Abstract
Objective
Impairment of transforming growth factor (TGF)-β1 signaling accelerates atherosclerosis in experimental mice. However it is uncertain whether increased TGF-β1 expression would retard atherosclerosis. The role of TGF-β1 in aneurysm formation is also controversial. We tested whether overexpression of active TGF-β1 in hyperlipidemic mice affects atherogenesis and aortic dilation.
Methods and Results
We generated apolipoprotein E-null mice with transgenes that allow regulated overexpression of active TGF-β1 in their hearts. Compared to littermate controls, these mice had elevated cardiac and plasma TGF-β1, less aortic root atherosclerosis (P ≤ 0.002), fewer lesions in the thoracic and abdominal aortae (P ≤ 0.01), less aortic root dilation (P < 0.001), and fewer pseudoaneurysms (P = 0.02). Mechanistic studies revealed no effect of TGF-β1 overexpression on plasma lipids or cytokines, or on peripheral lymphoid organ cells. However, aortae of TGF-β1-overexpressing mice had fewer T-lymphocytes, more collagen, less lipid, lower expression of inflammatory cytokines and matrix metalloproteinase-13, and higher expression of tissue inhibitor of metalloproteinase-2.
Conclusions
When overexpressed in the heart and plasma, TGF-β1 is an anti-atherogenic, vasculoprotective cytokine that limits atherosclerosis and prevents aortic dilation. These actions are associated with significant changes in cellularity, collagen and lipid accumulation, and gene expression in the artery wall.
Keywords: aneurysm, atherosclerosis, growth substances, inflammation, plaque
TGF-β1 is a pleiotropic cytokine that circulates in plasma and is produced by several cardiovascular cell types including smooth muscle and endothelial cells, monocytes, macrophages, and T cells.1, 2 Several studies associate variations in TGF-β1 expression or signaling with atherosclerosis and aneurysm formation; however, the precise relationships between TGF-β1, atherosclerosis, and aneurysm formation are incompletely understood.
Human studies largely support an anti-atherogenic role for TGF-β1, with most studies revealing a negative correlation between plasma TGF-β1 concentration and the presence or extent of atherosclerosis.3-6 Although some animal studies suggest that TGF-β1 could accelerate atherosclerosis by increasing vascular extracellular matrix accumulation and lipid retention,7-10 several studies in hyperlipidemic mice instead portray TGF-β1 as an anti-atherogenic cytokine that limits atherosclerosis largely through immunosuppressive effects. Almost all of these murine studies involve systemic suppression of TGF-β activity, for example by injection of antibodies to TGF-β, infusion of a soluble TGF-β receptor, or transgene-mediated abrogation of TGF-β signaling in T cells.11-14 These studies show that less systemic TGF-β activity accelerates atherosclerosis but they do not address whether enhancement of TGF-β1 signaling would be anti-atherogenic. A recent gene-transfer study suggests that elevated systemic expression of TGF-β1 might suppress atherosclerosis;15 however, because increased in vivo expression of TGF-β1 protein was not documented in this study, its interpretation is difficult.
The role of TGF-β signaling in regulating aortic lumen diameter and aneurysm formation is controversial. Evidence of increased TGF-β signaling was reported in aneurysmal aortae of humans with a familial aortic aneurysm syndrome and in a mouse model of Marfan syndrome.16, 17 In the mice, aneurysm formation was prevented by injection of neutralizing antibodies to TGF-β.16 However, in a xenograft model of aneurysm formation, TGF-β1 facilitated aneurysm healing.18 Similarly, in mice deficient in the extracellular matrix glycoprotein Emilin1, increased aortic TGF-β signaling was associated with aortic constriction.19 Because these studies are either correlational or involve a severe inflammatory reaction, they do not allow confident prediction of the effect of chronic overexpression of TGF-β1 on native aortic diameter. Both aneurysm formation and aortic constriction seem plausible.
To determine the effect of enhanced TGF-β1 expression on atherosclerosis and aneurysm formation, we used tetracycline-suppressible transgenes (i.e., the “tet-off” system)20 to achieve regulated, systemic overexpression of a constitutively active form of TGF-β1 in Apoe-/- mice. We measured the effect of enhanced TGF-β1 expression on aortic lesion size and composition, aortic structure and diameter, T-cell accumulation, and expression of selected genes in the aortic wall.
Methods
Atherosclerosis Studies
Mice with tetracycline-regulated cardiac-specific expression of constitutively active TGF-β120 were bred into the C57BL/6 Apoe-/- background. Atherosclerosis studies were performed on 3 groups of female mice: doubly transgenic (αMHC-tTA/tetO-TGF-β1) mice on and off doxycycline (“DT-on” and “DT-off”) and singly transgenic αMHC-tTA mice off doxycycline (“ST-off”). Mice were killed at 18 wks (after 12 wks of Western diet).
TGF-β1 Protein, Plasma Lipids, and Peripheral Blood Cell Counts
TGF-β1 protein was measured by ELISA of mouse plasma or explant culture media. Plasma lipids were measured.21
Tissue Processing and Histology
Atherosclerosis, aortic dilation, and pseudoaneurysm formation22 were evaluated using sections of aortic roots, computer-assisted planimetry, and both histochemical and immunohistochemical stains. Atherosclerosis was also measured on pinned aortae.23
Immunoblot for Phospho-Smad2
Phosphorylation of Smad2 was assessed by western analysis of extracts of hearts and aortae.
Peripheral Blood Cell Counts, Lymphocyte Markers, and Plasma Cytokines
Blood cells were counted by an outside laboratory. Flow cytometry was performed on splenocytes and lymph node cells. Plasma cytokines were measured by cytofluorometric bead assay.
