Abstract
Dysfunctions of the brain serotonin (5-HT) system are often associated with affective disorders, such as depression. The raphe nuclei target the limbic system and most forebrain areas and constitute the main source of 5-HT in the brain. All 5-HT neurons express tryptophan hydroxylase-2 (TPH2), the brain specific, rate-limiting enzyme for 5-HT synthesis. ERbeta agonists have been shown to attenuate anxiety-and despair-like behaviors in rodent models. Therefore, we tested the hypothesis that ERbeta may contribute to the regulation of gene expression in 5-HT neurons of the dorsal raphe nuclei (DRN) by examining the effects of systemic and local application of the selective ERbeta agonist diarylpropionitrile (DPN) on tph2 mRNA expression.
Ovariectomized (OVX) female rats were injected subcutaneously (s.c.) with DPN or vehicle once daily for 8 days. In situ hybridization revealed that systemic DPN-treatment elevated basal tph2 mRNA expression in the caudal and mid-dorsal DRN. Behavioral testing of all animals in the open field (OF) and on the elevated plus maze (EPM) on days 6 and 7 of treatment confirmed the anxiolytic nature of ERbeta activation.
Another cohort of female OVX rats was stereotaxically implanted bilaterally with hormone-containing wax pellets flanking the DRN. Pellets contained either 17-beta-estradiol (E), DPN, or no hormone. Both DPN and E significantly enhanced tph2 mRNA expression in the mid-dorsal DRN. DPN also increased tph2 mRNA in the caudal DRN. DPN- and E-treated rats displayed a more active stress-coping behavior in the forced-swim test (FST). No behavioral differences were found in the OF or on the EPM.
These data indicate that ERbeta acts at the level of the rat DRN to modulate tph2 mRNA expression and thereby influence 5-HT synthesis in DRN subregions. Our results also suggest that local activation of ERbeta neurons in the DRN may be sufficient to decrease despair-like behavior, but not anxiolytic behaviors.
Keywords: 5-HT, anxiety, depression, estradiol, raphe, TPH
INTRODUCTION
Major depressive disorder (MDD) affects about 17% of Americans (Kessler et al., 1994; Williams et al., 2007), and is a complex, heterogeneous disease (Winokur, 1997; Ellard, 2001; Weissman, 2002). A deficiency in 5-HT neurotransmission is a leading hypothesis regarding the development and pathophysiology of this disease (Owens and Nemeroff, 1994; Arango et al., 2002; Perlis et al., 2002; Lesch, 2004). An influence of gonadal hormones on the etiology of depression is indicated based on the incidence, duration, severity and rate of reoccurrence of depressive disorders, which are over twice as high in women compared to men (Earls, 1987; Angold and Worthman, 1993; Weissman et al., 1993; Kornstein et al., 1995). Moreover, women also tend to respond differently than men to common antidepressant treatments, such as selective serotonin-reuptake inhibitors (Kornstein, 1997; Gorman, 2006). These observations, together with animal studies reporting sex differences in the regulation of emotion (Steenbergen et al., 1990; Caldarone et al., 2003; Shors and Leuner, 2003; Toufexis, 2007) and interactions between estrogen and the 5-HT system (for review see (Amin et al., 2005)) suggest an involvement of estrogen receptors (ERs) in the etiology of MDD.
In animal models, estrogen can exert both anxiolytic, and anxiogenic actions depending on the behavioral context (Koss et al., 2004; Hiroi and Neumaier, 2006). This ambiguity may be explained by the existence of two different estrogen receptor systems, ERalpha and ERbeta. The endogenous ligand estradiol binds to and activates both receptor types with similar affinity (Kuiper et al., 1997). However, ERalpha-selective agonists are anxiogenic, while ERbeta-selective compounds are anxiolytic and anti-depressive (Walf et al., 2004; Lund et al., 2005; Weiser et al., 2009). Moreover, in flinders-sensitive rats, a strain selectively bred for depressive-like behaviors, ERbeta agonists reduce the animals’ passive floating and immobility behavior (Overstreet et al., 2006) during the forced swim test (FST), a test established to assess despair-like behavior in rodents (Porsolt et al., 1977). This rat strain also displays abnormal levels of 5-HT(2A) receptor mRNA in the perirhinal cortex, piriform cortex, medial anterodorsal amygdala and in the hippocampus, a phenotype that is reversed by estradiol-treatment (Osterlund et al., 1999).
The brainstem dorsal raphe nuclei (DRN) constitute the primary 5-HT system of the brain. Distinct DRN subdivisions give rise to axons that innervate most forebrain areas, including areas crucial for the regulation of emotion and stress-coping behavior, such as the amygdala and the paraventricular nucleus of the hypothalamus (Imai et al., 1986; Petrov et al., 1992). Other subregions of the DRN send projections to motivational areas like the prefrontal cortex (Lowry, 2002; Abrams et al., 2004), while axons from the caudal DRN target limbic structures, such as the hippocampus, the entorhinal cortex and the septum (Kohler and Steinbusch, 1982).
Tryptophan-hydroxylase 2 (TPH2), the brain-specific version of TPH (Walther et al., 2003; Zhang et al., 2004), catalyses the rate-limiting step of 5-HT synthesis. Disruption or dysfunction of the tph2 gene is strongly correlated with affective disorders (Zill et al., 2004; Zhang et al., 2005; Haghighi et al., 2008), and abnormal tph2 mRNA expression may be responsible for many of those pathologies.
Previous studies have demonstrated that ERbeta is robustly expressed within the DRN of rodents (Shughrue et al., 1997a; Lu et al., 2001; Mitra et al., 2003; Nomura et al., 2005; Vanderhorst et al., 2005), primates (Gundlah et al., 2000; Gundlah et al., 2001) and guinea pigs (Lu et al., 1999), whereas ERalpha is only expressed to a small extent in the DRN of these species. Given the importance of the 5-HT system in anxiety- and depressive disorders together with the anxiolytic nature of ERbeta and its presence in the raphe complex, we speculate that ERbeta activation may regulate gene expression in 5-HT neurons.
Results from earlier studies suggest that ERbeta activation can regulate transcription and neurotransmission in the brainstem. Alves et al. (2000) reported estradiol-induced progestin receptor expression in the DRN of ERalpha null mice, suggesting a role for ERbeta. Also, phytoestrogens that selectively bind ERbeta have been shown to improve mood and 5-HT neurotransmission in the cynomolgus monkey (Shively et al., 2003). In turn, ERbeta null mice are characterized by increased anxiety in conjunction with lower 5-HT content and decreased tph mRNA in the DRN (Imwalle et al., 2005; Nomura et al., 2005). In macaques, estradiol and a combination of the ovarian steroids progesterone and estradiol each caused an elevation of tph2 mRNA in the DRN (Sanchez et al., 2005). In rats, a recent study showed that estradiol itself increases tph2 mRNA expression specifically in those DRN subregions that are associated with attenuated anxiety (Hiroi et al., 2006).
These findings support the hypothesis that ERbeta activation in the DRN may be sufficient to alter behavioral parameters and 5-HT-neuronal gene expression in the DRN. Therefore, we examined the effects of chronic systemic versus local, intracerebral delivery of the ERbeta agonist, diarylpropionitrile (DPN), in OVX female rats on anxiety-and despair-like behaviors and on tph2 mRNA expression in the DRN.
EXPERIMENTAL PROCEDURES
Animals
All animal surgeries, behavioral tests and experimental protocols followed NIH guidelines and were approved by the Animal Care and Use Committee (ACUC) at Colorado State University. Young adult female Sprague-Dawley rats (200–250 g body weight, Charles River Laboratories, Wilmington, MA) were fed a phytoestrogen-free diet (modified AIN-93G with corn oil substituted for soy oil; Dyets, Philadelphia, PA), double housed, and kept under standard laboratory conditions (12:12 h light-dark cycle, lights on at 0600 h, 22 °C, 60 % humidity, and ad libitum access to water and food). The animals were handled and their weight monitored every other day for the duration of both experiments. Surgical procedures were performed under isoflurane- (for OVX) or ketamine-anaesthesia (100mg/kg of 93% ketamine / 5% xylazine / 2% acepromazine for stereotaxic surgeries).
Experimental design & Surgical procedures
One week after arrival, all rats underwent bilateral OVX to remove circulating gonadal steroids. This procedure also ensured a constant level of ERbeta expression within the brain because receptor levels are regulated by hormones (Suzuki and Handa, 2005). Chronic 8-day systemic (s.c.) or local (intracerebral) treatment with ER ligands began one week after OVX. All animals were double housed with a partner of the same treatment group.
