Abstract
Schizophrenia and autism both feature significant impairments in social cognition and social functioning, but the specificity and mechanisms of these deficits remain unknown. Recent research suggests that social cognitive deficits in both disorders may arise from dysfunctions in the neural systems that underlie social cognition. We explored the neural activation of discrete brain regions implicated in social cognitive and face processing in schizophrenia subgroups and autism spectrum disorders during complex social judgments of faces. Twelve individuals with autism spectrum disorders (ASD), 12 paranoid individuals with schizophrenia (P-SCZ), 12 non-paranoid individuals with schizophrenia (NP-SCZ), and 12 non-clinical healthy controls participated in this cross sectional study. Neural activation, as indexed by blood oxygenation level dependent (BOLD) contrast, was measured in a priori regions of interest while individuals rated faces for trustworthiness. All groups showed significant activation of a social cognitive network including the amygdala, fusiform face area (FFA), superior temporal sulcus (STS), and ventrolateral prefrontal cortex (VLPFC) while completing a task of complex social cognition (i.e. trustworthiness judgments). ASD and P-SCZ individuals showed significantly reduced neural activation in the right amygdala, FFA, and left VLPFC as compared to controls and in the left VLPFC as compared to NP-SCZ individuals during this task. These findings lend support to models hypothesizing well-defined neural substrates of social cognition and suggest a specific neural mechanism that may underlie social cognitive impairments in both autism and paranoid schizophrenia.
Keywords: Amygdala, Fusiform Face Area, Paranoia, fMRI, Schizophrenia, High-functioning Autism
1. Introduction
Social cognition is defined as “the mental operations underlying social interactions, which include the human ability to perceive the intentions and dispositions of others” (Brothers, 1990, p. 28). Neurobiological models of social cognition posit that a network of neural structures is critically involved in processing social stimuli (Adolphs, 2001; Brothers, 1990; Phillips et al., 2003a). These models focus on regions of the occipital and temporal cortices such as the Fusiform Gyrus (FG) and Superior Temporal Sulcus (STS) which underlie face processing (Haxby, Hoffmann, & Gobbini, 2000; Winston, Henson, Fine-Goulden, & Dolan, 2004) and the amygdala which plays a critical role in detecting threat, recognizing emotions, and making complex social judgments (Adolphs et al., 1994; Adolphs et al., 1998; Amaral et al., 2003; Winston et al., 2002). Such models provide a foundation for understanding the neural mechanisms underlying social deficits in several clinical disorders, particularly schizophrenia and autism.
Although schizophrenia and autism have different symptom presentations, ages of onset, and developmental courses, impaired social functioning is a hallmark characteristic of both disorders (DSM-IV-TR), and these social deficits are related to impairments in social cognition (Couture et al., 2006; Hughes et al., 1997; Klin et al., 2002; Pinkham et al., 2003). Behavioral data suggest both disorders show comparable social cognitive deficits, particularly on tasks requiring higher levels of social cognitive skill (i.e. complex social judgments; Craig et al., 2004; Pilowsky et al., 2000); however, our understanding of these deficits, and the potential similarities between disorders, remains incomplete. Specifically, behavioral findings are complicated by heterogeneity within disorders, particularly in schizophrenia, as individuals with persecutory delusions perform differently both at behavioral and neural levels on social cognitive tasks relative to individuals without persecutory delusions (Bentall et al., 2001; Davis and Gibson, 2000; Phillips et al., 1999; Ueno et al., 2004; Williams et al., 2004). Additionally, despite evidence of abnormal activation in the neural systems of social cognition in schizophrenia and autism (Pinkham et al., 2003; Pelphrey et al., 2004), no studies have used fMRI to directly compare the neural substrates underlying social cognitive performance in both disorders. Thus, comparing these two disorders may illuminate the general mechanisms underlying social cognitive deficits and inform the etiologies of social dysfunction seen in these disparate disorders.
We used event-related functional magnetic resonance imaging (fMRI) to measure neural activation during complex social judgments (i.e. trustworthiness) of faces in four groups: high-functioning individuals with autism spectrum disorders (ASD), paranoid individuals with schizophrenia (P-SCZ), non-paranoid individuals with schizophrenia (NP-SCZ), and non-clinical healthy controls. As previous research has demonstrated significantly greater amygdala activation in non-paranoid, relative to paranoid individuals with schizophrenia (Phillips et al., 1999; Williams et al., 2004), two separate groups of patients with schizophrenia were recruited in order to provide the most comprehensive comparison with ASD and to account for known differences between schizophrenia subgroups. Further, decisions of trustworthiness were employed due to previous work demonstrating that these judgments fully engage the neural regions implicated in social cognition in healthy individuals (Winston et al., 2002) and to the likelihood that these judgments would be particularly salient in assessing differences between paranoid and non-paranoid individuals.
Based on neurobiological models of social cognition and face processing, and previous behavioral and imaging work utilizing trustworthiness judgments (Adolphs et al., 1998; Winston et al., 2002), comparisons of neural activation were limited a priori to discrete, brain regions comprising a face processing/social cognitive neural circuit, which included the amygdala, fusiform face area of the FG, STS, and ventrolateral prefrontal cortex (VLPFC; BA 47). Although the VLPFC has not been included in all previous studies of face processing, recent evidence implicates this region in making evaluative judgments (Cunningham et al., 2003), and it has been found to modulate activation of the amygdala while viewing faces and labeling facial expressions (Cunningham et al., 2004; Hariri et al., 2003). As such, this region may play an important role in top-down processing of social stimuli.
We predicted healthy controls would show greater neural activity than all clinical groups in the amygdala, FFA, STS, and VLPFC when making complex social judgments. For the direct comparison between schizophrenia and ASD, one would expect the ASD group to differ from both the NP-SCZ and P-SCZ groups due to clinical distinctions between the disorders; however, careful examination of the social cognition literature actually suggests that ASD should be most similar to the P-SCZ group. Specifically, increased rates of paranoia are often seen clinically in individuals with Asperger’s syndrome (Hare, 1997; Wing, 1996), and two studies have found increased rates of paranoia in ASD as compared to healthy controls (Blackshaw et al., 2001; Craig et al., 2004). Further, only minimal differences in social cognition have been observed between individuals with autism and those with schizophrenia when the latter group was higher in paranoid symptoms (Craig et al., 2004; Pilowsky et al., 2000). Thus, based on this evidence and in conjunction with work suggesting greater amygdala activation in NP-SCZ relative to P-SCZ (Phillips et al., 1999; Williams et al., 2004), we tentatively hypothesized that the ASD and P-SCZ groups would show less amygdala activation than the NP-SCZ group.