Aortic Gene Expression
Aortic RNA was extracted, reverse transcribed and amplified.24
Statistical Analysis
Normally distributed data with equal group variances are presented as mean ± SEM and analyzed using ANOVA and unpaired Student's t test. Other data are presented as median (25 - 75% range) and analyzed with Kruskal-Wallis ANOVA and Mann-Whitney rank-sum test.
Results
Increased TGF-β1 Expression and Signaling in DT-Off Mice
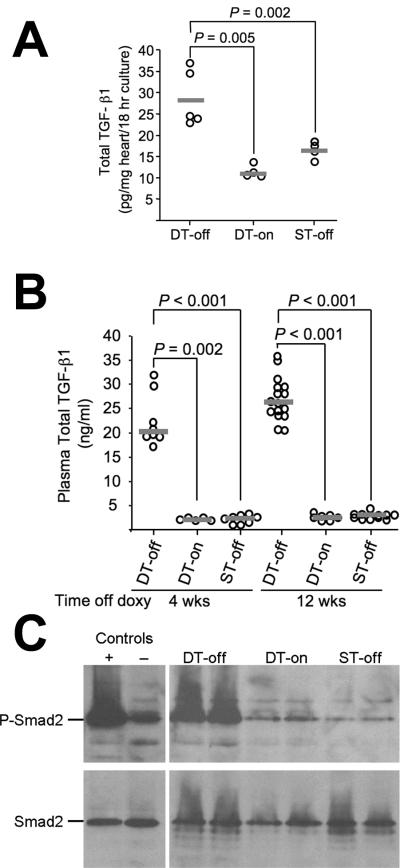
DT-off mice had doxycycline-regulated, cardiac overexpression of active TGF-β120 and elevated total cardiac and plasma TGF-β1 (Figure 1A-B). As with the DT-off Apoe+/+ mice reported previously,20 no active TGF-β1 was detected in any plasma sample. Elevated plasma total TGF-β1 was easily detected 4 wks after doxycycline withdrawal and continued for at least 12 wks. The 10-fold increase in plasma TGF-β1 in DT-off mice is within the range associated with a relative absence of severe multivessel coronary artery disease in humans.3
Figure 1.
Doxycycline-responsive expression of TGF-β1 in Apoe-/- mice. Increased cardiac expression of TGF-β1 after doxycycline withdrawal (A). Six wks after doxycycline withdrawal, hearts from doubly transgenic mice (DT-off), from doubly transgenic mice on doxycycline (DT-on) and from singly transgenic mice off doxycycline (ST-off) were placed in culture media, and TGF-β1 was detected by ELISA. Data points represent individual mice; bars are group medians. Elevated plasma TGF-β1 after doxycycline withdrawal (B). TGF-β1 was measured in plasma obtained at 4 and 12 wks after initiation of a high-fat diet (for all mice) and discontinuation of doxycycline (for DT-off and ST-off mice). Increased phosphorylation of Smad2 in hearts of TGF-β1-overexpressing mice (C). Hearts from TGF-β1-overexpressing mice (DT-off) and littermate controls (DT-on and ST-off) were studied after 6 wks of high-fat diet (all mice) and (for DT-off and ST-off mice) after 6 wks off doxycycline. Western blot of protein extracts was probed sequentially with antibodies against phospho-Smad2 and total Smad2. Control lanes contain extracts of cells treated with TGF-β1 (+) or vehicle (-). This experiment was repeated once with similar results.
DT-off hearts had evidence of increased TGF-β1 activity and signaling, shown by increased cardiac phosphorylated (phospho)-Smad2 in the absence of any change in total Smad2 protein (Figure 1C). Immunohistochemical staining of aortic root sections revealed surprisingly high baseline levels of both phospho-Smad2 and nucleus-localized Smad3 in control aortae, with no further increase in aortae of DT-off mice (Supplemental Figure I and data not shown). Similarly, western blotting of extracts of DT-off aortae did not show increased phospho-Smad2 compared to controls (Supplemental Figure II).
TGF-β1 Overexpression Limits Atherosclerosis, Aortic Root Dilation, and Pseudoaneurysm Formation
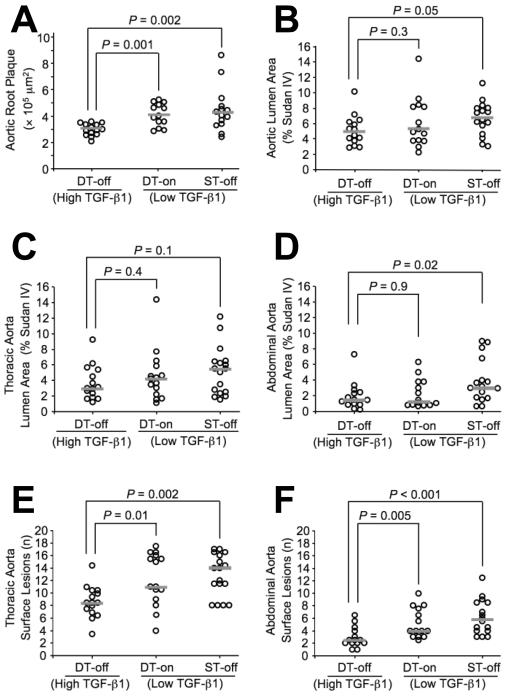
DT-off mice had significantly smaller aortic root intimal plaques (20 - 25% reduction; P ≤ 0.002 versus each of the control groups; Figure 2A). DT-off mice also had less total aortic surface atherosclerosis than either of the two control groups. This finding was of borderline significance versus the ST-off mice (25% reduction; P = 0.05) but not versus the DT-on group (P = 0.3; Figure 2B). The DT-off mice had less atherosclerosis in all segments of the aorta (Figure 2 and data not shown), but this difference reached statistical significance only in the abdominal aorta versus the ST-off mice (50% reduction; P = 0.02; Figure 2D). To measure lesion initiation, we counted the number of individual lesions in the abdominal and thoracic aortae. This analysis revealed a large and statistically significant anti-atherogenic effect of TGF-β1 overexpression: TGF-β1-overexpressing mice had fewer lesions in both the thoracic and abdominal aorta (approximately 30% decrease in thoracic aorta; P ≤ 0.01 versus both control groups; approximately 50% decrease in abdominal aorta; P ≤ 0.005 versus both control groups; Figure 2E-F).
