Abstract
We compared the effectiveness of two surgical interventions for improving word recognition ability in quiet of patients who presented with (i) bilateral, precipitously sloping, high-frequency hearing loss, (ii) relatively good auditory thresholds at and below 500 Hz and (iii) poor speech recognition. In one intervention (n=25), a conventional electrode array was inserted into one cochlea. As a consequence hearing was lost in the implanted ear. In the other intervention (n=22), a Nucleus Hybrid, short-electrode array was inserted 10 mm into one cochlea with the aim of preserving hearing in that ear. Both groups of patients had similar low-frequency hearing and speech understanding in the ear contralateral to the implant. Following surgery, both groups had significantly higher word recognition scores than before surgery. Between-group comparisons indicated that the conventional electrode-array group had higher word recognition scores than the 10 mm group when stimulation was presented to the operated ear and when stimulation was presented to both ears.
Keywords: cochlear implant, low-frequency hearing, EAS, Hybrid
The benefits of combining information from low-frequency acoustic hearing and from a cochlear implant have been well documented (e.g., Ching et al., 2004; Dorman et al., 2008; Gantz et al., 2005; Gstoettner et al., 2004; Kiefer et al., 2005; Kong et al., 2005; von Ilberg et al., 1999; Wilson et al., 2002). Both speech understanding in quiet and speech understanding in noise are significantly improved when patients have access to both acoustically and electrically stimulated information.
One surgical approach to produce combined acoustic and electric stimulation is to insert a conventional (long) electrode array into one cochlea of patients with bilateral, precipitously sloping hearing loss above 500 Hz. Hearing is lost in the implanted ear but patients can obtain low-frequency acoustic information from the contralateral ear. Dorman et al. (2008) report CNC scores averaging 74 % correct when both electrically and acoustically stimulated information is available to patients (see, also, Gifford et al., 2007 see, also, Gifford et al., 2008). For results from patients with varying amounts of residual acoustic hearing see Armstrong et al., 1997; Dunn et al., 2005; Ching et al., 2004; Hamzavi et al., 2004; Kong et al., 2005; Mok et al., 2006; Shallop et al., 1992; Tyler et al, 2002.
Another surgical approach to produce combined acoustic and electric stimulation is to insert an electrode array 10, 16 or 20 mm into the cochlea with the aim of preserving hearing in the implanted ear (Gantz et al., 2005; Gantz & Turner, 2004; Gstoettner et al., 2004; Kiefer et al., 2005; Leutje et al., 2007; Skarzynski et al., 2004; von Ilberg et al., 1999). When hearing is preserved, patients can use low-frequency acoustic stimulation from both ears when combining acoustic and electric hearing. For example, Gantz et al. (2005) studied 11 patients with 10 mm insertions and reported a mean CNC score of 69% correct in the combined electric and bilateral acoustic condition. Kiefer et al. (2005) studied 13 patients with 20 mm insertions and reported a mean monosyllabic word recognition score of 67% correct in the combined electric and bilateral acoustic condition.
In the research reported here we compared the effectiveness of two of the surgical interventions described above for improving the word recognition ability in quiet of patients who presented with (i) bilateral, precipitously sloping high-frequency hearing loss, (ii) relatively good auditory thresholds at and below 500 Hz and (iii) poor speech recognition. In one intervention, a conventional electrode array was inserted into one cochlea. As a consequence hearing was lost in the implanted ear. In the other intervention, a Nucleus Hybrid electrode array was inserted 10 mm into one cochlea with the aim of preserving hearing in that cochlea. Before surgery both groups of patients had similar low-frequency hearing and speech understanding in the ears ipsilateral and contralateral to the implant.
Methods
Subjects
Forty seven patients with bilateral, high-frequency hearing loss participated in this project. All patients met the audiometric and speech understanding criteria for entry into the Cochlear Corporation Nucleus Hybrid clinical trial, i.e., (i) thresholds at 500 Hz and below at less than or equal to 60 dB HL, (ii) thresholds at 2000 Hz and above at greater than or equal to 80 dB HL, (iii) monosyllabic word recognition less than or equal to 60% in the to-be-implanted-ear and no greater than 80% in the non-implanted ear.
One group was composed of twenty-two patients who received the Cochlear Corporation Nucleus Hybrid implant. The Hybrid electrode array is 10 mm in length. It has six electrode contacts spaced over the distal 4.3 mm of the array. The center frequency of the lowest input filter is variable and dependent on the degree of hearing preservation following surgery. Typically the center frequency of the lowest input filter is 774 Hz and center frequency of the highest filter is 6417 Hz. All electrode arrays were fully inserted by surgeon’s report. The patients were recruited from three implant centers in the United States. The patients ranged in age from 22 to 75 years with a mean age of 56. The patients’ experience with electrical stimulation ranged from 3 to 18 months with an average of 13 months.
