Abstract
The mammalian respiratory system, consisting of both trachea and lung, initiates from the foregut endoderm. The molecular program that instructs endodermal cells to adopt the respiratory fate is not fully understood. Here we show that conditional inactivation of β-Catenin (also termed Ctnnb1) in foregut endoderm leads to absence of both the trachea and lung due to a failure in maintaining the respiratory fate. In converse, conditional expression of an activated form of β-Catenin leads to expansion of Nkx2.1, an early marker for the trachea and lung, into adjacent endoderm including the stomach epithelium. Analyses of these mutants show that the loss or gain of trachea/lung progenitor identity is accompanied by an expansion or contraction of esophagus/stomach progenitor identity, respectively. Our findings reveal an early role for β-Catenin in the establishment of respiratory progenitors in mouse foregut endoderm.
Keywords: FGF, lung, progenitor cells, Wnt
Initiation of the respiratory system, namely the trachea and lung, is achieved in two steps: specification and morphogenesis. Specification is the process whereby respiratory progenitors become distinct from neighboring endodermal cells. In mouse, these progenitors reside in the ventral portion of the foregut tube adjacent to heart,and are first defined by their expression of the homeodomain-containing transcription factor gene Nkx2.1 (1, 2). Following the emergence of these progenitors, morphogenesis ensues. Epithelium at the caudal portion of the respiratory primordium evaginates into the surrounding splanchnic mesenchyme, forming the primary lung buds. Shortly thereafter, epithelium at the rostral portion of the primordium separates from the dorsal foregut and develops into a tube, forming the trachea.
In recent years, the molecular program that controls trachea/lung specification and morphogenesis has begun to be elucidated in mouse. In vitro culture experiments show that signals emanating from the cardiac mesoderm induce trachea/lung specification (3). In particular, fibroblast growth factors (FGFs) from the cardiac mesoderm serve to pattern the adjacent endoderm in a threshold-dependent manner. Cells closest to the cardiac mesoderm receive the highest amount of FGF signal and are induced to form trachea/lung. Cells positioned more caudally receive a lower amount of FGF signal and develop into the liver (4). Finally, cells out of range of the signal adopt the pancreas fate as a default. FGF signaling also plays a critical role in lung morphogenesis. FGF10, one of the FGF family members, is expressed in the mesenchyme adjacent to the lung primodium and likely acts as a chemoattractant for the evagination of the primary lung buds (5, 6). In support of this role, inactivation of either Fgf10, or its obligate receptor gene Fgf receptor 2 (Fgfr2, specifically splice variant IIIb) leads to a failure of lung budding even though respiratory specification occurs as indicated by the presence of the trachea (7–9). Recent data suggest that Fgf10 expression in the mesenchyme is regulated by retinoic acid (RA) and transforming growth factor β (TGFβ) signaling, implicating these pathways in trachea/lung initiation (10).
WNT signaling has been shown to play key roles in lung development. Several WNT ligands are expressed in the developing lung, including the Wnt2 (also known as Wnt2a), Wnt2b (also known as Wnt13), Wnt5a, Wnt7b, and Wnt11 genes (5, 11–15). These ligands have been shown to signal through different downstream pathways. In this study, we will focus on the WNT/β-Catenin (also referred to as canonical WNT) pathway where β-Catenin (also known as Ctnnb1-Mouse Genome Informatics) acts as a critical transcriptional mediator of WNT signaling (16). Inactivation of β-Catenin in lung epithelium after lung budding leads to aberrant epithelial branching and proximal-distal patterning (17, 18). Inactivation of β-Catenin in lung mesenchyme leads to decreased mesenchymal growth and a defect in endothelial differentiation (19, 20). Finally, a recent study shows that inactivation of β-Catenin during trachea/lung morphogenesis leads to shortening of the trachea and reduced lung size (21).
Although multiple studies have demonstrated the requirement for β-Catenin at later stages of lung development, whether it is required for initiation of the respiratory lineage has not been directly addressed. In this study, we show that inactivation of β-Catenin in the ventral foregut endoderm results in absence of both trachea and lung. Analysis of this phenotype led to the conclusion that β-Catenin is not required for cell survival or proliferation, but rather is essential for maintaining the respiratory fate. Furthermore, we show that conditional activation of β-Catenin in the endoderm leads to expansion of the respiratory characteristics into the anterior stomach. Our findings suggest that β-Catenin promotes the respiratory identity in mouse.
Results
Inactivation of β-Catenin in Early Mouse Foregut Endoderm.
