Abstract
Background
In 1937, Diven (1) reported that human fear responses to cues previously paired with shock progressively increase or incubate over 24 hours. Since then, fear incubation has been demonstrated in both humans and nonhumans. However, the difficulty of demonstrating long-lasting fear incubation in rodents has hampered the study of the underlying mechanisms of this incubation. Here, we describe a rat procedure where fear reliably incubates over time.
Methods
We trained food-restricted rats to lever-press for food pellets in daily 90-min sessions. We then gave each rat one-hundred 30-s tones co-terminating with a 0.5-s, 0.5 mA footshock over 10 days (10 pairings per day). Groups of rats (n=10-15) were then given 4 presentations of the tone (the fear cue) 2, 15, 31 or 61 days after fear conditioning training and were assessed for conditioned suppression of lever-pressing.
Results
We found that conditioned fear responses were significantly higher 31 and 61 days after fear training than after 2 or 15 days. In control experiments, we showed that extensive tone-shock pairing is necessary for the emergence of fear incubation, and that it is unlikely that non-associative factors contribute to this incubation.
Conclusions
We describe a procedure for generating reliable and long-lasting conditioned fear incubation. Our procedure can be used to study mechanisms of fear incubation, and may provide a model for studying the mechanisms of delayed-onset posttraumatic stress disorder that occur in a sub-population of people previously exposed to chronic stressors.
Keywords: Fear conditioning, anxiety, stress, incubation, post-traumatic stress disorder, animal models
For many years there has been great interest in using fear conditioning to model anxiety disorders (2-4), including posttraumatic stress disorder (PTSD) (5; 6). In fear conditioning, an initially neutral environment (context), or discrete cue (e.g., tone or spoken word) is paired with a noxious stimulus, usually an electric shock. Under certain experimental conditions, responses to fear conditioned cues can increase over time in the absence of further stress or cue exposure, a phenomenon termed conditioned fear incubation or fear incubation (7). In the human literature, fear incubation was most often observed during the first 24 h after stress exposure (1; 8; 9). However, to our knowledge, fear incubation has not been demonstrated experimentally in humans over periods longer than several days.
Fear incubation over the course of 24 h has also been well documented in rodents (10-12), but it is unclear whether fear in rodents reliably incubates over longer periods. Long-term fear incubation to environmental contexts associated with shock has been demonstrated over 60 days in rats (13) or over 14 days in mice (14; 15). However, in rats, this incubation has been inconsistent across age groups (13), and in mice it faded after 14 days and was inconsistent across strains (14; 15). Additionally, many investigators have reported that conditioned fear responses either remain stable over time or somewhat decrease (16-19). Consequently, the neurobiological mechanisms of fear incubation over long time periods have not been studied.
Here we describe the development of a reliable fear incubation protocol that allows for future neurobiological study of this phenomenon. We were inspired by an early observation of Millenson and Dent (20) in a single rat. They used an “overtraining” procedure in which a light-clicker cue was repeatedly paired with shock over 60 sessions until the fear response to the cue dissipated and then reappeared after a 30-day shock-free interval (see also Rosas and Alonso (21) for similar findings over a 20-day shock-free interval).
In our procedure, we gave each rat 100 30-s tone presentations co-terminating with a 0.5-s, 0.5-mA footshock over 10 days. Groups of rats were then given presentations of the tone 2, 15, 31 or 61 days after fear conditioning and assessed for conditioned suppression of lever-pressing. In additional experiments, we investigated whether lever-press suppression to tones that were never paired with shock would emerge over time, whether fear incubation would occur with repeated testing, and whether extended training is needed in order to demonstrate fear incubation.
