Abstract
Muscle protein metabolism is resistant to insulin’s anabolic effect in healthy older subjects. This is associated with reduced insulin vasodilation. We hypothesized that aerobic exercise restores muscle protein anabolism in response to insulin by improving vasodilation in older subjects. We measured blood flow, endothelin-1, Akt/mammalian target of rapamycin (mTOR) signaling, and muscle protein kinetics in response to physiological local hyperin-sulinemia in two groups of older subjects following a bout of aerobic exercise (EX group: aged 70 ± 2 years; 45-min treadmill walk, 70% heart rate max) or rest (CTRL group: aged 68 ± 1 years). Baseline endothelin-1 was lower and blood flow tended to be higher in the EX group, but protein kinetics was not different between groups. Insulin decreased endothelin-1 (P < 0.05) in both groups, but endothelin-1 remained higher in the CTRL group (P < 0.05) and blood flow increased only in the EX group (EX group: 3.8 ± 0.7 to 5.3 ± 0.8; CTRL group: 2.5 ± 0.2 to 2.6 ± 0.2 ml · min−1 · 100 ml leg−1). Insulin improved Akt phosphorylation in the EX group and increased mTOR/S6 kinase 1 phosphorylation and muscle protein synthesis (EX group: 49 ± 11 to 89 ± 23; CTRL group: 58 ± 8 to 57 ± 12 nmol · min−1 · 100 ml leg−1) in the EX group only (P < 0.05). Because breakdown did not change, net muscle protein balance became positive only in the EX group (P < 0.05). In conclusion, a bout of aerobic exercise restores the anabolic response of muscle proteins to insulin by improving endothelial function and Akt/mTOR signaling in older subjects.
Sarcopenia is an age-dependent loss of skeletal muscle mass, strength, and quality, which may lead to weakness of the lower extremities, slowing of gait speed, and increased risk of falls (1–3). Sarcopenia is a multifactorial disorder, and evidence is accumulating that a reduced response of skeletal muscle to anabolic stimuli is an important contributing factor. For example, we and others (4,5) have previously shown that healthy aging is associated with a blunted muscle protein anabolic response to hyperaminoacidemia with hyperinsulinemia. Nonetheless, muscle protein synthesis and breakdown in the fasting state may not necessarily change with age (6) and may respond normally to a simple amino acid stimulus (7–10).
Insulin is a potent anabolic stimulus for skeletal muscle. We have recently shown that physiological hyperinsulinemia increases skeletal muscle protein synthesis and anabolism in young healthy subjects, as long as blood flow and amino acid delivery to the muscle are stimulated by insulin (11). Thus, it appears that the insulin-induced modulation of muscle perfusion and nutrient availability is necessary for the anabolic response of muscle protein synthesis to insulin. Furthermore, insulin and amino acids both can stimulate the mammalian target of rapamycin (mTOR) signaling pathway (12). Specifically, insulin promotes the phosphorylation of Akt, an upstream regulator of mTOR, and enhances mTOR signaling to its downstream effectors 4E-binding protein 1 (4EBP1) and ribosomal S6 kinase 1 (S6K1), which are key regulators of translation initiation and protein synthesis (13). On the other hand, amino acids (in particular leucine) can directly activate mTOR kinase activity, resulting in an even more potent stimulation of translation initiation, elongation, and protein synthesis (13).
Healthy aging is also associated with a decreased insulin-induced vasodilation due to dysfunction of the endothelial-dependent vasodilation (14–16), which is normally initiated by insulin via activation of the endothelial NO synthase (17,18). In a recent study of healthy older subjects, we showed that such a vasomotor dysfunction is associated with increased endothelin-1 concentrations and a selective resistance of skeletal muscle protein synthesis to the anabolic action of insulin (16). This novel finding raises the question as to whether interventions that are known to improve endothelial-dependent vasodilation and/or insulin sensitivity may also improve muscle protein synthesis in response to insulin in older subjects.
Aerobic exercise improves endothelial function in older subjects (15) and is also important in the prevention and treatment of type 2 diabetes as it increases insulin sensitivity and glucose tolerance (19–21). These positive effects may be demonstrated in both patients with type 2 diabetes and normal subjects even after a single bout of aerobic exercise and persist for at least 48 h after exercise (21–27). On the other hand, as opposed to progressive resistance exercise, moderate or even strenuous aerobic exercise has only minimal direct effects on muscle protein metabolism, because it stimulates muscle protein synthesis only during the exercise bout, and these effects dissipate in the immediate postexercise period (28).
We hypothesized that a single bout of aerobic exercise performed before an insulin challenge will significantly improve endothelial function and insulin-induced vasodilation and, as a result, will improve the anabolic response of skeletal muscle protein synthesis to insulin in healthy older subjects. To test this hypothesis, we measured leg blood flow, endothelin-1 concentrations, phosphorylation of the Akt/mTOR signaling pathway, and muscle protein kinetics in healthy, nondiabetic, untrained older subjects at baseline and during an insulin challenge that was preceded by either a bout of moderate aerobic exercise (~20 h before the beginning of the measurements) or rest.
RESEARCH DESIGN AND METHODS
We studied 13 older subjects (10 men and 3 women) from the Los Angeles metropolitan area and the greater Houston/Galveston area. All subjects provided informed written consent before participating in the study, which was approved by the institutional review boards of the University of Southern California (Los Angeles, CA) and the University of Texas Medical Branch (Galveston, TX).
