Abstract
Acinetobacter species are emerging as an important nosocomial pathogen. Multidrug-resistant Acinetobacter spp. has limited the option for effective treatment. Although carbapenems are effective for the treatment of such infections, resistance to this drug has recently been reported. This study was undertaken to assess resistance to carbapenem in clinical isolates of Acinetobacter spp. from hospitalized patients by both disc-diffusion and minimum inhibitory concentration (MIC) methods. All clinical samples from suspected cases of nosocomial infections were processed, and 265 isolates were identified as Acinetobacter species. These isolates were tested for antibiotic resistance by the disc-diffusion method with 14 antimicrobials, including meropenem and imipenem. Thereafter, all Acinetobacter species were subjected to MIC for meropenem. More than 80% resistance to second- and third-generation cephalosporins, aminoglycosides, and quinolones was recorded. Thirty percent of the strains were resistant to cefoperazone/sulbactam. Resistance to meropenem was observed in 6.4% of Acinetobacter spp. while 8.3% of the isolates showed intermediate resistance detected by MIC. All carbapenem-resistant/intermediate strains were also resistant to other (>10) antibiotics tested by the disc-diffusion method. The rising trend of resistance to carbapenem poses an alarming threat to the treatment for such infections. Regular monitoring, judicious prescription, and early detection of resistance to carbapenem are necessary to check further dissemination of drug resistance in Acinetobacter spp.
Key words: Acinetobacter; Acinetobacter baumannii; Antibiotic resistance; Carbapenem; Cross-infections; Drug resistance, Microbial; Meropenem; India
INTRODUCTION
Although genus Acinetobacter was originally identified in the early 20th century, it was recognized as a ubiquitous pathogen only in the last decade (1). Acinetobacter baumannii, a member of the Acinetobacter calcoaceticus—A. baumannii complex, makes up to 73% of all Acinetobacter spp. and is the most commonly-involved pathogen in clinical infections (2). During the last decade, hospital-acquired infections involving multidrug-resistant A. baumannii isolates have been reported, often in association with contamination of hospital equipment or cross-contamination by colonized hands of personnel attending patients (1). Initial concern about multidrug-resistant and carbapenem-resistant Acinetobacter baumannii (CRAB)-associated infections began when the first hospitalwide outbreak occurred in New York city in 1991 (3). Since then, reports of CRAB have been accumulating from other parts of the world (4), including India (5). Currently, the spread in hospital populations of resistant microorganisms is of great concern worldwide, suggesting that we may be approaching the post-antimicrobial era (6). This study was undertaken to assess resistance to carbapenem in clinical isolates of Acinetobacter spp. from hospitalized patients by both disc-diffusion and minimum inhibitory concentration (MIC) methods.
MATERIALS AND METHODS
Duration and place of study
A three-year study (2003–2006) was conducted to determine the susceptibility of nosocomial isolates of Acinetobacter spp. to different antimicrobials, including imipenem and meropenem. Various specimens were collected from patients admitted to different wards and intensive care unit of S.S. Hospital, Banaras Hindu University, Varanasi, India.
Identification of Acinetobacter spp.
Isolation of Acinetobacter spp. was done. Briefly, all clinical specimens were initially processed to separate the oxidase-negative, non-fermenters from other gram-negative bacilli. Thereafter, identification was done to confirm Acinetobacter spp. by standard protocol (7).
In vitro susceptibility
Susceptibility to various antimicrobial agents was determined by the disc-diffusion method and MIC by the agar dilution method following the guidelines of Clinical and Laboratory Standards Institute (CLSI). Antimicrobial susceptibility testing was performed on Mueller Hinton agar by the disc-diffusion method for the following antimicrobial agents (Hi-Media, Mumbai, India) with their concentration given in parentheses: cefotaxime (30 μg), ceftazidime (30 μg), cefoperazone (75 μg), ciprofloxacin (05 μg), norfloxacin (10 μg), amikacin (30 μg), gentamicin (10 μg), tobramycin (10 μg), netilmicin (30 μg), piperacillin (100 μg), carbenicillin (100 μg), cefoperazone/sulbactam (75 μg/30 μg), meropenem (10 μg), and imipenem (10 μg) by the Kirby-Bauer method. Further in vitro susceptibility was determined for meropenem (AstraZeneca, India) by MIC with the agar dilution method, and results were interpreted according to the guidelines of CLSI (≤4 μg/mL=sensitive, 8 μg/mL=Intermediate, and ≥16 μg/mL=resistant). Quality control of susceptibility testing was done using ATCC 27853 Pseudomonas aeruginosa.