Methods and materials
Subjects and apparatus
Male Long-Evans rats (total n=155, Charles River, 250-370 g) were individually housed in a colony room under a reverse 12-h:12-h light-dark cycle with lights off at 9:30 or 9 am (after a move to a new building). The rats were maintained at 85% of free-feeding body weight, with free access to water. All procedures followed the guidelines outlined in the “Principles of Laboratory Animal Care” (NIH publication no. 85-23) and were approved by the local ACUC. Experiments were conducted in 8 self-administration chambers (Med Associates, Georgia, VT). Each chamber had two levers 9 cm above the floor, but only one lever (“active,” retractable lever) activated the pellet dispenser, delivering 45-mg food pellets (# F00021, Bioserv, Frenchtown, NJ). The chambers' grid floors were connected to electric shock generators.
Procedures
A fear incubation protocol (Figure 1A) was developed using 6 phases: magazine training (1 session), lever-press acquisition (5 sessions), fear conditioning (1 or 10 sessions), incubation period (1, 14, 30 or 60 days), lever-press re-stabilization (1 session), and test for cue-induced fear conditioning (1 session). Rats were trained during the dark cycle. Sessions began with extension of the active lever and illumination of a red houselight. Rats were weighed and fed after the daily sessions.
Figure 1. Fear incubation procedure.
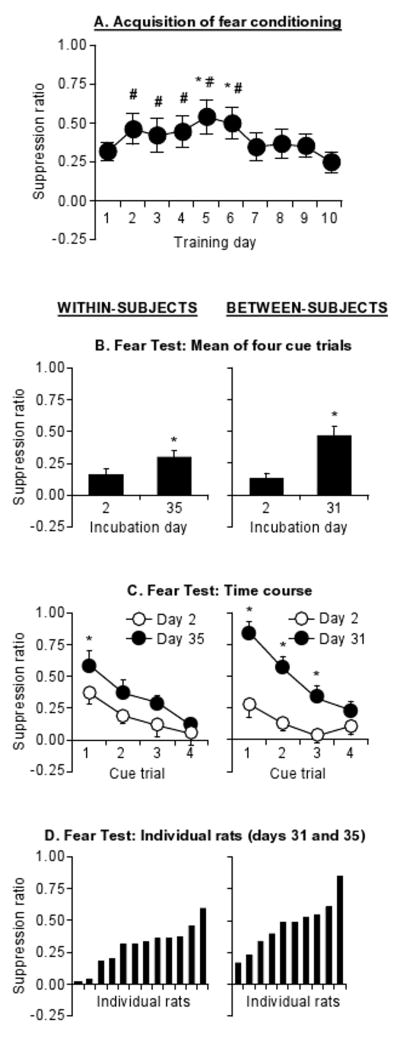
(A) Fear incubation protocol. (B) Acquisition of fear conditioning: suppression ratios (mean±sem) across 10 sessions of tone presentations for Exp. 1- 2. Predictable shock represents rats in Exp. 1 (n=52). Tone-only represents rats not exposed to shocks in Exp. 2 (n=21). Unpredictable shock represents rats given random tones and shocks in Exp. 2 (n=22). * and # p<0.05, different from Session 1 or Session 10, respectively.
Food self-administration training
On the first session, the rats were given 60-min magazine training (pellet delivery every 125 s). The following day, 2 fixed interval-1 (FI-1) schedule sessions (lever-presses could earn a pellet each sec) were run 2-4 h apart. These sessions ended when rats received 50 reinforcers (up to 1 h). A third session of up to 3 h was run immediately after the second session for rats that did not earn 50 pellets. All rats achieved 50 pellets in this session. The rats were then given one 90-min session in which pellets were earned under a variable interval-30 (VI-30) reinforcement schedule (pellet availability for lever-presses ranging from 1 to 59 s), and two daily 90-min sessions on a VI-60 schedule (pellet availability ranging from 1 to 119 s). Rats were maintained on the VI-60 schedule for the rest of the experiment.