2. Methods and Materials
2.1 Subjects
Participants comprised four groups: non-clinical control participants (n=12), individuals with schizophrenia or schizoaffective disorder without paranoid symptoms (NP-SCZ: n=12), individuals with schizophrenia or schizoaffective disorder with prominent paranoid symptoms (P-SCZ: n=12), and high-functioning individuals with ASD (ASD: n=12). All participants were male, between the ages of 18 and 35, reported no history of head injury, identified themselves as right-handed, had a visual acuity of 20/20 (natural or corrected via contact lenses), and did not meet current criteria for substance abuse or dependence. The University of North Carolina Behavioral IRB approved the research protocol, and all participants provided written informed consent.
Control participants were recruited via informational emails soliciting participation in research and from other studies conducted in our lab. These participants were screened for personal and family history of psychopathology to ensure that they, and their first-degree relatives, did not meet past or present criteria for any psychotic, affective, or developmental disorder.
Individuals in the schizophrenia groups had a diagnosis of schizophrenia or schizoaffective disorder based on the Structured Clinical Interview for DSM-IV (SCID-P) and chart review. Severity of symptoms was assessed with the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1992), administered by research assistants trained to adequate reliability (ICC of > .80 with a gold standard rater). Participants experiencing significant symptoms of paranoia at the time of scanning, scoring at least a 4 or above on the suspiciousness/persecution item, constituted the P-SCZ group, and participants scoring a 2 or below on this item, indicating absence or only sub-clinical levels of paranoia, constituted the NP-SCZ group.
Overall, the P-SCZ group received higher ratings for both positive (F(1,22)=33.2, p<.001) and general symptom clusters (F(1,22)=6.69, p=.017); however, these differences were not statistically significant after controlling for paranoia. All SCZ individuals adhered to a stable regimen of atypical antipsychotic medications for at least four weeks, and the mean Chlorpromazine equivalent dose did not significantly differ between these two groups (F(1,22)=1.51, p=ns) (Woods, 2003).
All individuals with ASD had documented DSM-IV diagnoses of autism or Asperger’s syndrome and were recruited through the TEACCH (Treatment and Education of Autistic and Related Communication Handicapped Children) program of Chapel Hill, NC. Diagnoses were confirmed where possible (n=8) with the Autism Diagnostic Interview-Revised ADI-R; Lord et al., 1994) and/or the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1999).
Groups did not differ in ethnicity (χ2=3.74, ns), age (F(3,44)=1.47, ns) or premorbid verbal IQ as assessed by the WRAT Reading subscale (F(3,44)=2.29, ns; Wickert et al., 2000). Education significantly differed between groups (F(3, 44)=8.03, p<.001); controls completed more years of education than all three clinical groups (p<.001 for all comparisons), who did not differ from each other (Table 1).
Table 1.
Demographic Information and Behavioral Data
| ASD (n=12) |
P-SCZ (n-12) |
NP-SCZ (n=12) |
Control (n=12) |
|
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Demographic Information | ||||
| Ethnicity | 10 | 10 | 11 | 10 |
| Caucasian | 1 | 2 | 1 | 2 |
| African American | 1 | 0 | 0 | 0 |
| Other | ||||
| Age | 24.08 (5.71) | 26.42 (5.25) | 28.0 (3.93) | 27.08 (3.99) |
| WRAT reading | 110.0 (10.91) | 103.83 (14.24) | 100.5 (15.37) | 112.58 (9.28) |
| Educationa,b,c | 13.5 (1.83) | 13.29 (2.73) | 13.29 (2.05) | 16.92 (1.98) |
| SCZ Diagnosis | ||||
| Schizophrenia | 8 | 9 | ||
| Schizoaffective Disorder | 4 | 3 | ||
| CPZ Equivalent | 404.86 (249.2) | 297.22 (173.03) | ||
| PANSS | ||||
| Positivef | 18.08 (4.34) | 9.83 (2.41) | ||
| Negative | 11.83 (6.16) | 10.67 (3.28) | ||
| Generalf | 31.0 (6.55) | 25.0 (4.65) | ||
| Paranoia Scale a,b,c,f | 48.25 (21.18) | 48.08 (20.48) | 33.82 (11.69) | 27.6 (5.27) |
| Behavioral Tasks | ||||
| Trustworthiness Task | ||||
| % Rated as Trustworthyg | 54.4 (15.3) | 46.3 (14.3) | 60.3 (13.3) | 60.7 (11.9) |
| Reaction Time (sec) | 2.49 (1.06) | 2.83 (1.20) | 2.71 (1.60) | 2.01 (.90) |
| Age Task | ||||
| % Rated as 30 or Under | 41.9 (08.3) | 37.3 (07.9) | 44.6 (18.5) | 37.2 (06.8) |
| Reaction Time (sec) | 2.34 (1.10) | 2.13 (.90) | 2.09 (1.13) | 1.67 (.65) |
Controls significantly different from ASD at p<.05
Controls significantly different from P-SCZ at p<.05
Controls significantly different from NP-SCZ at p<.05
ASD significantly different from P-SCZ at p<.05
ASD significantly different from NP-SCZ at p<.05
P-SCZ significant different from NP-SCZ at p<.05
The omnibus test of group differences was significant, but no pair-wise comparisons survived Tukey’s HSD poc-hoc tests. LSD pair-wise comparisons showed P-SCZ significantly different from controls (p=.014) and NP-SCZ (p=.017).
Finally, all participants completed the Paranoia Scale (PS; Fenigstein and Vanable, 1992), a self-report measure assessing sub-clinical paranoid thought. The PS is sensitive to subclinical levels of paranoia in normal populations (Combs and Penn, 2004), and correlates well with clinical ratings of paranoia in psychotic populations (Smari et al., 1994).