I. Experiment 1: Systemic DPN treatment
Rats were injected s.c. with the ERbeta agonist, diarylpropionitrile (DPN, 2 mg/kg; n=8) or vehicle (27% hydroxypropyl-beta-cyclodextrin from CTD Inc., High Springs, FL; n=8) in phosphate buffered saline once per day at 0600 h. DPN was synthesized de novo following an established protocol (Lund et al., 2005). In the morning of day 6, rats were tested for anxiety-like behavior in the OF, and on day 7 on the EPM. All animals were killed by decapitation on day 8 between 1000 and 1200 h under basal, non-stress conditions. This occurred 4 h after the last DPN injection to avoid potential acute effects of agonist treatment. For all animals, brains were removed from the skull, fresh-frozen in pre-cooled 2-methylbutane (−40° C) and stored at −80° C until sectioning.
II. Experiment 2: Local treatment with DPN or estradiol
Three groups of rats were stereotaxically implanted bilaterally with wax pellets (2.00 mm long, 0.25 mm in diameter) flanking the DRN at coordinates: 8.0 mm posterior to bregma, ± 1.5 mm lateral, 5.5 mm deep, at a 7° angle. Each pellet contained either 0.5 μM DPN (n=10), 0.5 μM 17-beta-estradiol (Sigma, St. Louis, MO; E, n=10) or beeswax only (VWR International, Bristol, CT; vehicle control, n=10). The dosage of DPN and E and the procedure for stereotaxic wax pellet implantation was based on previous studies by Lund et al. (2006). A second control group of OVX animals remained unoperated (C, n=7). All animals were tested in the OF, EPM and FST on days 5, 6 and 7 of treatment. Animals were returned to their home cage after each test. All animals were killed on day 8 between 1000 and 1200 h, their brains removed, fresh-frozen and stored at −80° C until sectioning.
Behavioral testing
To measure anxiety-like behaviors, all rats were tested in the OF and on the EPM on two consecutive days between 1000 and 1200 h for 5 min each. In the OF, the following parameters were scored: locomotor activity (total of square line crossings), number of rears at walls, time spent in center squares, time spent in outer squares, time spent grooming, and number of fecal boli. On the EPM, the latency until first open arm entry, the time spent in the open and closed arms, the number of closed and open arm entries (locomotor activity), the time spent grooming, and the number of fecal boli were recorded as described in Lund et al. (2005)
To measure despair-like behavior, rats were tested in the FST (Porsolt et al., 2001) for 5 min on day 7, using 25 °C tap water. In the afternoon of the previous day, all rats were trained for the FST for 3 min each, without recording their behavior. The time spent paddling (normal stress-coping behavior: slow-pace front and hind leg movements), the time spent struggling (active behavior: high-pace front leg paddling and strong hind leg strokes), the time spent floating (passive, despair-like behavior: minimal leg movements; stiff, floating body posture) and the number of dives (active behavior) was recorded.
Tph2 riboprobe design
For the production of a riboprobe specific for tph2 mRNA, total RNA was isolated from microdissected DRN tissue samples (Palkovits et al., 1975), following the protocol established by Chomczynski and Sacchi (1987). 1 μg RNA was reverse transcribed into 10 total cDNA with the reverse transcriptase MMLV-RT (Invitrogen, Carlsbad, CA) at 37° C for 50 min, using 1 μl oligo dT primers, dNTPs (100 mM each), 1st strand buffer (100 mM Tris–Cl–900 mM KCl–1 mM MgCl) and 2.5 mM DTT. Subsequently, a 583 bp fragment of tph2 cDNA was amplified by RT-PCR (forward primer: 5′-GGG GTG TTG TGT TTC GGG-3′, reverse primer: 5′-GTG GTG ATT AGG CAT TCC-3′). PCR conditions were: 45 s denaturation at 95° C, 45 s annealing at 55° C, 45 s elongation at 72° C, and a final 7-min elongation step at 72° C after 35 cycles. The 50 μl PCR reaction volume contained 1.5 mM Mg2+, 0.2 mM dNTPs, 0.2 μM forward and reverse primer, 50 ng template cDNA, and 1.0 unit Taq DNA polymerase (Eppendorf, Westbury, NY). After gel-purification (Qiagen, Valencia, CA), the PCR product was subcloned into the linearized 4.0 kb TOPO-vector pCR®II (Invitrogen, Carlsbad, CA), and amplified in chemically competent TOP10 bacterial cells (Invitrogen). Successful clones were verified via sequencing (Retrogen, San Diego, CA). Antisense and sense (control) tph2 cRNAs were transcribed from the plasmid in the presence of [35S]-UTP, following linearization with restriction enzymes, BlpI or XbaI respectively. As confirmed in two hybridization test runs, the antisense probe specifically detected tph2 mRNA in the dorsal and median raphe nuclei, but did not hybridize with tph1 mRNA in the pineal gland (Patel et al., 2004; Malek et al., 2005). There was no hybridization above background seen when using the sense-directed control probe.
Tissue preparation & In situ hybridization
A series of coronal 16-μm brainstem sections between bregma −6.5 mm to −9.5 mm (Paxinos and Watson, 1998) was cut at −20° C using a cryostat (Leica, Wetzlar, Germany) and thaw-mounted onto positively charged slides (Superfrost Plus, VWR Scientific, West Chester, PA). All sections were stored at −80° C until assayed. For in situ hybridization, tissue sections were thawed at room temperature, fixed within 10% paraformaldehyde, acetylated with 0.25% acetic anhydride, dehydrated in a graded series of alcohols, and air-dried. Next, sections were incubated with hybridization solution (50% formamide, 0.60 M NaCl, 0.02 M Tris, 0.01 M EDTA, 10% dextran sulfate, 2 M Denhart’s solution, 50 mM dithiothreitol, 0.2% SDS, 100 mg/ml salmon testis DNA, 500 mg/ml total yeast RNA, and 50 mg/ml yeast transfer RNA), containing radiolabeled cRNA at a concentration of 2 × 107 cpm/ml, in humidified chambers at 60° C overnight. After hybridization, slides were rinsed in 2 × SSC. Non-hybridized RNA was digested in a 30 mg/ml RNase A solution for 30 min at 37° C. A final high stringency wash (0.1 × SSC, room temperature) preceded dehydration in graded alcohols. Hybridization was first examined by opposing slides to a 35S-sensitive Biomax MR film (Kodak, Rochester, NY) for 14 hours. Subsequently, hybridization was detected using photographic emulsion-coated slide autoradiography (NTB-3; Kodak). After a 2 day-incubation at 4° C in the dark, all slides were developed (Kodak D-19) and counterstained with cresyl violet.
Validation of pellet implantation
We assumed that the compounds used in the present study successfully diffused into all rostro-caudal and medial-to-lateral subregions of the DRN if both pellets were placed within a maximal radius of 0.5 mm from the center of a coronal DRN section. This criterion was defined empirically by Lund et al. (2006) who found the diffusion of [3H]-labeled E to be confined within a 0.5 mm area surrounding the wax pellet. Based on this anatomical criterion, one individual of the vehicle-group had to be excluded from data analysis. Each section containing the DRN (12 sections per animal) was evaluated using bright-field microscopy and the center location of each pellet was estimated and mapped using a rat brain atlas (Paxinos and Watson, 1998).
Image analysis & Quantification of mRNA expression
Cytoplasmic detection of tph2 mRNA in individual 5-HT neurons was verified via photomicrographs. Counterstaining with cresyl violet allowed for distinction between silvergrain-labeled tph2 mRNA localized around purple-labeled nuclei. Dark-field images were captured by a Zeiss AxioCam HR camera on an Axioplan 2 microscope controlled by Axiovision, version 3.1, software. Three rostral, mid and caudal sections per animal were atlas matched (Paxinos and Watson, 1998) and used for analysis via ImageJ software (version 1.31). Matched dark-field images were inverted in order to cause silvergrains to appear as dark pixels in the inverted picture. Matrices in approximate shape of the DRN subregions of interest were then utilized to assess tph2 mRNA expression within the DRN. The density of black pixels was measured and expressed as arbitrary density units [AdU] for each subregion. After subtraction of background activity (determined in an adjacent area devoid of labeling), six (for the lateral DRN) or three values each (for the dorso-rostral, ventro-rostral, dorso-mid, ventro-mid, dorso-caudal, ventro-caudal DRN) were averaged per animal to obtain an individual value for statistical analysis.
Statistics
All data are expressed as the mean ± standard error of the mean (SEM). For studies with only two treatment groups, Student’s t- test was used for pair-wise data comparison. Results of all studies with more than two treatment groups were analyzed by one-way ANOVA (factor treatment) followed by Tukey’s post hoc test where appropriate, using SPSS 12.0 for Windows software. Results were considered significantly different when p < 0.05.
RESULTS
Experiment 1 (systemic treatment)
Weight gain
Vehicle animals gained 25.16 ± 2.79 g, DPN-treated animals 31.17 ± 3.90 g from day 1 to day 8 of treatment. There were no significant group differences in weight gain.