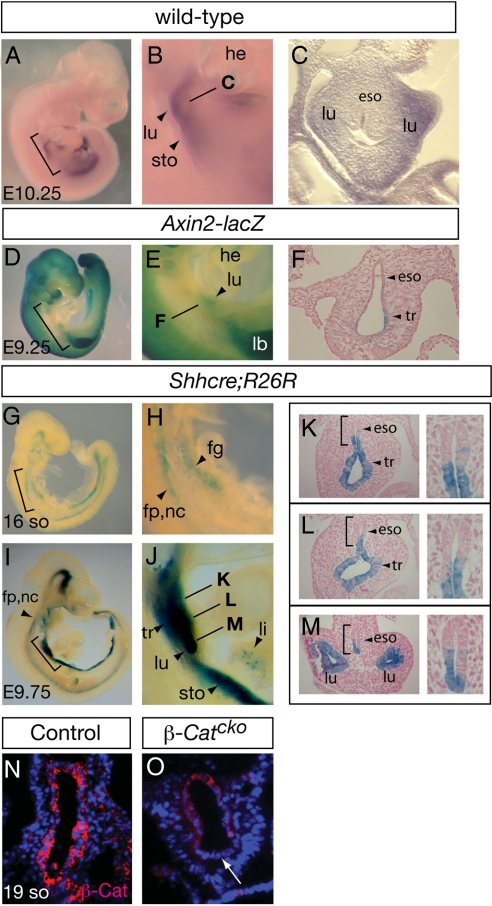
To determine whether WNT/β-Catenin signaling is active in mouse foregut during respiratory initiation, we examined the expression of a number of Wnt genes for their expression in the foregut region. Wnt2 has been shown to be expressed in lung mesenchyme at branching stages (22). We found that at the budding stage, it is present in the mesenchyme adjacent to nascent lung buds (Fig. 1 A–C), consistent with its earlier expression in the cardiac crescent next to the foregut (23). To further address if WNTs including WNT2 lead to productive signaling in the foregut, we used an Axin2-lacZ transgenic line, a reporter strain for WNT activity (24). We found that at embryonic day (E) 9.25 which is before lung initiation, β-galactosidase (β-gal) activity is detected in the prospective respiratory region of Axin2-lacZ embryos (Fig. 1 D–F). This result is consistent with observations made in other reporter strains of WNT activity shortly after lung budding (18, 25–28). Sections of Axin2-lacZ embryos show that β-gal activity is restricted to the ventral portion of the foregut which will form trachea/lung, but is absent in the dorsal portion, which will form the esophagus.
Fig. 1.
WNT/β-Catenin signaling and β-Catenin inactivation in the foregut using Shhcre. (A–C) Wnt2 expression as determined by RNA in situ hybridization in E10.25 embryos. Bracketed region in A is magnified in B, and line in B indicates the approximate level of transverse section shown in C. Axin2 is expressed in the ventral foregut mesenchyme adjacent to nascent lung buds. (D–M) β-galactosidase (β-gal) staining in Axin2-lacZ (D–F) or Shhcre;R26R embryos (G–M) at stages indicated. Bracketed regions in D, G, and I are enlarged in E, H, and J, respectively. Lines in E and J indicate approximate level of transverse sections shown in F and K–M. Bracketed regions in the left panels of K–M are magnified in the corresponding right panels. Arrowhead in E indicates Axin2-lacZ activity in the prospective respiratory region. Shhcre activity in the foregut is first detected at the 16-so stage (≈E8.75), around the time of specification. By E9.75 (after lung budding), its activity is detected in the primary lung buds, trachea, ventral esophagus, ventral stomach, intestine and isolated cells in the liver primordium. (N and O) Anti-β-Catenin antibody staining in transverse sections of 19-so stage embryos at the foregut level. Arrow in O indicates diminished β-Catenin staining in the ventral foregut epithelium of the β-Catcko mutant lung. For transverse sections shown in all figures, dorsal is up and ventral is down. Abbreviations: eso, esophagus; fg, foregut; fp, floor plate; he, heart; lb, limb bud; li, liver; lu, lung; nc, notochord; sto, stomach; tr, trachea.
To investigate the requirement for WNT/β-Catenin signaling in respiratory initiation, we disrupted β-Catenin function in foregut epithelium by conditional gene inactivation using Shhcre (29). By mating Shhcre mice to R26R cre reporter mice (30), we found that Shhcre is active in the foregut epithelium starting at the 16-somite (so) stage (≈E8.75) (Fig. 1 G and H), before lung budding around the 27-so stage (≈E9.5) (2). By E9.75, after lung budding and when most of the endoderm-derived primordia are identifiable, Shhcre-mediated recombination is detected in the endoderm of the trachea, lung, esophagus, stomach, intestine and isolated cells in the liver (Fig. 1 I–M). Focusing on the foregut, its activity is widespread in trachea and lung, but restricted to the ventral portion of the esophagus and stomach.
By mating Shhcre mice to mice carrying a conditional knockout allele of β-Catenin (Ctnnb1tm2.1Kem) (31), we generated Shhcre/+;Ctnnb1tm2.1Kem/tm2.1Kem (hereafter referred to as β-Catcko, for conditional knockout) mutant embryos. In the foregut of these embryos, we found that β-Catenin protein is severely reduced in the ventral foregut epithelium by the 19-so (≈9.0) stage, while it is clearly present in the dorsal foregut epithelium (Fig. 1 N and O). These data indicate that Shhcre is an effective tool for gene inactivation in the ventral foregut endoderm before lung budding.
Inactivation of β-Catenin Leads to Trachea and Lung Agenesis.