The second group was composed of twenty-five patients who received a conventional cochlear implant. All electrode arrays were fully inserted by surgeon’s report. The patients were recruited from four implant centers in the United States. Twelve patients used Cochlear Corporation devices and thirteen patients used Advanced Bionics Corporation devices. For the Cochlear Corporation devices the center frequency of the lowest input filter was 243 Hz and the center frequency of the highest input filter was 7421 Hz. The center frequency values cited here are the geometric means of the upper and lower frequency cutoffs of the input filters for the most apical and basal channels, respectively. For the Advanced Bionics devices the center frequency of the lowest input filter was 333 Hz and the center frequency of the highest input filter was 6665 Hz. Most of the patients had been identified as fitting the criteria for the clinical trial of the 20 mm Med El EAS device, but opted for a full insertion when the clinical trial was postponed. Another group of patients was identified by chart review. For these patients, complete pre-implant audiometric and speech data were not always available, e.g., audiometric thresholds at 125 Hz and bilateral, aided performance. The patients ranged in age from 45 to 85 years with a mean age of 68 years. The patients’ experience with electrical stimulation ranged from 5 to 98 months and averaged 20 months. This mean was influenced by two outliers -- patients with 98 and 51 months experience. For the remaining patients the mean was 15 months.
The prospectively identified patients were paid an hourly wage for participation. The research was approved by the IRBs at Arizona State University (encompassing the Arizona Ear Center), the Mayo Clinic in Rochester, the Midwest Ear Institute and the Carle Clinic.
Pre-implant audiograms
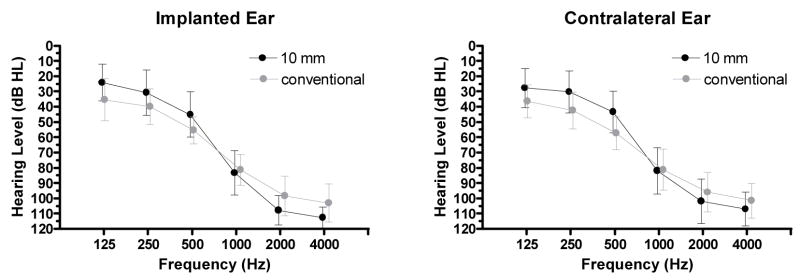
The mean pre-implant audiogram for the implanted ear of the 10 mm patients is shown in Figure 1 (left panel; black symbols). The mean pre-implant audiogram for the conventional electrode array patients is shown with grey symbols. The mean audiograms for the contralateral ears of the patients are shown in the right panel of Figure 1 using the same conventions.
Figure 1.
Pre-implant audiograms for patients in the 10 mm and conventional electrode array groups. The symbols are slightly offset at each frequency to make the plots easier to examine. Error bars indicate +/− 1 standard deviation.
Speech material
Monosyllabic word recognition performance in quiet was assessed using the Consonant Nucleus Consonant (CNC) word lists (Peterson and Lehiste, 1982). In accordance with the research protocol defined in the Nucleus Hybrid clinical trial, the following conditions were tested: Aided acoustic for the implanted ear (Ai); aided acoustic for the contralateral ear (Ac); binaural aided (Abin); electric only (E); electric plus ipsilateral acoustic (E + Ai or ‘Hybrid’); electric plus contralateral acoustic (E + Ac or ‘bimodal’); and electric plus binaural acoustic (E + Aic; or ‘combined’). Two 50-item lists were administered for each of the conditions with the reported score representing the average performance across the two lists. The CNC lists were assigned randomly for each subject and condition. All testing was achieved using recorded stimuli presented via a single loudspeaker at a calibrated level of 70 dB SPL. The loudspeaker was placed at 0° azimuth at a distance of 1 meter from the subject. When acoustic input to an ear was unwanted, e.g., to the ipsilateral ear in the E + Ac condition, that ear was occluded with a foam EAR plug.