2.2 Imaging Stimuli and fMRI Experiment
Functional magnetic resonance imaging (fMRI) was utilized while individuals viewed and evaluated 84 grayscale frontal images of faces taken from the Trustworthiness/Approachability Task (Adolphs et al., 1998). For the first 42 faces, which comprised the abbreviated version of this task, individuals made a forced choice of trustworthiness, rating each face as either trustworthy or untrustworthy, via a button-press response. In a secondary task, participants then made an age determination for the remaining 42 faces, classifying face stimuli as either “30 years of age or younger” or “over 30 years of age.” This procedure was based on that used by Winston et al. (2002). Button-press responses and reaction times were recorded as behavioral indices of task performance.
The imaging session was comprised of four functional runs, each containing 21 photographs. Each photograph was displayed for 2 seconds, followed by a 16 second inter-stimulus interval, during which participants were instructed to keep their eyes focused on a white fixation cross presented in the middle of the screen.
Following the Trustworthiness and Age tasks, individuals participated in a block design localizer session intended to isolate the face responsive region of the FG, or fusiform face area (FFA) (Kanwisher et al., 1997). Details of this task are provided in online supplemental material.
2.3 Image Acquisition
Data was collected using a Siemens Allegra 3T MRI scanner to acquire echo planar T2* weighted images with BOLD (blood oxygenation level dependent) contrast (EPI free induction decay, 2D; 32 transverse slices, voxel size 3.8×3.8×3.8 mm, matrix=64×64; FOV=243×243, TR=2 sec, TE=30ms, Flip angle = 80). All functional runs were collected using an interleaved sequence (bottom to top), and each functional run was preceded by four volumes that were discarded to allow for equilibration effects. A structural scan sequence (MPRAGE) was also conducted to obtain a T1 weighted anatomical image (128 slices, voxel size 1×1×1 mm, matrix=256×256, FOV=208×256, TR=1520 ms, TE=4.38 ms) for co-registration and display of functional data. Cushioned head restraints were used to control for movement.
Images were spatially preprocessed using SPM2 (Wellcome Department of Cognitive Neurology, Queen Square, London, United Kingdom). Data was corrected for slice-acquisition time and motion. Images were then normalized to an EPI template corresponding to standard MNI (Montreal Neurological Institute) space and smoothed using an 8-mm FWHM (full-width half-maximum) Gaussian kernel. In-plane anatomical images were co-registered to the functional images prior to normalization, and the same normalization parameters for the functional scans were then used to normalize the anatomical images.
2.4 Data Analysis
As the number of individuals in each group was somewhat limited, hypotheses were tested via region of interest (ROI) analyses conducted using a combination of SPM2 and the WFU Pick Atlas (Maldjian et al., 2003). First, for each subject, statistical contrast maps using a hemodynamic response function with the temporal derivative were generated to examine task dependent activation relative to fixation baseline during trustworthiness judgments. These contrast images were then used in one-sample t-tests conforming to random effects analyses. Statistical threshold was set at p<.05, family-wise error (FWE) corrected for multiple comparisons across a small volume of interest, using ROIs derived as detailed below.
A one-way ANOVA (group: control vs. ASD vs. NP-SCZ vs. P-SCZ) conforming to random effects analyses was then conducted to examine group differences on the Trustworthiness Task. Significant clusters of activation within each ROI were identified based on a statistical threshold of p<.05 (uncorrected) and a spatial extent of 3 contiguous voxels (Holt et al., 2006; Williams et al., 2004). Clusters showing a main effect across all four groups were explored with post-hoc paired comparisons. Here, the threshold for statistical significance was defined more liberally in order to be most sensitive to group differences.
As supplemental, exploratory analyses, contrasts were calculated for age judgments relative to baseline and for trust>age1. The later provides a preliminary examination of the specificity of the ROIs for processing complex social judgments relative to nonsocial judgments of faces. Within and between group activations were examined with the same procedure as that used for the primary analyses.
2.5 Masks Defining Regions of Interest
ROIs for right and left amygdala were defined by drawing a mask around the regions unilaterally using the software package MRIcro (Rorden and Brett, 2000) on a mean anatomical image of all participants in the study. All other regions required that they be defined functionally, as anatomical definitions of STS, VLPFC, and Fusiform gyrus encompass portions of the brain beyond the sub-areas involved specifically in social cognition. ROIs for bilateral STS and bilateral VLPFC were defined using statistical results from a one sample t-test of positive activation during trust judgments using all 48 participants (p<.05 FWE whole brain corrected) (Cannon et al., 2005). A region of interest for the FFA was defined by use of the localizer task in which data were combined across all participants, and significant activations from a faces > tools contrast (p<.001 uncorrected) were examined. Details are provided in supplemental online materials.
3. Results
3.1 Behavioral Data
One-way (group: control vs. ASD vs. NP-SCZ vs. P-SCZ) ANOVAs conducted on behavioral ratings made during scanning revealed significant between-group differences in trustworthiness (F(3, 44)=2.87, p=.047, Cohen’s f=.443), but not age (F(3, 44)=1.23, p=ns), judgments. For trustworthiness, Tukey’s HSD post hoc tests revealed trend level differences indicating that P-SCZ rated more faces untrustworthy than control and NP-SCZ groups (p=.064 and p=.076, respectively), who performed comparably to each other. Ratings from the ASD group did not significantly differ from any other group (Table 1, bottom). A repeated measures ANOVA on reaction time with type of judgment (trustworthiness vs. age) as the within-subjects factor and group as the between-subjects factor revealed a significant main effect for type of judgment (F(1, 44)=21.99, p<.001, Cohen’s f=.71); all groups responded faster when rating age, which suggests that trustworthiness judgments were indeed more complex than age judgments. There was no main effect for group (F(3, 44)=.99, ns), nor was there a significant interaction (F(3, 44)=1.71, ns).
A one-way ANOVA on PS scores revealed significant differences between groups (F(3, 41)=4.36, p=.009, Cohen’s f=.57). Post hoc tests indicated that both ASD and P-SCZ, who did not differ from each other, showed more agreement with paranoid statements than control and NP-SCZ, who also did not differ from each other (Table 1).
