Abstract
In mammalian skeletal muscle, an intimate association between the sarcoplasmic reticulum (SR) and mitochondria results in a symbiotic and privileged bidirectional communication between these organelles. Orthograde signaling reflects SR calcium release stimulating mitochondrial adenosine triphosphate (ATP) production via excitation-metabolism coupling. Retrograde signaling involves mitochondrial inhibition of local SR calcium release by controlling the redox environment of the calcium release unit.
SUMMARY
Bidirectional local sarcoplasmic reticulum (SR)-mitochondrial communication in skeletal muscle couples activity-dependent calcium signaling with mitochondrial bioenergetics and redox control.
Keywords: Calcium, calcium release unit, excitation-contraction coupling, reactive oxygen species, triad
INTRODUCTION
Excitation-contraction (EC) coupling in striated muscle is the process by which an electrical signal in the surface membrane (i.e., an action potential) is converted into an intracellular chemical signal (i.e., a calcium transient), which is then harnessed to do work by driving actomyosin cross bridge cycling. In skeletal muscle, this electrochemical conversion process is coordinated by a direct mechanical interaction between dihydropyridine receptors (DHPRs or L-type calcium channels) in the transverse tubule (T-tubule) membrane and ryanodine receptors (RyR1s or calcium release channels) located in the terminal cisternae of the sarcoplasmic reticulum (SR) (14). In adult skeletal muscle, RyR1 proteins, or feet, are arranged in ordered arrays within the SR terminal cisternae on either side of the T-tubule. Within these RyR1 arrays, every other calcium (Ca2+) release channel is associated with a “tetrad” of four DHPRs in the T-tubule membrane. The association of a T-tubule containing DHPR-tetrads, flanked on either side by two closely apposed SR terminal cisternae bearing RyR1 Ca2+ release channels, is referred to as the Ca2+ release unit (CRU) or triad (13). The signaling interaction between DHPR and RyR1 channels during skeletal muscle EC coupling is bidirectional. Orthograde coupling (DHPR to RyR1) refers to the mechanism by which depolarization of the T-tubule membrane triggers activation of RyR1-mediated SR Ca2+ release, while retrograde coupling (RyR1 to DHPR) reflects the influence of RyR1 on the Ca2+ conductance and gating properties of the DHPR (8).
Similar to EC coupling, signaling between the SR and mitochondria in adult mammalian skeletal muscle also involves a bidirectional “orthograde” (SR to mitochondrion) and “retrograde” (mitochondrion to SR) communication. Just as EC coupling is mediated by a mechanical interaction between DHPR and RyR1 proteins, bidirectional SR-mitochondrial signaling is dependent upon a physical association between players. The first part of this article addresses the structural basis of SR-mitochondrial communication, namely precise positioning of mitochondria adjacent to the triad in adult mammalian skeletal muscle. The second part considers the functional implications for muscle performance that result from this intimate inter-organelle communication.
The central hypothesis of this review article is that a structural association of mitochondria to the triad provides a basis for privileged bidirectional SR-mitochondrial communication that optimizes Ca2+ release and excitation-metabolism coupling during skeletal muscle activity. Specifically, orthograde SR-mitochondrial signaling entails enhancement of aerobic adenosine triphosphate (ATP) production by SR Ca2+ release during EC coupling through Ca2+-dependent activation of mitochondrial matrix dehydrogenases and the synthetic activity of the F1F0-ATPase (2, 4). On the other hand, retrograde mitochondrial-SR signaling involves suppression of localized SR Ca2+ release by modulation of the local redox environment of the CRU (16–18, 21). Finally, we consider the impact of orthograde (Ca2+-dependent activation of ATP production) and retrograde (local Ca2+ release inhibition) SR-mitochondrial signaling on excitation-metabolism coupling and skeletal muscle function during exercise and fatigue.
POSITIONING OF MITOCHONDRIA TO THE Ca2+ RELEASE UNIT IN MUSCLE
Ca2+ Release Unit Formation
Embryonic and postnatal development of CRUs and the EC coupling apparatus in mammalian skeletal muscle is well described in the literature (10, 11, 13, 27). The sequence of events follows a similar pattern with some variation in time-course between different species and skeletal muscle types. Following myoblast fusion during myogenesis, free SR is primarily aligned at the M-line, though its specific arrangement with respect to the myofibrils is largely unstructured (10). The first SR associations with exterior membranes in developing muscle, termed peripheral couplings, occur between the SR and surface membrane (13, 27). Shortly thereafter, electron dense RyR1-feet, detected in thin sections, start to form ordered arrays in the junctional gap between the SR and sarcolemma. Assembly of the RyR1 arrays provides the structural requirement for the resultant clustering of DHPRs into tetrads (13, 14). These peripheral junctions represent early stages in formation of the EC coupling apparatus. At this point, clear transverse invaginations of the sarcolemma, or T-tubules, are not readily discernable. Rather, primitive sarcolemmal branches, beginning at the periphery of the myofiber and exhibiting a longitudinal orientation, are the foundations of the future T-tubular network. Subsequent projection of these sarcolemmal invaginations further into the myofiber interior is accompanied by an increased association with the SR. Concurrently, the nonjunctional SR becomes a tighter network, located more exclusively at the Z-line and M-line. Further differentiation and development of these two membrane systems results in an increase in the formation of robust SR-T-tubule junctions, which are enriched in RyR1 arrays with alternately apposed DHPR tetrads (11, 13). Finally, the formation of the fully developed triad involves reorientation of the CRU from a longitudinal to a transverse location between myofibril bundles along the junction between the A- and I-bands (A–I) of the sarcomere in adult mammalian skeletal muscle (10, 13).
