Abstract
Objective
To test whether the level of hostility predicted the rate of cognitive decline in a community of older Blacks and Whites and whether the association varied as a function of race.
Methods
Over 4800 persons from a defined community in Chicago completed up to three structured interviews at approximately 3 year intervals over a period of up to 8.8 years (mean = 4.4 years). At the baseline interview, hostility was assessed with 8-items from the Cook-Medley Hostility Scale. Cognitive function was assessed at each interview with four cognitive function tests from which a composite measure of cognition was formed. Mixed effects models were used to assess change in cognition and its relation to hostility, controlling for age, sex, education, and race.
Results
The average score on the hostility scale at baseline was 3.0 (SD = 2.1). Higher levels of hostility were associated with lower cognitive scores (estimate = −0.028, SE = 0.004, p < .001). Cognition declined at a rate of 0.051 U per year on average, but hostility was not related to the rate of decline. Results were unchanged after controlling for depressive symptoms, chronic health, neuroticism, and social and cognitive activity patterns, or when persons with cognitive impairment at baseline were excluded. The association was similar in Blacks and Whites.
Conclusion
The results suggest that hostility is associated with level of cognitive function in older persons but not related to cognitive decline.
Keywords: cognitive decline, hostility, race, longitudinal
INTRODUCTION
Hostility is a relatively stable personality trait believed to have an adverse effect on common disease risk factors, chronic medical conditions, and mortality (1-3). It is generally defined in terms of cognitive (e.g., attitudes), behavioral (e.g., aggression), and emotional (e.g., anger) characteristics of an individual's negative orientation toward others (3). It has been one of the major constructs in the literature on the role of personality in disease processes and health outcomes (4). The majority of studies have focused on the relationship between hostility and cardiovascular disease. Previous studies in older populations have found that higher hostility scores are predictive of a range of cardiovascular outcomes, including myocardial infarction (2,5) and cardiovascular mortality (2,5,6) although negative studies have been reported as well (7,8). More recently, investigators have examined other health outcomes including fasting insulin and glucose (9), metabolic syndrome (10), inflammatory response (11), and lung function (12); in all cases reported associations revealed the expected pattern with hostility being related to higher levels and/or faster declines in function.
Given the large number of studies reporting an association between hostility and various health outcomes, particularly cardiovascular outcomes, it is striking that no study has directly examined the association of hostility to cognitive decline in old age. Why would hostility be a candidate risk factor for cognitive decline? First, it has been associated with other negative mood states or personality characteristics that have been implicated in studies of cognitive decline and dementia. For example, depression (13-15), neuroticism (16,17), and loneliness (18) have been well documented as important risk factors for cognitive decline or Alzheimer's disease. Further, other studies have found that higher levels of conscientiousness and extraversion are related to a reduced risk of Alzheimer's disease and mild cognitive impairment, and higher cognitive function (19-21). Second, as detailed above, it is strongly related to cardiovascular outcomes and there is a growing literature on the relationship of cardiovascular disease and cognitive impairment (22-24). However, we are unaware of any published studies of hostility and cognitive decline, although a few studies indirectly examined the association. One small study of 57 patients with memory complaints examined personality characteristics including neuroticism, extraversion, agreeableness, and depression, and memory impairment and found that only neuroticism and symptoms of depression interacted with memory performance (25). Another study of caregiver stress as a predictor of decline on a vocabulary test reported that caregivers declined by a small amount compared with noncaregivers, and that a higher hostile attribution and metabolic risk in caregivers mediated the decline (26).
In the current analysis, we had two objectives: 1) to examine the relationship of hostility and rate of cognitive decline in a population-based cohort of adults over the age of 65, and 2) to examine the extent to which there are racial differences in the relationship between hostility and cognition. Studies in the general population of racial differences in cognitive test performance find lower levels of test performance among Blacks as compared with Whites (e.g., 27,28), but no published population studies employ adequate numbers of identical measurements of cognition (three or more) to adequately compare decline across racial groups. Furthermore, information on racial differences in negative emotions is limited (17). Therefore, we hypothesized that higher levels of hostility would be associated with both lower cognitive function and a faster rate of cognitive decline, and that these associations would be similar in Blacks and Whites.
METHODS
Participants
Participants were residents of a geographically defined community of the south side of Chicago who enrolled in the Chicago Health and Aging Project, an ongoing population-based longitudinal study of risk factors for common-age related conditions, including Alzheimer's disease. The study started with a complete census of all households in the community area. All of the residents aged 65 years and older identified in the census were asked to participate. Of 7813 eligible residents in this original cohort, 6158 (78.9%) participated (non-Hispanic Blacks: 61.4%; non-Hispanic Whites: 37.7%; Hispanic and/or Other race or ethnicity unreported: 0.9%). Details of the study design have been described previously (29). Briefly, in-home baseline interviews were conducted from 1993 to 1997, followed by successive interview cycles at approximately 3-year intervals. Beginning with the third cycle (2000−2002), residents who had turned 65 since the inception of the study have also been invited to participate on a rolling basis. As they entered the study, each of these new participants received the same baseline interview as the original cohort members and is also re-interviewed at approximate 3-year intervals.