Systemic delivery of DPN decreases anxiety-like behavior
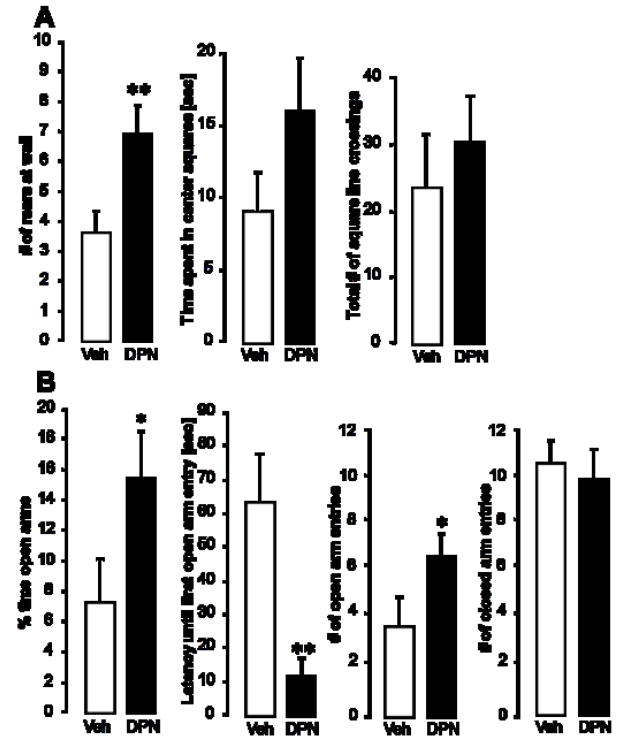
Animals were tested for anxiety-like behaviors in the open field (OF) and on the elevated plus maze (EPM). In the OF (Fig. 1A), DPN-treated animals displayed more rears at the walls than their vehicle-treated counterparts (p < 0.01). The total number of square line crossings (a measure of overall activity) did not differ between the two groups, suggesting that there was no overall effect on activity. The total time spent in inner or outer squares, the time spent grooming and the number of fecal boli were not significantly different between the two treatment groups.
Figure 1.
Effect of systemic delivery of the ERbeta agonist, DPN, on anxiety-like behaviors of female OVX rats. Animals were tested in the open field (A) and on the elevated plus maze (B). Panel A shows (left to right) the number of rears at the walls of the OF, the time the animals spent in the center squares, and the total number of square line crossings. Panel B displays the percent time the animals spent in the open arms of the EPM, the latency until the first open arm entry, the number of open arm entries and the number of closed arm entries (left to right). Each column represents the group mean ± SEM of 8 individuals per group. Veh = vehicle treated group, DPN = DPN-treated group. * (p < 0.05) and ** (p < 0.01) indicate significant differences versus vehicle controls (Student’s t-test).
On the EPM, DPN treatment caused the rats to enter the open arms sooner (p < 0.01), more often (p < 0.05), and stay on the open arms longer (p < 0.05) than vehicle controls (Fig. 1B). The number of entries into the closed arms did not differ between the two treatment groups, again indicating that DPN did not increase the rats’ overall activity or locomotor behavior. None of the other parameters recorded (time grooming, time in closed arms, fecal boli) revealed significant group differences.
Systemic DPN increases tph2 mRNA expression in the caudal and dorso-mid DRN
To test the hypothesis that systemic ERbeta activation may upregulate tph2 mRNA expression, in situ hybridization was performed, and the density of tph2 mRNA expression was measured in all subregions of the DRN. Fig. 2 displays representative dark-field photomicrographs of tph2 mRNA hybridization in the DRN of systemically treated rats. Compared to vehicle-treated animals, daily s.c. administration of DPN significantly enhanced tph2 mRNA levels in the dorso-mid (p < 0.05), the dorso- caudal (p < 0.05) and the ventro-caudal (p < 0.05) DRN (Fig. 3A). Accordingly, total tph2 mRNA levels in the entire caudal DRN were almost doubled in the DPN group (p < 0.01; Fig. 3B), compared to vehicle controls. Tph2 mRNA expression in the rostral DRN was not elevated by DPN. Fig. 3C illustrates in principle the matrix-based, digital analysis of inverted dark-field images that was used for all photomicrographs.
Figure 2.
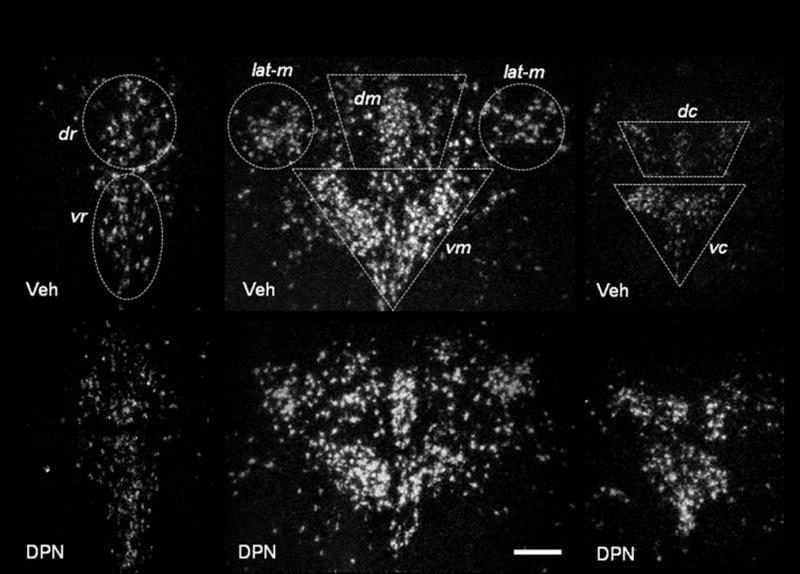
Representative dark-field photomicrographs from experiment 1. Shown are cells expressing tph2 mRNA in the rostral (left side, bregma −7.3), mid- (middle, bregma −8.0) and caudal DRN (right side, bregma −8.7) of systemically vehicle- or DPN-treated OVX animals. Vehicle-treated animals (Veh) are shown in the top row of panels, DPN-treated rats (DPN) in the lower row of panels. dr = dorso-rostral, vr = ventro-rostral, dm = dorso-mid, vm = ventro-mid, lat-m = lateral mid, dc = dorso-caudal, vc = ventro-caudal. Scale bar: 40 μm.
Figure 3.
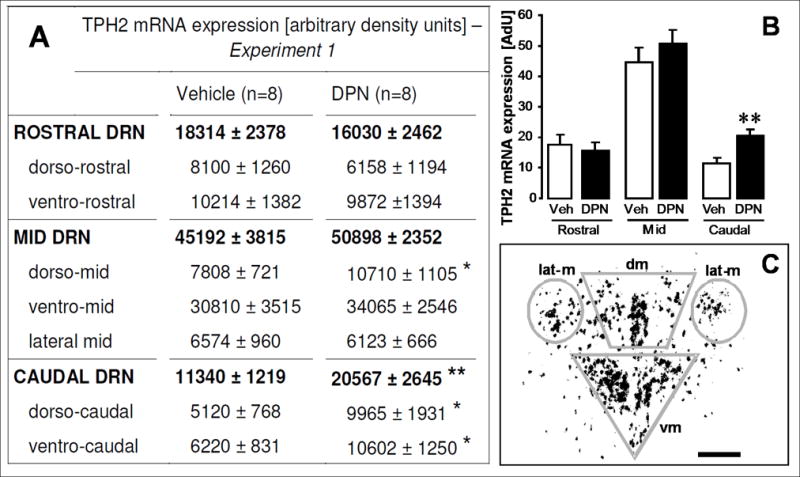
Effects of systemic DPN-treatment on tph2 mRNA in OVX females. DPN increases tph2 mRNA in the dorso-mid and in the dorso- and ventro-caudal DRN. The table in panel A lists densitometry-determined values for tph2 mRNA expression in seven subregions throughout the rostro-caudal extent of the DRN (regular letters). For the rostral, mid and caudal DRN, the sum of respective subregional values is displayed in bold letters. Animals were treated s.c. with DPN or vehicle for 8 days. Each value represents the mean ± SEM of 8 animals per group (numbers on parentheses). * (p < 0.05) and ** (p < 0.01) indicate significance versus vehicle controls. Data were analyzed by Student’s t-test for all regions. Panel B: This diagram is a graphic illustration of tph2 mRNA expression levels in the entire rostral, mid and caudal DRN, as listed in bold letters in Panel A. All columns represent means ± SEM of n = 8 per group. Veh = vehicle (hydroxypropyl-beta-cyclodextrin) group, DPN = DPN-treated group. AdU = arbitrary density units. ** (p < 0.01) indicates significance versus vehicle controls (Student’s t-test). Panel C: Schematic representation of matrix-based densitometry analysis of inverted, normalized dark-field pictures. Silvergrains appear as dark dots in the inverted picture. In this example, the densities of silver grains in the dorso-mid (dm), ventro-mid (vm) and lateral mid (lat-m) DRN were measured and subsequently summarized for total tph2 mRNA expression in the entire mid DRN. Data collection of rostral and caudal subregions of the DRN was performed accordingly. Scale bar: 40 μm.