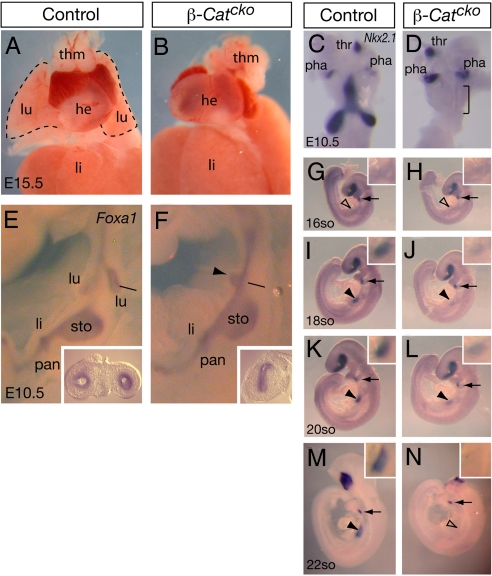
Gross examination indicated that β-Catcko mutant embryos are missing lungs, while other endoderm-derived internal organs are present (Fig. 2 A and B). To determine whether the defect arises during lung initiation, we examined the expression of Nkx2.1, the earliest marker that labels both trachea and lung (1). In normal embryos at E10.5, Nkx2.1 is expressed in the thyroid and pharyngeal pouches in addition to expression in nascent trachea and lung buds (Fig. 2C). In the β-Catcko mutant foregut, Nkx2.1 remains expressed in the thyroid and pharyngeal pouches. However, expression is not detected in the trachea/lung region (Fig. 2D). To address the possibility that this lack of expression is due to a general loss of the foregut epithelium at this stage, we examined the expression of Foxa1, a marker for endoderm-derived epithelial cells including those in the foregut. We detected Foxa1 expression in the mutant at normal intensity, suggesting that foregut epithelium is maintained (Fig. 2 E and F). Interesting, a majority of mutant samples exhibit an aberrant midline epithelial protrusion just anterior to the stomach (arrowhead in Fig. 2F). Although this protrusion is found at the equivalent level of the lung buds, it does not express Nkx2.1 in E10.5 embryos. These data together indicate that inactivation of β-Catenin in the foregut epithelium leads to a specific loss of the respiratory primordium.
Fig. 2.
Inactivation of β-Catenin leads to a failure in maintaining the respiratory fate. (A and B) Trachea and lung are absent in β-Catcko mutant at E15.5. (C, D, and G–N) Nkx2.1 expression as detected by RNA in situ hybridization. Ventral (C and D) or lateral (G–N) views are shown. Arrowheads in G–N indicate regions that are enlarged in the insets of each panel, with filled arrowheads indicate presence of expression and open arrowheads indicate absence of expression. Arrows indicate Nkx2.1 expression in the thyroid as a control that the staining intensity is equivalent in all embryos. Nkx2.1 is not detected in the prospective trachea/lung region in either the control or mutant at the 16-so stage. Later, at the 18- and 20-so stages, its expression is detected in both genotypes, although at a lower intensity in the mutant. This expression is absent by the 22-so stage in the mutant. (E and F) Foxa1 expression as detected by RNA in situ hybridization outlines the endoderm in E10.5 embryos. Lateral views are shown. Lines indicate the approximate level of transverse section shown in the insets. In the β-Catcko mutant, there is a common foregut tube and an aberrant bud is observed along the ventral midline of the presumptive trachea/lung region (arrowhead in F). Abbreviations: same as Fig. 1 with the addition of pha, pharyngeal pouch; thm, thymus.
To address whether specification of respiratory progenitors ever occurs in the β-Catcko mutant, we performed a time-course analysis of Nkx2.1 expression. We found that in the control foregut by RNA in situ hybridization, Nkx2.1 expression is not observed above background in the prospective respiratory region at the 16-so stage, but is clearly detected at the 18-so stage (≈E9.0) (Fig. 2 G and I). In the β-Catcko mutant, Nkx2.1 expression is first detected at approximately the same stage, albeit at lower intensity (n = 3) (Fig. 2 H and J). This lower level of expression persists at the 20-so stage, but is absent by the 22-so stage (n = 3) (Fig. 1 K–N). It is possible that the transient Nkx2.1 expression may be sustained by residual β-Catenin before its depletion in the β-Catcko foregut by Shhcre-mediated recombination (Fig. 1 N and O). We speculate that earlier endoderm-specific inactivation of β-Catenin may lead to a complete failure in the specification of the respiratory fate. Regardless, our results from the β-Catcko mutants suggest that while respiratory progenitors are first established, they are not maintained.
Cellular Mechanism Underlying Loss of Respiratory Progenitors.