3.2 Primary Neuroimaging Analyses
3.2.1 Trustworthiness Task: Overall activation and group differences
Completion of the trustworthiness task resulted in significant activation in each key structure implicated in social cognition. Averaged contrasts for trustworthiness judgments relative to baseline, revealed that each group showed significant activation of bilateral amygdala, bilateral VLPFC, STS, and right FFA (p<.05, FWE corrected; Table 2; Figure 1). Variation was seen in laterality of STS activation between groups; however, all other regions were consistently activated across groups.
Table 2.
Trustworthiness task overall activations and group differences
| Cerebral foci of activation within each ROI for trustworthiness judgments | ||||
|---|---|---|---|---|
| Coordinates (mm)^ |
||||
| Side | x, y, z | Cluster Size | Z Score | |
| ASD Group | ||||
| AMYG | right | 24, −3, −27 | 15 | 3.46* |
| left | −24, −3, −21 | 25 | 3.34* | |
| FFA | right | 48, −54, −30 | 8 | 3.26* |
| STS | left | −48, −48, 6 | 19 | 3.47* |
| VLPFC | right | 39, 18, 0 | 162 | 4.45* |
| left | −48, 15, −12 | 137 | 4.48* | |
| P-SCZ Group | ||||
| AMYG | right | 21, −3, −24 | 36 | 4.02* |
| left | −18, −6, −21 | 37 | 4.14* | |
| FFA | right | 45, −54, −27 | 8 | 3.76* |
| STS | right | 57, −36, −12 | 43 | 3.93* |
| left | −48, −48, −3 | 7 | 2.68* | |
| VLPFC | right | 39, 24, −9 | 174 | 4.79* |
| left | −42, 18, −9 | 83 | 4.17* | |
| NP-SCZ Group | ||||
| AMYG | right | 24, −3, −27 | 36 | 4.31* |
| left | −24, −3, −21 | 37 | 4.59* | |
| FFA | right | 45, −48, −30 | 9 | 4.73* |
| STS | right | 60, −42, −3 | 57 | 3.84* |
| VLPFC | right | 42, 15, −12 | 261 | 5.47* |
| left | −39, 21, −12 | 254 | 5.00* | |
| Control Group | ||||
| AMYG | right | 15, −6, −21 | 36 | 4.89* |
| left | −21, −3, −21 | 37 | 4.68* | |
| FFA | right | 48, −54, −30 | 9 | 4.39* |
| STS | right | 51, −39, −9 | 44 | 3.91* |
| VLPFC | right | 42, 21, −18 | 103 | 4.23* |
| left | −42, 21, −9 | 217 | 4.53* | |
|
Group differences in neural activation during trustworthiness judgments | ||||
| Coordinates (mm)^ |
||||
| Side | x, y, z | Cluster Size | Z Score | |
| Control > ASD | ||||
| AMYG | right | 15, −9, −21 | 21 | 3.40* |
| FFA | right | 45, −54, −30 | 5 | 2.50* |
| VLPFC | left | −33, 21, −18 | 85 | 2.87 |
| Control > P-SCZ | ||||
| AMYG | right | 18, −3, −21 | 17 | 2.60* |
| FFA | right | 45, −54, −30 | 5 | 2.74* |
| VLPFC | left | −33, 27, −18 | 47 | 3.17* |
| Control > NP-SCZ | ||||
| FFA | right | 45, −54, −30 | 7 | 3.09* |
| NP-SCZ > ASD | ||||
| AMYG | right | 21, −3, −27 | 9 | 2.80* |
| VLPFC | left | −36, 18, −21 | 47 | 2.59 |
| NP-SCZ > P-SCZ | ||||
| VLPFC | left | −33, 27, −18 | 27 | 2.69 |
All values, p<.05, uncorrected.
p<.05, FWE corrected for multiple comparisons across ROI.
Abbreviations: Amygdala (AMYG), Fusiform Face Area (FFA), Superior Temporal Sulcus (STS), Ventrolateral Preftronal Cortex (VLPFC)
The cluster with the largest number of voxels with each ROI is reported.
Talairach coordinates and Z scores refer to the voxel with the maximum signal change in each cluster.
Figure 1.
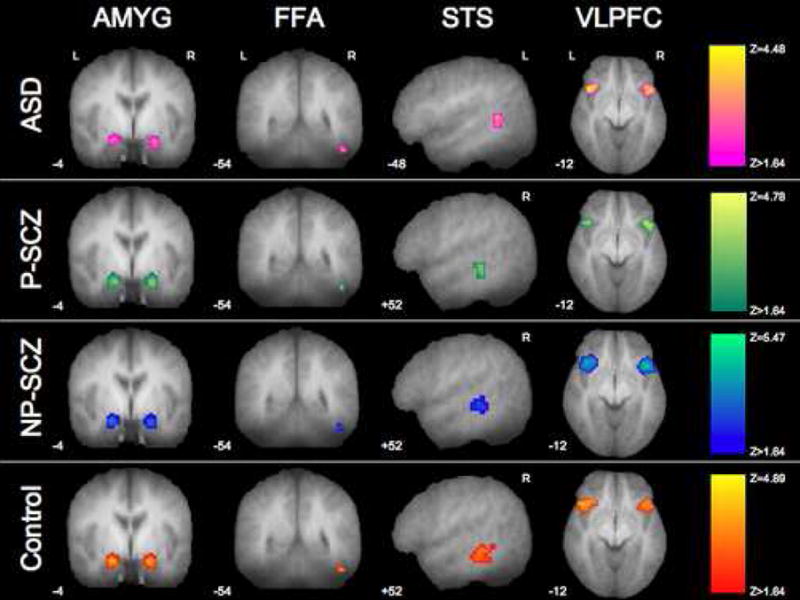
Activation in response to the Trustworthiness Task. Statistical parametric maps overlaid on the mean T1 anatomical image showing activation within each ROI for each group while rating faces for trustworthiness (significance and display threshold = p<.05, FWE corrected for multiple comparisons across a small volume of interest). Each column of images is masked by the specified ROI.