Pre-implant speech understanding
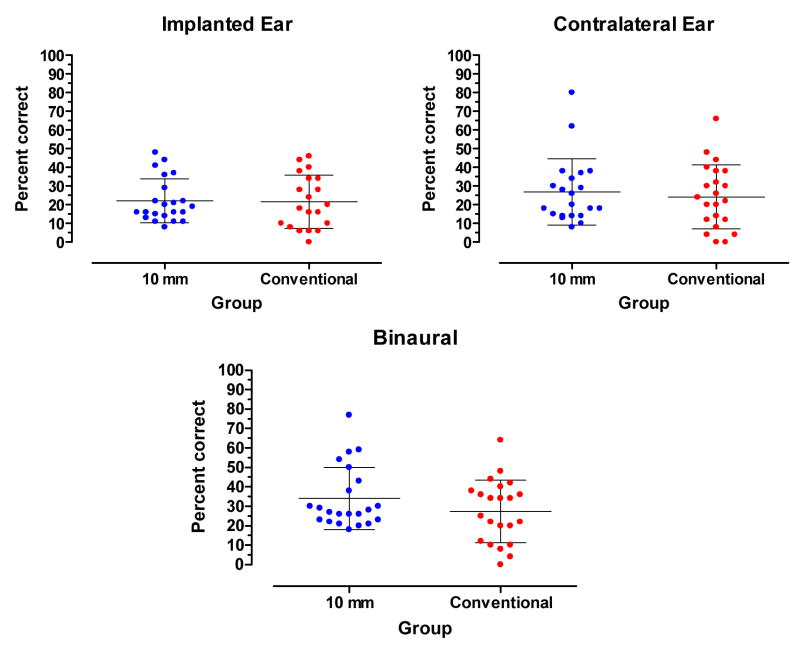
Prior to testing, all hearing aid settings for both the ipsilateral and contralateral ears were verified using the speechmap fitting system on the Audioscan Verifit real-ear mode (REM) to match NAL-NL1 targets for 70-dB-SPL speech. The pre-implant, aided CNC scores for stimulation directed to the to-be-implanted ear of the 10 mm patients and the conventional electrode array patients are shown in the left panel of Figure 2. The mean score for both groups was 22% correct. The scores for stimulation directed to the contralateral ear are shown in the right panel of Figure 2. The mean score for the 10 mm group was 27 % correct. The mean score for the conventional electrode-array group was 24 % correct. The mean scores were not significantly different (t41=0.5045, p.>.05). The scores for bilateral stimulation are shown in the bottom panel of Figure 2. The mean score for the 10 mm group was 34 % correct. The mean score for the conventional electrode-array group was 27 % correct. The mean scores were not significantly different (t42=1.4, p.>.05). Overall, there were no significant differences in pre-implant speech understanding between groups.
Figure 2.
Pre-implant, aided CNC scores for the 10 mm and conventional electrode array groups. The mean and +/− 1 standard deviation are indicated by horizontal lines.
Results
The results are organized into four sections. In the first we describe hearing preservation for the patients who received a 10 mm electrode array. In the second we describe word recognition outcomes for those patients. In the third we describe word recognition outcomes for patients with a conventional electrode array. In the fourth we compare levels of performance for the two groups of patients. In each section we discuss separately performance with stimulation directed to the implanted ear and performance with stimulation directed to both ears. Because we conducted multiple t-tests we adjusted our alpha (α) value using the Bonferroni correction. We report tests on 8 comparisons. The corrected α value is .05/8 or 0.0062.
Hearing preservation in the operated ear
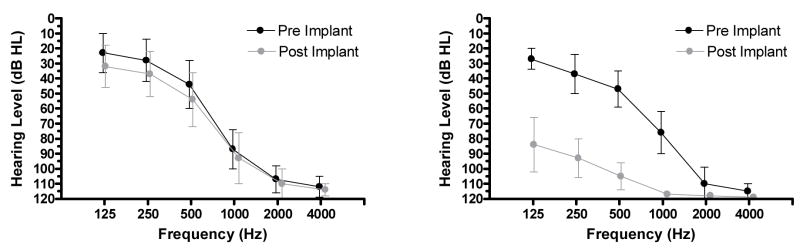
Hearing was well preserved in 15 of 22 patients. As shown in Figure 3 (left), for those 15 patients the average, pre-operative thresholds at 125, 250, 500, 1000, 2000 and 4000 Hz were 23, 28, 44, 87, 107 and 112 dB HL, respectively. Post-operative thresholds were 32, 37, 54, 93, 110 and 114 dB HL. Thus, the average hearing loss was approximately 10 dB over the low frequencies, i.e., 125–500 Hz. As shown in Figure 3 (right), for the remaining 7 patients the average, pre-operative hearing thresholds at 125, 250, 500, 1000, 2000 and 4000 Hz were 27, 37, 47, 76, 110 and 115 dB HL. Following surgery the thresholds were 84, 93, 105, 117, 118 and 119 dB HL. There were several patterns of hearing loss. Three patients lost hearing within 1 to 3 months following surgery. One patient lost hearing in the implanted ear over a period of 6 to 12 months following surgery. One patient lost hearing in both ears over a period of 6 to 12 months following surgery. Two patients lost hearing due to accidents not related to surgery. Overall, four of the seven losses appeared to be a consequence of surgery.