The interviews consisted of structured questions to elicit information on a wide range of sociodemographic characteristics, psychosocial variables, medical history, and physical and cognitive performance tests. The hostility measure was added during the second data collection period (1997−1999) and was completed by participants of the original cohort during their second interview and by newly added participants during their baseline interview. Data from these two sources were combined for the present analysis and includes the baseline interview and up to two follow-up interviews. All study procedures were approved by the Institutional Review Board of Rush University Medical Center and all participants provided written, informed consent.
Assessment of Hostility
Hostility was measured with eight factor-analytically derived items from the “cynicism subset” (2,3) of the Cook-Medley Hostility Scale (30). The Cook-Medley Hostility Scale is a commonly used measure that assesses trait tendencies toward interpersonal attitudes marked by cynical mistrust, suspiciousness, disparaging views of others, and anger responses (2,3). The abbreviated 8-item measure focuses specifically on a cynical outlook and attitude toward others and items are characterized by a general mistrust of others’ intentions and a perception that these intentions are targeted toward the respondent (3). Previous research with the scale has shown it to be a reliable, valid, and more specific measure of cynicism and distrust than the 50-item Cook-Medley Hostility Scale (2). Moreover, cynical hostility as measured with this scale has been associated with excess risk of cardiovascular disease in a number of studies (e.g., 2,5). Sample items include such statements as: It takes a lot of argument to convince most people of the truth; I think most people would lie to get ahead. All items have a true/false response option. Each true response was assigned 1 point and then summed across the items to yield a summary score ranging from 0 to 8, with higher scores indicating greater hostility. The coefficient alpha was 0.73, indicating a moderate level of internal consistency.
Assessment of Cognitive Function
Four brief tests of cognitive function were administered at each interview. There were two measures of episodic memory: immediate and delayed recall of 12 ideas contained in the East Boston Story (31). There was one test of perceptual speed: the oral version of the Symbol Digit Modalities Test (32), in which participants are given 90 seconds to identify as many digit-symbol matches as possible. The fourth test was the Mini-Mental State Examination (MMSE) (33) a commonly used measure of global cognition. A composite of all four tests was used in longitudinal analyses. As previously described (34), the raw scores on each test were converted to z scores, using the baseline mean and standard deviation (SD) in the population, and then the z scores were averaged, with higher scores indicating better cognitive function.
Assessment of Sociodemographic, Personality, and Mental Health Covariates
Other variables used in the analysis include age at baseline (based on date of birth and centered at age 75), gender, race (non-Hispanic Black or non-Hispanic White, hereinafter referred to as Black and White) and education (measured as years of schooling and centered at 12). A composite measure of lifetime socioeconomic status (SES) was constructed based on four components of SES that characterize different stages of the life course, as previously described (35). The four components included 1) a measure of childhood SES and was based on parents’ years of education, father's occupational prestige score, and financial status during childhood (36), 2) the participant's level of education, 3) occupational status at age 30, and 4) current income. We then calculated z-scores for each of the four components and computed the average of the nonmissing values of each component, as previously described (35).
We also considered other factors known to be related to hostility or cognitive function as covariates in the analysis. Depressive symptoms were assessed with a 10-item short form (37) of the Center for Epidemiological Studies Depression scale (38). Participants were asked if they had experienced each of 10 symptoms much of the time in the past week. The score is the number of symptoms experienced. Neuroticism, defined as the tendency for individuals to experience negative, distressing emotions (39) was assessed with four items from the 12-item Neuroticism scale of the NEO Five-Factor Inventory (40). Participants rated their level of agreement with each item, and scores (ranging from 0 to 4) were summed and then multiplied by three to make the score more comparable to the original 12-item version of the scale, as previously described (41). Frequency of participation in cognitively stimulating activities was quantified with a previously established scale (34). People rated how often they had participated in each of seven cognitive activities (e.g., reading a newspaper) in the past year on a 5-point scale. The mean score for the seven activities has been associated with cognitive decline and AD in this cohort (42). Social engagement was measured by four questions about participation in social and productive activities (43). Scores were summed across items, yielding a total score ranging from 0 to 8, with higher scores indicating greater social engagement. We used standard questions to quantify social network size as the number of friends and family members seen at least once per month, as previously described (43). We also controlled for seven chronic medical conditions that were reported by at least 5% of the population at baseline: hypertension, heart disease, diabetes, cancer, thyroid disease, stroke, and herpes zoster or shingles. We used the number of these conditions present at baseline as an indicator of chronic illness, as previously described (13).