To test the hypothesis that clinical groups would show reduced neural activation relative to controls, a voxel-based ANOVA was conducted across overall activation during the trustworthiness task. Significant group differences were revealed in right amygdala, FFA, and left VLPFC (effect sizes provided in supplemental materials). Post-hoc follow-up comparisons demonstrated that compared to controls, the ASD group had significantly reduced activation in right amygdala, FFA, and left VLPFC. The P-SCZ group showed a similar pattern of significantly less activation than controls in right amygdala, FFA, and left VLPFC. The NP-SCZ group also demonstrated significantly less activation in the FFA compared to controls. However, in contrast to the other groups, NP-SCZ showed comparable levels of activation to controls in all other regions (Figure 2), suggesting relatively intact neural functioning throughout the rest of this social cognition circuit.
Figure 2.
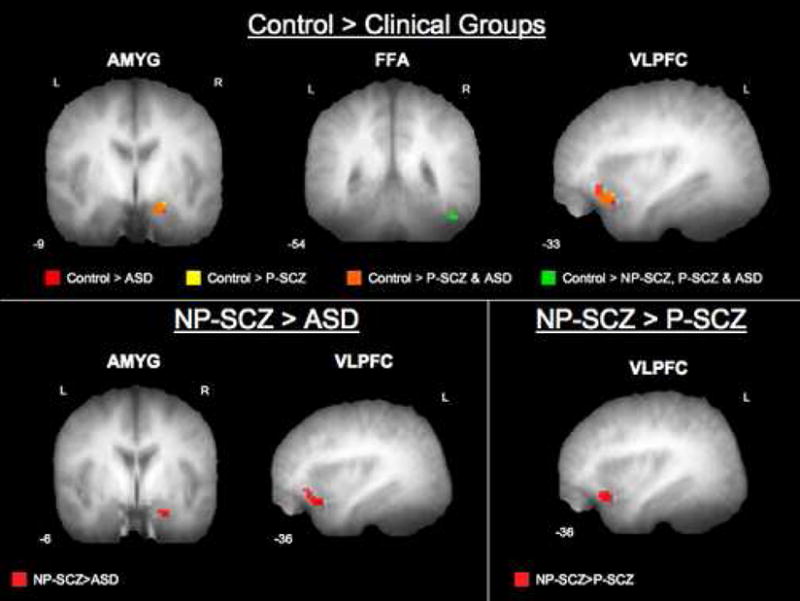
Group differences in activation while making trustworthiness judgments. Statistical parametric maps overlaid on the mean T1 anatomical image showing regions of greater activation for each comparison (statistical and display threshold = p<.05, uncorrected, spatial extent of 3 contiguous voxels). Images are masked by ROI.
Comparisons among clinical groups demonstrated greater activation for the NP-SCZ group in the right amygdala and left VLPFC as compared to the ASD group, and greater activation in left VLPFC as compared to the P-SCZ group. The direct comparison of the ASD and P-SCZ groups across ROIs identified in the omnibus test yielded no significant differences (all results summarized in Table 2).
3.3 Supplemental Neuroimaging Analyses
3.3.1 Age Task: Overall activation and group differences
Results from the age task showed activity throughout the social cognitive network for all 4 groups, with the exception of STS activation in controls. Differences in lateralization were apparent; notably, the three clinical groups continued to display bilateral amygdala activation whereas controls showed significant activation of right amygdala only (Table 3).
Table 3.
Age task overall activations and group differences
| Cerebral foci of activation within each ROI for age judgments | ||||
|---|---|---|---|---|
| Coordinates (mm)^ |
||||
| Side | x, y, z | Cluster Size | Z Score | |
| ASD Group | ||||
| AMYG | right | 21, −3, −21 | 10 | 3.38* |
| left | −21, −3, −18 | 31 | 4.33* | |
| FFA | right | 45, −54, −30 | 8 | 3.18* |
| STS | right | 51, −42, −18 | 6 | 4.02* |
| VLPFC | right | 45, 9, −3 | 184 | 4.67* |
| left | −36, 18, 3 | 68 | 4.14* | |
| P-SCZ Group | ||||
| AMYG | right | 24, −6, −18 | 36 | 4.17* |
| left | −15, −6, −24 | 37 | 3.72* | |
| FFA | right | 45, −51, −33 | 8 | 3.72* |
| STS | left | −48, −48, −3 | 7 | 2.76* |
| VLPFC | right | 36, 12, −6 | 194 | 5.47* |
| left | −39, 18, −6 | 140 | 4.31* | |
| NP-SCZ Group | ||||
| AMYG | right | 15, −6, −21 | 33 | 3.62* |
| left | −24, −9, −15 | 36 | 4.83* | |
| FFA | right | 45, −48, −30 | 9 | 4.42* |
| STS | right | 51, −45, −18 | 6 | 3.65* |
| VLPFC | right | 45, 24, −3 | 184 | 4.89* |
| left | −42, 18, −9 | 68 | 4.21* | |
| Control Group | ||||
| AMYG | right | 15, −9, −18 | 8 | 2.68* |
| FFA | right | 48, −51, −30 | 9 | 4.17* |
| VLPFC | right | 33, 12, −9 | 7 | 3.69* |
| left | −42, 18, −9 | 79 | 4.32* | |
| Group differences in neural activation during age judgments | ||||
| Coordinates (mm)^ |
Cluster | |||
| Side | x, y, z | Size | Z Score | |
| ASD > Control | ||||
| STS | left | −45, −51, 0 | 10 | 2.30* |
| VLPFC | right | 42, 6, −9 | 37 | 2.46 |
| P-SCZ > Control | ||||
| AMYG | left | −21, −9, −21 | 14 | 2.44* |
| STS | left | −48, −48, −3 | 14 | 3.31* |
| VLPFC | right | 51, 6, −6 | 103 | 3.59* |
| NP-SCZ > Control | ||||
| AMYG | left | −18, −9, −21 | 16 | 2.58* |
| VLPFC | right | 48, 3, −9 | 42 | 2.48 |
| NP-SCZ > ASD | ||||
| AMYG | left | −15, −6, −24 | 11 | 2.71* |
| VLPFC | right | 48, 21, −12 | 10 | 2.01 |
| P-SCZ > ASD | ||||
| AMYG | left | −15, −6, −24 | 6 | 2.30 |
| STS | left | −48, −48, −3 | 3 | 1.78 |
| VLPFC | right | 51, 24, −3 | 21 | 2.82 |
| NP-SCZ > P-SCZ | ||||
| VLPFC | right | 48, 18, −15 | 6 | 1.88 |
| P-SCZ > NP-SCZ | ||||
| STS | left | −48, −48, 0 | 3 | 1.99 |
All values, p<.05, uncorrected.
p<.05, FWE corrected for multiple comparisons across ROI.