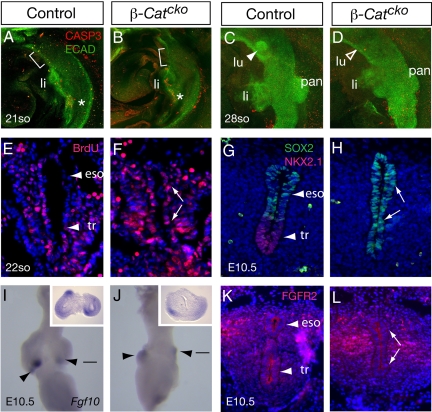
To determine the mechanism underlying the failure to maintain respiratory progenitors, we first addressed whether it is due to an increase in cell death in the mutant foregut. By detecting dying cells with an anti-cleaved Caspase 3 antibody, we found that there was no increase in Caspase 3 staining in the mutant foregut epithelium either during specification (≈19–21-so stage, n = 3) or during lung budding (≈28-so stage, n = 3) (Fig. 3 A–D). These data indicate that the loss of trachea/lung progenitors is not due to increased cell death.
Fig. 3.
Inactivation of β-Catenin leads to a defect in foregut patterning. (A–D) Double immunofluorescence staining with anti-cleaved Caspase 3 antibody that labels cells undergoing apoptosis (red) and anti-E-Cadherin antibody that labels foregut epithelium (green) at the 21-so stage (specification) or 28-so stage (budding morpohogenesis). Lateral views are shown. Brackets in A and B indicate prospective trachea/lung region. There is no detectable increase in cell death in the mutants compared to controls. Asterisks in A and B indicate cleaved Caspase 3-positive cells just posterior to the liver in both the mutant and the control to show that the assay is working. (E and F) Assays for BrdU incorporation in transverse sections of the foregut at the 22-so stage. No difference in the percentage of positive cells is detected. (G and H) Double immunofluorescence staining with anti-NKX2.1 antibody (red) and anti-SOX2 antibody (green) in transverse sections of the common trachea/esophageal tube at E10.5. In the β-Catcko mutant, NKX2.1 expression is downregulated, and SOX2 expression is expanded to the ventral epithelium. (I and J) Fgf10 expression as detected by RNA in situ hybridization in E10.5 foregut. Ventral views are shown. Filled arrowheads indicate presence of gene expression. Lines indicate approximate level of transverse sections shown in respective insets. (K and L) FGFR2 expression as detected by an anti-FGFR2 antibody in transverse sections of trachea/esophageal region at E10.5. In the β-Catcko mutant, FGFR2 remains expressed in both dorsal and ventral foregut endoderm as indicated by arrows. Abbreviations: same as Fig. 1 with the addition of CASP3, cleaved Caspase 3; ECAD, E-Cadherin.
We next investigated whether the failure to maintain respiratory progenitors is due to a decrease in cell proliferation. We examined this at the 22-so stage, shortly after the depletion of β-Catenin protein in the β-Catcko mutant and when Nkx2.1 expression is first absent. By labeling cells in S-phase using BrdU incorporation, we found that there was no statistically significant difference in the percentage of BrdU positive cells in the mutant foregut epithelium compared to control (Fig. 3 E and F, 49.4 ± 1.1% in mutant vs. 57.0 ± 5.5% in control, P = 0.130). This result indicates that the loss of respiratory progenitors is not due to a decrease in cell proliferation at this stage.
To address whether the failure to maintain respiratory progenitors is due to a defect in preserving proper foregut patterning, we examined whether loss of the respiratory lineage is accompanied by other cell fate changes in the foregut. We assayed for the expression of esophagus/stomach marker SOX2 in the common foregut tube before the tracheo-esophageal separation, and compared it to the expression domain of NKX2.1. In E10.5 control, we found that SOX2 expression was restricted to the dorsal portion of the foregut in the prospective esophageal cells, separate from NKX2.1-expressing cells in the ventral foregut (Fig. 2G). In E10.5 mutant, we found that accompanying the loss of NKX2.1 expression, SOX2 expression was expanded into the ventral foregut endoderm (Fig. 2H). This concordant down-regulation of a trachea/lung marker and up-regulation of an esophagus/stomach marker supports the conclusion that the primary requirement for β-Catenin in the foregut epithelium is to maintain respiratory identity and proper foregut patterning.
FGF10 and Its Principal Receptor Remain Expressed in the β-Catcko Mutant Foregut.
As genetic evidence demonstrates that FGF10 signaling via FGFR2 is essential for lung initiation (7–9), we sought to understand the relationship between WNT and FGF signaling in this process. We found that in β-Catcko mutants at E10.5, Fgf10 remains expressed in separated lateral domains in the lung mesenchyme (Fig. 3 I and J), even though the two primary lung buds do not form. Recent studies show that at later stages of lung development during branching morphogenesis, β-Catenin is required for Fgfr2 expression in lung epithelium and mesenchyme (18, 20). However, we found that FGFR2 protein is still present in the foregut endoderm of β-Catcko mutants at E10.5 (Fig. 3 K and L), indicating that β-Catenin is not required for FGFR2 expression during lung budding. Our results suggest that β-Catenin likely acts independent of FGF10/FGFR2 signaling during lung initiation.
Ectopic Activation of β-Catenin Leads to Expansion of a Respiratory Marker into the Anterior Stomach.