Figure 2.
Aortic atherosclerosis. TGF-β1-overexpressing (DT-off) mice and littermate controls (DT-on and ST-off) were fed a high-fat diet for 12 wks. Atherosclerosis was quantified by measuring intimal area on sections of aortic roots (A), percent Sudan IV-stained lesion area on pinned aortae (B-D) and counting individual lesions on pinned aortae (E-F). Data points represent individual aortae; bars are group medians.
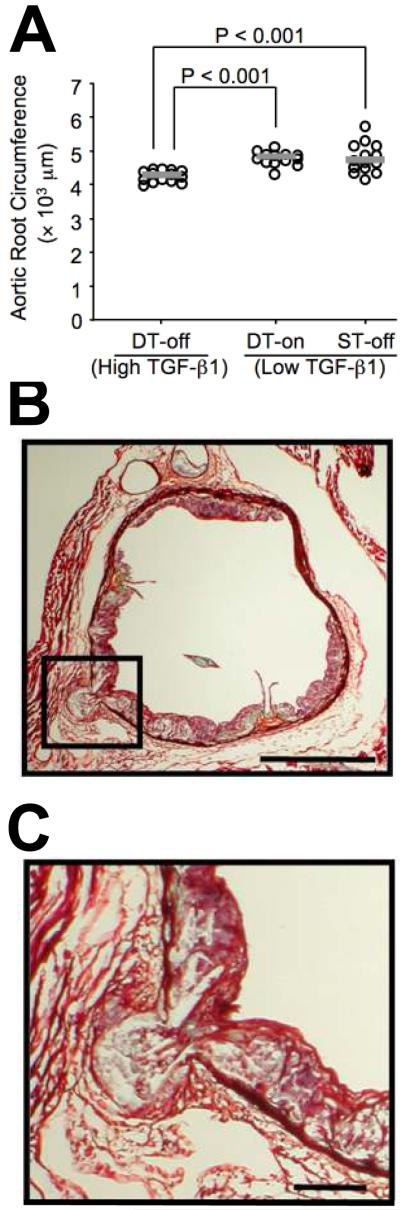
Overexpression of TGF-β1 in DT-off mice also decreased aortic root circumference and cross-sectional area. Aortic roots of DT-off mice had a 10% decrease in circumference and a 20% decrease in calculated area within the internal elastic lamina (P < 0.001 versus both control groups; Figure 3A and Table 1). Aortic roots of DT-off mice also had significantly fewer pseudoaneurysms (75% reduction; P = 0.02 versus both control groups; Table 1, Figure 3B-C). Total aortic luminal surface area was not different between DT-off mice and controls. However, mice on doxycycline had slightly less total aortic surface area (6% decrease; P = 0.05 versus all mice off doxycycline; Table 1).
Figure 3.
Aortic root circumference and pseudoaneurysm formation (A). Aortic root circumference was measured at the level of the internal elastic lamina in TGF-β1-overexpressing mice (DT-off) and littermate controls (DT-on and ST-off). Data points represent individual aortae; bars are group medians. Aortic root pseudoaneurysm (B-C). Section is from a control (ST-off) mouse, after 12 wks on high-fat diet. The pseudoaneurysm (box in B, enlarged in C), is defined as disruption of all medial elastic laminae with protrusion of intimal plaque contents abluminal to the external elastic lamina. Movat stain; Bars: 500 μm (B) and 100 μm (C).
Table 1.
Cardiovascular, Biochemical, and Cellular Effects of TGF-β1 Overexpression
| DT-off (n) | DT-on (n) | ST-off (n) | |
|---|---|---|---|
| Area within IEL (mm2) | 1.5 (1.4-1.6) (14) | 1.8 (1.8-2.0)* (13) | 1.8 (1.6-2.1)* (14) |
| Aortic root pseudoaneurysms† | 2/14 | 8/13‡ | 9/14‡ |
| Aortic lumen surface area (mm2) | 68 ± 5.7 (14) | 64 ± 4.7§ (14) | 68 ± 4.8 (16) |
| Heart weight (mg) | 116 ± 23 (15) | 126 ± 17 (15) | 129 ± 16 (15) |
| Body weight (g) | 22.3 ± 1.6 (15) | 21.6 ± 1.7 (15) | 22.8 ± 2.5 (15) |
| Heart/body weight ratio (mg/g) | 5.2 ± 0.8 (15) | 5.5 ± 0.2 (15) | 5.7 ± 0.8 (15) |
| Cardiomyocyte diameter (μm) | 8.8 (8.2-8.9) (7) | ND | 8.8 (8.4-9.1) (8) |
| Cholesterol (mg/dl) | 951 ± 163 (9) | 902 ± 115 (15) | 981 ± 96 (10) |
| Triglycerides (mg/dl) | 57 (52-71) (9) | 77 (59-127) (13) | 82 (70-96) (12) |
| Total leukocyte count (× 103) | 5.8 ± 1.6 (14) | 4.5 ± 1.7 (16) | 4.8 ± 1.5 (14) |
| Total lymphocyte count (× 103) | 5.0 ± 1.6 (14) | 3.9 ± 1.5 (16) | 4.1 ± 1.5 (14) |
| Intimal macrophage content (%) | 14 ± 5.2 (14) | 16 ± 6.9 (15) | 17 ± 5.3 (13) |
| Intimal SMC content (%) | 0.66 ± 0.34 (6) | ND | 0.71 ± 0.57 (4) |
Values are mean ± S.D. or median (25-75% range), followed by (number of mice) per group.