Fear conditioning
Fear conditioning training was performed over 1 or 10 90-min sessions during which the rats were given ten 30-s tones (2900 Hz, 20 dB above background), ranging from 3 to 14 min apart and co-terminating with an electric shock (0.5-s, 0.5-mA, amplitude adjusted for inter-chamber variability), unless otherwise noted. Conditioned inhibition of lever-pressing for food pellets was our measure of fear (22). Lever-presses were recorded during the 30 s prior to tone presentation (Precue) and during the 30-s tone presentation (Cue), and converted into a suppression ratio: Suppression ratio = ((Precue-Cue)/(Precue+Cue)). The suppression ratio normalizes lever-pressing during the tone for baseline Precue responding (23; 24). A value of 1 indicates total conditioned suppression of lever-pressing during tone presentation (high fear). A value of 0 reflects no lever-press suppression during tone presentation (no fear). When both precue and cue values were 0 on a trial or session, the rat's responding in the trials immediately before and after that trial or session was averaged to determine a value. The rats assigned to the different incubation days in the different experiments were matched for their mean suppression ratio during training.
Conditioned fear testing
During the incubation periods, the food-restricted rats were weighed and handled 5-6 times per week. On the last day of each incubation period, lever-pressing was re-stabilized in a 90-min test session with no tones or shocks. The following day, conditioned fear to the tone was tested by presenting 4 30-s tones, without shock, over 35 min. The first tone occurred after 6.5 min and subsequent tones occurred after inter-trial intervals of 4, 7 and 11 min. The suppression ratios across the 4 extinction trials were used as our measure of conditioned fear in order to assess the strength of the incubated fear response over repeated trials and to avoid a potential ceiling effect of a high fear response on the first trial.
Exp. 1: Time course of fear incubation
We studied the time course of fear incubation in 4 groups of rats (n=10-15 per group). The rats were trained for fear conditioning over 10 sessions of tone-shock pairings, and then tested for conditioned fear 2, 15, 31, and 61 days (±1 day for days 30 and 60) after the last tone and shock exposure.
Exp. 2: Response to the tone cue after training with unpredictable shock or tone alone
We studied in 4 groups of rats (n=9-13 per group) the response to the tone cue 2 or 31 days after 10 training sessions in which the rats were either given unpredictable tone and shock presentations or tone-only presentations. During the 10-day training in the ‘unpredictable shock’ condition, 5 shocks out of 100 occurred at a random time during a tone, with the remainder occurring at times when the tone was not present, such that the tone gave no predictive information about the delivery of the shocks (25). The rats were exposed to the shock and the tone 10 times per session. In the ‘tone only’ condition, tones were presented 10 times (every 3 to 14 min) during each session but no shocks were given.
Exp. 3: Fear incubation after repeated testing
We studied whether rats (n=12) tested for conditioned fear 2 days after the end of training would show increased fear when retested one month (33 days) later. The rats were trained over 10 sessions of tone-shock pairings, as in Exp. 1. They were then tested for conditioned fear 2 and 35 days after the end of training.
Exp. 4: Fear incubation after long versus short training
We studied conditioned fear in 4 groups of rats (n=12 per group) after either short (1 day, 10 tone-shock pairings) or prolonged (10 days, 100 tone-shock pairings) fear conditioning training. Tests were conducted 2 or 31 days after training.
Results
Experiment 1: Time course of fear incubation
Training
During training, the rats showed an increase in fear over the first 4 daily sessions, followed by a decline (Training Session: F(9,432)=7.4, p<0.01, Fig. 1B).
Conditioned fear testing
Conditioned fear increased over days with higher levels on incubation days 31 and 61 than on days 2 and 15 (Fig. 2A, B), with only a brief increase in fear on the first trial on Day 15. The statistical analysis included the between-subjects factor of Incubation Day (days 2, 15, 31, and 61), and the within-subjects factor of Test Trial (the 4 tone presentations). This analysis revealed significant effects of Incubation Day (F(3,48)=13.7, p<0.01), Test Trial (F(3,144)=51.4, p<0.01), and an interaction between these factors (F(9,144)=2.6, p<0.01). The interaction effect reflects increased resistance to extinction of the fear cue response on days 31 and 61. There were no group differences in precue response rates (Supplementary Table 1).