Experiment 2 (central treatment)
Weight gain
The average weight gain during experiment 2 was 28.57 ± 1.87 g for the unoperated control group, 25.00 ± 2.21 g for the vehicle control animals, 25.80 ± 3.30 g for E-treated animals, and 30.50 ± 2.25 g in the DPN group. No significant differences in weight gain were found between any of the treatment groups.
Evaluation of in situ hybridization and wax pellet placement
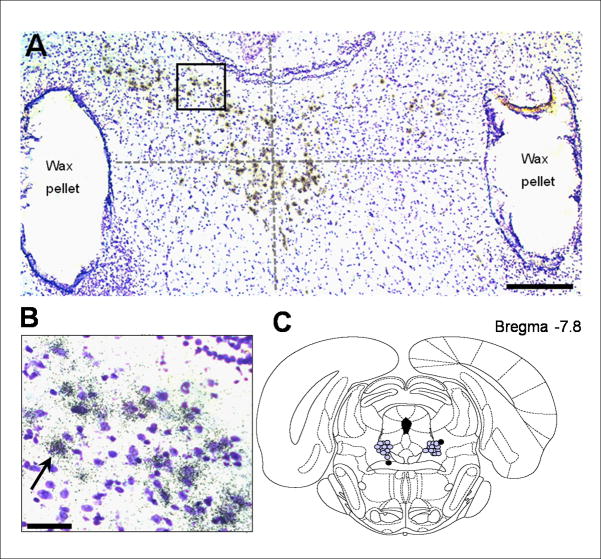
Fig. 4A shows a representative bright-field photomicrograph of the bilaterally implanted wax pellets placed to flank the DRN. To confirm the cellular silvergrain-labeling of neurons containing tph2 mRNA, brain tissue was counterstained with cresyl violet at an intensity sufficient to label the nucleus, but light enough to not alter or interfere with the identification of silvergrains (Fig. 4B). A schematic of actual bilateral wax pellet localization in the brainstem of all animals in experiment 2 is depicted in Fig. 4C. If the center of any of the two pellets was found to be more than 0.5 mm away from the estimated center of the DRN, the respective animal was excluded from all data analysis. This was true for one vehicle-treated rat (black dots in Fig. 4C), which reduced the size of this group from 10 to 9 animals.
Figure 4.
Localization of hormone containing pellets implanted in experiment 2. Wax pellets containing 0.5 μM DPN or 0.5 μM E were stereotaxically implanted lateral to the DRN. A: Bright-field image of silvergrain-labeled (small black grains) cells at bregma −8.2, hybridized with a riboprobe detecting tph2 mRNA, and counterstained with cresyl-violet (purple), are shown. The spread of a compound diffusing from a wax pellet was estimated to be confined within a radius of 0.5 mm (Lund et al. 2006). The predicted center of the DRN is indicated by the crossing point of the two dotted lines. Scale bar: 100 μm. B: Magnification of the outlined area from picture A. The arrow marks a concentration of silvergrain-labeled tph2 mRNA around a cell nucleus (purple). Scale bar: 20 μm. C: Schematic picture of the rat brainstem at Bregma −7.80 mm (Paxinos & Watson 1998). Each gray dot represents the center of an implanted wax pellet.
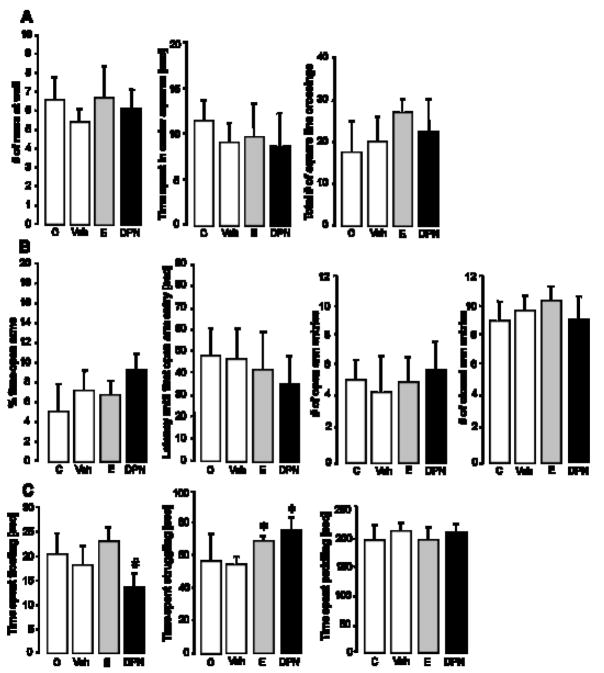
Local DPN or E administration does not alter anxiety-like behaviors, but enhances active stress-coping behavior
All animals were tested for anxiety-related behavior in the OF and on the EPM. Local, DRN-targeted delivery of neither DPN nor E altered any of the parameters measured in the OF and the EPM, compared to unoperated and vehicle controls (Fig. 5, A and B). In the FST, rats that were implanted with bilateral, DRN-flanking E- or DPN-pellets displayed a more active stress-coping strategy by spending more time actively struggling in the water than did vehicle controls (Fig. 5C; F3,32=4.628; p < 0.05). Animals of all treatment groups spent about the same time paddling (neutral stress-coping behavior). Neither E- nor DPN-treatment significantly altered the time spent floating, compared to controls. However, DPN-treated animals spent more time passively floating than their E-treated counterparts (Fig. 5C; F3,32=6.040; p<0.05). The number of dives did not differ between any of the groups.
Figure 5.
Effect of local DPN- or E-treatment on anxiety- and depressive-like behavior. Animals were tested in the OF (A) and on the EPM (B) for anxiety-related behavior, and in the FST (C) for depression-related behavior. Panel A shows (left to right) the number of rears at the walls of the OF, the time the animals spent in the center squares, and the total number of square line crossings. Panel B displays the percent time the animals spent in the open arms of the EPM, the latency until the first open arm entry, the number of open arm entries and the number of closed arm entries. Panel C depicts the time spent floating, struggling or paddling when the animals were forced to swim. Each column represents the mean ± SEM for 7–10 animals per group. * (p<0.05) indicates significant difference versus vehicle controls, # (p<0.05) versus E-treated animals. C = OVX control group without brain surgery (n=7), Veh = vehicle control group (blank wax pellets, n=9), E = estradiol-treated animals (n=10), DPN = DPN-treated animals (n=10). ANOVA (factor treatment) was performed, followed by Tukey’s post hoc test where appropriate.
Animals from the unoperated control group did not differ from the regular vehicle control group in any of the behavioral paradigms, indicating that there was no effect of brain surgery on anxiety-like behaviors.
Local exposure to E or DPN strongly elevates tph2 mRNA expression
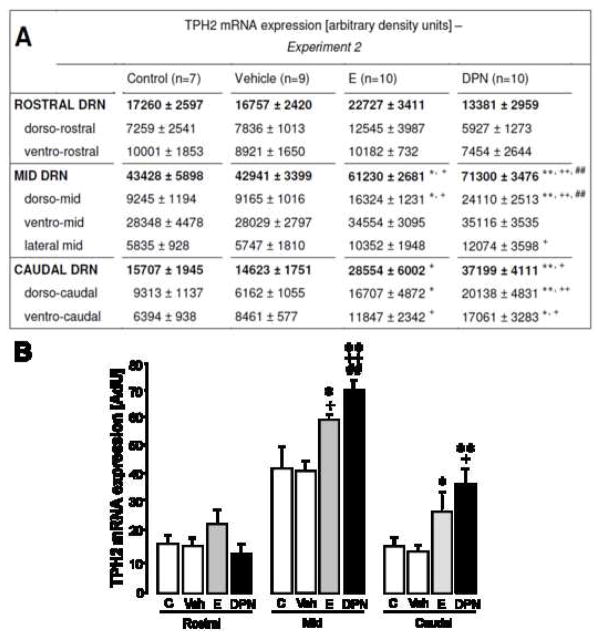
To investigate whether site-specific activation of ERs through local delivery of DPN or E itself is sufficient to enhance the expression of tph2 mRNA, in situ hybridization was performed. Fig. 6 displays representative dark-field photomicrographs of tph2 mRNA hybridization in the brainstem of locally DPN-, E-, or vehicle-treated rats. In the dorso-mid DRN, local DPN-administration elevated tph2 mRNA expression to approximately 2.5-fold of the level seen in controls (Fig 7A; F3,32=18.197; both p < 0.01), resulting in a more than 1.5-fold overall increase of tph2 mRNA expression for the entire mid-DRN, compared to vehicle and unoperated controls (Fig. 7A and B; F3,32=9.096; both p < 0.01). Regarding its effect on tph2 mRNA in the mid DRN, local DPN treatment also resulted in a stronger response than that of E (Fig. 7A and B; p < 0.01). In both the dorso- and ventro-caudal DRN, a 2- to 3-fold increase in tph2 mRNA expression (dorso-caudal: F3,32=13.720; p < 0.01; ventro-caudal: F3,32=14.962; p < 0.05) was seen when DPN-treated animals were compared to unoperated and vehicle-treated controls. In summary, local DPN-treatment more than doubled the expression of tph2 mRNA in the entire caudal DRN, compared to vehicle (Fig. 7A and B; F3,32=6.040; p < 0.01) and unoperated controls (p < 0.05). Tph2 mRNA expression in the rostral DRN was not elevated by DPN.