SMC = Smooth muscle cell. ND = not done.
Mice with ≥ 1 pseudoaneurysm/total mice examined.
For comparison to DT-off mice: P <0.001
For comparison to DT-off mice: P <0.05.
For comparison to DT-off and ST-off combined: P = 0.05.
Effects of TGF-β1 Overexpression on Cardiac and Systemic Parameters
Heart weight, body weight, heart/body weight ratios, and cardiomyocyte diameter were not affected by TGF-β1 overexpression (Table 1). DT-off hearts had mild perivascular and epicardial fibrosis and occasional patches of interstitial fibrosis but otherwise appeared normal histologically (Supplemental Figure III). There were no significant differences in plasma cholesterol among the 3 groups of mice in the atherosclerosis study (Table 1). Plasma triglycerides and FPLC profiles also did not differ among the groups (Table 1, Supplemental Figure IV). Blood pressure of DT-off mice was not significantly different from controls (Supplemental Figure V).
Total peripheral leukocyte and lymphocyte counts were not altered in DT-off mice versus either control group (Table 1). Three independent FACS analyses of splenocytes and lymph node cells revealed no consistent alterations in the number or proportion of CD4+, CD4+ CD25+, CD4+ CD44+, CD4+ CD69+, CD8+, CD8+ CD44+, or CD8+ CD69+ cells in DT-off mice compared to control ST-off mice (data not shown). Similarly, TGF-β1 overexpression did not alter the proportion of CD4+ or CD8+ cells that expressed IFN-γ, IL-2, TNF-α, or IL-4 and did not affect plasma levels of IFN-γ, TNF-α, IL-1β, IL-2, IL-5, or IL-12 (data not shown).
TGF-β Overexpression Alters Aortic Lipid and Collagen Accumulation, T-Cell Abundance, and Gene Expression
Because we found no systemic effects of TGF-β1 overexpression that could account for the highly significant reductions in atherosclerosis, aortic root dilation, and pseudoaneurysm formation in DT-off mice versus both control groups (Figures 2-3, Table 1), we looked for effects of increased plasma TGF-β1 on cellularity, matrix accumulation, lipid content, and gene expression in the aortic wall. Of the two control groups available for these studies, DT-on mice—but not ST-off mice—were exposed to several weeks of doxycycline, a compound that can affect vascular collagen deposition and cellularity, lipid accumulation, gene transcription, and cytokine expression.25-30 As noted above, there was a small, borderline significant effect of doxycycline on total aortic surface area. Therefore, for these experiments we used ST-off mice as littermate controls that differed from DT-off mice only in exposure to elevated TGF-β1.
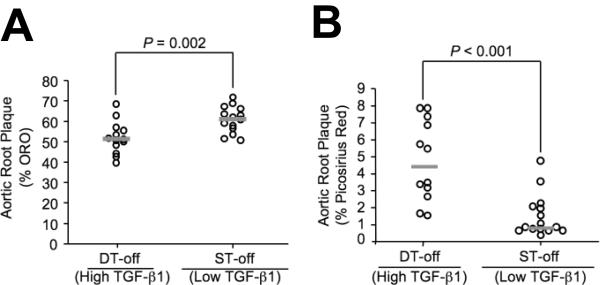
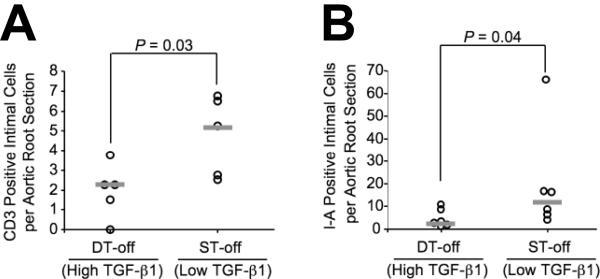
After 12 wks on high-fat diet, plaques in DT-off mice had a significantly different composition than plaques in ST-off mice. DT-off plaques had less lipid (52 versus 61% of plaque volume) and more collagen (4.7 versus 1.5% of plaque volume; P ≤ 0.002 for both; Figure 4A-B). These findings are of particular interest because they contrast with in vitro and correlational studies that associate TGF-β1 effects on vascular matrix with retention of lipid and worse atherosclerosis.9, 10 The groups did not differ in percentage of intimal plaque volume occupied by macrophages or smooth muscle cells (Table 1). DT-off intimal plaques also had less evidence of immune system activation. After 6 wks on diet, intimal T-cell accumulation was decreased in DT-off versus ST-off plaques [2.2 (1.1 - 2.6) versus 5.2 (2.7 - 6.6) intimal T cells per section; P = 0.03; Figure 5A] and I-A expression was reduced by 80% [2.8 (1.8 - 8.5) versus 12 (6.2 - 16) intimal I-A expressing cells per section; P = 0.04; Figure 5B]. I-A is a mouse MHC-II gene product expressed primarily by antigen-presenting cells such as macrophages, and most of the I-A positive cells appeared to be macrophages.
Figure 4.
Aortic root intimal plaque lipid and collagen content. Aortic root sections from TGF-β1-overexpressing mice (DT-off) and littermate controls (ST-off), all fed a high-fat diet for 12 wks, were analyzed for: plaque lipid content, determined by oil red O stain (A) and plaque collagen content, measured by picrosirius red birefringence (B). Data points represent individual aortae; bars are group medians.
Figure 5.
Cellular markers of inflammation in aortic root intima. Sections of aortic roots of TGF-β1-overexpressing mice (DT-off) and littermate controls (ST-off), all fed a high-fate diet for 6 wks, were stained for expression of CD3 (a T-cell marker) (A) and the histocompatibility antigen I-A (B). Data points represent mean values for aortae of individual mice; bars are group medians.