All subjects were healthy, nondiabetic, and physically active (no impairments in the activities of daily living or instrumental activities of daily living) but were not engaged in an exercise training program. Screening was performed with clinical history, physical examination, and laboratory tests including blood count, liver and kidney function tests, coagulation profile, fasting blood glucose, oral glucose tolerance tests, hepatitis B/C and HIV screening, thyroid-stimulating hormone, lipid profile, urinalysis, drug screening, and resting electrocardiogram. Subjects with normal results were randomly assigned to either the aerobic exercise group (EX group: 45-min treadmill walk at 70% of maximum heart rate [HRmax] performed the afternoon before the experiment) or a control group (CTRL group) not exercising on the day before the experiment. Subjects randomized to the EX group underwent exercise stress testing at least 1 week before the experiment to exclude individuals with silent cardiac ischemia and measure HRmax and oxygen consumption (VO2max). Characteristics of subjects meeting eligibility criteria are summarized in Table 1.
TABLE 1.
Characteristics of the study subjects
| Subject characteristics | Control | Exercise | P |
|---|---|---|---|
| n | 7 | 6 | |
| Sex (men/women) | 5/2 | 5/1 | 0.61 |
| AGE | 70 ± 2 | 68 ± 1 | 0.52 |
| Body weight (kg) | 80 ± 3 | 85 ± 3 | 0.41 |
| Height (cm) | 172 ± 5 | 180 ± 2 | 0.37 |
| BMI (kg/m2) | 27.7 ± 1.5 | 26.7 ± 1.1 | 0.73 |
| Leg volume (ml) | 9,547 ± 460 | 9,697 ± 414 | 0.87 |
Data are means ± SE.
We measured blood flow, muscle protein, amino acid, and glucose kinetics in the postabsorptive basal state (0–300 min) and during insulin infusion (300–480 min) in the EX and CTRL groups. The afternoon before the study, each subject was admitted to the University of Southern California or the University of Texas Medical Branch General Clinical Research Center. At 1600 h, subjects assigned to the EX group performed a 45-min treadmill walk at 70% HRmax. Briefly, the heart rate was continuously monitored throughout the exercise bout, while treadmill speed and inclination were adjusted to reach and maintain the heart rate at 70% HRmax. At 1830 h, all subjects were fed a standard dinner (one-third of their estimated daily caloric requirements) and given a snack at 2200 h, after which only water ad libitum was allowed until the end of the experiment. The next morning, polyethylene catheters were inserted into a forearm vein for tracer and dextrose infusion, in a contralateral hand or wrist vein for arterialized blood sampling, and in the femoral artery and vein of one leg for blood sampling. The arterial catheter was also used for infusion of insulin (Humulin R; Eli Lilly, Indianapolis, IN) and indocyanine green (ICG) (Akorn, Buffalo Grove, IL).
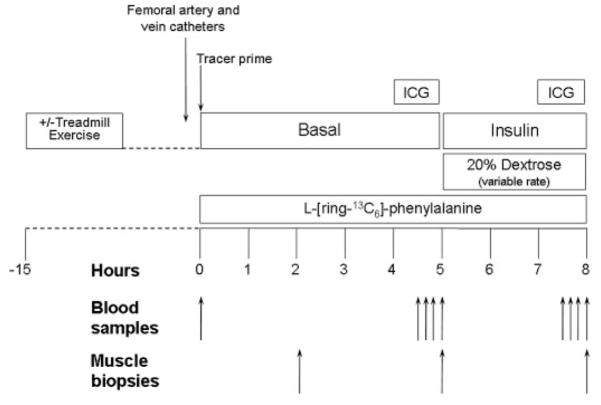
At 0730 h, after drawing a blood sample to measure background phenylalanine enrichment and ICG concentration, a primed (2 μmol/kg) continuous infusion of L-[ring-13C6]phenylalanine (0.05 μmol · kg−1 · min−1; Cambridge Isotope Laboratories, Andover, MA) was started and maintained at a constant rate until the end of the experiment (Fig. 1). After 2 h, the first muscle biopsy was taken from the lateral portion of the vastus lateralis of the leg with the femoral catheters, using a 5-mm Bergström biopsy needle, aseptic procedure, and local anesthesia with 1% lidocaine injected subcutaneously and on the muscle fascia. The muscle sample (150–400 mg) was quickly rinsed with ice-cold saline, blotted, and immediately frozen in liquid nitrogen and stored at −80°C until analysis. At 230 min, continuous ICG infusion was started in the femoral artery (0.5 mg/min) and maintained until 270 min. During ICG infusion, blood samples were taken four times, at 10-min intervals, from femoral and hand veins to measure ICG concentration. Subsequently, between 270 and 300 min, four blood samples were taken from the femoral artery and vein to measure glucose concentrations and phenylalanine concentrations and enrichments; four other samples were drawn from hand and femoral veins to measure systemic and femoral insulin concentration, respectively. At 300 min, a second muscle biopsy was taken as previously described.
FIG. 1.
Study design. Blood and muscle sampling are indicated by arrows. A detailed description of the study design is provided in the text.
Immediately after the second biopsy, an insulin infusion was initiated directly into the femoral artery (0.15 mU · min−1 · 100 ml leg−1) to expose the leg muscle to postprandial insulin concentrations. This technique allowed us to avoid a major systemic hyperinsulinemia and the consequent dramatic reduction in blood amino acid concentrations (11,29). During insulin infusion, blood samples (0.5 ml) were taken every 5–10 min to monitor plasma glucose concentration, and 20% dextrose was infused at a variable rate as necessary to maintain plasma glucose at the basal value (euglycemic clamp).
