reduce the risk of some reactions. However, it is sometimes difficult or impossible to assign a reaction to one type. For example, dose dependent (type A) nausea and vomiting due to erythromycin could also be classified as type B because it is not pharmacologically predictable.
Furthermore, other types of adverse reactions are not comfortably classified by the system. For example, osteoporosis from corticosteroids depends not only on dose but also on duration of treatment. And some reactions, such as asthma from β adrenoceptor antagonists, do not occur in all patients. The classification has gradually been extended to other alphabetically labelled types (see table A on bmj.com), including type C (dose and time dependent (chronic) reactions), type D (delayed reactions), type E (withdrawal reactions), and type F (failure of therapy).4 These modifications have mitigated some of the difficulties of the classification system but have introduced others.
The current classification is defined only by properties of the drug—its known pharmacology and the dose dependence of its effects. However, other criteria should be taken into account in a comprehensive classification, including properties of the reaction (the time course of its appearance and its severity) and properties of the individual (the genetic, pathological, and other biological differences that confer susceptibility). We therefore propose a three dimensional classification system based on dose relatedness, timing, and patient susceptibility (DoTS).
Figure 1.
Credit: LIANNE PAYNE
Dose relatedness
Traditionally, immunological and certain other adverse drug reactions have been considered not to be dose related. However, effects of drugs involve interactions between chemical entities and are therefore subject to the law of mass action. This implies that all drug effects, beneficial or adverse, are dose related. Examples of immunological reactions that are clearly dose dependent include hay fever in response to high pollen counts5; the immunogenic response to hepatitis B vaccine6; desensitisation by the use of increasing doses of antigen (for example, cephalosporins)7; and type IV hypersensitivity skin reactions.8
It is therefore misleading to suggest that type B adverse drug reactions are not dose dependent.2 In fact, it is clearer to divide adverse drug reactions into reactions that occur at supratherapeutic doses (toxic effects); reactions that occur at standard therapeutic doses (collateral effects); and reactions that occur at subtherapeutic doses in susceptible patients (hypersusceptibility reactions). We use the term collateral effects for reactions that occur at standard therapeutic doses because the term side effects is often colloquially used to refer to all adverse effects. Collateral effects include those that occur due to a different pharmacological effect from the therapeutic action and those that occur through the therapeutic pharmacological effect but in another tissue.
Time relatedness
Many pharmacological effects depend on both the concentration of the drug at the site of action and the time course of its appearance there. For example, a given dose of furosemide (frusemide) induces a greater diuresis when it is infused than when it is given as a bolus.9 And the toxicity of methotrexate is greater when a low dose is given repeatedly than when the same total amount is given as a single dose.10
We distinguish two patterns of time courses of adverse drug reactions, time dependent and time independent (see table B on bmj.com for details of the classification and its implications).
Time independent reactions
Time independent reactions occur at any time during treatment, independent of the duration of the course. They typically occur either when the concentration ofthe drug at the site of action changes (for example, digoxin toxicity when renal function worsens) or when the pharmacological response is altered without a change in concentration (for example, digoxin toxicity in association with potassium depletion). When such a reaction occurs, its time course may be affected by the kinetics of the drug, but that is not an aspect of its time dependency as defined here.
Time dependent reactions
There are six subtypes of time dependent reactions—rapid, first dose, early, intermediate, late, and delayed.
Rapid reactions occur only when a drug is administered too rapidly—for example, the red man syndrome with vancomycin.11
First dose reactions occur after the first dose of a course of treatment and not necessarily thereafter. Examples include hypotension after the first dose of an angiotensin converting enzyme inhibitor12 and type I hypersensitivity reactions. In type I hypersensitivity reactions the reaction occurs after the first dose of a course, whether or not previous exposure has been recorded; 30% of those who develop anaphylaxis with penicillin have no such record.13 We regard a previous sensitising exposure as causing a change in susceptibility.
Early reactions occur early in treatment then abate with continuing treatment. These are adverse drug reactions to which patients develop tolerance (such as nitrate induced headache).
Intermediate reactions occur after some delay; however, if a reaction has not occurred after a certain time, there is little or no risk that it will occur later. Examples are hypersensitivity reactions of type II (thrombocytopenia due to quinine), type III (interstitial nephritis with penicillins), and type IV (cutaneous hypersensitivity to antihistamines), and the ampicillin/amoxicillin pseudoallergic rash.14 Non-allergic reactions of this type include the increased risk of neutropenia with carbimazole and of venous thromboembolism with antipsychotic drugs. We believe that intermediate reactions occur in populations of individuals with different susceptibilities. Those at high risk have the reaction and stop taking the drug; those at low risk do not have the reaction and can be regarded as healthy survivors. Thus, after a time the population risk seems to fall.