Figure 6.
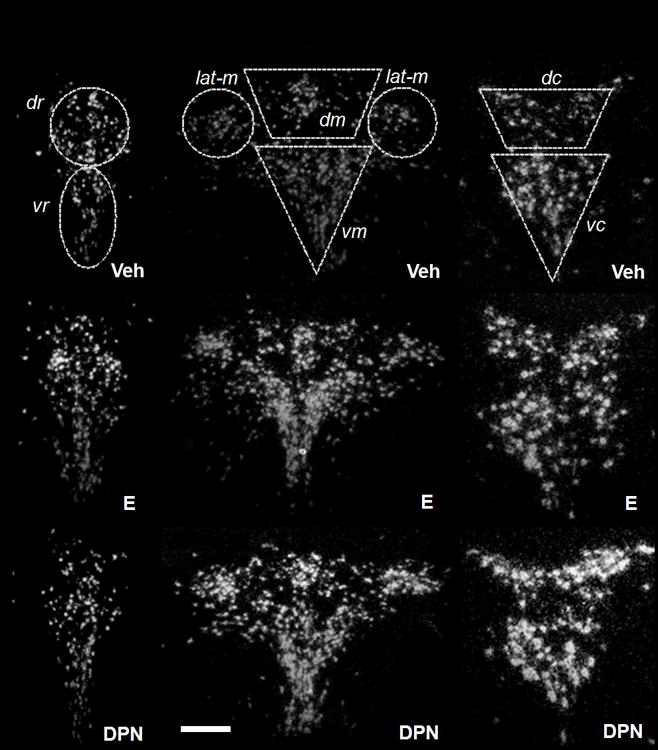
Representative dark-field photomicrographs from experiment 2. Shown are cells expressing tph2 mRNA in the rostral (left side, bregma −7.3), mid- (middle, bregma −8.0) and caudal (right side, bregma −8.7) DRN of female OVX rats stereotaxically implanted with vehicle-, E- or DPN-containing pellets. Vehicle-treated animals (Veh) are shown in the top row of panels, E-treated rats (E) in the middle row of panels, and DPN-treated rats (DPN) in the lower row of panels. dr = dorso-rostral, vr = ventro-rostral, dm = dorso-mid, vm = ventro-mid, lat-m = lateral mid, dc = dorso-caudal, vc = ventro-caudal. Scale bar: 40 μm.
Figure 7.
Effect of local DPN- or E-treatment on tph2 mRNA levels of OVX females. DPN and E both act locally to enhance tph2 expression in mid- and caudal subregions of the DRN. The table in panel A lists densitometry-determined values for tph2 mRNA expression in seven subregions throughout the rostro-caudal extent of the DRN (regular letters). For the rostral, mid and caudal DRN, the sum of respective subregional values is displayed in bold letters. OVX rats were implanted with DRN-flanking wax pellets containing nothing (vehicle group), E or DPN. An additional control group remained without brain surgery. Numbers in parentheses indicate group size. Each value represents the mean ± SEM. Panel B: This diagram is a graphic illustration of tph2 mRNA expression levels in the rostral, mid and caudal DRN, as listed in bold letters in Panel A. Each value represents the mean ± SEM. * (p < 0.05) and ** (p < 0.01) indicate significance versus vehicle controls, + (p < 0.05) and ++ (p < 0.01) versus unoperated controls, and # (p < 0.05) and ## (p < 0.01) versus E-treated animals. C = OVX control group without brain surgery (n=7), Veh = vehicle control group (blank wax pellets, n=9), E = estradiol-treated animals (n=10), DPN = DPN-treated animals (n=10). ANOVA (factor treatment) was performed, followed by Tukey’s post hoc test where appropriate.
Treatment with estradiol caused similar, but less intense, effects on tph2 mRNA expression compared to DPN. Local E-treatment caused tph2 mRNA levels in the dorso-mid DRN to be about 1.8-fold higher than in either control group (Fig. 7A; F3,32=10.007; p < 0.05), resulting in an overall 1.5-fold increase of tph2 mRNA in the entire mid DRN (Fig. 7A and B; F3,32=7.902; p < 0.05), compared to both control groups. In the dorso-caudal subregion of the DRN, local delivery of E caused a significant increase in tph2 mRNA compared to the vehicle control group (Fig. 7A; F3,32=14.665; p < 0.05), in the ventro-caudal part compared to the unoperated control group (Fig. 7A; F3,32=9.192; p < 0.05). Overall expression of tph2 mRNA in the caudal DRN was almost doubled by local E-treatment compared to vehicle controls (Fig. 7A and B; F3,32=8.360; p < 0.05).
Tph2 mRNA levels did not differ for any subregion between the vehicle- and the unoperated control group, ensuring that the implantation of wax pellets adjacent to the DRN did not alter tph2 gene expression.
DISCUSSION
The results of our studies show that both systemic and local activation of ERbeta in or around the DRN increased the expression of tph2 mRNA in a subregion-dependent manner. Estrogen treatment caused similar overall effects, but to a lesser extent than DPN. Furthermore, animals treated locally with DPN or E showed decreased despair-like behavior. However, only systemic delivery of DPN decreased anxiety-like behavior, while local administration of DPN failed to have the same effect.
The systemic delivery of DPN to OVX rats reduced several anxiety-related behaviors, confirming the anxiolytic nature of ERbeta (Krezel et al., 2001; Lund et al., 2005; Rocha et al., 2005; Weiser et al., 2009). In contrast, local DPN- or estradiol-treatment of the raphe nuclei failed to decrease anxiety-like behavior, indicating that estradiol’s action in regulating anxiety-like behaviors may primarily involve other brain areas, such as the hypothalamic PVN (Herman et al., 2002; Donner et al., 2007; Blume et al., 2008; Neumann, 2008), the lateral septum (Henry et al., 2006), the amygdala (Bosch et al., 2007), or the bed nucleus of the stria terminalis (Davis et al., 1997; Walker et al., 2003). Interestingly, our studies demonstrate that local delivery of DPN or estradiol is sufficient to decrease despair-like behaviors in the FST. It remains unclear why DPN-treated rats spent less time with passive floating behavior than estradiol-treated animals. Yet, in accordance with studies from other research groups, both estradiol and DPN increased the time rats spent actively struggling. While recent studies already revealed an antidepressant function of ERbeta (Walf et al., 2004; Rocha et al., 2005; Hughes et al., 2008), our experiments indicate a site of action for the observed effect. Thus, high anxiety and despair-like behavior may be closely related to the phenotype of depression (Chaby et al., 1993; Leibbrand et al., 1999; Farabaugh et al., 2005; Godart et al., 2006; Mittal et al., 2006), but the involvement of ERbeta may be through two distinct neuroanatomical regions. Further studies will be required to determine how ERbeta-mediated elevations of tph2 mRNA expression in the mid-dorsal and caudal DRN are coupled to the attenuation of despair-like behavior.