Figure 3.
Left panel: Pre- and post-implant audiograms for patients (n=15) with preserved hearing. Right panel: Pre- and post-implant audiograms for patients (n=7) with major loss of hearing. Error bars indicate +/− 1 standard deviation.
Hearing loss and the subsequent loss of speech information delivered acoustically to the operated ear is one of the risks of hearing-preservation surgery. In all but one of the analyses reported below all 22 patients were included in the statistical evaluations.
As noted above for pre-implant speech testing, post-implant testing was also conducted following verification of hearing aid settings for both the ipsilateral and contralateral ears using the speechmap fitting system on the Audioscan Verifit real-ear mode (REM) to match NAL-NL1 targets for 70-dB-SPL speech.
10 mm insertion with stimulation directed to the implanted ear
Figure 4 displays a within-group, pre- and post-implant comparison of performance for stimulation directed to the implanted ear of the 10 mm patients. The pre-implant, Ai mean score was 22% correct. The post-implant E mean score was 28% correct. These mean scores were not statistically different (t21 = 1.4, p.= 0.18). The post-implant E + Ai score was 39 %. Four patients achieved slightly poorer scores in the E + Ai condition than in the Ai condition. Nonetheless, the group mean score in the E + Ai condition was significantly higher than the pre-implant, Ai score (t21 = 4.01, p.= 0.006).
Figure 4.
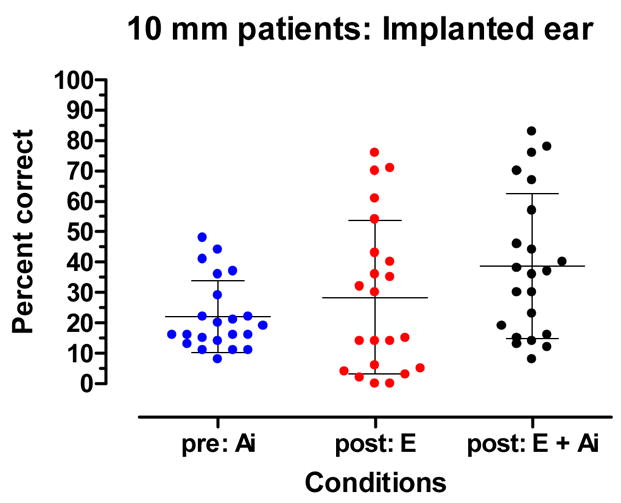
Pre-implant aided and post-implant, electric-only (E) and acoustic plus electric (E + Ai) recognition of CNC words. The mean and +/− 1 standard deviation are indicated by horizontal lines.
10 mm insertion with stimulation directed to both ears
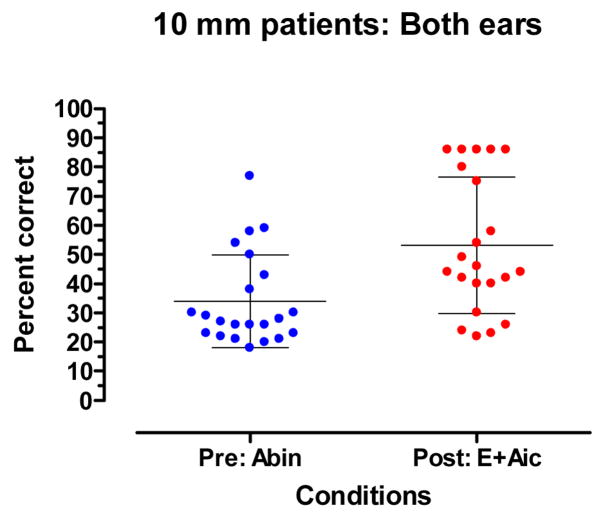
Figure 5 displays a within-group, pre- and post-implant comparison of performance with binaural inputs. The pre-implant, Abin score was 34 % correct. The post-implant E + Aic score was 53% correct. Two patients achieved poorer scores in the E + Aic condition than in the Abin condition. The mean scores for the Abin and E + Aic conditions were significantly different (t21=5.5, p. <0.001).
Figure 5.
Recognition of CNC words: Pre-implant, binaural aided (Abin) and post-implant E + Aic. The mean and +/− 1 standard deviation are indicated by horizontal lines.
