Abstract
Objective
To study the relationship between Alzheimer disease (AD) pathology and memory complaints proximate to death.
Methods
A group of 90 older persons underwent detailed clinical evaluations and brain autopsy at death. The evaluations included administration of questions on subjective memory complaints and clinical classification of dementia and AD. On postmortem examination, neuritic plaques, diffuse plaques, and neurofibrillary tangles in tissue samples from five cortical regions were counted, and a summary measure of overall AD pathology was derived. In addition, amyloid load and tau tangles were quantified in eight regions.
Results
In multiple linear regression models adjusted for age, sex, and education, memory complaints were associated with AD pathology, including both amyloid and tau tangles. Subsequent analyses demonstrated that the relationship between memory complaints and AD pathology was present in those with and without dementia, and could not be explained by the potentially confounding effects of depressive symptoms or coexisting common chronic health problems.
Conclusion
Memory complaints in older persons may indicate self awareness of a degenerative process.
Subjective memory complaints are common in older adults. Most studies that have attempted to address the significance of memory complaints have relied on clinical investigations that relate memory complaints to future cognitive decline or diagnosis of dementia. These studies have reported mixed results, with some,1-3 but not all,4,5 finding an association between memory complaints and dementia. Discrepant results may be due, in part, to the fact that memory complaints are associated with other common conditions of aging, such as depression,6,7 and there has been wide variability in how memory complaints are assessed. Interestingly, in spite of the controversy, some diagnostic criteria for mild cognitive impairment, a risk factor for dementia in older persons, include subjective memory complaints.8
Because Alzheimer disease (AD) pathology may be present in persons without dementia and may take years to express itself, the relationship between complaints and pathology may better address this issue. We are aware of only one study that examined whether memory complaints are related to AD pathology, specifically plaques and tangles, in the brain, but there were several years between assessment of memory complaints and autopsy.9 We used clinical and postmortem data from the Memory and Aging Project, a longitudinal clinicopathologic study of aging and dementia, to examine whether pathologic indices of AD were related to memory complaints determined proximate to death in older persons.
Methods
Participants
Subjects were participants in the Rush Memory and Aging Project, an ongoing longitudinal clinicopathologic study of common chronic conditions of old age. Participants were recruited from approximately 40 senior residential facilities throughout the Chicago metropolitan area. The study involves annual clinical evaluations and brain donation at death. It was approved by the Institutional Review Board of Rush University Medical Center.
Since September 1997, 1,061 persons have completed the baseline clinical evaluation and participation in follow-up has exceeded 91% in survivors. At the time of these analyses, 149 persons had died and 116 (78%) had undergone brain autopsy, and data from the postmortem evaluation were completed for the first 99 (there is a lag between death and completion of the postmortem evaluation).
Because we wanted to examine the relation between AD pathology and subjective memory proximate to death, only persons without dementia (n = 67) and those with clinically diagnosed AD (n = 24) were included in the analyses. Persons with clinical conditions other than AD (e.g., Parkinson disease, delirium, or stroke) thought to be causing or contributing to cognitive impairment (n = 3) or dementia (n = 6) were excluded from all analyses. One additional person was excluded due to missing data on memory complaints. Thus, of 99 with postmortem data, 90 men and women were included in these analyses.
Clinical evaluation
Details of the clinical evaluation have been reported previously.10 Briefly, at baseline, each participant had a uniform evaluation that included the procedures recommended by the Consortium to Establish a Registry for AD.11 Follow-up evaluations, identical in all essential details, were performed annually by examiners blinded to previously collected data. The evaluation included a medical history and neurologic and neuropsychological examinations. Diagnoses were made using a three-step process. First, neuropsychological tests were administered by trained technicians and scores were adjusted based on education. Second, a board-certified clinical neuropsychologist reviewed the cognitive results along with information on sensory and motor deficits, and effort, and made a clinical judgment regarding the presence of cognitive impairment. Finally, participants were evaluated by a physician with expertise in the evaluation of older adults with and without dementia. Based on review of all available data, participants were classified with respect to AD and other common neurologic disorders that can contribute to cognitive impairment using the criteria of the joint working group of the National Institute of Neurologic and Communicative Disorders and Stroke and the AD and Related Disorders Association.12 At the time of death, all available years of clinical data were reviewed blinded to postmortem data, and a summary diagnostic opinion was rendered regarding the most likely clinical diagnosis at the time of death.