The structure and arrangement of CRUs in a specific orientation with respect to the myofibrils contributes to the efficiency (10) and fidelity (1) of the EC coupling process, as muscle organization and physiology reach maturity in parallel. While triads rearrange from a longitudinal to transverse orientation and localize to the A–I junction, the speed of the contractile response increases and the time from the start of the action potential to the beginning of recorded muscle tension decreases (10). Conversely, failure of EC coupling following intense exercise, especially after lengthening contractions (i.e., eccentric exercise), is due in part to a disruption of triads. Prolonged downhill exercise leads to Z-line streaming and focal myofilament disruption (6). When the integrity of the contractile apparatus is compromised, damage to the SR and T-tubule networks ensues. For example, Takekura et al. (28) found significant swelling of the SR and T-tubules, increased appearance of longitudinal T-tubular elements, and a change in the normal direction and nature of triads following downhill treadmill running. Other studies revealed a decrease in SR Ca2+ reuptake in skeletal muscle homogenates following downhill exercise (6). This defect in SR Ca2+ reuptake following exercise-induced muscle damage leads to a prolongation of contractile relaxation and a decrease in SR Ca2+ load. Consequently, force production decreases due to the reduction in SR Ca2+ available for release during successive contractions (5). Over the long term, SR and T-tubular membrane damage and defective SR Ca2+ reuptake results in an elevation of myoplasmic Ca2+. Indeed, peak muscle damage occurs 1–3 days after strenuous exercise and most likely involves activation of Ca2+-dependent proteases (6).
Mitochondrial Localization
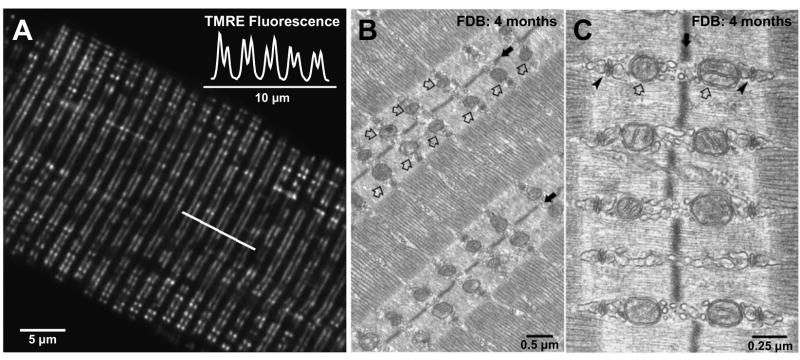
An equally striking characteristic of adult mammalian skeletal muscle is the precise positioning of mitochondria adjacent to the triad or CRUs (Fig. 1). Ogata and Yamasaki (23) originally described a population of inter-myofibrillar I-band-limited mitochondria in electron microscopy studies of adult rat leg muscle. All major muscle fiber types (red, white, and intermediate) exhibit pairs of slender mitochondria encircling the myofibrils within the I-band on either side of the Z-line (23). In addition, mitochondria are also found to cluster under the sarcolemma and occasionally stack in longitudinal columns between the myofibrils in red muscle, though columnar mitochondria are rarely observed in white muscle fibers (23). Quantitative analysis of non-fixed muscle fibers confirms the orientation of mitochondria in transverse double rows with a sarcomeric periodicity of ~2 μm (Fig. 1A; (29)), consistent with their close apposition to triads (Fig. 1B and C). This ordered positioning results in a transverse lattice of mitochondria in skeletal muscle fibers (29). A similar close CRU-mitochondrial juxtaposition is also observed in rat myocardium (25, 29), in which mitochondria occupy a much larger volume and also run the length of the sarcomere, forming a longitudinal lattice between the myofibrils (29).
Figure 1. Mitochondria are precisely localized adjacent to the calcium release unit in adult mammalian fast-twitch skeletal muscle.
A. Confocal image of a single flexor digitorum brevis (FDB) skeletal muscle fiber obtained from an adult mouse (4 months of age) stained with the mitochondrial-selective dye tetramethylrhodamine ethyl ester (TMRE). TMRE fluorescence along the line of interest marked in A shows characteristic doublets of fluorescence with a sarcomeric periodicity of ~2 μm (inset). B. and C. Representative low (B) and high (C) resolution electron micrographs of FDB skeletal muscle fibers obtained from an adult mouse (4 months of age). Mitochondria (open arrows) are aligned adjacent to the triad (arrowheads), on either size of the Z-line (solid arrows). Images kindly provided by Drs. Simona Boncompagni and Feliciano Protasi.