Data Analysis
We used mixed-effects regression models (44) to test the association of hostility score with baseline level of cognition and rate of cognitive decline. This approach to assessing change does not require participants to have the same number of observations or assume that time between observations is constant across persons or testing occasions. An additional advantage of this method compared with other models of longitudinal analysis such as generalized estimating equations is that initial level of function and rate of change are explicitly modeled as sources of random variability (45).
Modeling Hostility and Change in Cognitive Function
We first created a base model of change in cognitive function using data from up to three cycles. We included in the model time from the baseline interview (in years), as well as age, gender, education, race, and each of their interactions with time. After examining additional interactions and squared terms, we included education-squared, age × gender, age × education, and education × race in our final base model. Next, we tested the effect of hostility on baseline level of global cognition and annual rate of change by adding hostility and hostility × time to our base model. Subsequent models examined the effects of hostility on level and rate of change on the individual tests included in the global composite. The main effect of hostility reflects its effect on the starting level of cognitive function, and a significant interaction of hostility and time would indicate that hostility is related to rate of change in cognitive function.
Modeling the Effect of Covariates on Hostility and Decline
Next, we fit additional models with terms added for chronic health conditions, depressive symptoms, neuroticism, participation in cognitive and social activities, social network size, and their interactions with time to test the degree to which the effects of hostility on baseline cognitive function and annual rate of change were influenced by physical and mental health, participation in cognitively stimulating activities, or level of social interaction.
Secondary Analyses: Socio-Demographic Differences and Sensitivity Analyses
We next examined whether the effect of hostility on cognition varied as a function of self-reported race by adding a term for the interaction of hostility with race, and a three-way interaction of hostility, race, and time. To account for potential differences in the rate of change in cognitive decline as a function of other sociodemographic characteristics, we repeated the core model with a term for the interaction of age with hostility, and a three-way interaction of age, hostility and time, followed by identical analyses of 2- and 3-way interactions involving gender, education, and lifetime SES. We also performed a series of sensitivity analyses to a) test whether the results were affected by the inclusion of persons with cognitive impairment, by excluding, in separate models, persons who scored below 24 on the MMSE, the bottom 5%, and the bottom 10% of the sample with the lowest global cognitive scores at baseline, and b) whether excluding those without at least one follow-up evaluation may have biased the results. Model assumptions, particularly regarding normality of the random effects and residual error, were assessed graphically and analytically, and were adequately met (46).
RESULTS
Eligibility and Demographic Characteristics
There were 7669 persons with available data. Of these, 2667 were excluded from analyses as they had died before follow-up or had not yet been contacted for a follow-up interview. Of the remaining 5002, 50 (1%) were excluded due to other race/ethnicity, and 39 (<1%) due to missing hostility data, leaving 4913 eligible persons for the current analysis. The mean follow-up time was 4.42 years (SD = 1.56). Baseline descriptive data for Blacks and Whites in the analyses are presented in Table 1. The mean global cognitive function score was 0.26 (SD = 0.68, with a range of −3.08 to 1.66).
TABLE 1.
Participant Characteristics in the Chicago Health and Aging Project (N = 4913)
| Characteristic | Whites N = 1455 | Blacks N = 3458 | Total Sample N = 4913 |
|---|---|---|---|
| Women (%) | 62.5 | 62.0 | 62.2 |
| Age, mean (SD) | 74.9 (6.8) | 72.2 (5.5) | 73.0 (6.0) |
| Education, mean (SD) | 14.4 (3.2) | 11.6 (3.3) | 12.1 (1.0) |
| Global cognition, mean (SD) | 0.6 (0.6) | 0.1 (0.7) | 0.3 (0.7) |
| Hostility, mean (SD) | 2.0 (1.8) | 3.4 (2.1) | 3.0 (2.1) |
| CES-Da Score, mean (SD) | 1.0 (1.6) | 1.8 (2.1) | 1.6 (2.0) |
| Neuroticism | 7.1 (2.4) | 6.5 (2.4) | 6.7 (2.4) |
| Chronic health conditions | 1.2 (1.0) | 1.2 (1.0) | 1.2 (1.0) |
Center for Epidemiologic Studies Depression (Kohout et al., 1977).