Assessment of memory complaints
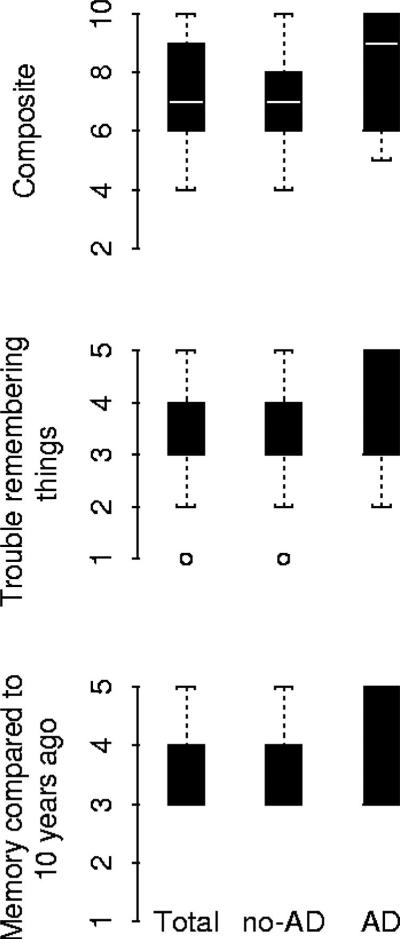
Two questions were used to assess memory complaints at each annual interview, both rated on a 5-point Likert scale: 1) How often do you have trouble remembering things, with responses ranging from 5 = very often to 1 = never; 2) How is your memory compared to 10 years ago, with responses ranging from 5 = much worse to 1 = much better. Previous studies have successfully used scales of this type with older adults.13,14 Although the memory complaints questions were asked every year, only the last valid scores proximate to death were used in analyses. To create a composite measure based on the scores of these two questions, we summed the two scores for each person (range of possible values was 2 to 10 but in the data we observed a range = 4 to 10); higher scores indicate more memory complaints (figure 1).
Figure.
Box plots showing the median values for the composite score and the two memory complaints questions in the total sample, no Alzheimer disease (AD), and AD subgroups.
Neuropathologic evaluation
Brains were removed in a standard fashion and cut coronally using a Plexiglas jig into 1 cm slabs as described previously.15,16 Slabs from one hemisphere were fixed for 3 days in 4% paraformaldehyde. Blocks of tissues from the hippocampus (CA-1), entorhinal cortex, midfrontal cortex, middle temporal cortex, and inferior parietal cortex were paraffin-embedded, sectioned at 6 μm, and stained with a modified Bielschowsky silver stain.15,16
Neuritic plaques, diffuse plaques, and neurofibrillary tangles were each counted in a 1 mm2 microscopic field using an Olympus BX41 microscope, 10× objective and 10× eyepiece graticule. In each region, the field with the greatest amount of pathology for that particular pathology was counted such that there were 3 measures from each slide and 18 counts total per case as previously described.15 In order to get an overall indicator of the burden of AD pathology with minimal measurement error, a summary measure of AD pathology was used in analyses. The summary measure was derived by converting the 15 raw scores from each case into standard scores. The standardized score for each pathologic index (e.g., midfrontal neuritic plaques) was obtained by dividing the raw score by the SD of the mean score for that index from that region from the entire deceased cohort. The scores across regions for that pathologic index were averaged to create a standardized score for each pathologic index. Finally, the summary scores across regions for each pathologic index (e.g., neuritic plaques, diffuse plaques, or neurofibrillary tangles) were averaged to yield the global measure of AD pathology.