We also measured aortic expression of genes encoding selected cytokines, matrix metalloproteinases (MMPs), and MMP inhibitors (Supplemental Table I). These analyses revealed 40 - 50% less mRNA for TNF-α, MIP-1α, and MIP-1β in DT-off versus ST-off aortae (P ≤ 0.015 for all). IFN-γ mRNA was reduced by 40%; however, this was of only borderline significance (P = 0.09). mRNA for IL-10, MIP-3α, and MIP-3β were not significantly altered (P ≥ 0.4 for all). In DT-off mice, aortic MMP-13 expression was reduced by 70% (P = 0.01), and TIMP-2 expression was increased by 230% (P = 0.03). Expression of MMP-9 was reduced by 70% and MMP-14 by 25%; these changes were of borderline significance (P = 0.1). Expression of MMP-2, -3, and -12, TIMP-1, and TIMP-3 were not significantly altered (P ≥ 0.2 for all).
Discussion
We generated Apoe-/- mice with regulated, cardiac-specific overexpression of active TGF-β1. These mice had: 1) Smaller aortic root intimal lesions, with less lipid and more collagen; 2) Fewer atherosclerotic lesions in their thoracic and abdominal aortae; 3) Unaltered peripheral blood leukocyte levels, peripheral lymphoid organ cell populations, and plasma cytokine levels; 4) Less evidence of immune activation within intimal plaques including fewer T cells and lower I-A expression; 5) Decreased aortic expression of TNF-α and the T-cell chemoattractant cytokines MIP-1α and MIP-1β; 6) Less aortic root dilation and pseudoaneurysm formation; and 7) lower aortic expression of MMP-13 and higher expression of TIMP-2. These data show that elevated plasma TGF-β1 limits atherosclerosis and prevents aneurysm formation. They also suggest that elevated plasma TGF-β1 acts directly on cells in the aortic wall.
Before initiating this study, we attempted both constitutive and conditional overexpression of TGF-β1 in the mouse vasculature. Constitutive overexpression of TGF-β1 was embryonically lethal31 and conditional postnatal vascular overexpression of TGF-β1 was unsuccessful.20 Therefore, we developed a conditional cardiac overexpression system that produces high plasma levels of TGF-β1.20 We reasoned that this system, applied in Apoe-/- mice, would deliver cardiac-derived active TGF-β1 locally to the adjacent aortic root lesions and potentially deliver blood-born TGF-β1 to the entire arterial tree. We anticipated that this model would test the hypothesis, derived from correlational human studies,3-6 that elevated plasma TGF-β1 suppresses atherogenesis.
Elevation of plasma TGF-β1 in Apoe-/- mice decreased the size and number of aortic atherosclerotic lesions and altered lesion composition towards a less inflammatory, more stable plaque phenotype. These results were not predictable from previous studies that almost exclusively used loss-of-function approaches to investigate the role of TGF-β signaling in murine atherosclerosis.11-14, 32 There are three major reasons for this. First, in general, it is not possible to predict the effect of an excess of a plasma factor solely from the effect of its deficiency. Second, in at least two of the TGF-β loss-of-function studies deficient TGF-β signaling caused systemic immune stimulation including splenocyte activation and elevated plasma cytokine levels.12, 13 These systemic effects rather than loss of a specific downstream effect of TGF-β signaling might be responsible for accelerated atherosclerosis. Third, three of the loss-of-function studies used germ-line manipulations that would alter TGF-β signaling during development, potentially yielding abnormal immune systems in the adult mice.12, 13, 32 Atherosclerosis studies performed in mice with abnormal immune systems might yield results that are not generally applicable. To avoid developmental perturbations, we used a conditional TGF-β overexpression approach that cannot affect immune system development because it is “turned on” only in adults. The lack, in the present study, of measurable effects of TGF-β1 overexpression on plasma cytokines and peripheral lymphoid organ cells suggests that we also avoided potentially confounding effects on systemic immunity. Nevertheless, we acknowledge the possibility that TGF-β1 overexpression in the DT-off mice could have caused immune system perturbations or other systemic effects that we failed to detect.
We investigated several potential mechanisms through which elevated plasma TGF-β1 could suppress atherosclerosis. After noting the absence of detectable effects of TGF-β1 overexpression on plasma lipids, cytokines, leukocytes, and peripheral lymphoid organ cells, we looked for evidence of increased TGF-β signaling in the aorta. Although we did not find increased aortic phospho-Smad2 (discussed below), we did find that TGF-β overexpression significantly altered aortic T-cell abundance, lipid and collagen content, and aortic wall expression of I-A, TNF-α, MIP-1α, MIP-1β, TIMP-2, and MMP-13. These specific effects on T cells, collagen, I-A, cytokines, TIMP-2 and MMP-13 are all plausibly related to TGF-β overexpression because each of them is a well-described consequence of increased TGF-β activity.13, 33-37 Moreover, suppression of T cells, I-A and TNF-α expression, and upregulation of TIMP-2 are all associated experimentally with decreased atherosclerosis.13, 38, 39 In addition, elevated aortic expression of MIP-1α and MIP-1β is correlated with plaque growth in Apoe-/- mice.40 Taken together, our data identify several plausible molecular and cellular mechanisms through which elevated TGF-β1 could suppress atherosclerosis at the level of the artery wall. Although we cannot exclude that plasma TGF-β1 acts at an extravascular site with indirect effects on atherosclerotic lesion growth, the simplest explanation of our results is that high levels of plasma TGF-β1 suppress atherosclerosis via direct actions on gene expression and consequently on cellularity and lipid accumulation in the artery wall.