Late reactions occur rarely or not at all at the beginning of treatment, but the risk increases with continued or repeated exposure. Examples include many of the adverse effects of corticosteroids and tardive dyskinesia with dopamine receptor antagonists. Withdrawal reactions are late reactions that occur when a drug is withdrawn or its dose is reduced after prolonged treatment. They include opiate and benzodiazepine withdrawal syndromes, hypertension after withdrawal of clonidine or methyldopa, and acute myocardial infarction after withdrawal of β blockers.
Delayed reactions are observed some time after exposure, even if the drug is withdrawn before the reaction appears. Examples are carcinogenesis (vaginal adenocarcinoma in women who were exposed to diethylstilbestrol in utero) and teratogenesis (phocomelia due to thalidomide).
Susceptibility
The risk of an adverse drug reaction differs among members of an exposed population. In some cases the risk of an adverse reaction will be present in susceptible subjects and absent in others. In other cases susceptibility follows a continuous distribution—for example, increasing susceptibility with increasing impairment of renal function. Although reasons for hypersusceptibility may be unknown, several types are recognised. These include genetic variation, age, sex, physiological variation, exogenous factors, and disease (table 1). More than one susceptibility factor can be present.
Table 1.
Sources of altered susceptibility to adverse drug reactions
| Source of susceptibility | Examples | Implications |
|---|---|---|
| Genetic | Porphyria | Screen for abnormalities; avoid specific drugs |
| Succinylcholine sensitivity
|
||
| Malignant hyperthermia
|
||
|
|
CYP isozyme polymorphisms
|
|
| Age | Neonates (chloramphenicol15) | Adjust doses according to age |
|
|
Elderly people (hypnotics16)
|
|
| Sex | Alcohol intoxication | Use different doses in men and women |
| Mefloquine, neuropsychiatric effects17
|
||
| Angiotensin converting enzyme inhibitors, cough
|
||
|
|
Lupus-like syndrome18
|
|
| Physiology altered | Phenytoin in pregnancy19 | Alter dose or avoid |
| Exogenous factors | Drug interactions | Alter dose or avoid co-administration |
|
|
Interactions with food (eg grapefruit juice with drugs cleared by CYP3A420)
|
|
| Disease | Renal insufficiency (eg lithium21) | Screen for abnormalities; avoid specific drugs; use reduced doses |
| Hepatic cirrhosis (eg morphine22) |
Using DoTS
To show how the categorical form of the classification works, the box gives examples of three adverse drug reactions that readers of draft versions of this paper have challenged us to classify. Classifying an adverse drug reaction in this way will allow doctors to consider the implications of managing it (see table B on bmj.com).
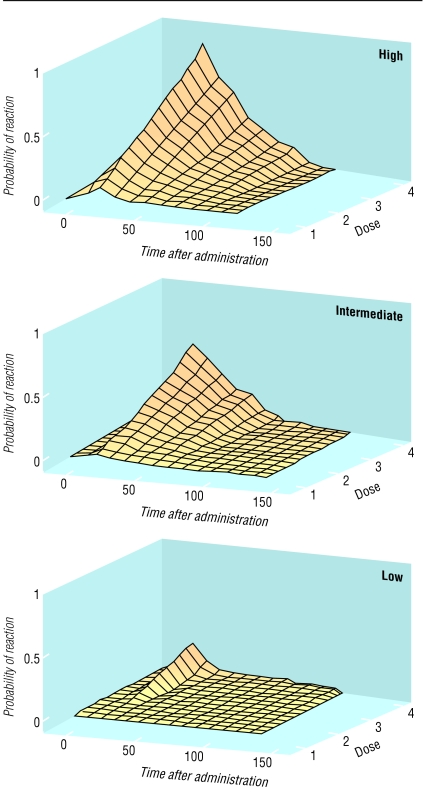
A more sophisticated probabilistic analysis is also possible, but we shall not discuss it in detail here. It requires an estimate of the probability of an adverse drug reaction at different doses and times after administration, for different degrees of susceptibility. The information can be displayed as a series of three dimensional graphs or equivalent nomograms (figure).
Figure 2.