Abbreviations: Amygdala (AMYG), Fusiform Face Area (FFA), Superior Temporal Sulcus (STS), Ventrolateral Preftronal Cortex (VLPFC)
The cluster with the largest number of voxels with each ROI is reported.
Talairach coordinates and Z scores refer to the voxel with the maximum signal change in each cluster.
A voxel-wise one-way ANOVA conducted to assess group differences during age judgments showed significant omnibus group differences in left amygdala, left STS, and right VLPFC. Follow-up comparisons revealed that in all three regions, the clinical groups displayed either similar or greater levels of activation than control participants. Comparisons between the clinical groups demonstrated that both SCZ groups showed greater activation of the left amygdala and right VLPFC than ASD (Table 3 lists all significant differences between groups in this circuit).
3.3.2 Trustworthiness vs. Age: With-in group comparisons
To explore if trust judgments resulted in greater activation of our ROIs than age judgments, a trust>age contrast was examined in each group. Control participants showed significantly increased BOLD responses (all p<.05 FWE small volume corrected) in bilateral amygdala (right: x,y,z = 15, −6, −24; Z=3.47; left: x,y,z = −18, −9, −24; Z=3.16;), bilateral STS (right: x,y,z = 51, −36, −9; Z=3.14; left: x,y,z = −48, −48, 0; Z=3.29;), and left VLPFC (x,y,z = −36, 24, −21; Z=3.53) indicating that greater responses in these areas were associated with judgments of trustworthiness (Figure 3). Activation in the right FFA did not differ between trust and age judgments. Within the NP-SCZ group, greater activation of left VLPFC (x,y,z = −30, 15, −15; Z=4.47; p<.05 FWE small volume corrected) was also associated with judgments of trustworthiness as compared to age. In contrast, both the ASD and P-SCZ groups failed to show greater activation during trustworthiness judgments compared to age judgments in any ROI.
Figure 3.
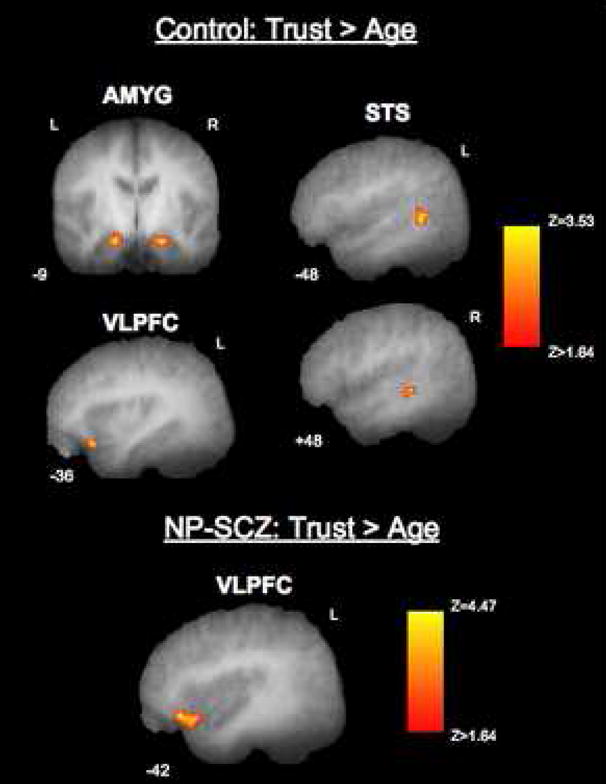
Social cognitive neural regions showing greater activation for trust judgments than age judgments in the control and NP-SCZ groups. Statistical parametric maps overlaid on mean T1 anatomical images showing activation in bilateral amygdala, bilateral STS, left VLPFC that is specific to judgments of trustworthiness as compared to judgments of age (statistical and display threshold = p<.05, FWE corrected for multiple comparisons across a small volume of interest). All images are masked by ROI.
To ensure that no ROIs were showing greater activation during age judgments, a contrast of age>trustworthiness was also examined. No regions within any group showed greater activation to age than trustworthiness judgments.
4. Discussion
Results of this study indicate that individuals with autism spectrum disorders and individuals with paranoid schizophrenia show significant reductions in neural activation compared to control and NP-SCZ individuals during tasks of complex social cognition. Reduced activation was evident in the amygdala, fusiform face area, and the ventrolateral prefrontal cortex. Reductions in amygdala and FFA activation in ASD and P-SCZ are consistent with several studies that have investigated these disorders independently, although primarily without examining schizophrenia subtypes, and while utilizing more basic tasks of social cognition such as viewing faces and recognizing emotion (Critchley et al., 2000, Hempel et al., 2003; Piggot et al., 2004; Quintana et al., 2003; note that Piggot et al., (2004) found reduced FG activation in ASD, but no difference in amygdala activation between controls and individuals with ASD). Overlapping reductions in the social cognitive network, across two distinct disorders, therefore suggest that social cognitive impairment may be subserved by specific neural abnormalities and that this pattern of neural abnormalities may be deficit specific rather than disorder specific. In other words, it is possible that any individual who shows specific social cognitive deficits may show the same pattern of neural abnormalities regardless of clinical diagnosis. Additionally, the lack of reduced activation in P-SCZ and ASD during age judgments suggests that reduced neural activity for complex social judgments cannot be attributed to overall reductions in activation, generalized deficits in social information processing, a failure to view the stimuli, or failure to engage in the task.