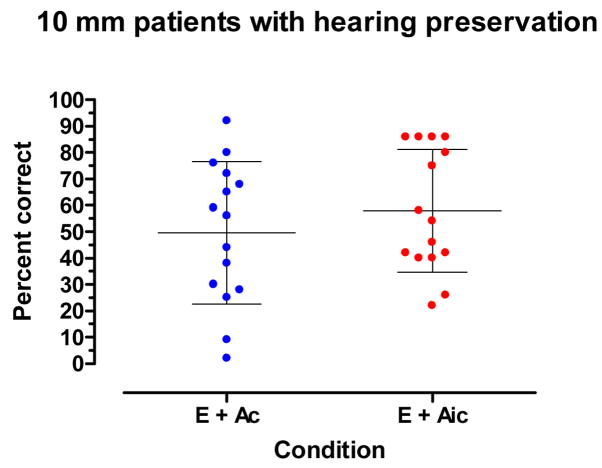
Figure 6 displays a within-group comparison for the 10 mm patients in the E + Ai and E + Aic conditions. This comparison speaks to the question of the benefit of preserving hearing in the implanted ear. In the E + Aic condition, or the ‘combined’ condition in the Nucleus Hybrid clinical trial, stimulation was directed to the implant and to both hearing ears. In the E + Ac, or bimodal condition, stimulation was directed to the implant and to the ear contralateral to the implant (the ipsilateral ear was plugged). The difference between the conditions is the availability of low-frequency information from the implanted ear in the E + Aic condition. For this analysis only patients with preserved hearing (n=15) were included.
Figure 6.
For patients (n=15) with hearing preservation: Recognition of CNC words in the E + Ac and E + Aic conditions. The mean and +/− 1 standard deviation are indicated by horizontal lines.
As shown in Figure 6 the mean score in the E + Ac condition was 50% correct. The mean score in the E + Aic condition was 58% correct. The mean scores were not statistically significant (t14=2.6, p.= 0.023) using the Bonferroni corrected α value of 0.006. If this had been the only condition tested, or we had not used the correction, the mean scores would have been significantly different.
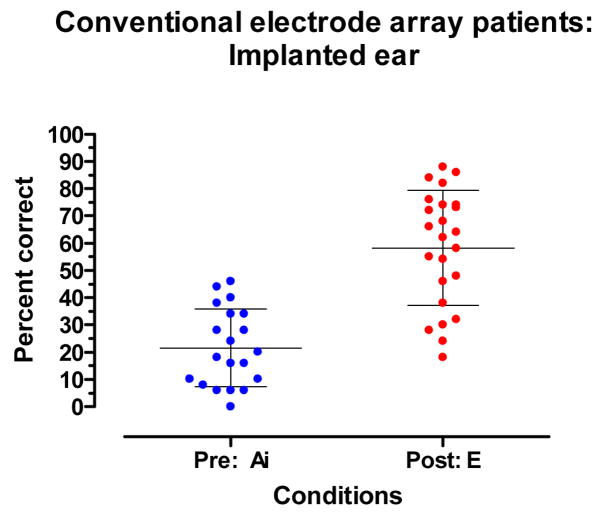
Full insertion with stimulation directed to the implanted ear
Figure 7 displays a within-group comparison of pre- and post-implant performance for stimulation directed to the implanted ear of patients who received a conventional electrode array. The mean pre-implant Ai score was 22% correct. The mean post-implant score for the E condition was 58 % correct. Two patients had slightly poorer E scores than Ai scores. The mean scores for the E and Ai conditions were significantly different (t18 = 5.67, p.< 0.001).
Figure 7.
Pre-implant aided and post-implant electric-only (E) CNC word recognition. The mean and +/− 1 standard deviation are indicated by horizontal lines.
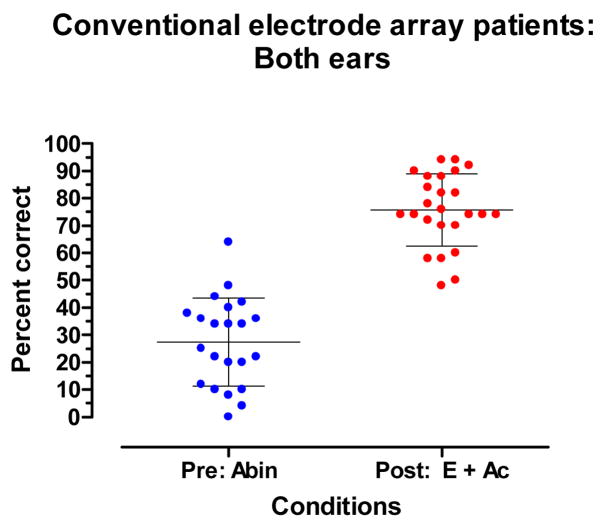
Full insertion with stimulation directed to both ears
Figure 8 displays a within-group, pre- and post-implant comparison of performance with binaural inputs. The pre-implant, Abin score was 27% correct. The post-implant E + Ac score was 76% correct. No patient had a poorer E + Ac score than an Abin score. The mean scores for the Abin and E + Ac conditions were significantly different (t21=13.55, p.< 0.0001).