Between 410 and 450 min, ICG was again infused to measure leg blood flow, and blood samples were taken between 420 and 450 min and 450 and 480 min to measure ICG concentrations, phenylalanine and glucose enrichments and concentrations, and insulin concentrations, as described for the basal period. At 480 min, before stopping the tracer and insulin infusion, a third muscle biopsy was taken as described above.
Analyses
Plasma insulin and endothelin-1 concentrations were determined by enzyme-linked immunosorbent assay (Linco Research, St. Charles, MO). Plasma glucose concentration was measured using an automated glucose analyzer (YSI, Yellow Springs, OH). Serum ICG concentration was measured spectrophotometrically (Beckman Coulter, Fullerton, CA) at λ = 805 nm (30,31).
Blood phenylalanine concentrations and enrichments were determined by gas chromatography–mass spectrometry (Agilent Technologies, Palo Alto, CA) as previously described (32). We measured only phenylalanine concentration because it is a good predictor of insulin-induced concentration changes in all essential amino acids (33).
Muscle tissue free amino acids and proteins were extracted as previously described (32). Intracellular free phenylalanine concentrations and enrichments of were determined by gas chromatography–mass spectrometry as previously described (32). Mixed muscle protein–bound phenylalanine enrichment was analyzed by gas chromatography–mass spectrometry after protein hydrolysis and amino acid extraction (32) using the external standard curve approach (34).
Total and phosphorylated Akt, mTOR, 4EBP1, and S6K1 in skeletal muscle samples were determined by SDS-PAGE and immunoblotting (BioRad, Hercules, CA) as previously described (35). The phospho and total primary antibodies used are listed in Fig. 3. Total content for each measured protein was detected using an antibody dilution of 1:1,000. Phosphorylation and total protein were normalized to a rodent standard (Precision Plus protein standard; BioRad). Final data were expressed as normalized protein phosphorylation relative to normalized total protein.
FIG. 3.
Phosphorylation of Akt/PKB, mTOR, 4E-BP1, and S6K1 in two groups of older subjects at baseline and during insulin infusion performed ~20 h following rest (Control: n = 5 for Akt/PKB and mTOR; n = 4 for 4E-BP1 and S6K1) or aerobic exercise (Exercise: n = 6). We used the following phospho and total primary antibodies (Cell Signaling, Beverly, MA): phospho-mTOR (Ser2448, cat. no. 2971, lot no. 9; 1:1,000), phospho-p70 S6K1 (Thr389, cat. no. 9234, lot no. 2; 1:500), phospho-Akt (Ser473, cat. no. 4058, lot no. 6; 1:500), and phospho-4EBP1 (Thr37/46, cat. no. 2971; 1:1,000). Anti-rabbit IgG horseradish peroxidase–conjugated secondary antibody was purchased from Amersham Bioscience (1:2,000). Values are means ± SE. *P < 0.05 vs. basal, #P < 0.05 vs. control. □, basal; ■, insulin. Bas, basal; Ins, insulin; MW, molecular weight.
Calculations
Muscle phenylalanine kinetic parameters were calculated using both the two- and the three-pool arteriovenous balance models because each model provides unique information regarding leg plasma (two-pool) and intracellular (three-pool) amino acid kinetics (11). The models’ assumptions and validation are extensively reviewed (36). The two- and three-pool model parameters were calculated as follows.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
where CA, CV is plasma phenylalanine concentrations in the femoral artery and vein, respectively. EA, EV, and EM are free phenylalanine enrichments (tracer-to-tracee ratio) in femoral artery and vein and in muscle, respectively. BF is leg blood flow calculated from steady-state ICG concentrations (30,31). Data were expressed per 100 ml of leg volume.
We also measured the fractional synthetic rate (FSR) of mixed muscle proteins by measuring the incorporation rate of the phenylalanine tracer into the proteins (ΔEp/t) and using the precursor-product model to calculate the synthesis rate as follows (37): FSR = (ΔEp/t)/([EM{1} + EM{2}]/2) × 60 × 100. ΔEp is the increment in protein-bound phenylalanine enrichment between two sequential biopsies, t is the time between the two sequential biopsies, EM{1} and EM{2} are the phenylalanine enrichments in the free intracellular pool in the two sequential biopsies. Data are expressed in the percentage per hour.
Leg glucose utilization was calculated as net glucose uptake across the leg: leg glucose uptake = BF × (CA − CV).
To determine the degree of muscle tissue exposure to insulin, we calculated the insulin delivery rate to the leg to account for recycling from the systemic circulation (16). Further, changes in blood flow can affect insulin concentration during constant infusion. Because insulin was infused in the artery, arterial insulin concentration was not measurable, and insulin delivery was estimated using the femoral insulin concentration (InsFV) as follows: insulin delivery = InsFV × BF.
Since some insulin is taken up by the muscle cells after binding to the insulin receptor, this method may slightly underestimate the total insulin delivery rate, but for the above reasons we found it preferable to simply relying on the calculated dose.
Statistical analysis
Sex differences between groups were analyzed using the χ2 test. Subjects’ characteristics and baseline values for all measured variables were analyzed using one-way ANOVA. The effects of the prior bout of exercise on the response variables of the basal period and during insulin infusion were compared using ANOVA for repeated measures, the main effects being group (the EX and CTRL groups) and time (basal and insulin infusion). Post hoc testing was performed using the Tukey-Kramer test. If baseline values were significantly different or tended to be different (P < 0.10), we also performed an ANCOVA using baseline values as covariates. Differences were considered significant at P < 0.05. P for trend was set at P < 0.10. Data are the means ± SE.