Not unlike the SR and mitochondria of adult striated muscle, domains of endoplasmic reticulum (ER) are closely apposed to mitochondria in a variety of other cell types, including smooth muscle cells, pancreatic acini, and hepatocytes (12). For example, mitochondria associated with lamellae of rough ER can be recovered following gentle subcellular fractionation procedures. This mitochondrial-associated rough ER actually represents a morphologically and functionally distinct subset of hepatic rough ER, and the isolated organelles retain a structural interaction similar to that seen in intact hepatocytes (22). The ER-mitochondrial association in hepatocytes is mediated by electron dense attachments or tethers (~10 nm to ~25 nm in length), which are susceptible to disruption by trypsin-induced proteolysis (7). Remarkably, the association of mitochondria with the ER could be strengthened by expression of a short (5 nm) synthetic linker to enhance the connection between the two compartments. Local Ca2+ signaling between the two organelles was augmented in accordance with the observed strengthening and reduction in ER-mitochondrial spacing. As a result, ER-mitochondrial distance was found to be a critical determinant of local inter-compartment Ca2+ signaling, mitochondrial Ca2+ overload, and apoptotic cell death (7).
Similarly, close association and tight coupling between mitochondria and the CRU in muscle is likely to markedly impact local inter-compartment Ca2+ signaling, metabolic coupling during normal muscle activity, and also possibly influence fatigability and muscle damage during eccentric exercise. Recently, a physical link between the outer mitochondrial membrane (OMM) and the face of the junctional SR opposite to that of the RyR1-feet has been suggested to anchor mitochondria to the CRU (3). Such a linkage would not only preserve SR-mitochondrial colocalization during muscle contraction and shortening, but also provide a structural basis for local signaling between these two organelles during exercise. In addition, alterations in intimate SR-mitochondrial coupling and local inter-organelle signaling could lead to defects in activity-dependent ATP production and mechanisms of Ca2+ release/removal, thus contributing to muscle dysfunction in aging, disease, or following muscle damage due to eccentric exercise.
BIDIRECTIONAL SR-MITOCHONDRIAL SIGNALING
Excitation-Metabolism Coupling (Orthograde Signaling)
Sufficient ATP reserves are required to drive both actomyosin cross bridge cycling and myoplasmic Ca2+ removal during striated muscle contraction. Following the powerstroke, ATP binding to myosin causes dissociation of the myosin head from actin, which is required for subsequent iterations of the cross bridge cycle. In addition, ATP also provides the energy used to drive Ca2+ reuptake by the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) pumps during contractile relaxation. In fact, the majority of ATP consumed during muscle contraction is used to fuel SERCA-mediated Ca2+ removal during contractile relaxation (1). In addition to ATP produced anaerobically via glycolysis and hydrolysis of phosphocreatine, aerobic metabolism within the mitochondria represents a major source of cellular ATP production in muscle.
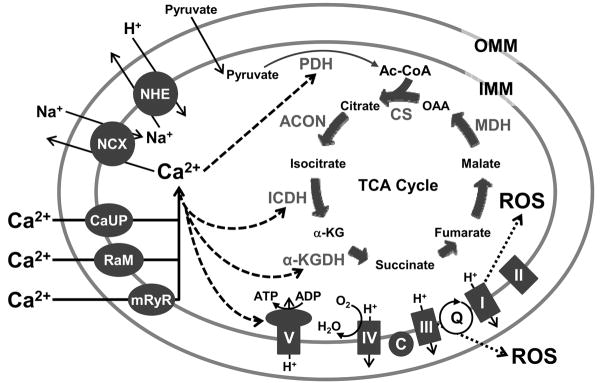
Anaerobic glucose metabolism in the myoplasm generates two molecules each of ATP and pyruvate per glucose. Following glycolysis, the matrix enzyme complex pyruvate dehydrogenase (PDH) catalyzes the decarboxylation of pyruvate, generating acetyl-CoA that is then funneled into the tricarboxylic acid (TCA) cycle (Fig. 2). The series of TCA cycle reactions produce reducing equivalents reduced nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) (4). These products enter the electron transport chain (ETC) at the NADH-dehydrogenase (complex I) and succinate dehydrogenase (complex II) complexes located within the inner mitochondrial membrane (IMM). Transfer of electrons from NADH to complex I results in accumulation of NAD+ which feeds back into the TCA cycle for continued NADH generation. Complex I and II transfer the electrons from NADH and FADH2, respectively, via Coenzyme Q (Q cycle), to complex III. Next, electrons are shuttled to complex IV by cytochrome C with molecular oxygen serving as the final electron acceptor (4). Electron transfer at complexes I, III, and IV is balanced by proton pumping from the matrix to the inter-mitochondrial membrane space (Figure 2). This process generates an electromotive proton gradient used to drive ATP synthesis from adenosine diphosphate (ADP) and inorganic phosphate (Pi) by the F1F0-ATPase (complex V) (4). The sum of reactions from the beginning of the ETC to ATP production by the F1F0-ATPase is commonly referred to as oxidative phosphorylation (OXPHOS) and results in a net production of 14 molecules of ATP per input pyruvate (4). In addition, a small percentage of electrons flowing through the respiratory chain can escape at complex I and the Q cycle and combine with molecular oxygen to produce superoxide anions which can then be converted to other reactive oxygen species (ROS) (Fig. 2).
Figure 2. Schematic representation of interactions between components of the mitochondrial calcium transport systems, the tricarboxylic acid (TCA) cycle, and the electron transport chain (ETC).