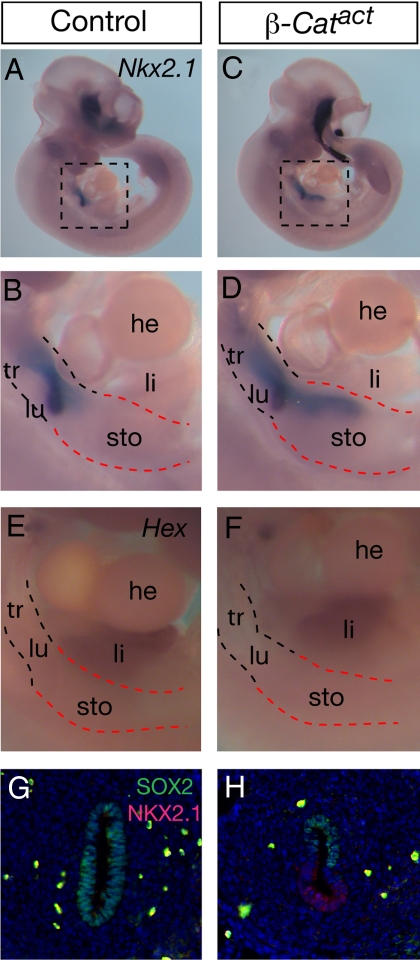
The requirement for β-Catenin in maintaining the identity of respiratory progenitors led to the question of whether it is capable of inducing respiratory fate in other regions of the foregut. To address this question, we overexpressed an activated form of β-Catenin in the early endoderm. This is achieved by mating the Shhcre mice with mice carrying a conditional activated allele of β-Catenin, Ctnnb1tm1Mmt (32), generating Shhcre/+;Ctnnb1tm1Mmt/+ (hereafter referred to as β-Catact for activated mutation). It has been shown that cre-mediated recombination of the Ctnnb1tm1Mmt allele results in deletion of the phosphorylation target sites in β-Catenin, leading to stabilization of the protein and dominant activation of the WNT/β-Catenin pathway. We found that in β-Catact embryos at E10.5, in addition to expression in the trachea and lung, Nkx2.1 expression is expanded into the anterior stomach (forestomach), but not the posterior stomach (glandular stomach) or more caudal regions of the endoderm (Fig. 4 A–D). A recent study shows that WNT signaling in zebrafish foregut promotes the liver fate (33). This led us to address if expression of activated β-Catenin in mouse would also lead to an expansion of the liver fate. We found that the expression of Hex, an early liver maker, remains restricted to the liver primordium in β-Catact embryos at E10.5 (Fig. 4 E and F). This suggests that in the mouse foregut, activated β-Catenin can promote respiratory, but not liver identity.
Fig. 4.
Ectopic activation of β-Catenin leads to expansion of a lung progenitor marker. (A–F) Nkx2.1 or Hex expression as detected by RNA in situ hybridization in E10.5 embryos. Boxed region in A and C are magnified in B and D, respectively. Similar magnified views are shown in E and F. In β-Catact embryos, expression of the activated form of β-Catenin leads to expansion of Nkx2.1 expression from the trachea/lung region (black dashed line) into the anterior ventral portion of the stomach (red dashed line). However, Hex expression remains not altered. (G and H) Double immunofluorescence staining with anti-NKX2.1 and anti-SOX2 antibodies in transverse sections of the anterior stomach region at E10.5. In β-Catact embryos, ectopic expression of NKX2.1 is accompanied by a down-regulation of SOX2 expression.
To address if the expansion of Nkx2.1 expression domain in β-Catact foregut is due to an increase in cell proliferation, we assayed for BrdU incorporation. We found that there is a slight decrease, rather than an increase, in the percentage of BrdU-positive cells in the β-Catact mutant compared to control at E10.5 (43.7 ± 5.3% in mutant vs. 57.6 ± 4.4% in control, P = 0.057), suggesting that the increase in Nkx2.1 expression domain is not likely a result of increased proliferation of respiratory progenitors. Similar to our analysis in β-Catcko mutants, we then addressed the effect of activated β-Catenin on foregut patterning by examining SOX2 and NKX2.1 expression domains in β-Catact mutants. We found that in transverse sections of E10.5 control embryos at the level of anterior stomach, SOX2 is expressed in the entire circumference, while NKX2.1 is not expressed (Fig. 4G). However, in E10.5 β-Catact mutant embryos, with the ectopic expression of NKX2.1 in the ventral epithelium of the anterior stomach due to activated β-Catenin expression in this domain, there is a concordant downregulation of SOX2 expression in this region (Fig. 4H). Interestingly, expression of activated β-Catenin does not lead to lung budding morphogenesis in the stomach region. Nor does activated β-Catenin lead to a full transformation of the anterior stomach into lung, as the expression of surfactant C (Sftpc) gene, a marker for differentiated type II pneumocytes, is not detected in the β-Catact anterior stomach. Nevertheless, the ectopic expression of NKX2.1 in the anterior stomach and concordant downregulation of SOX2 suggests that activation of β-Catenin can impose respiratory progenitor property to foregut epithelium outside of the trachea/lung region.