Figure 8.
Recognition of CNC words: Pre-implant, binaural aided (Abin) and post-implant, E + Ac. The mean and +/− 1 standard deviation are indicated by horizontal lines.
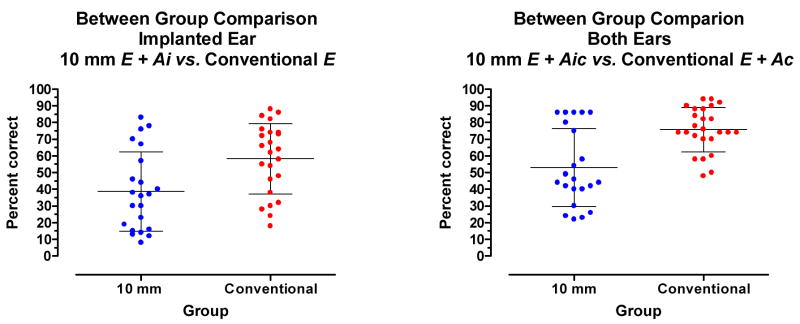
Comparing results between groups
Figure 9 (left panel) displays a between-group comparison of performance with stimulation directed to the implanted ear. The mean score for the 10 mm patients in the E+Ai condition was 39% correct. The mean score for the conventional electrode-array patients in the E condition was 58% correct. The mean scores were significantly different (t44=3.0, p.= 0.0049).
Figure 9.
Between-group comparisons for CNC word recognition. Left: Stimulation directed to the implanted ear. Right: Stimulation directed to both ears. The mean and +/−1 standard deviation are indicated by horizontal lines.
Figure 9 (right panel) displays a between-group comparison of performance with stimulation directed to both ears. The mean score for the 10 mm patients the E + Aic condition was 53 % correct. The mean score for the conventional electrode-array patients in the E + Ac condition was 76% correct. The mean scores were significantly different(t45=4.2, p.<0.0001).
Discussion
The aim of the research reported here was to assess the efficacy of two surgical interventions for improving the word recognition ability of patients who presented with (i) bilateral, precipitously sloping high-frequency hearing loss, (ii) relatively good auditory thresholds at and below 500 Hz and (iii) poor speech recognition. We have structured the discussion of outcomes around a set of questions.
Is word recognition improved for patients who receive a 10 mm electrode array with the aim of hearing preservation?
The answer is ‘yes’ for both the operated ear alone and for all inputs combined. This outcome is consistent with previous reports of benefit from hearing-preservation surgery with the Hybrid, 10 mm electrode array (e.g., Gantz et al., 2005; Luetje et al., 2007).
Is there benefit from preserving hearing in the implanted ear?
Because the patients had low-frequency hearing in both ears, the critical case, for the 10 mm patients, is performance in the E + Ac condition vs. performance in the E + Aic condition. The E + Aic condition allows acoustic hearing in the operated ear to contribute to word recognition. When looking only at patients with preserved acoustic hearing, there was a mean increase of 8 percentage points in the E + Aic condition. While this was not significant when using a Bonferroni corrected α value, it was significant at the .02 level.
It is useful to ask why we should expect any improvement in CNC word recognition when hearing is preserved in the operated ear. Consider first the preoperative condition of the patient. Both ears had relatively symmetrical high-frequency hearing loss and poor speech understanding. If there was a poorer ear, it was chosen for surgery. Following surgery, the hearing in the operated ear was reduced by a small amount -- an average of 10 dB at 125, 250 and 500 Hz (for patients with preserved hearing). Why would we expect a relatively poor ear to add to the speech understanding provided by a relatively good ear, i.e., the contralateral ear?
Consider the case of preoperative speech understanding when hearing in the operated ear had not yet been made just a little poorer. For the patients who were to receive a 10 mm electrode array, there was a 7 percentage point improvement in performance in the bilateral aided condition vs. the best single-ear, aided condition. For the patients who were to receive a conventional electrode array, there was a 3 percentage point improvement in performance in the bilateral vs. best single-ear, aided condition. Thus, there was little reason to believe that following surgery the poorer ear would add greatly to the better ear when both were combined with electric stimulation. And that is the outcome we obtained – an average of an 8 percentage point improvement in the E + Aic condition relative to the E + Ac condition. The statistical significance of this small improvement is open to question. If the Bonferroni correction is not necessary (e.g., Perneger, 1998; Rothman, 1990), then the improvement is statistically significant.