Elevations of calcium (Ca2+) in the mitochondrial matrix stimulate pyruvate dehydrogenase (PDH), isocitrate dehydrogenase (ICDH), α-ketoglutarate dehydrogenase (α-KGDH), and the adenosine triphosphate (ATP) synthetic activity of the F1F0-ATPase (complex V). Low levels of superoxide anions (reactive oxygen species, ROS) are generated as byproducts of electrons being passed to molecular oxygen (O2) at complex I and during the Q cycle (Q). OAA, oxaloacetate; Ac-CoA, acetyl coenzyme A; CS, citrate synthase; ACON, aconitase; α-KG, α-ketoglutarate; MDH, malate dehydrogenase; H+, hydrogen; I to V, complexes I to V; C, cytochrome c; ADP, adenosine diphosphate; H2O, water; OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane; Na+, sodium; NHE, sodium-hydrogen exchanger; NCX, sodium-calcium exchanger; CaUP, calcium uniporter; RaM; rapid mode calcium transporter; mRyR, mitochondrial ryanodine receptor.
The relationship between fatigability and reliance on aerobic metabolism for ATP generation between the different types of skeletal muscle underscores the critical role of OXPHOS in muscle function during exercise or sustained muscle activity. Specifically, slow-twitch muscle fibers are highly enriched in mitochondria, are more dependent on OXPHOS for ATP production, and exhibit significant fatigue resistance. On the other hand, fast-twitch glycolytic muscle fibers, with comparatively fewer mitochondria, rely primarily on ATP generation from glycolysis and hydrolysis of phosphocreatine. Since anaerobic ATP production is exhaustible and generates ATP at a much lower rate than OXPHOS, fast-twitch glycolytic fibers fatigue relatively quickly. Fast-twitch oxidative fibers, with a mitochondrial content that is intermediate between slow-twitch and fast-twitch glycolytic fibers, are relatively fatigue-resistant because they utilize a balance of aerobic and anaerobic ATP generation (1). While the cellular mechanisms of skeletal muscle fatigue are complex and beyond the scope of this review, a decline in cellular ATP levels during repetitive muscle activation clearly contributes to the development of fatigue. For example, as the level of ATP decreases during fatigue, the stimulatory effect of ATP on Ca2+ release and reuptake is lost (1), thus contributing to a reduction in muscle contraction. Therefore, cellular mechanisms that optimize ATP production with ATP utilization during muscle contraction serve to limit the development of muscle fatigue.
Cellular respiration and ATP production during skeletal muscle activity is controlled by a number of mechanisms including [ATP]/[ADP], creatine kinase activity, mitochondrial adenine nucleotide transport, and flux through the OXPHOS machinery (2, 4). Importantly, three of the mitochondrial dehydrogenases responsible for generating NADH (pyruvate, NAD+-isocitrate, and α-ketoglutarate dehydrogenases) during the TCA cycle are stimulated by elevations in matrix Ca2+ (Fig. 2). First, the phosphatase that converts inactive pyruvate dehydrogenase phosphate to the active pyruvate dehydrogenase is activated by Ca2+ in the high nM to low μM range (2). Two other enzymes, NAD+-isocitrate dehydrogenase (ICDH) and α-ketoglutarate dehydrogenase (α-KGDH), are activated directly by high nM to low μM Ca2+ (2). As reactions catalyzed by these enzymes may be rate-limiting for flux through the TCA cycle, Ca2+ stimulation of these enzymes increases production of reducing equivalents used by the electron transport chain. Finally, ATP generation via OXPHOS is further enhanced by Ca2+ activation of the ATP synthetic capacity of the F1F0-ATPase (2, 4). It is worth noting that while calcium-mediated enhancement of mitochondrial ATP production has been documented in cultured cells and isolated mitochondria, similar findings have yet to be demonstrated in adult skeletal muscle fibers. However, mitochondrial Ca2+ uptake during EC coupling would serve to stimulate aerobic ATP production in order to help keep pace with increased ATP consumption associated with cross bridge cycling and SERCA-mediated Ca2+ sequestration during muscle activity (i.e., excitation-metabolism coupling).