RESULTS
Insulin and glucose
Blood glucose and insulin concentrations and kinetics across the leg are shown in Table 2. Systemic and femoral vein insulin concentrations were not different at baseline and significantly increased in both groups during insulin infusion, but the change was larger in the CTRL group (P < 0.05). However, because of the differences in leg blood flow (see below), insulin delivery to the leg was not different between groups at baseline and significantly increased during insulin infusion in both groups, with no differences between groups. Plasma arterial glucose concentrations were not different between groups at baseline and did not change during insulin infusion with euglycemic clamp. However, the exogenous glucose infusion rate and leg glucose uptake during insulin infusion were significantly higher in the EX group (P < 0.05).
TABLE 2.
Endothelial function parameters, insulin and glucose concentrations, and kinetics at baseline and during insulin infusion
| CTRL group |
EX group |
P |
|||||
|---|---|---|---|---|---|---|---|
| Basal | Insulin | Basal | Insulin | Group | Time | Interaction | |
| Endothelial function | |||||||
| Endothelin-1 (pg/ml) | 3.53 ± 0.72 | 2.46 ± 0.39 | 1.85 ± 0.08 | 1.60 ± 0.10 | <0.01 | 0.02 | 0.14 |
| Blood flow (ml · min−1 · 100 ml leg−1) | 2.6 ± 0.2 | 2.7 ± 0.2 | 3.8 ± 0.7 | 5.3 ± 0.8 | 0.02 | <0.01 | <0.01 |
| Insulin | |||||||
| Systemic concentration (μU/ml) | 8.5 ± 1.0 | 24.0 ± 2.4 | 6.3 ± 0.8 | 15.0 ± 1.2 | <0.01 | <0.0001 | 0.06 |
| Femoral vein concentration (μU/ml) | 8.5 ± 1.0 | 83.4 ± 5.2 | 6.3 ± 0.8 | 46.7 ± 5.1 | <0.001 | <0.0001 | 0.001 |
| Delivery to the leg (μU · min−1 · 100 ml leg−1) | 22 ± 3 | 218 ± 11 | 23 ± 4 | 255 ± 57 | 0.48 | <0.0001 | 0.45 |
| Glucose | |||||||
| Femoral artery concentration (mmol/l) | 4.7 ± 0.2 | 4.5 ± 0.2 | 4.9 ± 0.1 | 5.0 ± 0.2 | 0.19 | 0.44 | 0.36 |
| Infusion rate (μmol · kg−1 · min−1)* | — | 10.4 ± 0.8 | — | 13.6 ± 0.9 | 0.02 | — | — |
| Leg uptake (μmol · min−1 · 100 ml leg−1)* | — | 1.8 ± 0.4 | — | 3.6 ± 0.6 | 0.03 | — | — |
Data are means ± SE. Statistical analysis performed using ANOVA with repeated measures for all parameters except those marked with *, which were analyzed using one-way ANOVA. Boldface data indicate statistical significance.
Endothelin-1 concentrations and leg blood flow
Plasma endothelin-1 concentrations in the femoral vein and leg blood flow are shown in Table 2 and Fig. 2. Endothelin-1 concentrations were significantly lower (P < 0.05) in the EX group at baseline and significantly decreased during insulin infusion in both groups (P = 0.02) with no time-by-group interaction. Leg blood flow at baseline tended to be higher in the EX than CTRL group (P = 0.08). In response to insulin, leg blood flow significantly increased only in the EX group (P < 0.05), while no change was observed in the CTRL group. ANCOVA using baseline values as covariates confirmed the results for both blood flow and endothelin-1.
FIG. 2.
Plasma endothelin-1 concentrations, blood flow, and muscle protein FSR in two groups of older subjects at baseline and during insulin infusion performed ~20 h following rest (Control: n = 7) or aerobic exercise (Exercise: n = 6). Values are means ± SE. *P < 0.05 vs. basal, #P < 0.05 vs. control. □, basal; ■, insulin.
Phenylalanine concentrations and enrichments
Average phenylalanine concentrations in the femoral artery and vein and in the muscle are reported in Table 3. Phenylalanine concentrations in the artery and in the vein were not different at baseline and slightly, but significantly, decreased in both groups with no differences between groups. Phenylalanine concentrations in muscle were significantly lower in the EX group at baseline (P < 0.01) and decreased during insulin infusion in both groups with no time-by-group interaction. ANCOVA using the baseline values as covariates confirmed these results. Phenylalanine enrichments in the femoral artery and vein and in the muscle tissue were not different between groups in the basal period and were at steady state during both sampling periods (data not shown). Phenylalanine enrichment in the femoral artery and vein increased slightly but significantly (P < 0.01) during insulin infusion in both groups, with no group differences. Phenylalanine enrichment in the muscle tissue tended to increase in both groups (P < 0.06) with no significant group differences either at baseline or during insulin infusion.
TABLE 3.
Phenylalanine concentrations at baseline and during insulin infusion
| CTRL group |
EX group |
P |
|||||
|---|---|---|---|---|---|---|---|
| Basal | Insulin | Basal | Insulin | Group | Time | Interaction | |
| Femoral artery (μmol/l) | 61 ± 2 | 56 ± 2 | 62 ± 2 | 59 ± 2 | 0.31 | <0.001 | 0.11 |
| Femoral vein (μmol/l) | 67 ± 2 | 56 ± 1 | 68 ± 1 | 58 ± 2 | 0.59 | <0.0001 | 0.65 |
| Muscle tissue (μmol/l) | 127 ± 11 | 95 ± 11 | 85 ± 5 | 81 ± 8 | 0.02 | 0.05 | 0.12 |
Data are means ± SE. Boldface data indicate statistical significance.