RESULTS
In total, 265 Acinetobacter spp. were isolated from 1,242 culture-positive samples from hospitalized patients and were identified up to species level as A. baumannii (91%) and A. Iwoffii (9%). On performing disc-diffusion for antimicrobial susceptibility, Acinetobacter spp. showed more than 80% resistance to third-generation cephalosporins. Among quinolones, 81% of the isolates were resistant to ciprofloxacin, while norfloxacin was inactive in 78% cases of nosocomial urinary tract infection (UTI) caused by Acinetobacter spp. Among aminoglycosides, although amikacin was relatively effective, still 74% of Acinetobacter spp. showed resistance to it. Cefoperazone/sulbactam combination was effective with an overall resistance of 31% while 98% of Acinetobacter spp. were resistant to piperacillin. Among carbapenems, 9.1% of the isolates were resistant to imipenem and 9.8% to meropenem (Table 1).
Table 1.
Antibiotic resistance pattern of Acinetobacter species isolated from different wards, expressed in percentage (%)
| Antibiotic | Post-operative and others (n=154) | ICU (n=89) | Burns (n=22) | Overall (n=265) |
|---|---|---|---|---|
| ß-lactams | ||||
| Piperacillin | 97.9 | 97.4 | 100.0 | 97.9 |
| Carbenicillin | 69.6 | 71.4 | 50.0 | 68.8 |
| Cefotaxime | 79.0 | 83.5 | 83.3 | 80.8 |
| Ceftazidime | 77.6 | 83.5 | 83.3 | 80.0 |
| Cefoperazone | 79.0 | 87.3 | 77.8 | 82.3 |
| Imipenem | 07.1 | 12.3 | 09.1 | 09.1 |
| Meropenem | 07.7 | 12.7 | 11.1 | 09.8 |
| Aminoglycosides | ||||
| Gentamicin | 83.9 | 87.3 | 94.4 | 85.8 |
| Tobramycin | 82.5 | 86.0 | 88.9 | 84.2 |
| Amikacin | 74.8 | 73.4 | 77.8 | 74.6 |
| Netilmicin | 78.3 | 83.5 | 83.3 | 80.4 |
| Quinolones | ||||
| Ciprofloxacin | 79.7 | 83.5 | 77.8 | 80.8 |
| Norfloxacin | 78.3 | 71.4 | 100.0 | 78.1 |
| Others | ||||
| Cefoperazone + sulbactam | 27.8 | 45.6 | 27.8 | 31.2 |
ICU=Intensive care unit
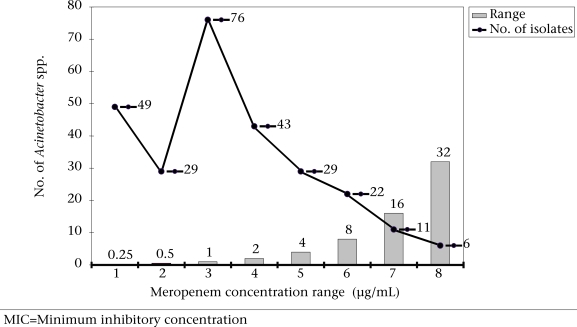
On performing MIC, 39 isolates of Acinetobacter spp., which were resistant to meropenem, showed 6.4% and 8.3% of absolute and intermediate resistance respectively. These 39 isolates were recovered from 34 patients whose clinical data revealed that most of these isolates were from patients admitted to intensive care units (Table 2). On further analysis, it was observed that 44.9% of the isolates were on borderline to the moderate/resistance range (Fig.). Interestingly, all carbapenem-resistant/intermediate strains of Acinetobacter spp. were also resistant to 12 other antibiotics tested by the disc-diffusion method.
Table 2.