Figure 2. Incubation of fear conditioning.
Suppression ratios (mean±sem) in different groups of rats at test. (A) Exp. 1. Mean of cue presentations 2, 15, 31, and 61 days after the last conditioning session (n=10-15 per day). (B) Exp. 1. Time course of individual cue presentations. (C) Exp. 2. Mean of cue presentations 2 or 31 days after the last conditioning session. Tone-only and Unpredictable shock represent rats not given shocks or rats given random tones and shocks during training, respectively (n=9-13 per day). * and # p<0.05, different from Day 2 and Day 31, respectively.
Exp. 2: Response to the tone cue after training with unpredictable shock or tone alone
Training
The rats trained with unpredictable shocks did not appear to show fear conditioning (Fig. 1B), but the analysis revealed a significant effect of Training Session (F(9,180)=1.9, p<0.05). This effect was due to a brief increase in conditioned suppression during Session 2. The rats trained with tones only did not change their response to the tone cue over days (p>0.05, Fig. 1B).
Conditioned fear testing
The response to the tone cue did not change over time in either the unpredictable shock condition or the tone only condition (Fig. 2C). The effects of Incubation Day, Test Trial or an interaction between the two factors were not significant for the unpredictable shock (p values>0.05) or the tone-only (p values>0.05). There was a small but significant difference in the precue lever-press rates in the unpredictable shock groups (supplementary Table 1); it is unlikely that this difference affected our results.
Experiment 3: Fear incubation with repeated testing
Training
During training, the rats showed increased fear until session 5, after which fear decreased (Training Session: F(9,99)=2.7, p<0.01, Fig. 3A).
Figure 3. Fear incubation with repeated testing.
Suppression ratios (mean±sem). (A) Acquisition of fear conditioning across 10 sessions of tone-shock presentations (n=12). * and # p<0.05, different from Session 1 and Session 10, respectively. (B) Mean of cue presentations 2 or 35 days after last training. (C) Time course of individual cue presentations in test. (D) Mean suppression ratio values of individual rats on Day 35. *p<0.05, different from Day 2 in (B) and (C). For comparison purposes, comparable data from a between-subjects assessment in Exp. 1 are provided in the right column.
Fear incubation testing
We found increases in fear over days with higher levels on incubation day 35 than on day 2 (Fig. 3B, C). The analysis revealed an effect of Incubation Day (F(1,11)=9.7, p<0.01) and Test Trial (F(3,33)=8.4, p<0.01) but no interaction (p>0.05).
Exp. 4: Fear incubation after long versus short training
Training
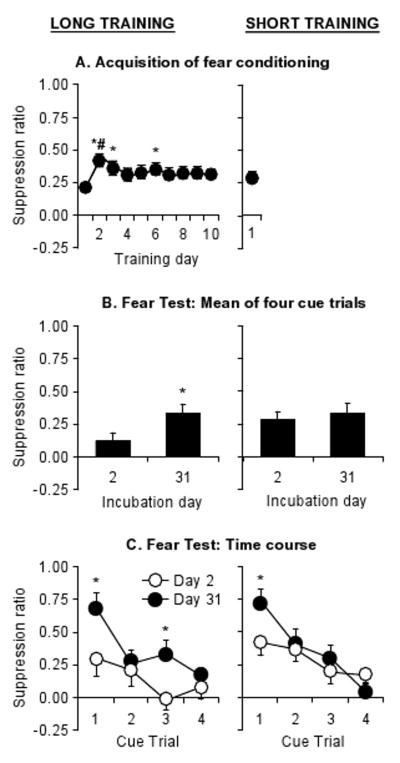
The rats in the long-training (10 d) condition showed increased fear over the first 2 daily sessions that modestly decreased over time (Fig. 4A). The analysis revealed an effect of Training Session (F(9,198)=2.3, p<0.05).