Amino acid kinetics
Leg and muscle phenylalanine kinetics are shown in Table 4. All kinetic parameters were not significantly different between groups in the basal period. With insulin infusion, phenylalanine delivery to the leg increased in the EX group only (P < 0.01). Phenylalanine output from the leg increased in the EX group and decreased in the CTRL group, resulting in a significant treatment-by-group interaction (P < 0.01). Because of a trend for basal phenylalanine delivery and output to be higher in the EX group (P = 0.07), an ANCOVA was carried out for both parameters using the basal values as covariates and confirmed the ANOVA results. Phenylalanine leg Ra significantly decreased in the CTRL group (P = 0.04), whereas no change was observed in the EX group. In contrast, phenylalanine leg Rd increased only in the EX group during insulin infusion (P < 0.01), whereas it did not change in the CTRL group.
TABLE 4.
Phenylalanine kinetics at baseline and during insulin infusion
| CTRL group |
EX group |
P |
|||||
|---|---|---|---|---|---|---|---|
| Basal | Insulin (nmol · min−1 · 100 ml leg−1) | Basal | Insulin (nmol · min−1 · 100 ml leg−1) | Group | Time | Interaction | |
| Delivery to the leg | 156 ± 12 | 145 ± 8 | 234 ± 43 | 309 ± 47 | 0.01 | <0.01 | <0.01 |
| Output from the leg | 171 ± 13 | 149 ± 10 | 255 ± 46 | 300 ± 45 | 0.02 | 0.23 | <0.01 |
| Release in the blood from proteolysis (Ra) | 63 ± 10 | 44 ± 9 | 66 ± 14 | 70 ± 19 | 0.32 | 0.21 | 0.04 |
| Disappearance from the blood (Rd) | 48 ± 9 | 40 ± 7 | 45 ± 11 | 79 ± 19 | 0.22 | 0.02 | <0.01 |
| Muscle inward transport | 113 ± 20 | 75 ± 11 | 193 ± 46 | 250 ± 47 | 0.01 | 0.47 | 0.02 |
| Muscle outward transport | 128 ± 21 | 79 ± 11 | 214 ± 48 | 241 ± 45 | 0.01 | 0.62 | 0.05 |
| Arteriovenous shunting | 43 ± 11 | 70 ± 12 | 41 ± 13 | 59 ± 28 | 0.76 | 0.06 | 0.77 |
| Intracellular availability | 186 ± 27 | 136 ± 21 | 263 ± 58 | 330 ± 60 | 0.03 | 0.49 | <0.01 |
| Release from muscle protein breakdown | 73 ± 9 | 61 ± 14 | 70 ± 14 | 80 ± 23 | 0.55 | 0.95 | 0.18 |
| Utilization for muscle protein synthesis | 58 ± 8 | 57 ± 12 | 49 ± 11 | 89 ± 23 | 0.44 | 0.02 | 0.03 |
| Net balance | −15 ± 2 | −2 ± 2 | −21 ± 4 | 9 ± 4 | 0.43 | <0.0001 | <0.01 |
Data are means ± SE. Boldface data indicate statistical significance.
Muscle phenylalanine inward and outward transport decreased in the CTRL group and increased in the EX group, resulting in significant treatment-by-group interactions (P < 0.05). ANCOVA performed on these two parameters, because of a trend for the baseline values to be higher in the EX group (P = 0.07), confirmed the ANOVA results. Phenylalanine intracellular availability increased with insulin in the EX group, whereas it decreased in the CTRL group, resulting in a significant time-by-group interaction (P < 0.01). Phenylalanine arteriovenous shunting increased significantly in both groups with no group differences. Phenylalanine release from proteolysis did not change significantly with insulin infusion in either group. Phenylalanine utilization for muscle protein synthesis increased significantly during insulin infusion in the EX group (P = 0.03), but it did not change in the CTRL group. As a result, phenylalanine net balance across the leg improved significantly in both groups in response to insulin (P < 0.0001), but the increase was significantly larger in the EX group such that net balance became positive only in the EX group (P < 0.01), indicating net muscle protein anabolism.
Mixed muscle FSR
The mixed muscle protein FSR (%/h) (Fig. 2) was not different between groups in the basal state (CTRL group: 0.063 ± 0.007; EX group: 0.062 ± 0.005). Insulin infusion significantly increased FSR in the EX group (P < 0.05) but not in the CTRL group (CTRL group: 0.062 ± 0.005; EX group: 0.099 ± 0.005).
Insulin and mTOR signaling proteins
Insulin and mTOR signaling is reported in Fig. 3. Akt/PKBSer473 phosphorylation increased significantly with insulin (time effect P < 0.05), but the response was significantly greater in the EX group (P < 0.05). Insulin infusion significantly increased mTORSer2448 phosphorylation only in the EX group (P < 0.05). 4EBP1Thr37/46 phosphorylation increased in both groups during insulin infusion (time effect P < 0.05), with no difference between groups. S6K1Thr389 phosphorylation increased significantly with insulin (time effect P < 0.05), but the increase was larger in the EX group (P < 0.05).