We were surprised to find increased phospho-Smad2/3 in hearts but not in aortae of DT-off mice. Three potential explanations for this are: 1) insensitivity of western analysis and immunostaining in a setting of high baseline phospho-Smad2/3; 2) compensatory downregulation of phospho-Smad2/3 via a negative feedback loop in response to TGF-β1-mediated phenotypic changes; and 3) Smad2/3-independent TGF-β1 signaling.41 Further studies are required to determine whether the DT-off mice have altered vascular cell signaling and, if so, which pathways are activated and when. These studies would also be aimed at identifying specific vascular cell types that respond to elevated plasma TGF-β1 and would likely include confocal microscopy to colocalize cell-type-specific antigens and markers of activated TGF-β signaling. Unfortunately, cell-type-specific deletion of TGF-β signaling cannot easily be used to investigate which vascular cells are responsive to TGF-β1 because deletion of TGF-β signaling in immune cells causes a lethal systemic inflammatory disease42 and loss of TGF-β signaling in SMC or EC is embryonically lethal.43, 44
Overexpression of TGF-β1 also prevented aortic root dilation and pseudoaneurysm formation. These vasculoprotective effects are all logical consequences of TGF-β1 stimulation of aortic TIMP-2 expression and suppression of MMP-13 expression. This TGF-β1-mediated protection against aortic root dilation contrasts with a study in fibrillin-1 null mice in which aortic root dilation was associated with increased aortic wall TGF-β signaling;16 and a report that familial aortic aneurysm syndromes are associated with type I and type II TGF-β receptor mutations that cause a paradoxical increase in TGF-β signaling.45 However, other studies are congruent with our finding that TGF-β1 can prevent aortic medial destruction and aneurysm formation. For example, overexpression of TGF-β1 in aortic xenografts stabilized already-formed aneurysms, downregulated metalloproteinase expression, decreased T-cell infiltration, and promoted vascular healing.18 Moreover, mice lacking Emilin1 had increased vascular TGF-β signaling that led to generalized blood vessel narrowing.19 It is difficult to reconcile all of these studies, which appear to show opposite effects of increased TGF-β signaling on aortic diameter. Further mechanistic studies are needed to explain these apparently context-specific effects of TGF-β1 on aortic diameter.
Another limitation of our study is that we could not—using only this line of tetO-TGF-β1 mice—determine the minimum level of TGF-β1 overexpression necessary to retard atherosclerosis and aneurysm formation. Identification of this minimum level will require generation of new lines of tetO-TGF-β1 mice that express TGF-β1 at lower levels and would include efforts to quantify TGF-β1 activity and signaling in target tissues such as the aorta.
In summary, elevated plasma TGF-β1 in Apoe-/- mice retards atherosclerosis, stabilizes plaque structure, prevents aortic dilation, decreases vascular inflammation, and limits pseudoaneurysm formation. In humans, the inverse correlation of plasma TGF-β1 and atherosclerosis3-6 could represent a cause-and-effect relationship. Enhancement of TGF-β1 signaling in the artery wall may be a promising approach to prevention and treatment of atherosclerosis and aneurysm formation in humans.18, 46
Supplementary Material
Acknowledgments
We thank Ming Xiao, Shi Hai-Kun, Kun Qian, Katherine Liang, and Justin Searns for technical assistance, Margo Weiss for administrative assistance, Tom McDonald for assistance with immunohistochemistry, Alexander Rudensky for helpful advice regarding FACS analyses, and Sunyoung Lee for generation and characterization of the transgenic mice. Dr. Marie's current address is: Inserm U758, Université Claude Bernard Lyon 1, IFR128 BioSciences Lyon-Gerland, Lyon F-69365, France.
Funding Sources This research was supported by a grant from the NIH (HL69063). A.D.F. was supported by NIH T32 HL07828 and the EVEREST Foundation, and A.S.-O. was supported by NIH grant HL070941.
Footnotes
Disclosures None
References
- 1.Bobik A. Transforming growth factor-betas and vascular disorders. Arterioscler Thromb Vasc Biol. 2006;26:1712–1720. doi: 10.1161/01.ATV.0000225287.20034.2c. [DOI] [PubMed] [Google Scholar]
- 2.Sarzani R, Brecher P, Chobanian AV. Growth factor expression in aorta of normotensive and hypertensive rats. J Clin Invest. 1989;83:1404–1408. doi: 10.1172/JCI114029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grainger DJ, Kemp PR, Metcalfe JC, Liu AC, Lawn RM, Williams NR, Grace AA, Schofield PM, Chauhan A. The serum concentration of active transforming growth factor-β is severely depressed in advanced atherosclerosis. Nat Med. 1995;1:74–79. doi: 10.1038/nm0195-74. [DOI] [PubMed] [Google Scholar]
- 4.Tashiro H, Shimokawa H, Sadamatu K, Yamamoto K. Prognostic significance of plasma concentrations of transforming growth factor-beta in patients with coronary artery disease. Coron Artery Dis. 2002;13:139–143. doi: 10.1097/00019501-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Erren M, Reinecke H, Junker R, Fobker M, Schulte H, Schurek JO, Kropf J, Kerber S, Breithardt G, Assmann G, Cullen P. Systemic inflammatory parameters in patients with atherosclerosis of the coronary and peripheral arteries. Arterioscler Thromb Vasc Biol. 1999;19:2355–2363. doi: 10.1161/01.atv.19.10.2355. [DOI] [PubMed] [Google Scholar]