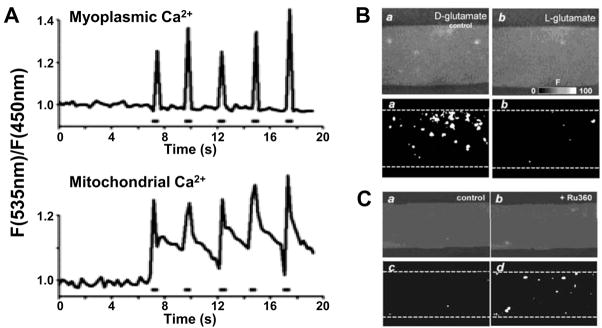
Localization of mitochondria close to sites of Ca2+ release in striated muscle (3, 23, 25, 29) provides a physical basis for excitation-metabolism coupling and localized SR-mitochondrial Ca2+ signaling. Though controversial, the concept of direct mitochondrial Ca2+ uptake of Ca2+ released during muscle EC coupling has recently garnered significant support (12). Since mitochondria colocalize to sites of Ca2+ release, high Ca2+ microdomains in these discrete regions coupled with the highly negative inner mitochondrial membrane potential (−180 mV) provide a sufficient driving force for Ca2+ uptake via the Ca2+ uniporter (CaUP), a rapid Ca2+ uptake mode (RaM), and the mitochondrial ryanodine receptor (mRyR) (see Fig 2 and (4)). In cardiac myocytes, rapid transient increases in mitochondrial Ca2+ during caffeine-induced SR Ca2+ release result in significant mitochondrial Ca2+ uptake that is blocked by inhibition of the CaUP with ruthenium red, but not by BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid), a rapid high affinity Ca2+ chelator (25). Similarly, Shkryl and Shirokova demonstrated rapid “Ca2+ tunneling” from the SR to the mitochondria in slow and fast-twitch skeletal muscle fibers during both electrical stimulation and caffeine exposure (26). However, since mitochondria are associated with the SR on the side opposite to sites of RyR1-mediated Ca2+ release (Fig. 1C), this most likely reflects a local “through-space” coupling mechanism rather than a structural tunneling between organelles. These data support the presence of highly localized or privileged Ca2+ signaling communication between the SR and mitochondria in striated muscle. Indeed, Rudolf et al. (24) provided striking evidence for efficient mitochondrial Ca2+ uptake during physiological elevations in myoplasmic Ca2+ in intact skeletal muscle (24). Specifically, this study demonstrated in vivo mitochondrial Ca2+ uptake that occurs in synchrony with SR Ca2+ release during muscle contraction following motor nerve stimulation (Fig. 3A and (24)).
Figure 3. Mitochondrial calcium uptake and calcium spark suppression in skeletal muscle.
A. Tibialis anterior muscles expressing myoplasmic and mitochondrial calcium (Ca2+)-sensitive cameleons were imaged in vivo with two-photon microscopy. Time course of myoplasmic (top) and mitochondrial (bottom) Ca2+ transients (fluorescence, F) elicited during high frequency stimulation (0.5 s, 50 Hz stimulation train). (Reprinted from Rudolf R, Mongillo M, Magalhaes PJ, Pozzan T. In vivo monitoring of Ca2+ uptake into mitochondria of mouse skeletal muscle during contraction. J Cell Biol. 2004;166(4):527–36. Copyright © 2004 Rockefeller University Press. Used with permission). B. (Upper) Confocal images obtained for permeabilized extensor digitorum longus (EDL) muscle fibers perfused with L-glutamate (right), a substrate for the TCA cycle, or D-glutamate (left), which is not a substrate for the tricarboxylic acid (TCA) cycle. (Lower) Corresponding cumulative binary images for regions with F/F0 > 3 S.D. Data reproduced with permission from J Physiol 2003;547(Pt 2):453–62 (16). C. (Upper) Confocal images obtained for a permeabilized EDL muscle fiber perfused with L-glutamate before (left) and after block of mitochondrial Ca2+ uptake with 20 μM Ru360 (right). (Lower) Corresponding cumulative binary images for regions with F/F0 > 3 S.D. (Reprinted from Isaeva EV, Shirokova N. Metabolic regulation of Ca2+ release in permeabilized mammalian skeletal muscle fibres. J Physiol. 2003;547(Pt 2):453–62. Copyright © 2003 Blackwell Publishing. Used with permission.)
In spite of the evidence discussed above, the relative importance of mitochondrial Ca2+ uptake in mammalian muscle during twitch and tetanic stimulation remains controversial (1). For example, Lannergren et al. (20) failed to detect significant mitochondrial Ca2+ uptake during SR Ca2+ release in mouse fast-twitch glycolytic fibers. In addition, even when mitochondrial Ca2+ uptake during tetanus and fatigue is detectable, its magnitude represents only a minor fraction of the total Ca2+ release, precluding a major role for mitochondria in shaping the global myoplasmic Ca2+ transient (1). Nevertheless, because of the relative paucity of Ca2+ buffers present in the mitochondrial matrix, even low levels of mitochondrial Ca2+ uptake may be sufficient to increase matrix Ca2+ to levels required to stimulate mitochondrial respiration and ATP production via excitation-metabolism coupling (orthograde SR-mitochondrial signaling). Thus, excitation-metabolism coupling is likely to be more important in mitochondria-enriched slow- and fast-twitch oxidative muscle and of less consequence in fast-twitch glycolytic muscle.
Mitochondrial Inhibition of Local SR Ca2+ Release (Retrograde Signaling)
Bidirectional SR-mitochondrial signaling extends beyond excitation-metabolism coupling. Local SR Ca2+ release, termed Ca2+ sparks, were first identified in cardiomyocytes, exhibit quantal spatiotemporal properties, and represent elementary events of RyR-mediated Ca2+ release. Specifically, Ca2+ sparks provide brief elevations in local Ca2+ release that last on the order of a few tens of milliseconds and exhibit a spatial width of ~1 μm(19). While Ca2+ sparks are not observed in mammalian skeletal muscle fibers under basal conditions, they are unmasked following sarcolemmal permeabilization, disruption of mitochondrial function, osmotic shock, and strenuous exercise (16, 17, 19, 21, 30, 31). Recent studies have demonstrated that Ca2+ spark onset in permeabilized skeletal muscle fibers is proportional to mitochondrial content and metabolic capacity, indicating that functional mitochondria inhibit Ca2+ spark genesis (16, 17). Importantly, Ca2+ spark properties and incidence are altered during muscle disease (30) and aging (31), suggesting that dysfunction in the mechanisms that control local Ca2+ release contribute to muscle deterioration under these conditions.