DISCUSSION
Our study indicates for the first time that a single bout of moderate aerobic exercise overcomes the muscle protein insulin resistance and restores the physiological anabolic response of muscle protein synthesis to insulin in older individuals. These exciting results indicate that a moderate-intensity aerobic exercise that can be performed by most ambulatory older individuals may be an important tool to combat sarcopenia and frailty of aging. The effect of aerobic exercise on muscle protein anabolism in these older individuals appears to be due, at least in part, to improvements in endothelial function, insulin-induced vasodilation, and nutrient delivery. Specifically, we found that muscle protein synthesis and net balance significantly increased during insulin infusion only if the infusion was preceded by a bout of aerobic exercise. Such an effect was directly associated with an increase in blood flow, which, in turn, was accompanied by a significant increase in amino acid delivery and transport into the muscle tissue, and by enhanced mTOR signaling. In contrast, insulin did not exert any effect on either blood flow or muscle amino acid and protein turnover in the subjects resting before the insulin challenge.
These novel data also indicate for the first time that insulin-induced vasodilation is an important contributor to muscle protein anabolism in older subjects. If we interpret these results in light of our recently published study (11) in young adults demonstrating that physiological hyperinsulinemia stimulates muscle protein synthesis as long as it increases blood flow and amino acid delivery to the muscle tissue, we conclude that vasodilation is a major mechanism by which insulin stimulates muscle protein synthesis throughout the adult life. Insulin-induced vasodilation appears to be more rate limiting for muscle protein anabolism than for glucose uptake. Studies on the regulation of vascular tone and glucose metabolism by insulin have uncovered a fascinating picture whereby insulin appears to rapidly recruit nutritive capillaries, which precedes the insulin’s effects on intracellular signaling and glucose uptake in muscle (38). However, the overall effect of insulin-induced vasodilation on muscle glucose metabolism is more evident when glucose uptake is high (i.e., when the artery-to-vein glucose concentration gradient is high) (39,40). This occurs because an increased blood flow reduces the arteriovenous glucose gradient and allows for a more uniform exposure of all muscle tissue to adequate blood glucose concentrations necessary to sustain the higher rate of uptake (41). The metabolic effects of insulin-induced vasodilation appear magnified when examining muscle amino acid uptake and utilization and appear to drive the overall muscle protein anabolic response (present study and 11,16,29).
The vascular tone is determined by the fine balance of a complex network of vasodilators and vasoconstrictors, many of which are directly produced by the endothelium (42). Insulin increases muscle perfusion and capillary recruitment by stimulating NO production via activation of endothelial NO synthase (17,18), and it is also well recognized that aging reduces endothelial-derived vasodilation (14–16). The negative effect of aging appears to be also associated with an increased plasma endothelin-1 concentration (16), which is another likely cause of the age-related endothelial dysfunction. Endothelin-1 is produced by vascular endothelial cells (43) and is one of the most powerful endothelial-derived vasoconstrictors (42). Interestingly, recent studies have shown that endothelin-1 receptor blockade enhances the vasodilatory action of insulin in humans (44), and aerobic exercise training for 3 months significantly reduces plasma endothelin-1 concentration in both younger and older subjects (45). Our current study is the first to report that a single bout of aerobic exercise reduces plasma endothelin-1 concentration to youthful levels and normalizes the vasodilatory and muscle protein anabolic response to insulin in older individuals. Considering all these data, it appears that endothelin-1 is potentially a suitable marker of age-related endothelial dysfunction and muscle protein insulin resistance. Further studies are needed to determine the sensitivity and specificity of endothelin-1 as a marker of muscle protein catabolism in older individuals.
Although our data strongly suggest that aerobic exercise normalized the anabolic response of muscle to insulin by improving endothelium-dependent vasodilation, we also found an improvement in insulin signaling. This was associated with a higher regional and whole-body insulin-stimulated glucose uptake in the exercise group, which is consistent with previous reports indicating that a single bout of aerobic exercise enhances insulin sensitivity for up to 48 h in both healthy subjects and in patients with type 2 diabetes (21–27). However, if we analyze the Akt/mTOR signaling results in light of the amino acid and protein kinetics data, interpretation becomes more complicated. In the control subjects, local hyperinsulinemia stimulated muscle AktSer473, 4EBP1Thr37/46, and S6K1Thr389 phosphorylation but had no effect on amino acid availability, mTORSer2448 phosphorylation, or muscle protein synthesis. This is consistent with previously published data (5,16) and suggests that increases in insulin signaling in the absence of increased amino acid availability may be insufficient to fully stimulate the mTOR signaling pathway (13). Aerobic exercise enhanced AktSer473 and S6K1Thr389 phosphorylation, amino acid availability (consequent to improved vasodilation), and mTORSer2448 phosphorylation in response to insulin, resulting in the stimulation of muscle protein synthesis. Thus, by merely considering the present data one can definitely conclude that aerobic exercise restores the normal muscle protein anabolic response to hyperinsulinemia. However, the question remains as to whether this effect of exercise on mTOR signaling and protein synthesis is due to its effect on vasodilation (and amino acid availability), insulin signaling, or both.
Some indirect answers can be derived from the analysis of recent data published by our group, which allow a better understanding of the complex mechanisms underlying the anabolic effect of insulin on muscle proteins. Insulin sensitivity of glucose metabolism is not correlated with the insulin sensitivity of protein metabolism, as the insulin-induced protein anabolic response in muscle is preserved in uncontrolled type 2 diabetes (46). Additionally, we have shown in healthy, insulin-sensitive, younger subjects that increasing insulin doses, which increase glucose uptake in a dose-dependent manner, are unable to stimulate muscle protein anabolism if muscle perfusion and amino acid availability are not simultaneously enhanced (11). Thus, improved insulin signaling following exercise is unlikely the only major mechanism allowing for the restoration of the physiological muscle protein anabolic response to insulin, since improvements in vasodilation and the consequent increases in amino acid availability appear to play an essential role as well.