Graphs showing how probability of adverse drug reaction (y axis) might vary with variations in time after administration (x axis, arbitrary units) and dose (z axis, arbitrary units) in people with high, medium, and low susceptibility having an adverse effect of intermediate type
A one dimensional classification system based on time course could meet all the requirements for a useful classification of adverse drug reactions (table 2). For example, the time course of a reaction is evident in each patient, so that all reactions can be classified by individual observation, supplemented, if necessary, by observations in the population. The association between halothane and hepatitis was first shown by a careful analysis of the time course of the reaction in individual cases.23 The time course also helps to distinguish similar adverse drug reactions, such as the two forms of heparin induced thrombocytopenia,24 the two forms of chloramphenicol induced anaemia,25 and photoallergic and phototoxic reactions.26 However, time course alone is an unsatisfactory basis for classification because it ignores important information on dose dependence and individual susceptibility. Our proposal for a three dimensional classification should provide important insights for drug development and regulation, for pharmacovigilance, for monitoring patients, and for the prevention, diagnosis, and treatment of adverse drug reactions.
Table 2.
How dose related, time related, and susceptibility related classifications of adverse drug reactions fulfil criteria for satisfactory classification
|
Classification
|
|||
|---|---|---|---|
| Criterion | Dose related | Time related | Susceptibility related |
| Allows classification on basis of clinical features | No; dose dependency is not always clear from clinical observations and dose ranging studies are not always available | Yes; the time course of a reaction can be directly observed in individual cases or populations | Sometimes, depending on type of susceptibility |
| Give insight into mechanism of reaction | No; only implies the range of doses at which it occurs | Yes; different mechanisms have different time courses | Yes; mechanism and susceptibility are often linked |
| Avoids assigning a reaction to more than one category | No | Yes | No; an adverse drug reaction may be associated with multiple susceptibility factors |
| Suggests how to monitor adverse reactions | Yes | Yes | Yes |
| Suggests population strategies for pharmacovigilance | Yes | Yes; also tells the patient when to be alert for an adverse reaction | Yes (can identify patients at high risk or low risk) |
| Helps in making decisions on treatment or avoiding adverse reactions | Only some types | Yes | Only some types |
| Guides drug development and regulation | Yes; can help in defining the therapeutic dosage range | Yes; suggests strategies for monitoring during drug development and after marketing | Yes; defines subgroups at high risk or low risk |
Examples of DoTS (dose-time-susceptibility) classification
Osteoporosis due to corticosteroids: Do—collateral effect; T—late; S—age, sex.
Anaphylaxis due to penicillin: Do—hypersusceptilbility; T—first dose; S—not understood; requires previous sensitisation
Hepatotoxicity due to isoniazid: Do—collateral effect; T—intermediate; S—genetic (drug metabolism), age, exogenous (alcohol), disease (malnutrition)
Summary points
The current classification of adverse drug reactions based on dose response is inadequate
The time course of the reaction and the susceptibility of the patient also need to be taken into account
A three dimensional approach to adverse drug reactions is proposed based on dose, time, and susceptibility (DoTS)
This approach would improve drug development and patient care
Supplementary Material
 Tables showing how classification of adverse drug reactions has evolved and time related classifaction are available on bmj.com
Tables showing how classification of adverse drug reactions has evolved and time related classifaction are available on bmj.com
We thank those who have commented on these ideas while we were developing them, in particular Stephen Evans and Ralph Edwards.
Contributors and sources: The authors are clinical pharmacologists with long experience of caring for patients with, and collating information on, adverse drug reactions. The ideas expressed in this paper have evolved through frequent discussions of the classification problems and by studying the nature of reported examples, as reviewed in the International Encyclopedia of Adverse Drug Reactions, Meyler's Side Effects of Drugs, and its annual update volumes (Side Effects of Drugs Annuals), both edited by JKA, and The Textbook of Adverse Drug Reactions, co-edited by REF.
Competing interests: JKA is vice chairman of the Medicines Commission and chairman of the joint (Committee on Safety of Medicines and Medicines Commission) working group on the prescribing, supply, and administration of medicines. REF is chair of the Medicines and Healthcare Products Regulatory Agency (MHRA) working group on electronic reporting of adverse drug reactions and a member of the Committee on Safety of Medicines expert advisory panel. However, the views expressed here should not necessarily be taken to reflect the views of the Medicines Commission, the CSM, or the MHRA.
References
- 1.Rawlins MD, Thompson JW. Pathogenesis of adverse drug reactions. In: Davies DM, ed. Textbook of adverse drug reactions. Oxford: Oxford University Press, 1977: 10.
- 2.Rawlins MD. Clinical pharmacology: adverse reactions to drugs. BMJ 1981;282: 974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawlins MD. Pharmacovigilance: paradise lost, regained or postponed? The William Withering lecture 1994. J R Coll Phys Lond 1995;29: 41-9. [PMC free article] [PubMed] [Google Scholar]
- 4.Aronson JK. Drug therapy. In: Haslett C, Chilvers ER, Boon NA, Colledge NR, Hunter JAA, eds. Davidson's principles and practice of medicine 19th ed. Edinburgh: Elsevier Science, 2002: 147-63.