Not only the route and site of drug administration (systemic versus local), but also the different time course of delivery (once daily s.c. versus constant intracerebral) may have contributed to the discrepancy regarding anxiety-related behaviors between our two experiments. The concentration of DPN in the plasma of male rats that received a 1mg/kg s.c. injection of DPN has been shown to peak after 1 hour and decrease rapidly to undetectable levels within 3 hours post injection (Patisaul et al., 2009). However, this doesn’t mean that the biological activity of DPN decreases within the same timeframe. Lund et al. (2005) used an in vitro binding approach and differential centrifugation for separation of bound and unbound receptors to estimate that DPN occupies neural ERs with a half life of about 8 hours. These and other studies indicate that DPN, phytoestrogens, and estradiol itself are likely to be sequestered by ERs in reproductive tissues and in the brain, and are able to exert physiological effects for many hours after incorporation (Blaustein et al., 1979; Dehennin et al., 1982; Morton et al., 1997; Walf et al., 2004; Walf and Frye, 2005). Although animals in our experiment weren’t sacrificed until 4 hours after the last s.c. injection of DPN, it is therefore not unlikely that the behavioral discrepancies observed were due to delayed acute effects of the last DPN injection, compared to a constant chronic exposure to the compound diffusing from locally implanted wax pellets. Chronic wax-mediated, application of DPN for 7 days around the paraventricular nucleus has previously proven sufficient to inhibit the neuronal and corticosterone response of the hypothalamo-pituitary adrenal axis to an acute stressor (Lund et al., 2006). The effects of local DPN-treatment on cellular parameters (tph2 mRNA) in our study further strengthen the assumption that DPN is stable and biologically active for a long time when dissolved and administered in beeswax. However, it would be of further interest to identify the biological stability and metabolic fate of DPN following administration.
The observed DPN- or estrogen-induced increase in tph2 mRNA expression was mainly restricted to the dorso-mid and the caudal DRN. These findings are consistent with observations by Hiroi et al. (2006), who reported elevated tph2 mRNA expression specifically in the dorso- and ventro-caudal DRN following systemic estrogen treatment in female OVX rats. Furthermore, recent studies in rats and mice indicate that depression-related behavioral paradigms (Keeney and Hogg, 1999; Becker et al., 2007) like social defeat (Gardner et al., 2005) or inescapable stress (Grahn et al., 1999; Amat et al., 2005) selectively activate the dorsal and caudal parts of the DRN. These subregions correspondingly give rise to projections targeting forebrain areas involved in the control of emotional behavior (Lowry et al., 2005; Lowry et al., 2008). The mid-dorsal DRN, for instance, sends out collateral projections to emotion- and stress-related brain areas, that could, for instance, simultaneously modulate the hypothalamic PVN and the basolateral or central nucleus of the amygdala (Lowry, 2002; Hale et al., 2008b; Hale et al., 2008a). 5-HT axons from the caudal DRN target limbic structures like the hippocampus, the entorhinal cortex and the septum (Kohler et al., 1982; Kohler and Steinbusch, 1982), indicating that an alteration in tph2 expression and 5-HT neurotransmission by estrogens may improve memory and learning deficits that are associated with depression (Shors et al., 1998; Burriss et al., 2008; Liu et al., 2008). Within the entire caudal DRN it is the dorso-caudal subdivision that has been suggested to play a crucial role in changes associated with affective disorders (Commons et al., 2003). In clinical studies of drug-free, depressed suicide victims, the dorso-caudal DRN subregions also exhibited elevated TPH2 protein and tph2 mRNA expression (Bonkale et al., 2006; Bach-Mizrachi et al., 2008). However, a pathological increase in tph2 expression may explain this apparent paradox. Pathologically elevated tph2 mRNA and protein may reflect a compensatory feedback response to low overall 5-HT concentrations in the brain of depressed patients (Mann et al., 1989; Owens and Nemeroff, 1994; Placidi et al., 2001). Also, most of the brains assessed in these clinical studies were derived from male individuals, not females. Consequently, more detailed studies quantifying TPH2 protein and local 5-HT release and turnover within the DRN itself (autoregulation) and in target areas of the DRN in both male and female animal models of anxiety and depression are required to answer this question.
Overall, local estradiol treatment had a similar, but less intense effect on tph2 mRNA expression and on despair-like behavior than the selective ERbeta agonist. This difference between E and DPN could be explained by the non-selective action of estradiol on both ERalpha and ERbeta. Our previous data and those of others suggest that ERalpha and ERbeta have opposing actions on stress related behaviors (Liu et al., 2002; Lund et al., 2005; Toufexis et al., 2007; Weiser et al., 2009). While increased ERalpha mRNA and single nucleotide polymorphisms (SNPs) in the gene coding for ERalpha are associated with mental illness, specifically with depression (Perlman et al., 2005; Mill et al., 2008), ERbeta-mediated actions have been found to exert anxiolytic and antidepressant effects in various animal models (Imwalle et al., 2005; Lund et al., 2005; Rocha et al., 2005). Since estradiol can bind to both receptor types with equal affinity (Kuiper et al., 1997; Lund et al., 2005), the possibility exists that it could activate two functionally opposing mechanisms, both ultimately balancing tph2 mRNA expression and 5-HT-dependent behaviors.
At present, the exact patterns for ERbeta expression in DRN 5-HT neurons are controversial. Lu et al. (2001) demonstrated that 5-HT neurons of the DRN of rats contain ERbeta, whereas Sheng et al. (2004) were unable to identify ERbeta in 5-HT neurons. In mice, Nomura et al. (2005) revealed that ERbeta, but not ERalpha is located within 5-HT neurons. ERbeta has also been shown in 5-HT neurons of the guinea pig (Lu et al., 1999) and rhesus monkey (Gundlah et al., 2001). Furthermore, ERbeta2 (Chung et al., 2007), a novel splice variant carrying an 18-amino acid insert between the fifth and the sixth exon in the ligand-binding domain of ERbeta, has been shown in the DRN of female rats. In contrast, ERalpha may only be expressed in non-5-HT-, but possibly GABAergic interneurons (Hart et al., 2001; Su et al., 2001), placing ERalpha in a position to interfere with the negative feedback regulation of 5-HT-neuronal function (Haddjeri et al., 2000; Liu et al., 2000). Differences in the expression of ERalpha versus ERbeta in the midbrain of rats (Shughrue et al., 1997a; Shughrue et al., 1997b; Lu et al., 2001), mice (Nomura et al., 2005; Vanderhorst et al., 2005), guinea pigs (Lu et al., 1999; Warembourg and Leroy, 2004), and cats (VanderHorst et al., 1998) suggest that species differences may exist in the modulation of the 5-HT system by gonadal steroids. In non-human primates, ERbeta, but not ERalpha, seems to be the predominant ER expressed in raphe 5-HT neurons (Gundlah et al., 2000; Gundlah et al., 2001; Vanderhorst et al., 2009). While ER expression in the human DRN remains to be fully described, estrogens would be expected to exert mainly anxiolytic and anti-depressive actions in humans if our expression profile of ERbeta in the brain resembles the pattern found in other primates.
The molecular mechanisms by which ERbeta and ERalpha may directly or indirectly modulate tph2 gene expression are still unknown. Although most ER-induced changes in gene transcription are due to classic effects of the steroid receptors acting as nuclear transcription factors, it is possible that other mechanisms may be present, particularly given the recent studies showing rapid, membrane mediated mechanisms of ER action (Cato et al., 2002; Mhyre and Dorsa, 2006; Levin, 2008).
In conclusion, our results show that chronic, local activation of ERbeta alters tph2 mRNA expression in the DRN in a subregion-dependent manner, and, at the same time, facilitates active stress-coping behavior. Interactions between ERbeta and 5-HT neurons of the DRN may be key regulators of anti-depressive behavior, whereas other brain circuits seem to be necessary for ERbeta to exert its anxiolytic actions. Our observations also raise the question whether physiological changes in circulating estradiol can differentially influence behaviors in women across the menstrual cycle. One possibility is that an altered ratio of ERalpha versus ERbeta expression or a disruption of normal ER-regulation of tph2 expression in the midbrain might contribute to mood disorders like premenstrual syndrome (Rubinow, 1992; Arpels, 1996; Schmidt et al., 1998) or premenstrual dysphoric disorder (Gorman, 2006). These data demonstrate the potential of ERbeta as a pharmaceutical target for treating affective disorders. The future development of an ERbeta agonist for clinical use that facilitates 5-HT function and emotional stability in menopausal women without the risk of breast- or gynecological cancer associated with ERalpha-mediated actions (Chen et al., 2008) would be highly beneficial.
Acknowledgments
The authors are grateful to Dr. Michael Weiser for de novo synthesis of diarylpropionitrile (DPN) and for help with stereotaxic surgeries.