Clinical data of patients producing carbapenem-resistant Acinetobacters
| Sl. no. | Sample | Ward/ICU | Age (years) | Sex | Clinical diagnosis |
|---|---|---|---|---|---|
| 1 | Pus | FSW | 45 | F | Gall bladder perforation |
| 2 | Pus | MSW | 34 | M | Non-healing ulcer |
| 3 | Pus | MSW | 33 | F | Polytrauma |
| 4 | Pus | Spl | 50 | M | Diabetic foot |
| 5 | Blood | NICU | 1 | M | Neonatal septicaemia |
| 6 | Pus | Tr | 32 | M | Crush injury |
| 7 | Pus | Ortho | 7 | F | Abscess Rt knee |
| 8 | Pus | MSW | 33 | M | Non-healing ulcer |
| 9 | Pus | Gyn | 28 | F | PO infection |
| 10 | Swab | ICU | 26 | M | Multiple fracture |
| 11 | ETT | ICU | 50 | F | Renal failure |
| 12 | ETT | ICU | 61 | M | ARDS, with PUO |
| 13 | ETT | ICU | 21 | F | Respiratory failure |
| 14 | Urine | NICU | 12 days | M | UTI |
| 15 | ETT | ICU | 35 | M | COPD |
| 16 | Pus | MSW | 55 | M | Necrotizing fascitis |
| 17 | Pus | ICU | 45 | M | Road traffic accident |
| 18 | Pus | FSW | 35 | M | Abdominal surgery |
| 19 | Pus | MSW | 41 | M | Laparotomy |
| 20 | Pus | Burns | 70 | M | 90% burn |
| 21 | Pus | NICU | 1 month | M | Cellulitis |
| 22 | ETT | ICU | 10 | F | Head injury |
| 23 | ETT | ICU | 35 | M | Bronchial asthma |
| 24 | Tr. tube | ICU | 30 | M | Pneumonia |
| 25 | Pus | CTVS | 45 | M | Infective endocarditis |
| 26 | Pus | Burns | 30 | F | 70% burn |
| 27 | Urine | Gyn | 22 | F | PO infection |
| 28 | ETT | ICU | 32 | M | COPD, complications |
| 29 | Pus | MSW | 44 | M | Abdominal surgery |
| 30 | Tr. tube | ICU | 43 | F | Bronchial asthma |
| 31 | ETT | ICU | 70 | M | Pneumonia |
| 32 | Pus | Spl | 48 | M | Bracheal artey injury |
| 33 | Urine | ICU | 29 | M | Laparatomy |
| 34 | Pus | Surg | 27 | F | Deglobing injury scalp |
| 35 | Cat. tip | ICU | 48 | F | Opium poisoning |
| 36 | ETT | ICU | 35 | F | GI bleeding, pneumonia |
| 37 | Swab | ICU | 22 | M | Renal failure |
| 38 | ETT | ICU | 43 | F | Pneumonia |
| 39 | Blood | ICU | 47 | M | Septicaemia |
ARDS=Acute respiratory distress syndrome; Cat=Catheter; COPD=Chronic obstructive pulmonary disease; CTVS=Cardiovascular thoracic surgery; ETT=Endotracheal tube; FSW=Female surgical ward; GI=Gastrointestinal; Gyn=Gynaecology; ICU=Intensive care unit; MSW=Male surgical ward, NICU=Neonatal ICU, PO=Postoperative; Ortho=Orthopaedics; PUO =Pyrexia of unknown origin; Spl =Special ward; Tr=Tracheostomy; UTI=Urinary tract infection
Fig.
Response of Acinetobacter spp. to various concentration ranges of meropenem by MIC
DISCUSSION
In the present study, an overall 18% isolation of Acinetobacter species in nosocomial colonization/infections was observed. Acinetobacter species accounted for 1.4% of all nosocomial infections during 1971–1981 in a university hospital in the United States (8). A more recent study in a university hospital found that hospitalization in an intensive care unit and previous administration of antibiotics were associated with Acinetobacter colonization at various sites of the body in 3.2–10.8 per 1,000 patients (9). Contrary to the previous studies, a higher prevalence of Acinetobacter spp. in the region could be due to lack of good infection-control practices, personal hygiene, over-crowding situations in infirmary, and heavy patient load.
In this study, more than 75% of the isolates were resistant to third-generation cephalosporins, aminoglycosides, and quinolones. Other studies on Acinetobacters have depicted similar results with respect to these antibiotics (10-13). Thirty-one percent of these isolates was resistant to cefoperazone-sulbactam; the efficacy of this drug was significant (p<0.001) compared to other groups of antimicrobials. However, another study showed 46% resistance to cefoperazone-sulbactam by the disc-diffusion method (11).