- 6.Stefoni S, Cianciolo G, Donati G, Dormi A, Silvestri MG, Coli L, De Pascalis A, Iannelli S. Low TGF-beta1 serum levels are a risk factor for atherosclerosis disease in ESRD patients. Kidney Int. 2002;61:324–335. doi: 10.1046/j.1523-1755.2002.00119.x. [DOI] [PubMed] [Google Scholar]
- 7.Majesky MW, Lindner V, Twardzik DR, Schwartz SM, Reidy MA. Production of transforming growth factor β1 during repair of arterial injury. J Clin Invest. 1991;88:904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulick AH, Taylor AJ, Zuo W, Qiu C-B, Dong G, Woodward RN, Agah R, Roberts AB, Virmani R, Dichek DA. Overexpression of transforming growth factor β1 in arterial endothelium causes hyperplasia, apoptosis, and cartilaginous metaplasia. Proc Natl Acad Sci U S A. 1998;95:6983–6988. doi: 10.1073/pnas.95.12.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little PJ, Tannock L, Olin KL, Chait A, Wight TN. Proteoglycans synthesized by arterial smooth muscle cells in the presence of transforming growth factor-beta1 exhibit increased binding to LDLs. Arterioscler Thromb Vasc Biol. 2002;22:55–60. doi: 10.1161/hq0102.101100. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien KD, Olin KL, Alpers CE, Chiu W, Ferguson M, Hudkins K, Wight TN, Chait A. Comparison of apolipoprotein and proteoglycan deposits in human coronary atherosclerotic plaques. Colocalization of biglycan with apolipoproteins. Circulation. 1998;98:519–527. doi: 10.1161/01.cir.98.6.519. [DOI] [PubMed] [Google Scholar]
- 11.Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, Fradelizi D, Tedgui A. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- 12.Gojova A, Brun V, Esposito B, Cottrez F, Gourdy P, Ardouin P, Tedgui A, Mallat Z, Groux H. Specific abrogation of transforming growth factor-{beta} signaling in T cells alters atherosclerotic lesion size and composition in mice. Blood. 2003;102:4052–4058. doi: 10.1182/blood-2003-05-1729. [DOI] [PubMed] [Google Scholar]
- 13.Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutgens E, Gijbels M, Smook M, Heeringa P, Gotwals P, Koteliansky VE, Daemen MJ. Transforming growth factor-beta mediates balance between inflammation and fibrosis during plaque progression. Arterioscler Thromb Vasc Biol. 2002;22:975–982. doi: 10.1161/01.atv.0000019729.39500.2f. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Liu Y, Chen J, Velchala N, Amani F, Nemarkommula A, Chen K, Rayaz H, Zhang D, Liu H, Sinha AK, Romeo F, Hermonat PL, Mehta JL. Suppression of atherogenesis by delivery of TGFbeta1ACT using adeno-associated virus type 2 in LDLR knockout mice. Biochem Biophys Res Commun. 2006;344:701–707. doi: 10.1016/j.bbrc.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 18.Dai J, Losy F, Guinault AM, Pages C, Anegon I, Desgranges P, Becquemin JP, Allaire E. Overexpression of transforming growth factor-beta1 stabilizes already-formed aortic aneurysms: a first approach to induction of functional healing by endovascular gene therapy. Circulation. 2005;112:1008–1015. doi: 10.1161/CIRCULATIONAHA.104.523357. [DOI] [PubMed] [Google Scholar]
- 19.Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, Braghetta P, Marino G, Selvetella G, Aretini A, Colonnese C, Bettarini U, Russo G, Soligo S, Adorno M, Bonaldo P, Volpin D, Piccolo S, Lembo G, Bressan GM. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell. 2006;124:929–942. doi: 10.1016/j.cell.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Agah R, Xiao M, Frutkin AD, Kremen M, Shi H, Dichek DA. In vivo expression of a conditional TGF-beta1 transgene: no evidence for TGF-beta1 transgene expression in SM22alpha-tTA transgenic mice. J Mol Cell Cardiol. 2006;40:148–156. doi: 10.1016/j.yjmcc.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dichek HL, Johnson SM, Akeefe H, Lo GT, Sage E, Yap CE, Mahley RW. Hepatic lipase overexpression lowers remnant and LDL levels by a noncatalytic mechanism in LDL receptor-deficient mice. J Lipid Res. 2001;42:201–210. [PubMed] [Google Scholar]
- 22.Lemaitre V, Soloway PD, D'Armiento J. Increased medial degradation with pseudo-aneurysm formation in apolipoprotein E-knockout mice deficient in tissue inhibitor of metalloproteinases-1. Circulation. 2003;107:333–338. doi: 10.1161/01.cir.0000044915.37074.5c. [DOI] [PubMed] [Google Scholar]
- 23.Cozen AE, Moriwaki H, Kremen M, DeYoung MB, Dichek HL, Slezicki KI, Young SG, Veniant M, Dichek DA. Macrophage-targeted overexpression of urokinase causes accelerated atherosclerosis, coronary artery occlusions, and premature death. Circulation. 2004;109:2129–2135. doi: 10.1161/01.CIR.0000127369.24127.03. [DOI] [PubMed] [Google Scholar]
- 24.Nuttall RK, Pennington CJ, Taplin J, Wheal A, Yong VW, Forsyth PA, Edwards DR. Elevated membrane-type matrix metalloproteinases in gliomas revealed by profiling proteases and inhibitors in human cancer cells. Mol Cancer Res. 2003;1:333–345. [PubMed] [Google Scholar]