Discussion
Genetic evidence from both the β-Catcko and β-Catact mutants establishes β-Catenin as a key player responsible for initiating the respiratory development program. We show that respiratory progenitor identity is not maintained in β-Catcko mutants, while it is ectopically induced in β-Catact mutants. Neither of these phenotypes can be explained by changes in cell proliferation and/or cell survival in these mutants. Rather, the coordinated up or down-regulations of NKX2.1 versus SOX2 in the early foregut endoderm of these mutants support a role of β-Catenin in controlling the balance between the respiratory (trachea/lung) versus digestive (esophagus/stomach) progenitor identities.
This role is consistent with the function of β-Catenin in adult lung stem cells in mice. Recent studies show that conditional activation of β-Catenin in adult lung epithelium leads to an increase in bronchioalveolar stem cell (BASC) number (34, 35). Further analysis of these adult mutant lungs show that there is no change in cell proliferation that would account for the phenotype, similar to our finding in β-Catact embryonic foregut. Rather, the increase of BASCs in the adult mutant lungs is due to the ability of β-Catenin in maintaining these cells in the progenitor state (34). This finding and our data together suggest that β-Catenin promotes respiratory progenitor characteristics both during lung initiation in the fetal lung and in stem cell maintenance in the adult lung.
In addition to being a key mediator of canonical WNT signaling, β-Catenin has also been implicated in cell adhesion events independent of WNT signaling (36). Although our data do not exclude the possibility that β-Catenin functions outside of the WNT pathway to promote respiratory progenitors, three lines of evidence suggest that β-Catenin likely acts as a WNT mediator in this process. First, we and others have found that Wnt2 and Wnt2b are expressed in the mesenchyme adjacent to the respiratory primordium (Fig. 1 A–C) (15, 23). Second, we show that Axin2-lacZ, a WNT reporter, is active in the ventral foregut (Fig. 1 D–F), possibly as a result of WNT2/2b signaling. This result indicates that canonical WNT signaling is active in the prospective respiratory region. Third, recent data from Dr. Morrisey's laboratory show that mice homozygous for null alleles of both Wnt2 and Wnt2b exhibit a specific loss of trachea/lung, resembling the phenotype in β-Catcko mutants (personal communication). These data together suggest that WNT2 and WNT2b likely function through β-Catenin to promote respiratory progenitor identity in the foregut endoderm.
This role of WNT/β-Catenin signaling is unexpected based on recent findings from zebrafish and Xenopus studies. In zebrafish, it was shown that a mutation in Wnt2b leads to a delay/reduction in liver specification with no other morphological defects (33). Our conditional β-Catcko mutants do not allow us to address the requirement for β-Catenin in liver specification in mouse, as Shhcre only recombines in sporadic cells in the liver (Fig. 1J). However, in Wnt2;Wnt2b null mutants (E. Morrisey, personal communication), the liver is present, even though trachea and lung are absent. This result indicates that in the mouse foregut, WNT2 signaling is required for the establishment of the respiratory, but not the liver lineage. Furthermore, in our β-Catact mouse mutants, the liver marker Hex is not ectopically expressed as a result of β-Catenin activation, unlike Nkx2.1. These data suggest that in the mouse foregut, WNT/β-Catenin signaling promotes respiratory, but not liver fate. We speculate that the differences in the findings from mouse and zebrafish may be due to distinct requirements for β-Catenin in the foregut of these organisms. Previous studies show that RA signaling is essential for specifying the pancreas in zebrafish, but not in mouse (37–39), providing precedence that there are species-specific requirements in the molecular mechanisms essential for organ initiation.
Findings from a recent Xenopus study also appear to differ from our results in mice. In Xenopus, it was shown that WNT-induced activation of β-Catenin signaling in the endoderm leads to reduction of markers for all foregut organs including the lung, liver and pancreas (40). This result is different from the phenotype in β-Catact mouse mutants, and could reflect species-specific control of organ formation. An alternative explanation is that β-Catenin may play different roles at distinct stages of lung development. In the Xenopus study, β-Catenin activation is induced at the gastrula stage during the emergence of the endoderm. However in our study, we manipulate β-Catenin function after the establishment of foregut endoderm. At an even later time window during lung branching morphogenesis, overexpression of a constitutively active β-Catenin-Lef1 fusion protein leads to transformation from lung to intestinal fates (26). This finding further supports context-dependence of β-Catenin function in the foregut.
Our data also emphasize the view that this crucial contribution of β-Catenin to respiratory development is dependent on additional players in the foregut. Results from β-Catact mutants show that activated β-Catenin induces Nkx2.1 in the anterior, but not posterior stomach. This boundary coincides with many molecular and cellular differences that have been documented in these two regions of the stomach (41), and suggest that some of these differences may account for distinct responses to activated β-Catenin protein. Furthermore, in the anterior stomach of β-Catact mutants, even though activated β-Catenin induces respiratory progenitor characteristics, it does not direct later steps of lung development, including lung budding morphogenesis and epithelial differentiation. It is likely that additional obligatory partners in the endoderm and/or signals from the mesenchyme are required to implement the remainder of the respiratory program. For example, FGF10 signaling is essential for lung budding morphogenesis (8, 9). Fgf10 is expressed at a low level in the anterior stomach compared to surrounding regions of the mouse foregut (42). This raises the possibility that in the anterior stomach of β-Catact mutants, the amount of FGF10 present may not be sufficient to drive budding following ectopic induction of Nkx2.1. Our findings that β-Catenin is capable of inducing early respiratory progenitor identity, and is required to maintain this identify demonstrate that β-Catenin functions at or near the top of the genetic hierarchy that executes the lung development program. Thus discovery and characterization of the partners and targets of β-Catenin in the context of respiratory initiation will be an informative future direction of research.