Several mechanisms have been proposed to explain the absence or suppression of Ca2+ sparks in intact adult mammalian skeletal muscle under basal conditions. A degeneration of the T-tubular network in de-differentiating cultured adult skeletal muscle cells correlates with an increase in Ca2+ spark frequency (19), consistent with a structural component to Ca2+ spark suppression in adult muscle. Similarly, Ca2+ spark activity following strenuous exercise may involve a physical disruption of the DHPR-RyR1 interaction (30). However, Ca2+ sparks are also readily observed in mechanically skinned fibers in which the DHPR-RyR1 interaction is preserved (19). Therefore, mechanisms other than tight DHPR-RyR1 coupling likely contribute to Ca2+ spark suppression in skeletal muscle.
Results from Shirokova and colleagues indicate a strong contribution of functional mitochondria to Ca2+ spark suppression in adult mammalian skeletal muscle (16, 17, 21). The potential role of strong mitochondrial Ca2+ buffering in spark suppression has been neither supported nor disproven. Certainly, evidence presented in the previous section indicates that mitochondria are capable of physiological Ca2+ uptake. However, total mitochondrial Ca2+ buffering capacity is limited, and the degree to which mitochondria accumulate Ca2+ during a spark remains to be determined. Nonetheless, Ca2+ spark activity is markedly decreased by interventions that energize mitochondria (Fig. 3B) and increased by interventions that disrupt mitochondrial Ca2+ uptake (Fig. 3C). For example, addition of TCA cycle substrates diminishes Ca2+ spark activity (Fig. 3B and (16)). Moreover, the onset of Ca2+ sparks in chemically permeabilized fibers occurs slowly in mitochondria-rich slow-twitch oxidative muscle fibers, yet occurs rapidly in mitochondria-poor fast glycolytic fibers (17). In addition, spark activity in both fiber types is augmented by mitochondrial Ca2+ uptake blockers, protonophores that dissipate the mitochondrial membrane potential, and following complex III inhibition with antimycin A (16, 17). Furthermore, oxidative insult produced by H2O2 application accelerates the onset and increases the frequency of Ca2+ sparks (17), whereas reactive oxygen species (ROS) scavengers delay and/or suppress Ca2+ spark activity (17, 21). Clearly, mitochondrial Ca2+ uptake and proper redox balance are critical determinants of Ca2+ spark suppression in both skeletal (16, 17, 21) and cardiac (18) muscle.
How does mitochondrial content/activity inhibit local SR Ca2+ release? Since oxidation/nitrosylation of RyR1 destabilizes channel interactions with accessory proteins (e.g., calmodulin and FKBP12) and increases Ca2+ activation and the probability of RyR1 channel opening (9, 15), Ca2+ sparks in muscle may be unmasked under conditions that increase the oxidative environment around the CRU (17, 21). Mitochondria are both intimately associated with the CRU (Fig. 1) and provide a primary means of cellular ROS production and scavenging/detoxification (Fig. 2). Specifically, while mitochondria produce superoxide ions at complex I and during the Q cycle, this ROS production is balanced by robust ROS scavenging (superoxide dismutase and catalase) mechanisms, as well as glutathione- and thioredoxin-mediated ROS detoxification. Consequently, mitochondria play a critical role in maintaining proper balance of the cellular redox potential, and given their localization at the triad (Fig. 1), may influence Ca2+ spark activity by regulating the local redox environment of the CRU. Accordingly, under basal conditions, mitochondria inhibit local Ca2+ release by ensuring that the local redox environment around the CRU is sufficiently reduced. On the other hand, Ca2+ spark activity is increased under conditions that overwhelm this local redox control mechanism and promote RyR1 oxidation either directly (e.g., H2O2 application) or indirectly (low mitochondrial content, addition of mitochondrial uncouplers, inhibition of mitochondrial Ca2+ uptake) (16, 17, 21). Accordingly, Ca2+ spark activation following prolonged or fatiguing muscle activity (30) could result from unabated oxidation of the local SR-mitochondrial microenvironment. Indeed, ROS-mediated dysfunction in SR Ca2+ release/reuptake is thought to contribute to contractile decline during fatigue, suggesting that antioxidants may correct defects in local Ca2+ release and increase fatigue resistance (1).