From the standpoint of speech information it is unclear why even an 8 percentage point improvement was obtained. There should have been no information from the operated ear that wasn’t contained in the signal from the contralateral ear. Given that the speech signal was presented at a high level from a single speaker at 0 degrees azimuth, binaural summation for loudness should not have played a role in performance. Perhaps it is useful to have two ‘looks’ at the same information even if the second ‘look’ (from the operated ear) is of lower fidelity than the ‘look’ from the better ear.
Is word recognition better post-implant than pre-implant in patients who receive a conventional electrode array in one ear?
The answer is ‘yes’ both for the case of inputs to the operated ear and for the case of inputs to both ears. This finding is consistent with our previous reports with smaller samples (Dorman et al., 2008; Gifford, et al., 2007).
Do the interventions differ as an aid to word recognition?
For both the case of stimulation directed to the operated ear and stimulation directed to both ears, patients with a conventional electrode array achieved higher scores than patients with a 10 mm array. For stimulation directed to the implanted ear the mean scores were 58% correct and 39% correct for the conventional electrode array and 10 mm groups, respectively. For stimulation directed to both ears, the mean scores were 76% correct and 53% correct. The significant differences in performance remained when patients who had large hearing losses in the ear ipsilateral to the implant were removed from the sample.
What accounts for the differences in performance for the conventional electrode array and 10 mm patients?
Because both groups had access to similar acoustic information in the ear contralateral to the implant, and because preserved hearing in the implanted ear added little to intelligibility, we suggest that the difference in overall performance between groups was due largely to differences in the information made available by the two electrode arrays.
For the 10 mm patients, the signal was first high-pass filtered and then delivered to a 6-electrode array covering an extent of 4.3 mm. In contrast, the conventional electrode-array patients received a more broadband signal that was delivered to electrode arrays with 16 or 22 contacts that covered an extent of approximately 14–17 mm. From this point of view it is not surprising that the conventional electrode-array patients achieved higher scores than the 10 mm patients.
On the other hand, it is surprising that three 10 mm patients achieved high scores -- 70, 71 and 76% correct -- in the E-condition. While not as high as the best E-scores with a conventional electrode-array (four scores between 82 and 88 % correct), the scores document that patients can ‘remap’ electrical stimulation at the base of the cochlea in the service of speech understanding. If there had been more patients in our sample who could remap, then our mean scores might have been closer to the mean scores reported by Gantz et al. (2005). And, if remapping takes place over a long time period, then scores at more than two years after surgery might be higher than the scores we have recorded.
On our view, the most significant issue in hearing preservation surgery is not whether hearing is preserved in the operated ear. Instead, it is the ability of a basally positioned, shortened electrode array to convey speech information. It will be of interest to see whether deeper insertions – 16 and 20 mm -- using longer electrode arrays (as used in ongoing clinical trials of hearing preservation surgery) will allow the same level of speech understanding as conventional electrode arrays. If they do, then there will be little ‘risk’ in hearing preservation surgery in terms of speech understanding because, even if hearing is lost, performance will be no worse than with a conventional electrode array.
How can we best assess the benefits of hearing preservation surgery?
As noted above, there is little reason for a second, partially hearing ear (the implanted ear with preserved hearing) to be very useful for word recognition in quiet or in noise when (i) signals are presented from a single loudspeaker and when (ii) signals are presented at suprathreshold levels. For the case of signals at threshold, having two partially hearing ears could result in binaural summation that could be of benefit to a listener. However, the practical benefits of small changes in speech reception threshold are debatable. If large differences in speech understanding are to be found for patients with two partially hearing ears vs. one partially hearing ear, then the differences will likely to be found in complex sound environments characterized by separate noise and signal sources -- in which low-frequency, binaural cues could aid speech recognition.
Acknowledgments
This work was supported by R01 DC 00654-15 to MFD from the National Institute for Deafness and Other Communication Disorders.