Methods
Generation of β-Catenin Mutants.
Mice carrying a conditional loss-of-function allele of β-Catenin (Ctnnb1tm2.1Kem) or a conditional gain-of-function allele of β-Catenin (Ctnnb1tm1Mmt) were mated to mice carrying the Shhcre allele to generate Shhcre/+; Ctnnb1tm2.1Kem/tm2.1Kem (β-Catcko) or Shhcre/+;Ctnnb1tm1Mmt/+ (β-Catact) mutant embryos, respectively (29, 31, 32). Offspring were genotyped using the following PCR primer pairs: for Cre, 5′-TGATGAGGTTCGCAAGAACC-3′ and 5′-CCATGAGTGAACGAACCTGG-3′, product size 420 bp; for Ctnnb1tm2.1Kem, 5′-AAGGTAGAGTGATGAAAGTTGTT-3′ and 5′-CACCATGTCCTCTGTCTATTC-3′, product sizes 324 bp from the Ctnnb1tm2.1Kem allele and 221 bp from the wild-type allele, For Ctnnb1tm1Mmt allele, 5′-GCTGCGTGGACAATGGCTACTCAA-3′ and 5′-GCCATGTCCAACTCCATCAGGTCA-3′, product sizes 525 bp from the Ctnnb1tm1Mmt allele and 300 bp from the wild-type allele.
Embryo Isolation and Ohenotype Analyses.
Embryos were dissected from time-mated mice, counting noon on the day the vaginal plug was found as E0.5. As Shhcre/+; Ctnnb1tm2.1Kem/+ or Shh+/+;Ctnnb1tm1Mmt/+ littermates were indistinguishable from wild-type embryos, they were used as controls for respective β-Catcko or β-Catact experiments. To assay for cre activity through β–gal expression, the R26R reporter line (30) was introduced into the background of the Shhcre line (29). β–gal activity was detected using a standard protocol. Stained embryos were embedded in JB-4 plastic resin (Polysciences Inc.) according to the manufacturer's instructions. Sections were prepared at 5 μm and counterstained with 1% eosin. Whole-mount in situ hybridization using digoxigenin-labeled RNA probes was performed as previously described (43).
To determine the extent of β-Catenin inactivation, immunofluorescence staining was performed using a mouse anti-β-Catenin antibody (BD Transduction Laboratories, 1:50 dilution) on paraffin sections (5 μm). Mouse-on-Mouse reagent (Vector Laboratories) was used in the blocking step before addition of antibody. Sections were counterstained with DAPI to label cell nuclei. To determine the extent of programmed cell death in the foregut epithelium, immunofluorescence staining was performed using a rabbit anti-cleaved Caspase-3 antibody (Cell Signaling, 1:500 dilution) to label dying cells and a rat anti-E-Cadherin antibody (Sigma, 1:500 dilution) to label the epithelium. To determine foregut patterning, immunofluorescence staining was performed using a mouse anti-NKX2.1 (also called anti-TTF1, clone 8G7G3/1, Neomarkers, used at 1:100 dilution) and a rabbit anti-SOX2 (Novus Biologicals, used at 1:1,000 dilution) on paraffin sections. To determine FGFR2 expression, a rabbit anti-FGFR2 antibody (Santa Cruz Biotechnology) was used at 1:100 dilution. Staining was performed using a recently described protocol (44).
Cell Proliferation Assay.
Pregnant females received an i.p. injection of 100 μg BrdU (Sigma) per gram bodyweight 1 h before sacrifice. Embryos were fixed, processed and stained as described above. After immunostaining, slides were counterstained with DAPI. For each 20× field of view, the number of BrdU+ nuclei relative to the number of nuclei in the foregut endoderm was calculated. The percentage BrdU+ nuclei between control and mutant was compared using the Student's t test. Results were considered statistically significant if P ≤ 0.05.
Acknowledgments.