CONCLUSIONS
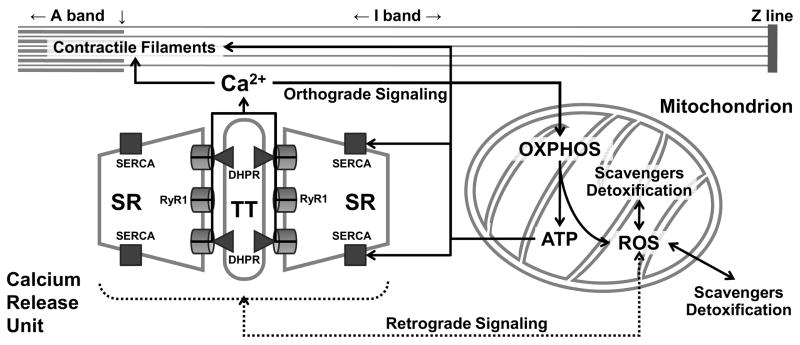
In summary, precise colocalization of CRUs and mitochondria in skeletal muscle provides a physical basis for a symbiotic bidirectional crosstalk between these two organelles (Fig. 4). Orthograde SR-mitochondrial signaling involves mitochondrial Ca2+ uptake following SR Ca2+ release during EC coupling that subsequently stimulates mitochondrial respiration and ATP production to help meet increased energetic demand during muscle activity (excitation-metabolism coupling). On the other hand, functionally intact mitochondria inhibit undesired localized SR Ca2+ release by controlling the local redox environment of the CRU. Thus, bidirectional SR-mitochondrial communication provides a powerful local control mechanism for integrating Ca2+ release/reuptake and ATP utilization during muscle contraction with ATP production and skeletal muscle bioenergetics. Defects in this local control mechanism can lead to excessive mitochondrial ROS production that disrupts the cellular redox state in a manner that contributes to altered Ca2+ release/reuptake during aging, fatigue, and certain forms of muscle disease (9, 30, 31).
Figure 4. Bidirectional SR-mitochondrial signaling in skeletal muscle.
Orthograde sarcoplasmic reticulm (SR)-mitochondrial signaling (solid lines) involves calcium (Ca2+) release during excitation-contraction (EC) coupling being taken up into adjacent mitochondria to stimulate oxidative phosphorylation (OXPHOS) and adenosine triphosphate (ATP) production. Retrograde mitochondrial-SR signaling (broken lines) involves the influence of mitochondrial reactive oxygen species (ROS) production and scavenging/detoxification on the local redox environment and local Ca2+ spark activity of the Ca2+ release unit (CRU). SR, sarcoplasmic reticulum; RyR1, type 1 ryanodine receptor; DHPR, dihydropyridine receptor; TT, transverse tubule; SERCA, sarco(endo)plasmic reticulum Ca2+-ATPase.
Acknowledgments
The authors wish to thank Feliciano Protasi Ph.D., for constructive feedback on this review.
This work was supported by a research grant from the National Institutes of Health (AR44657 to R.T.D.) and a National Institute of Dental and Craniofacial Research training grant (T32-DE07202 to A.E.R.).
References
- 1.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: Cellular mechanisms. Physiol Rev. 2008;88(1):287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 2.Balaban RS. Cardiac energy metabolism homeostasis: Role of cytosolic calcium. J Mol Cell Cardiol. 2002;34(10):1259–71. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- 3.Boncompagni S, Protasi F. Tethers: Structural connections between SR and the outer mitochondrial membrane. Biophys J. 2007;92:313a. [Google Scholar]
- 4.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287(4):C817–C33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 5.Byrd SK. Alterations in the sarcoplasmic reticulum: A possible link to exercise-induced muscle damage. Med Sci Sports Exerc. 1992;24(5):531–6. [PubMed] [Google Scholar]
- 6.Chen W, Ruell PA, Ghoddusi M, Kee A, Hardeman EC, Hoffman KM, Thompson MW. Ultrastructural changes and sarcoplasmic reticulum Ca2+ regulation in red vastus muscle following eccentric exercise in the rat. Exp Physiol. 2007;92(2):437–47. doi: 10.1113/expphysiol.2006.036442. [DOI] [PubMed] [Google Scholar]
- 7.Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174(7):915–21. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dirksen RT. Bi-directional coupling between dihydropyridine receptors and ryanodine receptors. Front Biosci. 2002;7:d659–d70. doi: 10.2741/A802. [DOI] [PubMed] [Google Scholar]
- 9.Durham WJ, Aracena-Parks P, Long C, Rossi AE, Goonasekera SA, Boncompagni S, Galvan DL, Gilman CP, Baker MR, Shirokova N, Protasi F, Dirksen R, Hamilton SL. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133(1):53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edge MB. Development of apposed sarcoplasmic reticulum at the t system and sarcolemma and the change in orientation of triads in rat skeletal muscle. Dev Biol. 1970;23(4):634–50. doi: 10.1016/0012-1606(70)90144-2. [DOI] [PubMed] [Google Scholar]
- 11.Flucher BE, Takekura H, Franzini-Armstrong C. Development of the excitation-contraction coupling apparatus in skeletal muscle: Association of sarcoplasmic reticulum and transverse tubules with myofibrils. Dev Biol. 1993;160(1):135–47. doi: 10.1006/dbio.1993.1292. [DOI] [PubMed] [Google Scholar]
- 12.Franzini-Armstrong C. ER-mitochondria communication. How privileged? Physiology (Bethesda) 2007;22:261–8. doi: 10.1152/physiol.00017.2007. [DOI] [PubMed] [Google Scholar]