References
- Armstrong M, Pegg P, James C, Blamey P. Speech perception in noise with implant and hearing aid. A J Oto. 1997;18:S140–S141. [PubMed] [Google Scholar]
- Ching T, Incerti P, Hill M. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear Hear. 2004;25:9–21. doi: 10.1097/01.AUD.0000111261.84611.C8. [DOI] [PubMed] [Google Scholar]
- Dorman M, Gifford R, Spahr A, McKarns S. The benefits of combining acoustic and electric stimulation for the recognition of speech, voice and melodies. Audiol Neuroto. 2008;13:105–112. doi: 10.1159/000111782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C, Tyler R, Witt S. Benefit of wearing a hearing aid on the unimplanted ear in adult users of a cochlear implant. JSLHR. 2005;48:668–680. doi: 10.1044/1092-4388(2005/046). [DOI] [PubMed] [Google Scholar]
- Gantz B, Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus hybrid implant. Acta Oto. 2004;124:344–347. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- Gantz B, Turner C, Gfeller K, Lowder M. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- Gifford R, Dorman M, McKarns S, Spahr A. Combined electric and contralateral acoustic hearing: word and sentence intelligibility with bimodal hearing. J Sp Lang Hear Res. 2007;50:835–843. doi: 10.1044/1092-4388(2007/058). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford R, Shallop J, Peterson A. Speech recognition materials and ceiling effects: Considerations for cochlear implant programs. Aud Neuroto. 2008;13:193–205. doi: 10.1159/000113510. [DOI] [PubMed] [Google Scholar]
- Gstoettner WK, Helbig S, Maier N, Kiefer J, Radeloff A, Adunka OF. Ipsilateral electric acoustic stimulation of the auditory system: results of long-term hearing preservation. Audiol Neurootol. 2006;11:49–56. doi: 10.1159/000095614. [DOI] [PubMed] [Google Scholar]
- Gstoettner W, Kiefer J, Baumgartner W, Pok S, Peters S, Adunka O. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Oto. 2004;124:348–352. doi: 10.1080/00016480410016432. [DOI] [PubMed] [Google Scholar]
- Hamzavi J, Pok S, Gstoettner W, Baumgartner WD. Speech perception with a cochlear implant used in conjunction with a hearing aid in the opposite ear. I J Audiology. 2004;43:61–65. [PubMed] [Google Scholar]
- Kiefer J, Pok M, Adunka O, Sturzebecher E, Baumgartner W, Schmidt M, Tillein J, Ye Q, Gstoettner W. Combined electric and acoustic stimulation of the auditory system: results of a clinical study. Audiol Neuro-Otol. 2005;10(3):134–144. doi: 10.1159/000084023. [DOI] [PubMed] [Google Scholar]
- Kong Y-L, Stickney G, Zeng F-G. Speech and melody recognition in binaurally combined acoustic and electric hearing. J Acoust Soc Am. 2005;117(3):1351–1361. doi: 10.1121/1.1857526. [DOI] [PubMed] [Google Scholar]
- Luetje C, Thedinger B, Buckler L, Dawson K, Lisbona K. Hybrid cochlear implantation: Clinical results and critical review in 13 cases. Oto Neuroto. 2007;28:473–478. doi: 10.1097/RMR.0b013e3180423aed. [DOI] [PubMed] [Google Scholar]
- Mok M, Grayden D, Dowell R, Lawrence D. Speech perception for adults who use hearing aids in conjunction with cochlear implants in opposite ears. J Sp Lang Hear Res. 2006;49:338–351. doi: 10.1044/1092-4388(2006/027). [DOI] [PubMed] [Google Scholar]
- Peterson G, Lehiste I. Revised CNC lists for auditory tests. J Sp Hear Dis. 1962;17:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- Perneger T. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Shallop J, Arndt, Turnacliff K. Expanded indications for cochlear implantation: perceptual results in seven adults with residual hearing. J Sp Lang Path Audio. 1992;16:141–148. [Google Scholar]
- Skarzynski H, Lorens A, Piotrowska A. Preservation of low-frequency hearing in partial deafness cochlear implantation. International Congress Series. 2004;1273:239–242. [Google Scholar]
- Tyler R, Parkinson A, Wilson B, Witt S, Preece J, Noble W. Patients utilizing a hearing aid and a cochlear implant: speech perception and localization. Ear Hear. 2002;23:98–105. doi: 10.1097/00003446-200204000-00003. [DOI] [PubMed] [Google Scholar]
- vIlberg C, Kiefer J, Tillein J, Pfenningdorff T, Hartmann R, Sturzebecker E, Klinke R. Electric-acoustic stimulation of the auditory system. ORL. 1999;61:334–340. doi: 10.1159/000027695. [DOI] [PubMed] [Google Scholar]
- Wilson B, Wolford R, Lawson D, Schatzer R. Speech processors for auditory prostheses: Third QPR on NIH project N01-DC-2-1002. 2002:1–25. [Google Scholar]