Figure 4. Fear incubation after short and long training.
Suppression ratios (mean±sem). (A) Acquisition of fear conditioning over 10 sessions (long training) or 1 session (short training) of tone-shock pairings (n=12 per group). * and # p<0.05, different from Session 1 and Session 10, respectively. (B) Mean of cue presentations 2 or 31 days after the last training session in the long (left column) and short (right column) training conditions. (C) Time course of individual cue presentations in test in long and short training conditions. *p<0.05, different from Day 2 in (B) and (C).
Fear incubation testing
Conditioned fear increased over days with higher values on Incubation Day 31 than on Day 2; the time-dependent increases in the fear response were significant after long (10 d) but not short (1 d) training (Fig. 4B). The analysis revealed significant effects of Incubation Day (F(1,44)=4.8, p<0.05), Test Trial (F(3,132)=22.3, p<0.01) and an interaction of Incubation Day × Test Trial (F(3,132)=4.8, p<0.01), but no effect of Training Length or other interactions (p values>0.05). A post-hoc analysis of the two groups in the long training condition (Fig. 4C) revealed significant effects of Incubation Day (F(1,22)=5.0, p<0.05) and Test Trial (F(3,66)=7.2, p<0.01), but no interaction (p>0.05). Analysis of the two groups in the short training condition (Fig. 4D) did not reveal a significant effect of Incubation Day (p>0.05), but did reveal significant effects of Test Trial (F(3,66)=20.2, p<0.01) and Incubation Day×Test Trial (F(3,66)=4.1, p<0.01); this interaction reflects the difference in suppression ratios on trial 1 that disappeared and even reversed on later trials. This rapid extinction suggests that unlike the robust fear incubation after long training, the fear incubation seen after short-training is weaker and shorter-lived.
Discussion
We described a procedure in which reliable and long-lasting fear incubation is observed for up to 2 months after training for fear conditioning. This fear incubation is also observed, albeit to a lesser degree, when rats are repeatedly tested. A critical variable for the emergence of fear incubation is the duration of fear conditioning training: sustained fear incubation is not reliably observed after short (1 day) training, an observation in agreement with previous reports (16; 18). We also showed that the time-dependent increases in the response to the tone cue are not due to non-associative factors.
Potential mechanisms of fear incubation
One potential mechanism for fear incubation is a spontaneous recovery-like process. In this regard, Golin and colleagues (8; 26) and Mednick (27) suggested that the emergence of fear incubation is due to time-dependent recovery from adaptation to repeated exposure to aversive stimuli. It is likely that adaptation to shock exposure during training occurred in our experiments, because responding to the tone cue gradually decreased during training days 4 to 10 (Fig 1B). How might decreased responding to shock and shock cues during training lead to incubation of fear?
Based on work of Bouton and colleagues (28), we speculate that the underlying mechanism is a process akin to spontaneous recovery; the resumption of the extinguished conditioned response that occurs after time has passed following the conclusion of extinction training (29; 30). Specifically, when rats become less sensitive to shocks after repeated exposure, the repeated pairing of tone with these less effective shocks causes reduced responding to the cue, similar to that which occurs during extinction when rats are exposed to tone without shock. This results in decreased responding to the tone cue late in training, as well as during day 2 fear testing. On incubation days 31 and 61, the fear response spontaneously recovered (similar to resumption of fear in traditional spontaneous recovery after extinction), leading to the emergence of fear incubation.