Hostility, Baseline Cognition, and Rate of Cognitive Decline
In the initial mixed effects models, we examined the relation of hostility to initial level of cognitive function and rate of cognitive decline, while adjusting for the effects of age, gender, education, and race, using the terms included in the base model described in the Methods section (Table 2, Models 1 and 2). Level of hostility was negatively associated with global cognition at baseline, as shown by the term for hostility in Table 2. That is, there was an average 0.028 U lower baseline global cognitive score (estimate = −0.028, SE = 0.004, p < .001) for each 1-point higher hostility score. Thus a person with a low level of hostility (score = 0, 10th percentile) had a predicted baseline cognitive score of 0.279 compared with a cognitive score of 0.109 for a person with a high level of hostility (score = 6, 90th percentile), a difference in cognition that is roughly equivalent to being 4 years older at baseline. Figure 1 shows the effect size in terms of SD unit changes. Global cognition declined an average of 0.050 standard unit per year as shown by the term for time in Table 2 (Model 1). Hostility was not associated with rate of cognitive decline as shown by the lack of a significant interaction term for hostility and time (Table 2, Model 2). Analyses of the four individual cognitive function tests revealed a similar pattern of results for the two memory measures and the MMSE, with a nearly significant longitudinal effect of hostility and Symbol Digit Modality Test (data not shown).
TABLE 2.
Effects of Time and Hostility on Global Cognition as Estimated From Mixed Models
| Model Term | Model 1 Estimate (SE) p |
Model 2 Estimate (SE) p |
Model 3a Estimate (SE) p |
Model 4b Estimate (SE) p |
|---|---|---|---|---|
| Time (yr)c | −0.050 (0.005) <.001 | −0.048 (0.005) <.001 | −0.048 (0.005) <.001 | −0.048 (0.005) <.001 |
| Hostility | −0.029 (0.004) <.001 | −0.028 (0.004) <.001 | −0.028 (0.004) <.001 | −0.025 (0.004) <.001 |
| Hostility × time | −0.001 (0.001) .30 | −0.001 (0.001) .29 | −0.001 (0.001) .29 |
All models are adjusted for time, age, age × time, gender, gender × time, education, education × time, education-squared, race, race × time, age × gender, age × education, and education × race.
Model 3 includes terms to adjust for the effects of CES-D and chronic health conditions.
Model 4 adjusts for neuroticism.
Age and education were centered at 75 and 12 yr, respectively. The effect of time, therefore, represents the rate of change in global cognition (in standard units) over a 1-yr period for a 75-yr-old White woman with 12 yr of education.
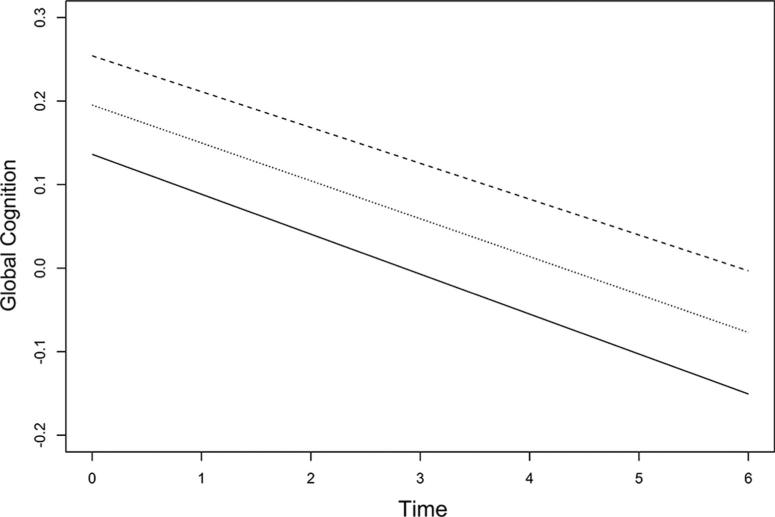
Figure 1.
Predicted paths of global cognitive decline associated with hostility. The middle line represents the mean hostility score and the upper and lower lines represent one standard deviation change above and below the mean.
Controlling for Covariates
In subsequent models, the main effect of hostility on level of baseline cognition was unchanged after controlling for the potentially confounding effects of depressive symptoms (hostility estimate = −0.028, SE = 0.004, p < .001), chronic health conditions (hostility estimate = −0.027, SE = 0.004, p < .001), or both depressive symptoms and chronic health conditions together in the same model (hostility estimate = −0.028, SE = 0.004, p < .001). Because previous studies have shown that hostility is moderately correlated with neuroticism (47), we repeated the core model and included a term for neuroticism. Again, the association of hostility on level of baseline cognition was unchanged (hostility estimate = −0.025, SE = 0.004, p < .001, Model 4). Both activity patterns and social conditions have been shown to vary by race (34,43), and are associated with hostility (48,49) and cognitive function (42,43). Therefore, we added terms for frequency of social engagement, frequency of cognitive activity, and size of social network in separate models. There was no change in the association of hostility on level of baseline cognition in either model (data not shown).