However, it is also important to underscore how the current data make it apparent that increases in tissue perfusion and amino acid availability are necessary, but not sufficient, to induce an anabolic response of muscle proteins. Indeed, the EX group tended to have a higher baseline blood flow and amino acid delivery, but this did not result in a higher baseline mTOR signaling or muscle protein synthesis. Therefore, it is likely that for insulin to acutely increase human skeletal muscle protein synthesis and anabolism it has to increase not only muscle perfusion and amino acid delivery but also provide adequate signals for an increase in muscle protein translation. Additionally, the higher baseline blood flow with no differences in muscle protein turnover or signaling also indicates that the effect of exercise on the vascular tone is direct and not mediated by increases in insulin sensitivity or muscle metabolism.
Finally, our finding of enhanced Akt signaling following exercise is consistent with some (47,48), but not all (49,50), previous reports. However, in the negative studies (49,50) insulin signaling had been measured immediately after exercise (<6 h), while we measured it 20 h postexercise (48). These data suggest that insulin signaling may be enhanced by aerobic exercise in a time-dependent manner. Future studies on the time course of exercise-induced enhancement of insulin signaling are warranted.
In summary, a single bout of aerobic exercise overcomes the age-related insulin resistance of muscle protein synthesis by reducing endothelial dysfunction, improving muscle perfusion and amino acid availability for the muscle tissue, and enhancing activation of the Akt/mTOR signaling pathway and muscle protein synthesis. Clinical studies are needed to investigate whether aerobic exercise may prevent the loss of muscle mass with aging by improving the muscle anabolic response during feeding.
Acknowledgments
This study was supported by the National Institute on Aging (grants R01 AG018311 and P30 AG024832); the Robert E. and May R. Wright Foundation; the National Institute for Arthritis and Musculoskeletal and Skin Diseases (grant R01 AR049877); the Shared Instrumentation Grant Program, National Center for Research Resources, NIH (grant S10 RR16650); and General Clinical Research Center Grants from the National Center for Research Resources, NIH (grants M01 RR00043 [University of Southern California] and M01 RR00073 [University of Texas Medical Branch]).
We thank the study subjects for their participation, Jeanine Cordero for technical assistance, and the nurses and personnel of the General Clinical Research Center of the University of Southern California and the University of Texas Medical Branch for their assistance with the clinical conduct of this study. Preliminary data from this study were presented at the Experimental Biology Meeting 2005.
- 4EBP1
4E-binding protein 1
- FSR
fractional synthetic rate
- ICG
indocyanine green
- mTOR
mammalian target of rapamycin
- S6K1
S6 kinase 1
References
- 1.Volpi E, Nazemi R, Fujita S. Muscle tissue changes with ageing. Curr Opin Clin Nutr Metab Care. 2004;7:405–410. doi: 10.1097/01.mco.0000134362.76653.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337:1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 3.Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci. 1995;50(64–67) doi: 10.1093/gerona/50a.special_issue.64. [DOI] [PubMed] [Google Scholar]
- 4.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the olderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guillet C, Prod’homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in olderly humans. FASEB J. 2004;18:1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 6.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286:1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the olderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the olderly despite higher first pass splanchnic extraction. Am J Physiol Endocrinol Metab. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 9.Volpi E, Kobayashi H, Mittendorfer B, Sheffield-Moore M, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy olderly adults. Am J Clin Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and olderly. Am J Physiol Endocrinol Metab. 2004;286:E321–E328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 11.Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. The effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab. 2006;291:E745–E754. doi: 10.1152/ajpendo.00271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proud CG. Regulation of protein synthesis by insulin. Biochem Soc Trans. 2006;34:2–6. doi: 10.1042/BST20060213. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology. 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 14.Meneilly GS, Elliot T, Bryer-Ash M, Floras JS. Insulin-mediated increase in blood flow is impaired in the olderly. J Clin Endocrinol Metab. 1995;80:1899–1903. doi: 10.1210/jcem.80.6.7775638. [DOI] [PubMed] [Google Scholar]
- 15.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent: a novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherrer U, Randin D, Vollenweider P, Vollenweider L, Nicod P. Nitric oxide release accounts for insulin’s vascular effects in humans. J Clin Invest. 1994;94:2511–2515. doi: 10.1172/JCI117621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM the Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M the Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 21.Henriksen EJ. Invited review: effects of acute exercise and exercise training on insulin resistance. J Appl Physiol. 2002;93:788–796. doi: 10.1152/japplphysiol.01219.2001. [DOI] [PubMed] [Google Scholar]