A spontaneous recovery-like interpretation of our data is a speculative notion, because our rats did not undergo extinction. However, this notion is in agreement with data of Bouton et al. (28), suggesting that factors causing resumption of conditioned responses after extinction also cause resumption of conditioned responses that are decreased during extensive CS-UCS pairing (often termed overtraining). Bouton et al. (28) reported that overtraining caused reduction in responding to shock cues, and this responding was restored when rats were exposed to the fear cue in a different context. This observation resembles the renewal phenomenon, the recovery of extinguished conditioned behavior that occurs when the context is changed after extinction (29). The putative spontaneous recovery in our fear incubation procedure and the renewal of the conditioned response with context switch in the overtraining experiment of Bouton et al. (28) may be related phenomena. As Bouton (31) suggested for resumption of extinction responding, spontaneous recovery is a special case of the renewal phenomenon in which time functions as a context.
The mechanisms underlying potential adaptation to shock during training are unknown. One potential mechanism is that shocks (32) or cues that predict shock (33) induce analgesia, which decreases the impact of additional shocks. Additionally, decreased overall responding to the cue may have occurred over sessions because rats have learned that shocks coincide with termination of the 30-sec tone, and thus only suppressed responding at the end of each tone presentation but kept normal responding during most of the tone presentation period (21; 34). However, we found no evidence for such graded responding during the cue presentations: in Exp. 1, lever-presses were similar in the first and last 10 sec of the tone (13.37±1.53 and 13.38±1.52 presses/min, respectively) on training session 10.
A potential neurobiological explanation for our finding of fear incubation is that after fear conditioning there are time-dependent increases in “gain control”, or amplification of neuronal responses to the fear cue (13), in brain areas responsible for the expression of fear, such as the central amygdala (CeA). The CeA is critical for conditioned suppression (35). Thus, time-dependent increases in CeA neurons' responsiveness to fear cues could cause increased fear responding. This idea is intriguing, because the CeA is involved in another incubation phenomenon termed incubation of cocaine or morphine craving, in which time-dependent increases in cue-induced drug seeking after withdrawal are observed (36-38). More generally, a question for future research is whether neurobiological mechanisms of incubation of reward craving (39-42) also contribute to fear incubation.
Fear incubation as a model of delayed-onset PTSD
Pavlovian fear conditioning and extinction procedures in rodents are regarded as valid animal models of PTSD (43), because PTSD is characterized by exaggerated response to trauma-associated cues (44; 45), and impaired fear extinction (46; 47). Support for this notion comes from the findings that dysregulated neuronal activity and anatomical abnormalities in two brain sites critical for conditioned fear and its extinction in rodents, the amygdala and ventral medial prefrontal cortex (48-50), are associated with human PTSD symptoms (51; 52).
However, standard fear conditioning procedures where rodents are exposed to acute stress on a single day and maximal conditioned fear emerges within 1-24 h after cue-shock exposure (48-50) may not be a suitable model for delayed-onset PTSD, which occurs in almost 40% of military PTSD cases and 15% of civilian cases (53). Interestingly, the literature suggests that the causes of civilian PTSD are primarily one-time traumatic events, whereas traumatic events during combat exposure that lead to PTSD tend to be chronic (53). Thus, we propose that our fear incubation procedure, in which rats are exposed to stress chronically over days and fear responses increase over time, can model symptoms of delayed-onset PTSD, in which mild symptoms become more severe over time (53). A word of caution, however, is that while our fear incubation procedure mimics one critical aspect of delayed-onset PTSD, a time-dependent increase in response to trauma-associated cues over time, it is unknown whether our procedure captures other delayed-onset PTSD symptoms, such as hyperarousal, exaggerated responses to trauma unrelated stressors, and social withdrawal (43; 44).
Finally, to the degree that standard conditioned fear procedures and fear incubation procedures model immediate-onset PTSD and delayed-onset PTSD, respectively, results from fear incubation studies can provide new information on whether similar or different mechanisms underlie these two variations of PTSD. Such knowledge can have implications for development of medications to treat PTSD and for understanding the variability in the response of PTSD patients to existing medications.