Hostility and Interactions with Race and Other Demographics
In secondary analyses, neither the two-way interaction of race and hostility nor the three-way interaction of race, hostility, and time was significant, suggesting that the association between hostility and cognitive decline did not vary by race (Table 3, Model 1). To explore this issue further, we examined lifetime SES given the well-documented differences in SES between Blacks and Whites. Including the lifetime SES measure in the model did not change the results (data not shown), nor did the inclusion of a term for the interaction of hostility and lifetime SES. There also was no interaction between hostility and education. However, there were significant interactions between hostility and age (Table 3, Model 2), and hostility and gender (Table 3, Model 3) on level of baseline cognition. The effect of hostility on cognition was stronger for older persons (hostility × age estimate = −0.002, SE = 0.001, p = .008), and weaker among men (hostility × male gender estimate = 0.016, SE = 0.008, p = .04).
TABLE 3.
Effects of Demographic Features on Hostility and its Association With Global Cognition as Estimated From Mixed Models
| Model Term | Model 1 (Black Race) Model Term (SE) p |
Model 2 (Age) Model Term (SE) p |
Model 3 (Male Gender) Model Term (SE) p |
Model 4 (Education) Model Term (SE) p |
|---|---|---|---|---|
| Hostility | −0.037 (0.008) <0.001 | −0.031 (0.004) <0.001 | −0.034 (0.005) <0.001 | −0.028 (0.004) <0.001 |
| Hostility* (demographic feature) | 0.011 (0.009) 0.22 | −0.002 (0.001) 0.008 | 0.016 (0.008) 0.035 | 0.000 (0.001) 0.926 |
All models are adjusted for time, age, age × time, gender, gender × time, education, education × time, education-squared, hostility × time, race, race × time, age × gender, age × education, education × race, CES-D, and chronic health conditions.
Sensitivity Analyses
Although we controlled for baseline level of global cognition, we considered whether hostility would be associated with change in cognitive function among relatively unimpaired persons. In separate models, we excluded persons whose score on the MMSE was below 24 points at baseline, a commonly used cut-point for screening for cognitive impairment (33). The association between hostility and baseline cognition was smaller but remained significant (estimate = −0.015, SE = 0.003, p < .001), and there was no relationship between hostility and change in cognitive function. However, some studies suggest that the MMSE has limited specificity in persons with low levels of education and minorities (50), therefore we conducted additional sensitivity analyses by excluding persons whose average score on the composite measure of cognitive function was at or below the 5th and 10th percentile at baseline. Although the strength of the association between hostility and baseline cognition was weakened slightly with each increasing percentile group (hostility estimate = −0.022 and −0.017, respectively), excluding those persons with low levels of cognitive function at baseline did not alter the association of hostility with baseline cognition or cognitive decline. In another sensitivity analysis we repeated the core model after removing the restriction of having at least one nonmissing cognitive function follow-up observation. The results were the same as in the primary model: hostility was associated with level of cognitive function (estimate = −0.027, SE = 0.004, p < .001), but not with change in cognition (estimate = −0.001, SE = 0.001 p = .18).
DISCUSSION
In a population-based study of older Blacks and Whites we found that a measure of cynical hostility was associated with lower cognitive function, but not with cognitive decline over about 4.4 years of follow-up. The association of hostility with level of cognition was robust, however, and remained essentially unchanged after controlling for education, chronic medical conditions, depressive symptoms, neuroticism, and social and cognitive activity patterns, and after excluding those with low levels of cognitive function at baseline. There were no racial differences in the association of hostility with level of cognition. The results suggest that hostility may contribute to level of cognition in a diverse population of older adults.
We are not aware of previous studies of the relation of hostility to level of or rate of change in cognition. But our results are consistent with studies that have examined other aspects of negative affect, negative emotional states, or personality. For example, depressive symptoms have been shown to be related to impaired cognition and cognitive decline in most (13,14), but not all (15) large-scale epidemiologic studies. Neuroticism, a measure of distress proneness, has also been related to impaired cognition (41). Thus, hostility may reflect an additional psychological characteristic that negatively affects cognition in old age.
Although the relationship between hostility and cognition remains poorly understood, there have been links between hostility and other health outcomes. A number of studies have shown that hostility is related to a range of adverse health outcomes including various cardiovascular disease outcomes (2,5), inflammation (11), and mortality (2,5,6). Several mechanisms have been proposed to explain the association of hostility to these adverse health outcomes. One mechanism suggests that hostility confers risk via exaggerated cardiovascular and/or neuroendocrine responses to potential stressors (51). Consistent with this notion, Shapiro et al. (52) reported that persons high in hostility had reduced blood flow to the medial prefrontal cortex during mental arithmetic compared with those low in hostility. Another possibility is that hostility may increase proneness to experience chronic stress (53), which has also been found to increase risk for memory impairment (54). Other studies have proposed psychosocial variables, such as social support or social stressors (55), or poor health behaviors (2) as potential mediators between hostility and health. The basis of the association of hostility with level of cognition is uncertain but the mechanisms may be similar to those proposed for the link between hostility and cardiovascular disease. Hostility increases risk for clinical and subclinical cardiovascular conditions, including carotid atherosclerosis, myocardial infarction, and coronary heart disease (2,5,56). As cardiovascular disease is a potentially important risk factor for cognitive decline and Alzheimer's disease (22-24), it is possible that hostility may influence level of cognition through its association with cardiovascular disease. However, adjustment for chronic health conditions, including cardiovascular disease, did not seem to account for the association between hostility and cognitive function, suggesting that cardiovascular disease is not an important mediator of this relationship. Future studies are needed to determine the mechanisms linking hostility with level of cognition.