Resistance to meropenem was observed in 9.8% of Acinetobacter spp. by the disc-diffusion met-hod while 6.4% and 8.3% of the isolates were resistant and intermediate respectively by MIC. Till date, there are limited reports from India on resistance to carbapenem, confirmed by MIC, in the nosocomial isolates of Acinetobacter species (5,14). Taneja et al. reported a high incidence (>20%) of resistance to carbapenem among Acinetobacters in India. However, a report from France showed that 17% of Acinetobacter spp. was resistant to meropenem by the agar dilution method while a study in the UK reported 10% resistance which is quite similar to our results (15,16).
Other studies have shown a high incidence of resistance to carbapenem among Acinetobacters from patients in intensive care units, suggesting that intensive care units are the epicentre for carbapenem-resistant Acinetobacters (17,18).
Meropenem-resistant Acinetobacter spp. was also found to be resistant to all other antimicrobials (Pandrug-resistant Acinetobacter baumannii) (19). This disturbing situation could be attributed to the increased use of antibiotics which has to be controlled by a strict policy for use of antibiotics, in the face of aggressive marketing by the pharmaceuticals. Effective strategies, such as strict infection-control measures, judicious prescriptions of antibiotics, antimicrobial resistance surveillance programmes, and antibiotic cycling have all been tried successfully to control drug resistance in some countries (20).
Carbapenems have become the drugs of choice in Acinetobacter-associated infections in many centres but are slowly being compromised by the emergence of carbapenem-hydrolyzing-lactamases of molecular class B and D (19). Class B carbapenemases found so far in Acinetobacters include various IMP and VIM types; class D enzymes include members of the OXA-23- and OXA-24-related families and various unsequenced types (20). Loss of porins, PBP with reduced affinity, efflux pump, AmpC, and different class B and D ß-lactamases have been associated with resistance to carbapenems in clinical strains of Acinetobacter spp. (21,22). A report from India on mechanisms of carbapenem resistance (phenotypic method) among Acinetobacters has suggested that AmpC is responsible for such resistance (5).
Despite the low prevalence of carbapenem resistance in this study, caution has to be exercised in its use in critically-ill hospitalized patients to check any further increase in the resistance to carbapenems. It is notable that almost 45% isolates of Acinetobacter species were on the borderline to moderate/resistant range to carbapenem. Regular monitoring and documentation of carbapenem resistance is, therefore, crucial while developing strategies to control infections due to Acinetobacter spp. in hospitalized patients.
Acknowledgments
The authors are grateful to the Indian Council of Medical Research, New Delhi, for extending financial support to conduct the present study. The authors do not directly own any stock/share in a company that might be financially affected by the conclusion of this article.
REFERENCES
- 1.Bergogne-Bérézin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148–65. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouvet PJM, Grimont PAD. Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., Acinetobacter junii sp. nov., and emended descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int J Syst Bacteriol. 1986;36:228–40. [Google Scholar]
- 3.Urban C, Go E, Mariano N, Berger BJ, Avraham I, Rubin D, Rahal JJ. Effect of sulbactam on infections caused by imipenem-resistant Acinetobacter calcoaceticus biotype anitratus. J Infect Dis. 1993;167:448–51. doi: 10.1093/infdis/167.2.448. [DOI] [PubMed] [Google Scholar]
- 4.Bou G, Cerveró G, Domínguez MA, Quereda C, Martínez-Beltrán J. Characterization of a nosocomial outbreak caused by a multiresistant Acinetobacter baumannii strain with a carbapenem-hydrolyzing enzyme: high-level carbapenem resistance in A. baumannii is not due solely to the presence of beta-lactamases. J Clin Microbiol. 2000;38:3299–305. doi: 10.1128/jcm.38.9.3299-3305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha M, Srinivasa H. Mechanisms of resistance to carbapenems in meropenem resistant Acinetobacter isolates from clinical samples. Indian J Med Microbiol. 2007;25:121–5. doi: 10.4103/0255-0857.32717. [DOI] [PubMed] [Google Scholar]