Additionally, exploratory analyses suggest that unlike control participants, and to lesser extent NP-SCZ participants, ASD and P-SCZ failed to show greater activation in face processing/social cognitive neural regions during trustworthiness, as compared to age, judgments. Although this finding needs to be interpreted cautiously as trustworthiness judgments always preceded age judgments, the lack of neural modulation seen in P-SCZ and ASD may suggest that these individuals are processing all judgments of faces in a similar manner, irrespective of their social nature or complexity. Given that the amygdala has been linked to associating stimuli with social and emotional value (Adolphs, 2001), it is possible that individuals with ASD and P-SCZ are not assigning appropriate emotional significance to facial stimuli when asked to make a complex social judgment. This may, in turn, contribute to social dysfunction. Though speculative, these findings suggest a mechanism for social impairments in schizophrenia and ASD that warrants further research.
It should also be noted that the ASD group did not differ from controls on behavioral ratings of faces despite reduced activation of the amygdala, FFA, and VLPFC. While somewhat counterintuitive, this may be explained by the fact that the ASD group showed normative STS activation. Such differential activation across regions within the social cognitive network is consistent with a systemizing strategy in which individuals with ASD use feature- and rule-based strategies for processing faces (Ashwin et al., 2007). Thus, these findings may provide further evidence that individuals with ASD do not assign emotional significance to faces.
Interestingly, both ASD and P-SCZ showed comparably increased levels of paranoid ideation. Similar levels of paranoia may explain the observed neural and behavioral similarities in this study. As noted previously, behavioral comparisons between schizophrenia and autism have yielded few to no differences in social cognitive performance when the schizophrenia group shows prominent paranoid symptoms (Craig et al., 2004; Pilowsky et al., 2000; for an exception see Bolte and Poustka (2003); however in this study the symptom presentation of the schizophrenia sample was not detailed, and an effort to recruit only individuals with paranoid symptoms was not reported). These results underscore the importance of a symptom-based approach in the study of clinical disorders. As applied here, a symptom based approach suggests that a long-standing paranoid perceptual process may serve as the mechanism for the equifinality found between the two disorders in social cognitive neural activation.
The present study also extends previous work by highlighting an important distinction between schizophrenia subgroups. More normative activation of social cognitive regions for non-paranoid relative to paranoid individuals is consistent with previous work showing normal levels of amygdala activation in non-paranoid schizophrenia during passive viewing of emotional facial expressions (Williams et al., 2004) and may help explain why individuals with non-paranoid schizophrenia rated faces similarly to healthy individuals. The finding that this differentiation between subgroups is also present during complex social information processing and in other regions implicated in social cognition underscores the vital importance of symptoms and sub-typing for fully understanding social cognitive deficits in schizophrenia.
Although the present study provides new data regarding mechanisms of impaired social cognition in two distinct clinical populations, a number of issues require further clarification. First, the effect of medication was not addressed here, as all SCZ individuals were taking neuroleptic medication at the time of the study and it is possible that type of medication or duration of neuroleptic exposure may influence neural activation. It is unlikely however that our results were due solely to medication effects given the SCZ groups did not differ in medication dosage but did differ in neural activity. Furthermore, ASD individuals were not taking neuroleptics but showed similar neural patterns to the P-SCZ group. Second, while the P-SCZ and ASD groups reported a similar amount of paranoid ideation, we are unable to determine if these symptoms are qualitatively similar, and thus future work will be necessary to clarify this interesting overlap between disorders. Third, the trustworthiness vs. age results are complicated by the fact that these conditions were not counterbalanced. It is possible that patients may have continued to assess faces for trustworthiness during the age task; however, this seems unlikely as there were no behavioral differences, and more importantly, no differences in reaction times, between the groups on age judgments. If the clinical groups were continuing to process trustworthiness in addition to age, this should have been reflected in longer reaction times than those seen in the trustworthiness task. Fourth, while 48 individuals were examined, the size of each group was relatively small and only right handed male participants were included. These factors limit the generalizability of the results (e.g. to females and individuals who are left handed or lack handedness, which may be more common in autism (Cornish & McManus, 1996)) and suggest that replication with a more diverse sample is required. Finally, given recent work demonstrating that neural activation is related to how individuals visually scan face stimuli (Dalton et al., 2005), it is possible that our results may in part reflect abnormal visual face scanning in the clinical groups. Here, we investigated the natural viewing of faces by these groups, but future fMRI work should employ concurrent eye-tracking to investigate these effects.
Supplementary Material
Acknowledgments
We are grateful to Kathy Wilber, B.S., RT(R)(MR) and Weili Lin, Ph.D. of the UNC Magnetic Resonance Imaging Research Center for their invaluable assistance with data collection and to Gary Mesibov, Ph.D. for providing his expertise in autism and for his assistance recruiting participants. We would also like to thank Dr. Ralph Adolphs who provided us with the Trustworthiness Task, and finally, would like to thank all the individuals who participated in this study.