The validity of the global AD pathology summary measure was assessed previously by comparing it to other established methods commonly used to stage and classify AD pathology, including the CERAD system,17 NIA Reagan Institute criteria,18 and Braak staging,19 and found to be a reliable and metrically sound method of summarizing the traditional pathologic hallmarks of AD.
We also dissected blocks of paraformaldehyde fixed tissue from the hippocampus (CA-1/subiculum), entorhinal cortex, anterior cingulate, midfrontal cortex, superior frontal cortex, inferior temporal cortex, angular gyrus, and calcarine cortex. These were paraffin-embedded, sectioned at 20 μm, and stained with antibodies to amyloid beta protein (10D5, 1:1,000, courtesy of Elan Pharmaceuticals) and to the tau protein, paired helical filament (AT8; 1:1,000, Pierce Endogen). Amyloid load is quantified by image analysis and tau tangles by stereology as previously described.15 Briefly, for both procedures, the region of interest is outlined at low power using computer-driven videomicroscopy (MicroBright-field StereoInvestigator 5.0 software, Cochester, VT) and a systematic sampling grid is overlaid on the video image. For amyloid quantification, the microscope lens objective is raised to 20× and an image at each grid intersection is captured to give approximately 50 to 70 images per slide. The percent area occupied by amyloid is measured by an automatic segmentation of these lesions using an algorithm, an approach that relies on automatic arbitration between two intensity-based thresholders. For tangles, a video random systematic sampling grid is used along with an optical fractionation counting box using Stereoinvestigator version 5. The measures for the percent area of amyloid and density of tangles are averaged across regions to create summary measures for both amyloid and tangles.
Other variables
Other variables used in the analysis include age, sex, and education. We also considered potential risk factors for subjective memory complaints, including depressive symptoms and a measure of chronic health. Depressive symptoms were assessed with a 10-item short form20 of the Center for Epidemiologic Studies Depression (CES-D) scale.21 Because some persons may have had AD at the last clinical evaluation, and therefore may not have been able to reliably report on their mood, we used an average score for each participant across all follow-up evaluations in analyses. Additional analyses using the first and last CES-D score in place of the average CES-D score yielded similar results. Seven medical conditions were identified in at least 5% of study participants at baseline: hypertension, diabetes, heart disease, cancer, thyroid disease, head injury with loss of consciousness, and cerebrovascular disease. The number of these conditions present at any time during the study was used as an index of chronic illness, as previously described.22 We used 19 cognitive function tests to assess functions commonly affected by aging and AD (e.g., episodic memory, working memory). To minimize floor and ceiling effects and other sources of measurement error, we used a composite measure of all 19 tests in analyses. We converted raw scores on each individual test to z scores, using the baseline mean and SD of all participants, and averaged the z scores. Further information about the individual tests and the derivation of the global cognitive measure is published elsewhere.15,16,23
Statistical analysis
Analyses were carried out in SAS,24 and models were validated graphically and analytically. We constructed multiple linear regression models with memory complaints as the outcome and the composite measure of AD pathology as the predictor. All regression models included terms for the potentially confounding effects of age, sex, and education.
Multiple linear regression analyses were performed to examine the relation of AD pathology to memory complaints. The primary model included terms for AD pathology, age, sex, and education. In subsequent models, we examined other factors that may confound the relation between AD pathology and memory complaints. In separate models, each with terms for age, sex, education, and global AD pathology, we added terms for number of depressive symptoms and number of chronic health problems. Additional multiple linear regression analyses were performed to determine whether memory complaints were related to the specific deposition of amyloid or tau tangles. Because the interval between the last clinical evaluation and the autopsy may affect measurement of AD pathology, we repeated each of these models and included a term for clinical interval.
To determine whether memory complaints may differ in persons with and without AD, core models were repeated in those two groups. As an additional test of this hypothesis, a Pearson correlation was conducted to examine the relation between memory complaints and level of global cognitive function proximate to death.