Hostility was only associated with level of cognition but not with cognitive decline. One possibility is that hostility negatively affects test taking performance. Another possibility is that hostility, a relatively stable personality trait, affected cognitive development earlier in life or selection into less cognitively stimulating occupations or lifestyles, leading to a lower level of cognitive function in older age. However, we did find that the association between hostility and cognition was stronger in older adults, making this hypothesis less likely. Prospective studies representing a broader spectrum of ages ranging from young adulthood into middle age and old age are needed to address this issue.
Our study has several notable strengths. First, these data come from a geographically defined population of older persons with representation across a wide spectrum of SES in both Blacks and Whites. Second, we had three waves of data over an average of 4.4 years, allowing greater precision in measuring change over time. Third, we used a composite measure of cognitive function that assessed a range of cognitive abilities, rather than individual tests, decreasing the possibility of floor and ceiling effects or other sources of measurement error. Finally, we considered other important factors in our analysis that might have influenced the association between hostility and change in cognitive function, including depressive symptoms, neuroticism, and chronic health conditions.
Our study also had limitations. First, we used a brief measure of hostility that only assessed cynical hostility. It is possible that other aspects of hostility may have had a stronger relation with change in cognition over time. Second, our population is from an urban setting in the Midwest, and so the findings may not be generalizable to aging populations in other types of settings in the United States.
Overall, our results indicate that hostility is associated with baseline level of cognition in older adults, but not with rate of decline, and the association seems to be similar among older Blacks and Whites.
Acknowledgments
Supported by National Institute on Aging (AG11101 and AG22018) and the National Institute of Environmental Health Science (ES10902).
Glossary
- SES
socioeconomic status
- MMSE
mini-mental state examination
- SD
standard deviation
- SE
standard error
REFERENCES
- 1.Barefoot JC, Peterson BL, Dahlstrom WG, Siegler IC, Anderson NB, Williams RB., Jr Hostility patterns and health implications: correlates of Cook-Medley Hostility Scale scores in a national survey. Health Psychol. 1991;10:18–24. doi: 10.1037//0278-6133.10.1.18. [DOI] [PubMed] [Google Scholar]
- 2.Everson SA, Kauhanen J, Kaplan GA, Kaplan GA, Goldberg DE, Julkunen J, Tuomilehto J, Salonen JT. Hostility and increased risk of mortality and acute myocardial infarction: the mediating role of behavioral risk factors. Am J Epidemiol. 1997;146:142–52. doi: 10.1093/oxfordjournals.aje.a009245. [DOI] [PubMed] [Google Scholar]
- 3.Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB., Jr The Cook-Medley hostility scale: item content and ability to predict survival. Psychosom Med. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Siegler IC, Costa PT, Burmmett BH, Helms MJ, Barefoot JC, Williams RB, Dahlstrom WG, Kaplan BH, Vitaliano PP, Nichaman MZ, Day RS, Rimer BK. Patterns of change in hostility from college to midlife in the UNC Alumni Heart Study predict high risk status. Psychosom Med. 2003;65:738–45. doi: 10.1097/01.psy.0000088583.25140.9c. [DOI] [PubMed] [Google Scholar]
- 5.Barefoot JC, Larsen S, von der LL, Schroll M. Hostility, incidence of acute myocardial infarction, and mortality in a sample of older Danish men and women. Am J Epidemiol. 1995;142:477–84. doi: 10.1093/oxfordjournals.aje.a117663. [DOI] [PubMed] [Google Scholar]
- 6.Barefoot JC, Siegler IC, Nowlin JB, Peterson BL, Haney TL, Williams RB., Jr Suspiciousness, health, and mortality: a follow-up study of 500 older adults. Psychosom Med. 1987;49:450–7. doi: 10.1097/00006842-198709000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Stewart JC, Janicki DL, Muldoon MF, Sutton-Tyrrell K, Kamarck TW. Negative emotions and 3-year progression of subclinical atherosclerosis. Arch Gen Psychiatry. 2007;64:225–33. doi: 10.1001/archpsyc.64.2.225. [DOI] [PubMed] [Google Scholar]