- 13.Franzini-Armstrong C, Jorgensen AO. Structure and development of E-C coupling units in skeletal muscle. Annu Rev Physiol. 1994;56:509–34. doi: 10.1146/annurev.ph.56.030194.002453. [DOI] [PubMed] [Google Scholar]
- 14.Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: A complex channel capable of multiple interactions. Physiol Rev. 1997;77(3):699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- 15.Hidalgo C, Donoso P, Carrasco MA. The ryanodine receptors Ca2+ release channels: Cellular redox sensors? IUBMB Life. 2005;57(4–5):315–22. doi: 10.1080/15216540500092328. [DOI] [PubMed] [Google Scholar]
- 16.Isaeva EV, Shirokova N. Metabolic regulation of Ca2+ release in permeabilized mammalian skeletal muscle fibres. J Physiol. 2003;547(Pt 2):453–62. doi: 10.1113/jphysiol.2002.036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaeva EV, Shkryl VM, Shirokova N. Mitochondrial redox state and Ca2+ sparks in permeabilized mammalian skeletal muscle. J Physiol. 2005;565(Pt 3):855–72. doi: 10.1113/jphysiol.2005.086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung C, Martins AS, Niggli E, Shirokova N. Dystrophic cardiomyopathy: Amplification of cellular damage by Ca2+ signaling and reactive oxygen species-generating pathways. Cardiovasc Res. 2008;77(4):766–73. doi: 10.1093/cvr/cvm089. [DOI] [PubMed] [Google Scholar]
- 19.Klein MG, Schneider MF. Ca2+ sparks in skeletal muscle. Prog Biophys Mol Biol. 2006;92(3):308–32. doi: 10.1016/j.pbiomolbio.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Lannergren J, Westerblad H, Bruton JD. Changes in mitochondrial Ca2+ detected with rhod-2 in single frog and mouse skeletal muscle fibres during and after repeated tetanic contractions. J Muscle Res Cell Motil. 2001;22(3):265–75. doi: 10.1023/a:1012227009544. [DOI] [PubMed] [Google Scholar]
- 21.Martins AS, Shkryl VM, Nowycky MC, Shirokova N. Reactive oxygen species contribute to Ca2+ signals produced by osmotic stress in mouse skeletal muscle fibres. J Physiol. 2008;586(1):197–210. doi: 10.1113/jphysiol.2007.146571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meier PJ, Spycher MA, Meyer UA. Isolation and characterization of rough endoplasmic reticulum associated with mitochondria from normal rat liver. Biochim Biophys Acta. 1981;646(2):283–97. doi: 10.1016/0005-2736(81)90335-7. [DOI] [PubMed] [Google Scholar]
- 23.Ogata T, Yamasaki Y. Scanning electron-microscopic studies on the three-dimensional structure of mitochondria in the mammalian red, white and intermediate muscle fibers. Cell Tissue Res. 1985;241(2):251–6. doi: 10.1007/BF00217168. [DOI] [PubMed] [Google Scholar]
- 24.Rudolf R, Mongillo M, Magalhaes PJ, Pozzan T. In vivo monitoring of Ca2+ uptake into mitochondria of mouse skeletal muscle during contraction. J Cell Biol. 2004;166(4):527–36. doi: 10.1083/jcb.200403102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma VK, Ramesh V, Franzini-Armstrong C, Sheu SS. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J Bioenerg Biomembr. 2000;32(1):97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- 26.Shkryl VM, Shirokova N. Transfer and tunneling of Ca2+ from sarcoplasmic reticulum to mitochondria in skeletal muscle. J Biol Chem. 2006;281(3):1547–54. doi: 10.1074/jbc.M505024200. [DOI] [PubMed] [Google Scholar]
- 27.Takekura H, Flucher BE, Franzini-Armstrong C. Sequential docking, molecular differentiation, and positioning of t-tubule/SR junctions in developing mouse skeletal muscle. Dev Biol. 2001;239(2):204–14. doi: 10.1006/dbio.2001.0437. [DOI] [PubMed] [Google Scholar]
- 28.Takekura H, Fujinami N, Nishizawa T, Ogasawara H, Kasuga N. Eccentric exercise-induced morphological changes in the membrane systems involved in excitation-contraction coupling in rat skeletal muscle. J Physiol. 2001;533(Pt 2):571–83. doi: 10.1111/j.1469-7793.2001.0571a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vendelin M, Beraud N, Guerrero K, Andrienko T, Kuznetsov AV, Olivares J, Kay L, Saks VA. Mitochondrial regular arrangement in muscle cells: A “Crystal-like” Pattern. Am J Physiol Cell Physiol. 2005;288(3):C757–C67. doi: 10.1152/ajpcell.00281.2004. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Weisleder N, Collet C, Zhou J, Chu Y, Hirata Y, Zhao X, Pan Z, Brotto M, Cheng H, Ma J. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat Cell Biol. 2005;7(5):525–30. doi: 10.1038/ncb1254. [DOI] [PubMed] [Google Scholar]
- 31.Weisleder N, Brotto M, Komazaki S, Pan Z, Zhao X, Nosek T, Parness J, Takeshima H, Ma J. Muscle aging is associated with compromised Ca2+ spark signaling and segregated intracellular Ca2+ release. J Cell Biol. 2006;174(5):639–45. doi: 10.1083/jcb.200604166. [DOI] [PMC free article] [PubMed] [Google Scholar]