Footnotes
The trustworthiness task was always administered before the age task because it was unclear if all participants in all clinical populations would be able to complete the entire MRI session and the primary goal of this experiment was to assess neural activation during a social cognitive task (i.e., trustworthiness). Although all participants did complete the session, we view the age task analyses as exploratory due to this lack of task-counterbalancing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol . 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H. The human amygdala in social judgment. Nature . 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expression following bilateral damage to the human amygdala. Nature . 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: implications from non-human primate studies. Genes Brain Beh . 2003;2:295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Ashwin C, Baron-Cohen S, Wheelwright S, O’Riordan M, Bullmore ET. Differential activation of the amygdala and the ‘social brain’ during fearful face-processing in Asperger Syndrome. Neuropsychologia . 2007;45:2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Bentall RP, Corcoran R, Robert H, Blackwood N, Kinderman P. Persecutory delusions: a review and theoretical integration. Clin Psychol Rev . 2001;21:1143–1192. doi: 10.1016/s0272-7358(01)00106-4. [DOI] [PubMed] [Google Scholar]
- Blackshaw AJ, Kinderman P, Hare DJ, Hatton C. Theory of mind, causal attributions and paranoia in Asperger’s syndrome. Autism . 2001;5:147–163. doi: 10.1177/1362361301005002005. [DOI] [PubMed] [Google Scholar]
- Bolte S, Poustka F. The recognition of facial affect in autistic and schizophrenic subjects and their first-degree relatives. Psychol Med . 2003;33:907–915. doi: 10.1017/s0033291703007438. [DOI] [PubMed] [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci . 1990;1:27–51. [Google Scholar]
- Cannon TD, Glahn DC, Kim J, Van Erp TGM, Karlsgodt K, Cohen MS, et al. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry . 2005;62:1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- Combs DR, Penn DL. The role of sub-clinical paranoia on social perception and behavior. Schizophr Res . 2004;69:93–104. doi: 10.1016/S0920-9964(03)00051-3. [DOI] [PubMed] [Google Scholar]
- Cornish KM, McManus IC. Hand preference and hand skill in children with autism. J Autism Dev Disord . 1996;26:597–609. doi: 10.1007/BF02172349. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: A review. Schizophr Bull. 2006;32(suppl 1):44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JS, Hatton C, Craig FB, Bentall RP. Persecutory beliefs, attributions and theory of mind: comparison of patients with paranoid delusions, Asperger’s syndrome and healthy controls. Schizophr Res . 2004;69:29–33. doi: 10.1016/S0920-9964(03)00154-3. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SCR, Amelsvoort TV, Robertson DM, et al. The functional neuroanatomy of social behavior: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain . 2000;123:2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Gatenby JC, Gore JC, Banaji MR. Neural components of social evaluation. J Pers Soc Psychol . 2003;85:639–649. doi: 10.1037/0022-3514.85.4.639. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Gatenby JC, Gore JC, Banaji MR. Separable neural components in the processing of black and white faces. Psychol Sci . 2004;15:806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci . 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PJ, Gibson MG. Recognition of posed and genuine facial expressions of emotion in paranoid and nonparanoid schizophrenia. J Abnorm Psychol . 2000;109:445–450. [PubMed] [Google Scholar]
- Fenigstein A, Vanable PA. Paranoia and self-consciousness. J Pers Soc Psychol . 1992;62:129–38. doi: 10.1037//0022-3514.62.1.129. [DOI] [PubMed] [Google Scholar]
- Hare DJ. The use of cognitive behavioral therapy with people with Asperger’s syndrome: A case study. Autism . 1997;1:215–225. [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala in response to fearful stimuli. Biol Psychiatry . 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffmann EA, Gobbini MI. The distributed human neural system for face perception. Trends Cog Sci . 2000;4:223–232. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hempel A, Hempel E, Schonknecht P, Stippich C, Schroder J. Impairment in basal limbic function in schizophrenia during affect recognition. Psychiatry Res . 2003;122:115–124. doi: 10.1016/s0925-4927(02)00126-9. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Kunkel L, Weiss AP, Goff DC, Wright CI, Shin LM, et al. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr Res . 2006;82:153–162. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Hughes C, Soares-Boucaud I, Hochmann J, Frith U. Social behaviour in pervasive developmental disorders: Effects of informant, group, and “theory of mind.” Eur. Child Adolesc Psychiatry . 1997;6:191–198. doi: 10.1007/BF00539925. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci . 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale: Manual. Mulit-Health Systems, Inc.; 1992. [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry . 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord . 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage . 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabil Res Rev . 2004;10:259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry . 2003a;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry . 2003b;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams L, Senior C, Bullmore BT, Brammer MJ, Andrew C, et al. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res . 1999;92:11–31. doi: 10.1016/s0925-4927(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Piggot J, Kwon H, Mobbs D, Blasey C, Lotspeich L, Menon V, et al. Emotional attribution in high-functioning individuals with autistic spectrum disorder: a functional imaging study. J Am Acad Child Adolesc Psychiatry . 2004;43:473–480. doi: 10.1097/00004583-200404000-00014. [DOI] [PubMed] [Google Scholar]
- Pilowsky T, Yirmiya N, Arbelle S, Mozes T. Theory of mind abilities in children with schizophrenia, children with autism, and normally developing children. Schizophr Res . 2000;42:145–155. doi: 10.1016/s0920-9964(99)00101-2. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Perkins DO, Lieberman J. Implications for the neural basis of social cognition for the study of schizophrenia. Am J Psychiatry . 2003;160:815–824. doi: 10.1176/appi.ajp.160.5.815. [DOI] [PubMed] [Google Scholar]
- Quintana J, Wong T, Ortiz-Portillo E, Marder SR, Mazziotta JC. Right lateral fusiform gyrus dysfunction during facial information processing in schizophrenia. Biol Psychiatry . 2003;53:1099–1112. doi: 10.1016/s0006-3223(02)01784-5. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol . 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Smari J, Stefansson S, Thorgilsson H. Paranoia, self-consciousness, and social cognition in schizophrenics. Cognitive Therapy and Res . 1994;18:387–399. [Google Scholar]
- Ueno T, Morita K, Shoji Y, Yamamoto M, Yamamoto H, Maeda H. Recognition of facial expression and visual P300 in schizophrenic patients: differences between paranoid type patients and non-paranoid patients. Psychiatry Clin Neurosci . 2004;58:585–592. doi: 10.1111/j.1440-1819.2004.01307.x. [DOI] [PubMed] [Google Scholar]
- Van Rijn S, Swaab B, Aleman A, Khan RS. X chromosomal effects on social cognitive processing and emotion regulation: A study with Klinefelter men (47, XXY) Schizophr Res . 2006;84:194–203. doi: 10.1016/j.schres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Harris AWF, Liddell BB, Brammer MJ, Olivieri G, et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry . 2004;161:480–489. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- Wing L. The Autistic Spectrum: A Guide for Parents and Professionals. London: St. Edmundsbury Press.; 1996. [Google Scholar]
- Winston JS, Henson RNA, Fine-Goulden MR, Dolan RJ. fMRI-Adaptation reveals dissociable neural representations of identity and expression in face perception. J Neurophysiol . 2004;92:1830–1839. doi: 10.1152/jn.00155.2004. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional responses during evaluation of trustworthiness from faces. Nat Neurosci . 2002;5:277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry . 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