- 8.Surtees PG, Wainwright NW, Luben R, Day NE, Khaw KT. Prospective cohort study of hostility and the risk of cardiovascular disease mortality. Int J Cardiol. 2005;100:155–61. doi: 10.1016/j.ijcard.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Shen BJ, Countryman AJ, Spiro A, 3rd, Niaura R. The prospective contribution of hostility characteristics to high fasting glucose levels: the moderating role of marital status. Diabetes Care. 2008;31:1293–8. doi: 10.2337/dc07-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niaura R, Banks SM, Ward KD, Stoney CM, Spiro A, 3rd, Aldwin CM, Landsberg L, Weiss ST. Hostility and the metabolic syndrome in older males: the normative aging study. Psychosom Med. 2000;62:7–16. doi: 10.1097/00006842-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Graham JE, Robles TF, Kiecolt-Glaser JK, Malarkey WB, Bissell MG, Glaser R. Hostility and pain are related to inflammation in older adults. Brain Behav Immun. 2006;20:389–400. doi: 10.1016/j.bbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Kubzansky LD, Sparrow D, Jackson B, Cohen S, Weiss ST, Wright RJ. Angry breathing: a prospective study of hostility and lung function in the Normative Aging Study. Thorax. 2006;61:863–8. doi: 10.1136/thx.2005.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson RS, Barnes LL, Mendes de Leon CF, Aggarwal NT, Schneider JS, Bach J, Pilat J, Beckett LA, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59:364–70. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 14.Chodosh J, Kado DM, Seeman TE, Karlamangla AS. Depressive symptoms as a predictor of cognitive decline: MacArthur studies of successful aging. Am J Geriatr Psychiatry. 2007;15:406–15. doi: 10.1097/01.JGP.0b013e31802c0c63. [DOI] [PubMed] [Google Scholar]
- 15.Ganguli M, Du Y, Dodge HH, Ratcliff GG, Chang CC. Depressive symptoms and cognitive decline in late life: a prospective epidemiological study. Arch Gen Psychiatry. 2006;63:153–60. doi: 10.1001/archpsyc.63.2.153. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer's disease in old age. Neuroepidemiology. 2006;27:143–53. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- 17.Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, Evans DA. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology. 2005;64:380–2. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, Tang Y, Bennett DA. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64:234–40. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- 19.Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Arch Gen Psychiatry. 2007;64:1204–12. doi: 10.1001/archpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]
- 20.Booth JE, Schinka JA, Brown LM, Mortimer JA, Borenstein AR. Five-factor personality dimensions, mood states, and cognitive performance in older adults. J Clin Exp Neuropsychol. 2006;28:676–83. doi: 10.1080/13803390590954209. [DOI] [PubMed] [Google Scholar]
- 21.Crowe M, Andel R, Pedersen NL, Fratiglioni L, Gatz M. Personality and risk of cognitive impairment 25 years later. Psychol Aging. 2006;21:573–80. doi: 10.1037/0882-7974.21.3.573. [DOI] [PubMed] [Google Scholar]
- 22.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–6. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 23.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274:1846–51. [PubMed] [Google Scholar]
- 24.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–63. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 25.Vestberg S, Passant U, Risberg J, Elfgren C. Personality characteristics and affective status related to cognitive test performance and gender in patients with memory complaints. J Int Neuropsychol Soc. 2007;13:911–9. doi: 10.1017/S1355617707071159. [DOI] [PubMed] [Google Scholar]
- 26.Vitaliano PP, Echeverria D, Yi J, Phillips PE, Young H, Siegler IC. Psychophysiological mediators of caregiver stress and differential cognitive decline. Psychol Aging. 2005;20:402–11. doi: 10.1037/0882-7974.20.3.402. [DOI] [PubMed] [Google Scholar]
- 27.Manly JJ, Jacobs DM, Sano M, Bell K, Merchant CA, Small SA, Stern Y. Effect of literacy on neuropsychological test performance in nondemented, education-matched elders. J Int Neuropsychol Soc. 1999;5:191–202. doi: 10.1017/s135561779953302x. [DOI] [PubMed] [Google Scholar]
- 28.Fillenbaum GG, Hughes DC, Heyman A, George LK, Blazer DG. Relationship of health and demographic characteristics to Mini-Mental State examination score among community residents. Pscychol Med. 1988;18:719–26. doi: 10.1017/s0033291700008412. [DOI] [PubMed] [Google Scholar]
- 29.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP). J Alzheimers Dis. 2003;5:349–55. doi: 10.3233/jad-2003-5501. [DOI] [PubMed] [Google Scholar]
- 30.Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. J Appl Psychol. 1954;38:414–8. [Google Scholar]
- 31.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci. 1991;57:167–78. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 32.Smith A. Symbol Digit modalities test manual–revised. Western Psychological Services; Los Angeles: 1982. [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Wilson RS, Bennett DA, Beckett LA, Morris MC, Gilley DW, Bienias JL, Scherr PA, Evans DA. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci. 1999;54B:P155–60. doi: 10.1093/geronb/54b.3.p155. [DOI] [PubMed] [Google Scholar]