ABBREVIATIONS
- TPH2
Tryptophan hydroxylase 2
- DRN
dorsal raphe nuclei
- DPN
diarylpropionitrile
- E
17-beta-estradiol
- ER
estrogen receptor
- 5-HT
serotonin
- EPM
elevated plus maze
- OF
open filed
- FST
forced swim test
- MDD
major depressive disorder
- OVX
ovariectomy/ovariectomized
- s.c.
subcutaneous
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Alves SE, McEwen BS, Hayashi S, Korach KS, Pfaff DW, Ogawa S. Estrogen-regulated progestin receptors are found in the midbrain raphe but not hippocampus of estrogen receptor alpha (ER alpha) gene-disrupted mice. J Comp Neurol. 2000;427:185–195. doi: 10.1002/1096-9861(20001113)427:2<185::aid-cne2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- Angold A, Worthman CW. Puberty onset of gender differences in rates of depression: a developmental, epidemiologic and neuroendocrine perspective. J Affect Disord. 1993;29:145–158. doi: 10.1016/0165-0327(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–453. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol Psychiatry. 2008;13:507–513. 465. doi: 10.1038/sj.mp.4002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Zeau B, Rivat C, Blugeot A, Hamon M, Benoliel JJ. Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002097. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Dudley SD, Gray JM, Roy EJ, Wade GN. Long-term retention of estradiol by brain cell nuclei and female rat sexual behavior. Brain Res. 1979;173:355–359. doi: 10.1016/0006-8993(79)90637-1. [DOI] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2008;27:1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- Bonkale WL, Turecki G, Austin MC. Increased tryptophan hydroxylase immunoreactivity in the dorsal raphe nucleus of alcohol-dependent, depressed suicide subjects is restricted to the dorsal subnucleus. Synapse. 2006;60:81–85. doi: 10.1002/syn.20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Sartori SB, Singewald N, Neumann ID. Extracellular amino acid levels in the paraventricular nucleus and the central amygdala in high- and low-anxiety dams rats during maternal aggression: regulation by oxytocin. Stress. 2007;10:261–270. doi: 10.1080/10253890701223197. [DOI] [PubMed] [Google Scholar]
- Burriss L, Ayers E, Ginsberg J, Powell DA. Learning and memory impairment in PTSD: relationship to depression. Depress Anxiety. 2008;25:149–157. doi: 10.1002/da.20291. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, Karthigeyan K, Harrist A, Hunsberger JG, Wittmack E, King SL, Jatlow P, Picciotto MR. Sex differences in response to oral amitriptyline in three animal models of depression in C57BL/6J mice. Psychopharmacology (Berl) 2003;170:94–101. doi: 10.1007/s00213-003-1518-7. [DOI] [PubMed] [Google Scholar]
- Cato AC, Nestl A, Mink S. Rapid actions of steroid receptors in cellular signaling pathways. Sci STKE. 2002;2002:RE9. doi: 10.1126/stke.2002.138.re9. [DOI] [PubMed] [Google Scholar]
- Chaby L, Grinsztein A, Weitzman JJ, de Bodinat C, Dagens V. Anxiety-related and depressive disorders in women during the premenopausal and menopausal period. Study of the efficacy and acceptability of tianeptine versus maprotiline. Presse Med. 1993;22:1133–1138. [PubMed] [Google Scholar]
- Chen GG, Zeng Q, Tse GM. Estrogen and its receptors in cancer. Medicinal Research Reviews. 2008;28:954–974. doi: 10.1002/med.20131. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chung WC, Pak TR, Suzuki S, Pouliot WA, Andersen ME, Handa RJ. Detection and localization of an estrogen receptor beta splice variant protein (ERbeta2) in the adult female rat forebrain and midbrain regions. J Comp Neurol. 2007;505:249–267. doi: 10.1002/cne.21490. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Ann N Y Acad Sci. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Dehennin L, Reiffsteck A, Jondet M, Thibier M. Identification and quantitative estimation of a lignan in human and bovine semen. J Reprod Fertil. 1982;66:305–309. doi: 10.1530/jrf.0.0660305. [DOI] [PubMed] [Google Scholar]
- Donner N, Bredewold R, Maloumby R, Neumann ID. Chronic intracerebral prolactin attenuates neuronal stress circuitries in virgin rats. Eur J Neurosci. 2007;25:1804–1814. doi: 10.1111/j.1460-9568.2007.05416.x. [DOI] [PubMed] [Google Scholar]
- Earls F. Sex differences in psychiatric disorders: origins and developmental influences. Psychiatr Dev. 1987;5:1–23. [PubMed] [Google Scholar]
- Ellard JH. Depression is complex and heterogeneous. Med J Aust. 2001;174:543–544. doi: 10.5694/j.1326-5377.2001.tb143418.x. [DOI] [PubMed] [Google Scholar]
- Farabaugh A, Fava M, Mischoulon D, Sklarsky K, Petersen T, Alpert J. Relationships between major depressive disorder and comorbid anxiety and personality disorders. Compr Psychiatry. 2005;46:266–271. doi: 10.1016/j.comppsych.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Godart NT, Perdereau F, Curt F, Rein Z, Lang F, Venisse JL, Halfon O, Bizouard P, Loas G, Corcos M, Jeammet P, Flament MF. Is major depressive episode related to anxiety disorders in anorexics and bulimics? Compr Psychiatry. 2006;47:91–98. doi: 10.1016/j.comppsych.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Gorman JM. Gender differences in depression and response to psychotropic medication. Gend Med. 2006;3:93–109. doi: 10.1016/s1550-8579(06)80199-3. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Mirkes SJ, Bethea CL. Estrogen receptor beta (ER[beta]) mRNA and protein in serotonin neurons of macaques. Molecular Brain Research. 2001;91:14–22. doi: 10.1016/s0169-328x(01)00108-5. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Lucas G, Blier P. Role of cholinergic and GABAergic systems in the feedback inhibition of dorsal raphe 5-HT neurons. Neuroreport. 2000;11:3397–3401. doi: 10.1097/00001756-200010200-00026. [DOI] [PubMed] [Google Scholar]
- Haghighi F, Bach-Mizrachi H, Huang YY, Arango V, Shi S, Dwork AJ, Rosoklija G, Sheng HT, Morozova I, Ju J, Russo JJ, Mann JJ. Genetic architecture of the human tryptophan hydroxylase 2 Gene: existence of neural isoforms and relevance for major depression. Mol Psychiatry. 2008;13:813–820. doi: 10.1038/sj.mp.4002127. [DOI] [PubMed] [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Shekhar A, Lowry CA. Exposure to an open-field arena increases c-Fos expression in a distributed anxiety-related system projecting to the basolateral amygdaloid complex. Neuroscience. 2008a;155:659–672. doi: 10.1016/j.neuroscience.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Bouwknecht JA, Evans AK, Stamper CE, Shekhar A, Lowry CA. Exposure to an open-field arena increases c-Fos expression in a subpopulation of neurons in the dorsal raphe nucleus, including neurons projecting to the basolateral amygdaloid complex. Neuroscience. 2008b;157:733–748. doi: 10.1016/j.neuroscience.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SA, Patton JD, Woolley CS. Quantitative analysis of ER alpha and GAD colocalization in the hippocampus of the adult female rat. J Comp Neurol. 2001;440:144–155. doi: 10.1002/cne.1376. [DOI] [PubMed] [Google Scholar]
- Henry B, Vale W, Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16:381–385. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF. Differential effects of ovarian steroids on anxiety versus fear as measured by open field test and fear-potentiated startle. Behav Brain Res. 2006;166:93–100. doi: 10.1016/j.bbr.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol Psychiatry. 2006;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Hughes ZA, Liu F, Platt BJ, Dwyer JM, Pulicicchio CM, Zhang G, Schechter LE, Rosenzweig-Lipson S, Day M. WAY-200070, a selective agonist of estrogen receptor beta as a potential novel anxiolytic/antidepressant agent. Neuropharmacology. 2008;54:1136–1142. doi: 10.1016/j.neuropharm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol. 1986;243:363–380. doi: 10.1002/cne.902430307. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84:157–163. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Keeney AJ, Hogg S. Behavioural consequences of repeated social defeat in the mouse: preliminary evaluation of a potential animal model of depression. Behav Pharmacol. 1999;10:753–764. doi: 10.1097/00008877-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kohler C, Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982;7:951–975. doi: 10.1016/0306-4522(82)90054-9. [DOI] [PubMed] [Google Scholar]
- Kohler C, Chan-Palay V, Steinbusch H. The distribution and origin of serotonin-containing fibers in the septal area: a combined immunohistochemical and fluorescent retrograde tracing study in the rat. J Comp Neurol. 1982;209:91–111. doi: 10.1002/cne.902090109. [DOI] [PubMed] [Google Scholar]
- Kornstein SG. Gender differences in depression: implications for treatment. J Clin Psychiatry. 1997;58 Suppl 15:12–18. [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Yonkers KA, Thase ME, Keitner GI, Ryan CE, Schlager D. Gender differences in presentation of chronic major depression. Psychopharmacol Bull. 1995;31:711–718. [PubMed] [Google Scholar]