Supplementary Material
Acknowledgments
Research was supported by the National Institute on Drug Abuse, Intramural Research Program. The authors report no biomedical financial interests or potential conflicts of interest. We thank Drs. Mark Bouton, Brian Wiltgen, Gonzalo Urcelay, and Dan Wheeler for helpful comments on issues related to experimental procedures and data interpretation and Drs. Roger Pitman and Markus Heilig for helpful discussions on the applicability of the fear incubation model to PTSD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- 1.Diven K. Certain determinants in the conditioning of anxiety reactions. J Psychol Interdisciplinary and Applied. 1937;3:291–308. [Google Scholar]
- 2.Eysenck HJ. The conditioning model of neurosis. Behav Brain Sci. 1979;2:155–199. [Google Scholar]
- 3.Mowrer OH. A stimulus-response analysis of anxiety and its role as a reinforcing agent. Psychol Rev. 1939;46:553–565. [Google Scholar]
- 4.Watson JB, Rayner R. Conditioned emotional responses. J Exp Psychol. 1920;3:1–14. doi: 10.1037//0003-066x.55.3.313. [DOI] [PubMed] [Google Scholar]
- 5.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 7.McAllister DE, McAllister WR. Incubation of fear: an examination of the concept. J Exp Res Personality. 1967;2:180–190. [Google Scholar]
- 8.Golin S. Incubation effect: role of awareness in an immediate versus delayed test of conditioned emotionality. J Abnorm Soc Psychol. 1961;63:534–539. doi: 10.1037/h0043261. [DOI] [PubMed] [Google Scholar]
- 9.Bindra D, Cameron L. Changes in experimentallly poroduced anxiety with the passage of time: incubation effect. J Exp Psychol. 1953;45:197–203. doi: 10.1037/h0055125. [DOI] [PubMed] [Google Scholar]
- 10.Pinel JP, Mucha RF. Incubation and Kamin effects in the rat: changes in activity and reactivity after footshock. J Comp Physiol Psychol. 1973;84:661–668. doi: 10.1037/h0034890. [DOI] [PubMed] [Google Scholar]
- 11.McMichael JS. Incubation of anxiety and instrumental behavior. J Comp Physiol Psychol. 1966;61:208–211. doi: 10.1037/h0023148. [DOI] [PubMed] [Google Scholar]
- 12.Zammit-Montebello A, Black M, Marquis HA, Suboski MD. Incubation of passive avoidance in rats: shock intensity and pretraining. J Comp Physiol Psychol. 1969;69:579–582. [Google Scholar]
- 13.Houston FP, Stevenson GD, McNaughton BL, Barnes CA. Effects of age on the generalization and incubation of memory in the F344 rat. Learn Mem. 1999;6:111–119. [PMC free article] [PubMed] [Google Scholar]
- 14.Balogh SA, Radcliffe RA, Logue SF, Wehner JM. Contextual and cued fear conditioning in C57BL/6J and DBA/2J mice: context discrimination and the effects of retention interval. Behav Neurosci. 2002;116:947–957. doi: 10.1037//0735-7044.116.6.947. [DOI] [PubMed] [Google Scholar]
- 15.Balogh SA, Wehner JM. Inbred mouse strain differences in the establishment of long-term fear memory. Behav Brain Res. 2003;140:97–106. doi: 10.1016/s0166-4328(02)00279-6. [DOI] [PubMed] [Google Scholar]
- 16.Gleitman H, Holmes PA. Retention of incompletely learned CER in rats. Psychon Sci. 1967;7:19–20. [Google Scholar]
- 17.Hendersen RW. Forgetting and conditioned fear inhibition. Learn Motiv. 1978;9:16–30. [Google Scholar]
- 18.Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millenson JR, Dent JG. Habituation of conditioned suppression. Q J Exp Psychol. 1971;23:126–134. doi: 10.1080/00335557143000130. [DOI] [PubMed] [Google Scholar]
- 21.Rosas JM, Alonso G. The effect of context change upon long-term memory of CS duration. Behav Processes. 1997;39:69–76. doi: 10.1016/s0376-6357(96)00045-9. [DOI] [PubMed] [Google Scholar]
- 22.Estes WK, Skinner BF. Some quantitative properties of anxiety. J Exp Psychol. 1941;29:390–400. [Google Scholar]