Results
Sample characteristics are shown in table 1. The mean age at death was slightly over 87 years and more than half of the cohort were women. The median subjective memory score was 7.0 (range = 4 to 10). The average interval from last clinical interview and autopsy was 6.8 months (SD = 4.03; range = 0.2 to 17.3).
Table 1.
Selected characteristics of 90 deceased participants separated by dementia status
| Characteristics | AD, n = 23 | Non-AD, n = 67 | Total sample, n = 90 |
|---|---|---|---|
| Mean age at death, y (SD) | 89.9 (4.9) | 86.1 (5.9) | 87.1 (5.9) |
| Women, % | 48 | 58 | 56 |
| Mean education, y (SD) | 14.9 (3.3) | 14.1 (3.3) | 14.3 (3.3) |
| Mean PMI, mo (SD) | 8.1 (9.5) | 9.3 (10.5) | 6.8 (4.0) |
| Mean clinical interval,* mo (SD) | 12.6 (10.6) | 8.3 (5.9) | 9.4 (7.6) |
| Median memory complaints score (range) | 9.0 (5−10) | 7.0 (4−10) | 7.0 (4−10) |
| Mean no. of depressive symptoms (SD) | 1.8 (2.0) | 1.7 (1.6) | 1.8 (1.7) |
| Mean no. of chronic health conditions (SD) | 1.1 (1.0) | 1.8 (1.0) | 1.6 (1.1) |
| Mean AD pathology summary score (SD) | 1.3 (0.56) | 0.51 (0.53) | 0.70 (0.64) |
| Mean amyloid, % burden (SD) | 6.4 (3.9) | 2.9 (4.1) | 3.8 (4.3) |
| Mean tau tangles, no./mm2 (SD) | 13.7 (11.6) | 3.6 (4.4) | 6.2 (8.3) |
Interval between last clinical evaluation and death.
PMI = postmortem interval; AD = Alzheimer disease.
We examined the relation of AD pathology to memory complaints proximate to death using linear regression models adjusted for age, sex, and education (table 2, model A). Each unit of AD pathology was associated with about a one point higher memory complaint score (p = 0.002) proximate to death, and this did not vary by age, education, or sex. Findings were essentially unchanged after accounting for depressive symptoms (table 2, model B) and number of chronic health conditions (table 2, model C).
Table 2.
Summary of linear regression models examining the association of Alzheimer disease pathology and memory complaints in 90 persons with and without dementia
| Model term* | Estimated effect of AD pathology | SE | p Value |
|---|---|---|---|
| Model A: AD pathology | 0.88 | 0.28 | 0.002 |
| Model B: Adjustment for depressive symptoms | 0.87 | 0.28 | 0.003 |
| Model C: Adjustment for chronic health problems | 0.83 | 0.29 | 0.006 |
| Model D: Amyloid (%) | 0.12 | 0.05 | 0.01 |
| Model E: Tau tangles (no./mm2) | 0.10 | 0.02 | <0.001 |
See text for derivation of summary measures for Alzheimer disease (AD) pathology, amyloid, and tau tangles. All models control for age, sex, and education. Memory complaints is the outcome variable.
To assess whether the relation between AD pathology and memory complaints was related specifically to deposition of one or both of the pathologic markers of AD, we repeated the analyses using a summary measure for amyloid deposits and tangles in place of the global measure of AD pathology. As shown in table 2, each 1% increase in amyloid load and each tau-positive neurofibrillary tangle was associated with about a tenth of a point higher memory complaint score (p = 0.01 and p < 0.001). These associations were essentially unchanged in analyses that controlled for depressive symptoms and number of chronic health conditions (data not shown).
Because the interval between the last clinical evaluation and the autopsy may have affected measurement of AD pathology, we repeated each of the core models and included a term for clinical interval. The results were essentially unchanged.