- 6.Acar JF, Goldstein FW. Consequences of increasing resistance to antimicrobial agents. Clin Infect Dis. ((Suppl 1)) 1998;27:S125–S30. doi: 10.1086/514913. [DOI] [PubMed] [Google Scholar]
- 7.Baron EJ, Peterson LR, Finegold SM. In: Non-fermentative Gram-negative bacilli and coccobacilli. Bailey and Scott's diagnostic microbiology. 9th ed. St. Louis, MO: Mosby; 1994. Genus Acinetobacter; pp. 401–2. [Google Scholar]
- 8.Larson E. A decade of nosocomial Acinetobacter. Am J Infect Control. 1984;12:14–8. doi: 10.1016/0196-6553(84)90067-1. [DOI] [PubMed] [Google Scholar]
- 9.Struelens MJ, Carlier E, Maes N, Serruys E, Quint WG, van Belkum A. Nosocomial colonization and infection with multiresistant Acinetobacter baumannii: outbreak delineation using DNA macrorestriction analysis and PCR-fingerprinting. J Hosp Infect. 1993;25:15–32. doi: 10.1016/0195-6701(93)90005-k. [DOI] [PubMed] [Google Scholar]
- 10.Prashanth K, Badrinath S. In vitro susceptibility pattern of acinetobacter species to commonly used cephalosporins, quinolones and aminoglycosides. Indian J Med Microbiol. 2004;22:97–103. [PubMed] [Google Scholar]
- 11.Kucukates E, Kocazeybek B. High resistance rate against 15 different antibiotics in aerobic gram-negative bacteria isolates of cardiology intensive care unit patients. Indian J Med Microbiol. 2002;20:208–10. [PubMed] [Google Scholar]
- 12.Anstey NM, Currie BJ, Withnall KM. Community-acquired Acinetobacter pneumonia in the Northern Territory of Australia. Clin Infect Dis. 1992;14:83–91. doi: 10.1093/clinids/14.1.83. [DOI] [PubMed] [Google Scholar]
- 13.Singh AK, Sen MR, Anupurba S, Bhattacharya P. Antibiotic sensitivity pattern of the bacteria isolated from nosocomial infections in ICU. J Commun Dis. 2002;34:257–63. [PubMed] [Google Scholar]
- 14.Taneja N, Maharwal S, Sharma M. Imipenem resistance in nonfermenters causing nosocomial urinary tract infections. Indian J Med Sci. 2003;57:294–9. [PubMed] [Google Scholar]
- 15.Aubert G, Guichard D, Vedel G. In-vitro activity of cephalosporins alone and combined with sulbactam against various strains of Acinetobacter baumannii with different antibiotic resistance profiles. J Antimicrob Chemother. 1996;37:155–60. doi: 10.1093/jac/37.1.155. [DOI] [PubMed] [Google Scholar]
- 16.Henwood CJ, Gatward T, Warner M, James D, Stockdale MW, Spence RP, et al. Antibiotic resistance among clinical isolates of Acinetobacter in the UK, and in vitro evaluation of tigecycline (GAR-936) J Antimicrob Chemother. 2002;49:479–87. doi: 10.1093/jac/49.3.479. [DOI] [PubMed] [Google Scholar]
- 17.Manikal VM, Landman D, Saurina G, Oydna E, Lal H, Quale J. Endemic carbapenem-resistant Acinetobacter species in Brooklyn, New York: citywide prevalence, interinstitutional spread, and relation to antibiotic usage. Clin Infect Dis. 2000;31:101–6. doi: 10.1086/313902. [DOI] [PubMed] [Google Scholar]
- 18.Corbella X, Montero A, Pujol, M, Domínguez MA, Ayats J, Argerich MJ, et al. Emergence and rapid spread of carbapenem resistance during large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J Clin Microbiol. 2000;38:4086–95. doi: 10.1128/jcm.38.11.4086-4095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Go ES, Urban C, Burns J, Kreiswirth B, Eisner W, Mariano N, et al. Clinical and molecular epidemiology of acinetobacter infections sensitive only to polymyxin B and sulbactam. Lancet. 1994;344:1329–32. doi: 10.1016/s0140-6736(94)90694-7. [DOI] [PubMed] [Google Scholar]
- 20.Merz LR, Warren DK, Kollef MH, Fraser VJ. Effects of an antibiotic cycling program on antibiotic prescribing practices in an intensive care unit. Antimicrob Agents Chemother. 2004;48:2861–5. doi: 10.1128/AAC.48.8.2861-2865.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livermore DM. The impact of carbapenemases on antimicrobial development and therapy. Curr Opin Investing Drugs. 2002;3:218–24. [PubMed] [Google Scholar]
- 22.Poirel L, Nordmann P. Acquired carbapenem-hydrolyzing beta-lactamases and their genetic support. Curr Pharm Biotechnol. 2002;3:117–27. doi: 10.2174/1389201023378427. [DOI] [PubMed] [Google Scholar]