- 25.Amin AR, Attur MG, Thakker GD, Patel PD, Vyas PR, Patel RN, Patel IR, Abramson SB. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc Natl Acad Sci U S A. 1996;93:14014–14019. doi: 10.1073/pnas.93.24.14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieillard-Baron A, Frisdal E, Eddahibi S, Deprez I, Baker AH, Newby AC, Berger P, Levame M, Raffestin B, Adnot S, d'Ortho MP. Inhibition of matrix metalloproteinases by lung TIMP-1 gene transfer or doxycycline aggravates pulmonary hypertension in rats. Circ Res. 2000;87:418–425. doi: 10.1161/01.res.87.5.418. [DOI] [PubMed] [Google Scholar]
- 27.Bendeck MP, Conte M, Zhang M, Nili N, Strauss BH, Farwell SM. Doxycycline modulates smooth muscle cell growth, migration, and matrix remodeling after arterial injury. Am J Pathol. 2002;160:1089–1095. doi: 10.1016/S0002-9440(10)64929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23:483–488. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- 29.Bernal-Mizrachi C, Gates AC, Weng S, Imamura T, Knutsen RH, DeSantis P, Coleman T, Townsend RR, Muglia LJ, Semenkovich CF. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature. 2005;435:502–506. doi: 10.1038/nature03527. [DOI] [PubMed] [Google Scholar]
- 30.Madan M, Bishayi B, Hoge M, Messas E, Amar S. Doxycycline affects diet- and bacteria-associated atherosclerosis in an ApoE heterozygote murine model: cytokine profiling implications. Atherosclerosis. 2007;190:62–72. doi: 10.1016/j.atherosclerosis.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Agah R, Prasad KSS, Linnemann R, Firpo MT, Quertermous T, Dichek DA. Cardiovascular overexpression of transforming growth factor-β1 causes abnormal yolk sac vasculogenesis and early embryonic death. Circ Res. 2000;86:1024–1030. doi: 10.1161/01.res.86.10.1024. [DOI] [PubMed] [Google Scholar]
- 32.Grainger DJ, Mosedale DE, Metcalfe JC, Bottinger EP. Dietary fat and reduced levels of TGFbeta1 act synergistically to promote activation of the vascular endothelium and formation of lipid lesions. J Cell Sci. 2000;113(Pt 13):2355–2361. doi: 10.1242/jcs.113.13.2355. [DOI] [PubMed] [Google Scholar]
- 33.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 34.Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. J Hepatol. 1999;30:48–60. doi: 10.1016/s0168-8278(99)80007-5. [DOI] [PubMed] [Google Scholar]
- 35.Lechuga CG, Hernandez-Nazara ZH, Dominguez Rosales JA, Morris ER, Rincon AR, Rivas-Estilla AM, Esteban-Gamboa A, Rojkind M. TGF-beta1 modulates matrix metalloproteinase-13 expression in hepatic stellate cells by complex mechanisms involving p38MAPK, PI3-kinase, AKT, and p70S6k. Am J Physiol Gastrointest Liver Physiol. 2004;287:G974–G987. doi: 10.1152/ajpgi.00264.2003. [DOI] [PubMed] [Google Scholar]
- 36.Seeland U, Haeuseler C, Hinrichs R, Rosenkranz S, Pfitzner T, Scharffetter-Kochanek K, Bohm M. Myocardial fibrosis in transforming growth factor-beta(1) (TGF-beta(1)) transgenic mice is associated with inhibition of interstitial collagenase. Eur J Clin Invest. 2002;32:295–303. doi: 10.1046/j.1365-2362.2002.00985.x. [DOI] [PubMed] [Google Scholar]
- 37.Maltman J, Pragnell IB, Graham GJ. Specificity and reciprocity in the interactions between TGF-beta and macrophage inflammatory protein-1 alpha. J Immunol. 1996;156:1566–1571. [PubMed] [Google Scholar]
- 38.Johnson JL, Baker AH, Oka K, Chan L, Newby AC, Jackson CL, George SJ. Suppression of atherosclerotic plaque progression and instability by tissue inhibitor of metalloproteinase-2: involvement of macrophage migration and apoptosis. Circulation. 2006;113:2435–2444. doi: 10.1161/CIRCULATIONAHA.106.613281. [DOI] [PubMed] [Google Scholar]
- 39.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 40.Lutgens E, Faber B, Schapira K, Evelo CT, van Haaften R, Heeneman S, Cleutjens KB, Bijnens AP, Beckers L, Porter JG, Mackay CR, Rennert P, Bailly V, Jarpe M, Dolinski B, Koteliansky V, de Fougerolles T, Daemen MJ. Gene profiling in atherosclerosis reveals a key role for small inducible cytokines: validation using a novel monocyte chemoattractant protein monoclonal antibody. Circulation. 2005;111:3443–3452. doi: 10.1161/CIRCULATIONAHA.104.510073. [DOI] [PubMed] [Google Scholar]
- 41.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 42.Leveen P, Larsson J, Ehinger M, Cilio CM, Sundler M, Sjostrand LJ, Holmdahl R, Karlsson S. Induced disruption of the transforming growth factor beta type II receptor gene in mice causes a lethal inflammatory disorder that is transplantable. Blood. 2002;100:560–568. doi: 10.1182/blood.v100.2.560. [DOI] [PubMed] [Google Scholar]
- 43.Frutkin AD, Shi H, Otsuka G, Leveen P, Karlsson S, Dichek DA. A critical developmental role for tgfbr2 in myogenic cell lineages is revealed in mice expressing SM22-Cre, not SMMHC-Cre. J Mol Cell Cardiol. 2006;41:724–731. doi: 10.1016/j.yjmcc.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 44.Carvalho RL, Itoh F, Goumans MJ, Lebrin F, Kato M, Takahashi S, Ema M, Itoh S, van Rooijen M, Bertolino P, Ten Dijke P, Mummery CL. Compensatory signalling induced in the yolk sac vasculature by deletion of TGFbeta receptors in mice. J Cell Sci. 2007;120:4269–4277. doi: 10.1242/jcs.013169. [DOI] [PubMed] [Google Scholar]
- 45.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 46.Grainger DJ. Transforming growth factor beta and atherosclerosis: so far, so good for the protective cytokine hypothesis. Arterioscler Thromb Vasc Biol. 2004;24:399–404. doi: 10.1161/01.ATV.0000114567.76772.33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.