Both the magnitude and significance of memory complaints may differ in persons with and without AD. Therefore, we examined the relationship between AD pathology and memory complaints in the group with no dementia and, separately, among those with clinically diagnosed AD proximate to death. Twenty-three persons met clinical criteria for probable AD, and 67 people did not have dementia proximate to death. Using multiple linear regression analyses that controlled for age, sex, and education, we found in the 67 persons without dementia, AD pathology was related to memory complaints (estimate = 0.69, SE = 0.35, p = 0.05), and the results were not substantially changed after controlling for depressive symptoms or chronic health conditions (results not shown). Similarly, both amyloid and tau tangles were associated with memory complaints in the non-demented group (amyloid estimate = 0.10, SE = 0.05, p = 0.05; tangles estimate = 0.09, SE = 0.04, p = 0.03). In secondary analyses that controlled for the interval between the last clinical evaluation and autopsy, the results were similar for the association between AD pathology and memory complaints and amyloid and memory complaints, but each only approached significance. The results for tau tangles and memory complaints were essentially unchanged after controlling for clinical interval (p = 0.02).
We repeated these analyses in persons with dementia. Although we did not find a relationship between the summary measure of AD pathology (estimate = 0.60, SE = 0.79, p = 0.44) or amyloid load (estimate = 0.17, SE = 0.11, p = 0.12) with memory complaints in persons with clinically diagnosed AD, we did find that the number of tau-positive neurofibrillary tangles was associated with memory complaints (estimate = 0.09, SE = 0.03, p = 0.02). This finding was not substantially changed after controlling for depressive symptoms, chronic health conditions, or interval between last clinical evaluation and autopsy (results not shown).
These results suggest that older persons both with and without dementia possess insight to their level of cognitive functioning. To explore this hypothesis further, we examined the correlation between level of global cognitive function proximate to death and memory complaints in both the demented and non-demented groups. The relation was significant for both groups (non-demented r = −0.34; demented r = −0.49).
Discussion
In this clinicopathologic study of 90 older persons, we found that subjective memory complaints were associated with AD pathology, assessed with histochemistry, and with measures of amyloid and tau tangles using molecular specific antibody immunohistochemistry. This association was not accounted for by depressive symptomatology or chronic health conditions. In secondary analyses, we found that an association between memory complaints and AD pathology was present in both persons with and without clinically diagnosed AD. These findings suggest that memory complaints in older persons should be taken seriously because they may indicate self-awareness of a degenerative pathologic process.
Most previous studies have investigated memory complaints and future dementia with neuroimaging, and results have been mixed.1-5,25-28 These studies differed widely on how memory complaints were measured, what confounding variables were included in the analyses, and how cognitive impairment or dementia was assessed. To our knowledge, only one previous study has examined the relation between memory complaints and neuropathologically diagnosed AD.9 Similar to our study, they found that persons with memory complaints who were free from dementia or cognitive impairment were more likely to have neuropathologically diagnosed AD at autopsy. However, their study consisted only of Japanese American men and assessed memory complaints only at baseline, an average of 6 years before autopsy. In the current study, we assessed both men and women and memory complaints were assessed on average 9 months proximate to death.
While opinions have differed in the research community as to the importance of subjective memory complaints in older adults, they are often assumed to be related to depression, stress, or other personality traits26,29,30 rather than signifying the presence of underlying brain disease. Our results suggest that community-dwelling older persons with and without dementia possess some insight to their level of functioning, and this insight is related to actual changes in the brain. Consistent with this idea, one imaging study found that memory complaints in patients without any cognitive impairment were associated with smaller hippocampal volumes25 (although another study did not find a relationship26), suggesting that memory complaints may reflect actual brain disease.28 Awareness of this relationship may allow for earlier intervention by family members for safety and planning and by physicians for diagnosis and treatment. The association between memory complaints and AD pathology will become especially important as we develop better preventions and therapies that target early disease.