- 22.Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- 23.Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. International Journal of Sports Medicine. 2000;21:1–12. doi: 10.1055/s-2000-8847. [DOI] [PubMed] [Google Scholar]
- 24.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 25.Mikines KJ, Sonne B, Tronier B, Galbo H. Effects of acute exercise and detraining on insulin action in trained men. Journal of Applied Physiology. 1989;66:704–711. doi: 10.1152/jappl.1989.66.2.704. [DOI] [PubMed] [Google Scholar]
- 26.Wojtaszewski JF, Hansen BF, Kiens B, Richter EA. Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes. 1997;46:1775–1781. doi: 10.2337/diab.46.11.1775. [DOI] [PubMed] [Google Scholar]
- 27.Wojtaszewski JF, Hansen BF, Gade Kiens B, Markuns JF, Goodyear LJ, Richter EA. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49:325–331. doi: 10.2337/diabetes.49.3.325. [DOI] [PubMed] [Google Scholar]
- 28.Sheffield-Moore M, Yeckel CW, Volpi E, Wolf SE, Morio B, Chinkes DL, Paddon-Jones D, Wolfe RR. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab. 2004;287:E513–E522. doi: 10.1152/ajpendo.00334.2003. [DOI] [PubMed] [Google Scholar]
- 29.Bell JA, Fujita S, Volpi E, Cadenas JG, Rasmussen BB. Short-term insulin and nutritional energy provision does not stimulate muscle protein synthesis if blood amino acid availability decreases. Am J Physiol Endocrinol Metab. 2005;289:999–1006. doi: 10.1152/ajpendo.00170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jorfeldt L, Juhlin-Dannfelt A. The influence of ethanol on splanchnic and skeletal muscle metabolism in man. Metabolism. 1978;27:97–106. doi: 10.1016/0026-0495(78)90128-2. [DOI] [PubMed] [Google Scholar]
- 31.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss; 1992. [Google Scholar]
- 33.Denne SC, Liechty EA, Liu YM, Brechtel G, Baron AD. Proteolysis in skeletal muscle and whole body in response to euglycemic hyperinsulinemia in normal adults. Am J Physiol. 1991;261:E809–E814. doi: 10.1152/ajpendo.1991.261.6.E809. [DOI] [PubMed] [Google Scholar]
- 34.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Sp. 1992;6:421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- 35.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol (Lond) 2006;576:2–24. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 37.Toffolo G, Foster DM, Cobelli C. Estimation of protein fractional synthetic rate from tracer data. Am J Physiol. 1993;264:E128–E135. doi: 10.1152/ajpendo.1993.264.1.E128. [DOI] [PubMed] [Google Scholar]
- 38.Vincent MA, Dawson D, Clark AD, Lindner JR, Rattigan S, Clark MG, Barrett EJ. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes. 2002;51:42–48. doi: 10.2337/diabetes.51.1.42. [DOI] [PubMed] [Google Scholar]
- 39.Baron AD, Tarshoby M, Hook G, Lazaridis EN, Cronin J, Johnson A, Steinberg HO. Interaction between insulin sensitivity and muscle perfusion on glucose uptake in human skeletal muscle: evidence for capillary recruitment. Diabetes. 2000;49:768–774. doi: 10.2337/diabetes.49.5.768. [DOI] [PubMed] [Google Scholar]
- 40.Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418–1423. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- 41.Clerk LH, Vincent MA, Lindner JR, Clark MG, Rattigan S, Barrett EJ. The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes Metab Res Rev. 2004;20:3–12. doi: 10.1002/dmrr.414. [DOI] [PubMed] [Google Scholar]
- 42.Cardillo C, Kilcoyne CM, Cannon RO, III, Panza JA. Interactions between nitric oxide and endothelin in the regulation of vascular tone of human resistance vessels in vivo. Hypertension. 2000;35:1237–1241. doi: 10.1161/01.hyp.35.6.1237. [DOI] [PubMed] [Google Scholar]
- 43.Boulanger C, Luscher TF. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mather KJ, Mirzamohammadi B, Lteif A, Steinberg HO, Baron AD. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes. 2002;51:3517–3523. doi: 10.2337/diabetes.51.12.3517. [DOI] [PubMed] [Google Scholar]
- 45.Maeda S, Tanabe T, Miyauchi T, Otsuki T, Sugawara J, Iemitsu M, Kuno S, Ajisaka R, Yamaguchi I, Matsuda M. Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J Appl Physiol. 2003;95:336–341. doi: 10.1152/japplphysiol.01016.2002. [DOI] [PubMed] [Google Scholar]
- 46.Bell JA, Volpi E, Fujita S, Cadenas JG, Sheffield-Moore M, Rasmussen BB. Skeletal muscle protein anabolic response to increased energy and insulin is preserved in poorly controlled type 2 diabetes. J Nutr. 2006;136:1249–1255. doi: 10.1093/jn/136.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christ-Roberts CY, Pratipanawatr T, Pratipanawatr W, Berria R, Belfort R, Mandarino LJ. Increased insulin receptor signaling and glycogen synthase activity contribute to the synergistic effect of exercise on insulin action. J Appl Physiol. 2003;95:2519–2529. doi: 10.1152/japplphysiol.00605.2003. [DOI] [PubMed] [Google Scholar]
- 48.Wadley GD, Konstantopoulos N, Macaulay L, Howlett K, Garnham A, Hargreaves M, Cameron-Smith D. Increased insulin-stimulated Akt pSer473 and cytosolic SHP2 protein abundance in human skeletal muscle following acute exercise and short-term training. J Appl Physiol. 2007;102:1624–1631. doi: 10.1152/japplphysiol.00821.2006. [DOI] [PubMed] [Google Scholar]
- 49.Wojtaszewski JF, Hansen BF, Gade Kiens B, Markuns JF, Goodyear LJ, Richter EA. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49:325–331. doi: 10.2337/diabetes.49.3.325. [DOI] [PubMed] [Google Scholar]
- 50.Howlett KF, Sakamoto K, Yu H, Goodyear LJ, Hargreaves M. Insulin-stimulated insulin receptor substrate-2-associated phosphatidylinositol 3-kinase activity is enhanced in human skeletal muscle after exercise. Metabolism. 2006;55:1046–1052. doi: 10.1016/j.metabol.2006.03.016. o:p. [DOI] [PubMed] [Google Scholar]