- 23.Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE. Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cereb Cortex. 1997;7:157–165. doi: 10.1093/cercor/7.2.157. [DOI] [PubMed] [Google Scholar]
- 24.Annau Z, Kamin LJ. The conditioned emotional response as a function of intensity of the US. J Comp Physiol Psychol. 1961;54:428–432. doi: 10.1037/h0042199. [DOI] [PubMed] [Google Scholar]
- 25.Rescorla RA. Pavlovian conditioning and its proper control procedures. Psychol Rev. 1967;74:71–80. doi: 10.1037/h0024109. [DOI] [PubMed] [Google Scholar]
- 26.Golin S, Golin AK. Incubation and inhibition. J Exp Psychol. 1966;71:208–211. doi: 10.1037/h0022827. [DOI] [PubMed] [Google Scholar]
- 27.Mednick MT. Mediated generalization and the incubation effect as a function of manifest anxiety. J Abnorm Psychol. 1957;55:315–321. doi: 10.1037/h0044440. [DOI] [PubMed] [Google Scholar]
- 28.Bouton ME, Frohardt RJ, Sunsay C, Waddell J, Morris RW. Contextual control of inhibition with reinforcement: Adaptation and timing mechanisms. J Exp Psychol Anim Behav Process. 2008;34:223–236. doi: 10.1037/0097-7403.34.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouton ME, Swartzentruber D. Sources of relapse after extinction in Pavlovian and instrumental learning. Clin Psychol Rev. 1991;11:123–140. [Google Scholar]
- 30.Pavlov IP. Conditioned reflexes. London: Oxford University Press; 1927. [Google Scholar]
- 31.Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- 32.Maier SF. Determinants of the nature of environmentally induced hypoalgesia. Behav Neurosci. 1989;103:131–143. doi: 10.1037//0735-7044.103.1.131. [DOI] [PubMed] [Google Scholar]
- 33.MacLennan AJ, Jackson RL, Maier SF. Conditioned analgesia in the rat. Bull Psychon Soc. 1980;15:387–390. [Google Scholar]
- 34.Zielinski K. “Inhibition of delay” as a mechanism of the gradual weakening of the conditioned emotional response. Acta Biol Exp (Warsz) 1966;26:407–418. [PubMed] [Google Scholar]
- 35.Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- 36.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 37.Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM, et al. Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci. 2008;28:13248–13257. doi: 10.1523/JNEUROSCI.3027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56 1:177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47 1:214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 42.Uejima JL, Bossert JM, Poles GC, Lu L. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res. 2007;181:292–296. doi: 10.1016/j.bbr.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 43.Siegmund A, Wotjak CT. Toward an animal model of posttraumatic stress disorder. Ann N Y Acad Sci. 2006;1071:324–334. doi: 10.1196/annals.1364.025. [DOI] [PubMed] [Google Scholar]
- 44.APA. Diagnostic and statistical manual of mental disorders. 4th. 4th Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 45.Pitman RK, Orr SP, Shalev AY, Metzger LJ, Mellman TA. Psychophysiological alterations in post-traumatic stress disorder. Semin Clin Neuropsychiatry. 1999;4:234–241. doi: 10.153/SCNP00400234. [DOI] [PubMed] [Google Scholar]
- 46.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- 47.Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- 48.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 50.Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci. 1992;13:35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- 51.Bremner JD. Functional neuroimaging in post-traumatic stress disorder. Expert Rev Neurother. 2007;7:393–405. doi: 10.1586/14737175.7.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. Am J Psychiatry. 2007;164:1319–1326. doi: 10.1176/appi.ajp.2007.06091491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.