- 5.Frenz DA. Interpreting atmospheric pollen counts for use in clinical allergy: allergic symptomology. Ann Allergy Asthma Immunol 2001;86: 150-7. [DOI] [PubMed] [Google Scholar]
- 6.Troisi CL, Heiberg DA, Hollinger FB. Normal immune response to hepatitis B vaccine in patients with Down's syndrome. A basis for immunization guidelines. JAMA 1985;254: 3196-9. [PubMed] [Google Scholar]
- 7.Kelkar PS, Li JT. Cephalosporin allergy. N Engl J Med 2001;345: 804-9. [DOI] [PubMed] [Google Scholar]
- 8.Friedmann PS, Moss C, Shuster S, Simpson JM. Quantitative relationships between sensitising dose of DNCB and reactivity in normal subjects. Clin Exp Immunol 1983;53: 709-15. [PMC free article] [PubMed] [Google Scholar]
- 9.Wakelkamp M, Alvan G, Paintaud G. Natriuretic efficiency of frusemide as a consequence of drug input rate. Br J Clin Pharmacol 1997;43: 481-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinkerton CR, Milla PJ. Methotrexate enterotoxicity: influence of drug dose and timing in the rat. Br J Cancer 1984;49: 97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace MR, Mascola JR, Oldfield EC. Red man syndrome: incidence, etiology and prophylaxis. J Infect Dis 1991;164: 1180-5. [DOI] [PubMed] [Google Scholar]
- 12.Alderman CP. Adverse effects of the angiotensin-converting enzyme inhibitors. Ann Pharmacother 1996;30: 55-61. [DOI] [PubMed] [Google Scholar]
- 13.Saxon A, Beall GN, Rohr AS, Adelman DC. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med 1987;107: 204-15. [DOI] [PubMed] [Google Scholar]
- 14.Geyman JP, Erickson S. The ampicillin rash as a diagnostic and management problem: case reports and literature review. J Fam Pract 1978;7: 493-6. [PubMed] [Google Scholar]
- 15.Mulhall A, de Louvois J, Hurley R. Chloramphenicol toxicity in neonates: its incidence and prevention. BMJ 1983;287: 1424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenblatt DJ, Divoll M, Harmatz JS, MacLaughlin DS, Shader RI. Kinetics and clinical effects of flurazepam in young and elderly noninsomniacs. Clin Pharmacol Ther 1981;30: 475-86. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz E, Potasman I, Rotenberg M, Almog S, Sadetzki S. Serious adverse events of mefloquine in relation to blood level and gender. Am J Trop Med Hyg 2001;65: 189-92. [DOI] [PubMed] [Google Scholar]
- 18.Batchelor JR, Welsh KI, Tinoco RM, Dollery CT, Hughes GR, Bernstein R, et al. Hydralazine-induced systemic lupus erythematosus: influence of HLA-DR and sex on susceptibility. Lancet 1980;i: 1107-9. [DOI] [PubMed] [Google Scholar]
- 19.Dickinson RG, Hooper WD, Wood B, Lander CM, Eadie MJ. The effect of pregnancy in humans on the pharmacokinetics of stable isotope labelled phenytoin. Br J Clin Pharmacol 1989;28: 17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aronson JK. Forbidden fruit. Nature Med 2001;7: 7-8. [DOI] [PubMed] [Google Scholar]
- 21.Clericetti N, Beretta-Piccoli C. Lithium clearance in patients with chronic renal diseases. Clin Nephrol 1991;36: 281-9. [PubMed] [Google Scholar]
- 22.Hasselstrom J, Eriksson S, Persson A, Rane A, Svensson JO, Sawe J. The metabolism and bioavailability of morphine in patients with severe liver cirrhosis. Br J Clin Pharmacol 1990;29: 289-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inman WHW, Mushin WW. Jaundice after repeated exposure to halothane: an analysis of reports to the Committee on Safety of Medicines. BMJ 1974;i: 45-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andréjak M, Gars V. Heparin. In: Dukes MNG, Aronson JK, eds. Meyler's side effects of drugs. 14th ed. Amsterdam: Elsevier, 2000: 1177-8.
- 25.Yunis AA. Chloramphenicol toxicity: 25 years of research. Am J Med 1989;87: 44-8N. [PubMed] [Google Scholar]
- 26.Roujeau J-C. Cutaneous reactions. In: Bénichou C, ed. Adverse drug reactions. A practical guide to diagnosis and management. Chichester: John Wiley, 1992: 44-7.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.