- 35.Mendes de Leon CF, Barnes LL, Bienias JL, Skarupski KA, Evans DA. Racial disparities in disability: recent evidence from self-reported and performance-based disability measures in a population-based study of older adults. J Gerontol Psychol Sci Soc Sci. 2005;60:263–71. doi: 10.1093/geronb/60.5.s263. [DOI] [PubMed] [Google Scholar]
- 36.Everson-Rose SA, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Early life conditions and cognitive functioning in later life. Am J Epidemiol. 2003;158:1083–9. doi: 10.1093/aje/kwg263. [DOI] [PubMed] [Google Scholar]
- 37.Kohout F, Berkman L, Evans D, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–93. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 38.Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 39.Salovey P, Rothman AJ, Detweiler JB, Steward WT. Emotional states and physical health. Am Psychol. 2000;55:110–21. doi: 10.1037//0003-066x.55.1.110. [DOI] [PubMed] [Google Scholar]
- 40.Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Psychological Assessment Resources; Odessa, FL: 1992. [Google Scholar]
- 41.Wilson RS, Bennett DA, Mendes de Leon CF, Bienias JL, Morris MC, Evans DA. Distress proneness and cognitive decline in a population of older persons. Psychoneuroendocrinol. 2005;30:11–7. doi: 10.1016/j.psyneuen.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003;61:812–6. doi: 10.1212/01.wnl.0000083989.44027.05. [DOI] [PubMed] [Google Scholar]
- 43.Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63:2322–6. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- 44.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 45.Blackwell E, Mendes de Leon CF, Miller GE. Applying mixed regression models to the analysis of repeated-measures data in psychosomatic medicine. Psychosom Med. 2006;68:870–8. doi: 10.1097/01.psy.0000239144.91689.ca. [DOI] [PubMed] [Google Scholar]
- 46.Bienias JL, Hall CB, Bang W. Diagnostics for random coefficient mixed models with unweighted and weighted data.. Proceedings of the Annual Meeting of the American Statistical Association, Biometrics Section [CD-ROM].; Alexandria, VA: American Statistical Association. 2002. Available at: http://www.amstat.org. [Google Scholar]
- 47.Johnson M. The vulnerability status of neuroticism: over-reporting or genuine complaints? Personality and Individual Differences. 2003;35:877–87. [Google Scholar]
- 48.Vella EJ, Kamarck TW, Shiffman S. Hostility moderates the effects of social support and intimacy on blood pressure in daily social interactions. Health Psychol. 2008;27:155–62. doi: 10.1037/0278-6133.27.2(Suppl.).S155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guyll M, Contrada RJ. Trait hostility and ambulatory cardiovascular activity: responses to social interaction. Health Psychol. 1998;17:30–9. doi: 10.1037//0278-6133.17.1.30. [DOI] [PubMed] [Google Scholar]
- 50.Brayne C, Calloway P. The association of education and socioeconomic status with the Mini Mental State Examination and the clinical diagnosis of dementia in elderly people. Age Ageing. 1990;19:91–6. doi: 10.1093/ageing/19.2.91. [DOI] [PubMed] [Google Scholar]
- 51.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Shapiro PA, Sloan RP, Bagiella E, Kuhl JP, Anjilvel S, Mann JJ. Cerebral activation, hostility, and cardiovascular control during mental stress. J Psychosom Res. 2000;48:485–91. doi: 10.1016/s0022-3999(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 53.Steptoe A, Marmot M. Burden of psychosocial adversity and vulnerability in middle age: associations with biobehavioral risk factors and quality of life. Psychosom Med. 2003;65:1029–37. doi: 10.1097/01.psy.0000097347.57237.2d. [DOI] [PubMed] [Google Scholar]
- 54.Lupien SJ, Gaudreau S, Tchiteya BM, Maheu F, Sharma S, Nair NP, Hauger RL, McEwen BS, Meaney MJ. Stress-induced declarative memory impairment in healthy elderly subjects: relationship to cortisol reactivity. J Clin Endocrinol Metab. 1997;82:2070–5. doi: 10.1210/jcem.82.7.4075. [DOI] [PubMed] [Google Scholar]
- 55.Smith TW, Gallo LC. Hostility and cardiovascular reactivity during marital interaction. Psychosom Med. 1999;61:436–45. doi: 10.1097/00006842-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Everson-Rose SA, Lewis TT, Karavolos K, Matthews KA, Sutton-Tyrrell K, Powell LH. Cynical hostility and carotid atherosclerosis in African American and white women: the Study of Women's Health Across the Nation (SWAN) Heart Study. Am Heart J. 2006;152:982–13. doi: 10.1016/j.ahj.2006.08.010. [DOI] [PubMed] [Google Scholar]