Several factors increase confidence in the findings. First, participants were community-dwelling men and women from a single cohort study, with high follow-up participation and brain autopsy. Second, analyses controlled for potentially confounding demographic (i.e., age, education, sex) and health (i.e., depressive symptoms, chronic conditions) factors. Third, uniform structured procedures were followed with blinding to previously collected clinical data as well as to postmortem data, reducing the potential for bias. Finally, both traditional silver staining and more sensitive immunohistochemical techniques were used to ascertain AD pathology, further strengthening confidence in our results.
This study has limitations. First, our sample was relatively small. A larger sample size, particularly for persons with clinically diagnosed AD, would have increased our power and ability to detect an association between memory complaints and AD pathology. It is also possible that ceiling effects on the memory complaints composite measure, at least in the AD subgroup, limited our ability to detect an association between complaints and AD pathology. Second, clinicopathologic studies are subject to survival bias. Persons who die with memory complaints in a clinicopathologic study may represent an unusual subset of persons, and therefore may limit our ability to generalize to the wider population.
Acknowledgment
The authors thank the residents from the following groups participating in the Rush Memory and Aging Project: Fairview Village, Wyndemere, Luther Village, The Holmstad, Windsor Park Manor, Covenant Village, Bethlehem Woods, King-Bruwaert House, Friendship Village, Mayslake Village, The Moorings, WA Jane Smith, Victory Lakes, Village Woods, Franciscan Village, Victorian Village, The Breakers of Edgewater, The Oaks, St. Paul Home, The Imperial, Frances Manor, Peace Village, Alden Waterford, Marian Village, The Birches, Elgin Housing Authority, Renaissance, Holland Home, Trinity United Church Of Christ, St. Andrews-Phoenix, Green Castle, Kingston Manor, Lawrence Manor, Community Renewal-Senior Ministry, Garden House, and the residents of the Chicago metropolitan area. They also thank Traci Colvin, MPH, and Tracy Hagman for coordinating the study, Hye-Jin Nicole Kim and Todd Beck, MS, for statistical programming, and George Dombrowski, MS, and Greg Klein for data management.
Supported by National Institute on Aging Grants R01AG17917 and R01AG22018, and the Illinois Department of Public Health.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Schmand B, Jonker C, Hooijer C, Lindeboom J. Subjective memory complaints may announce dementia. Neurology. 1996;46:121–125. doi: 10.1212/wnl.46.1.121. [DOI] [PubMed] [Google Scholar]
- 2.Schmand B, Jonker C, Geerlings MI, Lindeboom J. Subjective memory complaints in the elderly: depressive symptoms and future dementia. Br J Psychiatry. 1997;171:373–376. doi: 10.1192/bjp.171.4.373. [DOI] [PubMed] [Google Scholar]
- 3.Geerlings MI, Jonker C, Bouter LM, Ader HJ, Schmand B. Association between memory complaints and incident Alzheimer's disease in elderly people with normal baseline cognition. Am J Psychiatry. 1999;156:531–537. doi: 10.1176/ajp.156.4.531. [DOI] [PubMed] [Google Scholar]
- 4.Schofield PW, Jacobs D, Marder K, Sano M, Stern Y. The validity of new memory complaints in the elderly. Arch Neurol. 1997;54:756–759. doi: 10.1001/archneur.1997.00550180064014. [DOI] [PubMed] [Google Scholar]
- 5.Jorm AF, Christensen H, Korten AE, Henderson AS, Jacomb PA, Mackinnon A. Do cognitive complaints either predict future cognitive decline or reflect past cognitive decline? A longitudinal study of an elderly community sample. Psychol Med. 1997;27:91–98. doi: 10.1017/s0033291796003923. [DOI] [PubMed] [Google Scholar]
- 6.McGlone J, Gupta S, Humphrey D, Oppenheimer S, Mirsen T, Evans DR. Screening for early dementia using memory complaints from patients and relatives. Arch Neurol. 1990;47:1189–1193. doi: 10.1001/archneur.1990.00530110043015. [DOI] [PubMed] [Google Scholar]