We thank Dr. E. Morrisey and his laboratory for sharing unpublished data; members of the X.S. laboratory and Drs. Caroline Alexander and Grace Boekhoff-Falk for insightful discussions and critical reading of the manuscript; Dr. Terry Yamaguchi (NCI, Maryland) for sharing WNT pathway reagents; Drs. Makoto Mark Taketo (Kyoto University, Kyoto) for sharing the Ctnnb1tm1Mmt mice; Brian Harfe (University of Florida) and Cliff Tabin (Harvard University, Boston) for sharing the Shhcre mice; Dr. Rolf Kemler (Max Planck Institute of Immunology) for sharing the Ctnnb1tm2.1Kem mice; and R. Beddington, S. Bellusci (University of Southern California), K. Kastner (University of Pennsylvania), J. Rubenstein (University of California, San Francisco), and L.T. Williams (Five Prime Therapeutics, Inc., California) for providing plasmids from which RNA in situ probes were prepared. We thank Amber Lashua and Minghui Zhao for technical assistance. This work was supported by David and Lucille Packard Foundation Graduate Scholars Fellowship 2003-24989 (to K.S.H-J.), National Science Foundation Graduate Research Fellowship 2008044659 (to E.T.D.), and National Institutes of Health/National Institute of Environmental Health Sciences Grant National Reseasrch Service Award Postdoctoral Fellowship F32ES014284 (to C.M.V.). This work was supported by Burroughs-Wellcome Career Award 1002361, American Heart Grant 0950041G, and a University of Wisconsin Medical Education Research Committee young investigator award (to X.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Kimura S, et al. The T/ebp null mouse: Thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso WV, Lu J. Regulation of early lung morphogenesis: Questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 3.Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132:35–47. doi: 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- 4.Calmont A, et al. An FGF response pathway that mediates hepatic gene induction in embryonic endoderm cells. Dev Cell. 2006;11:339–348. doi: 10.1016/j.devcel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- 6.Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development. 2000;127:2695–2704. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- 7.De Moerlooze L, et al. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signaling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- 8.Min H, et al. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekine K, et al. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 10.Chen F, et al. Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134:2969–2979. doi: 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- 11.Lako M, et al. Isolation, characterisation and embryonic expression of WNT11, a gene which maps to 11q13.5 and has possible roles in the development of skeleton, kidney and lung. Gene. 1998;219:101–110. doi: 10.1016/s0378-1119(98)00393-x. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopal J, et al. Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development. 2008;135:1625–1634. doi: 10.1242/dev.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katoh M, Hirai M, Sugimura T, Terada M. Cloning, expression and chromosomal localization of Wnt-13, a novel member of the Wnt gene family. Oncogene. 1996;13:873–876. [PubMed] [Google Scholar]
- 15.Zakin LD, et al. Structure and expression of Wnt13, a novel mouse Wnt2 related gene. Mech Dev. 1998;73:107–116. doi: 10.1016/s0925-4773(98)00040-9. [DOI] [PubMed] [Google Scholar]
- 16.Moon RT. Wnt/beta-catenin pathway. Sci STKE. 2005;271:cm1. doi: 10.1126/stke.2712005cm1. [DOI] [PubMed] [Google Scholar]
- 17.Mucenski ML, et al. β-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem. 2003;278:40231–40238. doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- 18.Shu W, et al. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005;283:226–239. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 19.De Langhe SP, et al. Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PLoS ONE. 2008;3:e1516. doi: 10.1371/journal.pone.0001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin Y, et al. An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev Biol. 2008;319:426–436. doi: 10.1016/j.ydbio.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Gordon J, Manley NR, Litingtung Y, Chiang C. Bmp4 is required for tracheal formation: A novel mouse model for tracheal agenesis. Dev Biol. 2008;322:145–155. doi: 10.1016/j.ydbio.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122:1693–1702. doi: 10.1242/dev.122.6.1693. [DOI] [PubMed] [Google Scholar]
- 23.Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122:3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- 24.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Langhe SP, et al. Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev Biol. 2005;277:316–331. doi: 10.1016/j.ydbio.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Okubo T, Hogan B. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maretto S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pongracz JE, Stockley RA. Wnt signalling in lung development and diseases. Respir Res. 2006;7:15. doi: 10.1186/1465-9921-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harfe BD, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 31.Brault V, et al. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 32.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signaling positively regulates liver specification. Nature. 2006;442:688–691. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds SD, et al. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells. 2008;26:1337–1346. doi: 10.1634/stemcells.2008-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, et al. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet. 2008;40:862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Desai TJ, et al. Distinct roles for retinoic acid receptors alpha and beta in early lung morphogenesis. Dev Biol. 2006;291:12–24. doi: 10.1016/j.ydbio.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 38.Molotkov A, Molotkova N, Duester G. Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev Dyn. 2005;232:950–957. doi: 10.1002/dvdy.20256. [DOI] [PubMed] [Google Scholar]
- 39.Stafford D, Prince VE. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol. 2002;12:1215–1220. doi: 10.1016/s0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- 40.McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- 41.Que J, et al. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nyeng P, Norgaard GA, Kobberup S, Jensen J. FGF10 signaling controls stomach morphogenesis. Dev Biol. 2007;303:295–310. doi: 10.1016/j.ydbio.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neubuser A, Peters H, Balling R, Martin GR. Antagonistic interactions between FGF and BMP signaling pathways: A mechanism for positioning the sites of tooth formation. Cell. 1997;90:247–255. doi: 10.1016/s0092-8674(00)80333-5. [DOI] [PubMed] [Google Scholar]
- 44.Yi L, Domyan ET, Lewandoski M, Sun X. Fibroblast growth factor 9 signaling inhibits airway smooth muscle differentiation in mouse lung. Dev Dyn. 2009;238:123–137. doi: 10.1002/dvdy.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]