- 7.Grut M, Jorm AF, Fratiglioni L, Forsell Y, Viitanen M, Winblad B. Memory complaints of elderly people in a population survey: variation according to dementia stage and depression. J Am Geriatr Soc. 1993;41:1295–1300. doi: 10.1111/j.1532-5415.1993.tb06478.x. [DOI] [PubMed] [Google Scholar]
- 8.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 9.Jorm AF, Masaki KH, Davis DG, et al. Memory complaints in nondemented men predict future pathologic diagnosis of Alzheimer disease. Neurology. 2004;63:1960–1961. doi: 10.1212/01.wnl.0000144348.70643.f2. [DOI] [PubMed] [Google Scholar]
- 10.Bennett DA, Schneider JA, Buchman AS, Mendes de LC, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 11.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDSADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, van Belle G, Crane PK, et al. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc. 2004;52:2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x. [DOI] [PubMed] [Google Scholar]
- 14.Bunch TJ, White RD, Smith GE, et al. Long-term subjective memory function in ventricular fibrillation out-of-hospital cardiac arrest survivors resuscitated by early defibrillation. Resuscitation. 2004;60:189–195. doi: 10.1016/j.resuscitation.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Bennett DA, Schneider JA, Arnold SE, Tang Y, Wilson RS. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 16.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based clinical-pathologic studies. Neurology. 2006 doi: 10.1212/01.wnl.0000219668.47116.e6. (in press) [DOI] [PubMed] [Google Scholar]
- 17.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 18.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 19.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 20.Kohout F, Berkman L, Evans D, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 21.Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 22.Wilson RS, Mendes de Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 23.Wilson RS, Barnes LL, Kreuger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11:400–407. [PubMed] [Google Scholar]
- 24.SAS Institute Inc. SAS OnlineDocR 9.1.3. SAS Institute Inc.; Cary, NC: 2004. [Google Scholar]
- 25.van der Flier WM, van Buchem MA, Weverling-Rijnsburger AW, et al. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol. 2004;251:671–675. doi: 10.1007/s00415-004-0390-7. [DOI] [PubMed] [Google Scholar]
- 26.Jorm AF, Butterworth P, Anstey KJ, et al. Memory complaints in a community sample aged 60−64 years: associations with cognitive functioning, psychiatric symptoms, medical conditions, APOE genotype, hippocampus and amygdala volumes, and white-matter hyperintensities. Psychol Med. 2004;34:1495–1506. doi: 10.1017/s0033291704003162. [DOI] [PubMed] [Google Scholar]
- 27.Minett TS, Dean JL, Firbank M, English P, O'Brien JT. Subjective memory complaints, white-matter lesions, depressive symptoms, and cognition in elderly patients. Am J Geriatr Psychiatry. 2005;13:665–671. doi: 10.1176/appi.ajgp.13.8.665. [DOI] [PubMed] [Google Scholar]
- 28.Ercoli L, Siddarth P, Huang SC, et al. Perceived loss of memory ability and cerebral metabolic decline in persons with the Apolipoprotein E-IV genetic risk for Alzheimer Disease. Arch Gen Psychiatry. 2006;63:442–448. doi: 10.1001/archpsyc.63.4.442. [DOI] [PubMed] [Google Scholar]
- 29.Wolf OT, Dziobek I, McHugh P, et al. Subjective memory complaints in aging are associated with elevated cortisol levels. Neurobiol Aging. 2005;26:1357–1363. doi: 10.1016/j.neurobiolaging.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Smith GE, Petersen RC, Ivnik RJ, Malec JF, Tangalos EG. Subjective memory complaints, psychological distress, and longitudinal change in objective memory performance. Psychol Aging. 1996;11:272–279. doi: 10.1037//0882-7974.11.2.272. [DOI] [PubMed] [Google Scholar]