- Koss WA, Gehlert DR, Shekhar A. Different effects of subchronic doses of 17-beta estradiol in two ethologically based models of anxiety utilizing female rats. Horm Behav. 2004;46:158–164. doi: 10.1016/j.yhbeh.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta -deficient mice. Proc Natl Acad Sci U S A. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Leibbrand R, Hiller W, Fichter MM. Effect of comorbid anxiety, depressive, and personality disorders on treatment outcome of somatoform disorders. Compr Psychiatry. 1999;40:203–209. doi: 10.1016/s0010-440x(99)90004-4. [DOI] [PubMed] [Google Scholar]
- Lesch KP. Gene-environment interaction and the genetics of depression. J Psychiatry Neurosci. 2004;29:174–184. [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Rapid Signaling by Steroid Receptors. Am J Physiol Regul Integr Comp Physiol. 2008 doi: 10.1152/ajpregu.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Liu MM, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, Price RH, Jr, Pestell RG, Kushner PJ. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem. 2002;277:24353–24360. doi: 10.1074/jbc.M201829200. [DOI] [PubMed] [Google Scholar]
- Liu R, Jolas T, Aghajanian G. Serotonin 5-HT(2) receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- Lowry C, Evans A, Gasser P, Hale M, Staub D, Shekhar A. Topographical organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus in Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Basel; Birkhauser: 2008. [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Lu H, Ozawa H, Nishi M, Ito T, Kawata M. Serotonergic neurones in the dorsal raphe nucleus that project into the medial preoptic area contain oestrogen receptor beta. J Neuroendocrinol. 2001;13:839–845. doi: 10.1046/j.1365-2826.2001.00695.x. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Shlaes TA, Gundlah C, Dziennis SE, Lyle RE, Bethea CL. Ovarian steroid action on tryptophan hydroxylase protein and serotonin compared to localization of ovarian steroid receptors in midbrain of guinea pigs. Endocrine. 1999;11:257–267. doi: 10.1385/ENDO:11:3:257. [DOI] [PubMed] [Google Scholar]
- Lund T, Rovis T, Chung W, Handa R. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek ZS, Dardente H, Pevet P, Raison S. Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. Eur J Neurosci. 2005;22:895–901. doi: 10.1111/j.1460-9568.2005.04264.x. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Arango V, Marzuk PM, Theccanat S, Reis DJ. Evidence for the 5-HT hypothesis of suicide. A review of post-mortem studies. Br J Psychiatry. 1989;Suppl:7–14. [PubMed] [Google Scholar]
- Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: role of estrogen receptor alpha and estrogen receptor beta in neurons and glia. Neuroscience. 2006;138:851–858. doi: 10.1016/j.neuroscience.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Mill J, Kiss E, Baji I, Kapornai K, Daroczy G, Vetro A, Kennedy J, Kovacs M, Barr C. Association study of the estrogen receptor alpha gene (ESR1) and childhood-onset mood disorders. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1323–1326. doi: 10.1002/ajmg.b.30751. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mittal D, Fortney JC, Pyne JM, Edlund MJ, Wetherell JL. Impact of comorbid anxiety disorders on health-related quality of life among patients with major depressive disorder. Psychiatr Serv. 2006;57:1731–1737. doi: 10.1176/ps.2006.57.12.1731. [DOI] [PubMed] [Google Scholar]
- Morton MS, Chan PS, Cheng C, Blacklock N, Matos-Ferreira A, Abranches-Monteiro L, Correia R, Lloyd S, Griffiths K. Lignans and isoflavonoids in plasma and prostatic fluid in men: samples from Portugal, Hong Kong, and the United Kingdom. Prostate. 1997;32:122–128. doi: 10.1002/(sici)1097-0045(19970701)32:2<122::aid-pros7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Nomura M, Akama K, Alves S, Korach K, Gustafsson J, Pfaff D, Ogawa S. Differential distribution of estrogen receptor (ER)-alpha and ER-beta in the midbrain raphe nuclei and periaqueductal gray in male mouse: Predominant role of ER-beta in midbrain serotonergic systems. Neuroscience. 2005;130:445–456. doi: 10.1016/j.neuroscience.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Overstreet DH, Hurd YL. The flinders sensitive line rats, a genetic model of depression, show abnormal serotonin receptor mRNA expression in the brain that is reversed by 17beta-estradiol. Brain Res Mol Brain Res. 1999;74:158–166. doi: 10.1016/s0169-328x(99)00274-0. [DOI] [PubMed] [Google Scholar]
- Overstreet D, Osterlund M, Dahllund J, Appelqvist T, Lindstrom E, Ryan C, Witt M. Estrogen receptor beta agonists reduce exaggerated swim test immobility in a genetic animal model for depression. Society for Neuroscience 36th Annual Meeting; Atlanta, GA. 2006. Abstract 476.11. [Google Scholar]
- Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40:288–295. [PubMed] [Google Scholar]
- Palkovits M, Kobayashi R, Kizer J, Jacobowitz D, Kopin I. Effects of stress on catecholamines and tyrosine hydroxylase activity of individual hypothalamic nuclei. Neuroendocrinology. 1975;18:144–153. doi: 10.1159/000122394. [DOI] [PubMed] [Google Scholar]
- Patel PD, Pontrello C, Burke S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biological Psychiatry. 2004;55:428–433. doi: 10.1016/j.biopsych.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Burke KT, Hinkle RE, Adewale HB, Shea D. Systemic administration of diarylpropionitrile (DPN) or phytoestrogens does not affect anxiety-related behaviors in gonadally intact male rats. Hormones and Behavior. 2009;55:319–328. doi: 10.1016/j.yhbeh.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotactic coordinates. Academic Press; 1998. [Google Scholar]
- Perlis M, Smith M, Orff H. Major Depressive Disorder (MDD) is associated with a primary defect within the serotonergic system. Sleep Med Rev. 2002;6:353–357. discussion 359. [PubMed] [Google Scholar]
- Perlman WR, Tomaskovic-Crook E, Montague DM, Webster MJ, Rubinow DR, Kleinman JE, Weickert CS. Alteration in estrogen receptor alpha mRNA levels in frontal cortex and hippocampus of patients with major mental illness. Biol Psychiatry. 2005;58:812–824. doi: 10.1016/j.biopsych.2005.04.047. [DOI] [PubMed] [Google Scholar]
- Petrov T, Krukoff TL, Jhamandas JH. The hypothalamic paraventricular and lateral parabrachial nuclei receive collaterals from raphe nucleus neurons: a combined double retrograde and immunocytochemical study. J Comp Neurol. 1992;318:18–26. doi: 10.1002/cne.903180103. [DOI] [PubMed] [Google Scholar]
- Placidi GP, Oquendo MA, Malone KM, Huang YY, Ellis SP, Mann JJ. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol Psychiatry. 2001;50:783–791. doi: 10.1016/s0006-3223(01)01170-2. [DOI] [PubMed] [Google Scholar]
- Porsolt R, Brossard G, Hautbois C, Roux H, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2001;Chapter 8(Unit 810A) doi: 10.1002/0471142301.ns0810as14. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17 Beta-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-beta knockout (BERKO) mice. Psychopharmacology (Berl) 2005;179:637–643. doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Molecular Brain Research. 2005;135:194–203. doi: 10.1016/j.molbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Kawano J, Yanai A, Fujinaga R, Tanaka M, Watanabe Y, Shinoda K. Expression of estrogen receptors (alpha, beta) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neurosci Res. 2004;49:185–196. doi: 10.1016/j.neures.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Shively CA, Mirkes SJ, Lu NZ, Henderson JA, Bethea CL. Soy and social stress affect serotonin neurotransmission in primates. Pharmacogenomics J. 2003;3:114–121. doi: 10.1038/sj.tpj.6500166. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. J Affect Disord. 2003;74:85–96. doi: 10.1016/s0165-0327(02)00428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9:419–423. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- Shughrue P, Lane M, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. The Journal of Comparative Neurology. 1997a;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I. The distribution of estrogen receptor-beta mRNA in forebrain regions of the estrogen receptor-alpha knockout mouse. Endocrinology. 1997b;138:5649–5652. doi: 10.1210/endo.138.12.5712. [DOI] [PubMed] [Google Scholar]
- Steenbergen HL, Heinsbroek RP, Van Hest A, Van de Poll NE. Sex-dependent effects of inescapable shock administration on shuttlebox-escape performance and elevated plus-maze behavior. Physiol Behav. 1990;48:571–576. doi: 10.1016/0031-9384(90)90302-k. [DOI] [PubMed] [Google Scholar]
- Su JD, Qiu J, Zhong YP, Chen YZ. Expression of estrogen receptor -alpha and -beta immunoreactivity in the cultured neonatal suprachiasmatic nucleus: with special attention to GABAergic neurons. Neuroreport. 2001;12:1955–1959. doi: 10.1097/00001756-200107030-00036. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Handa R. Estrogen receptor-beta, but not estrogen receptor-alpha, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol. 2005;484:28–42. doi: 10.1002/cne.20457. [DOI] [PubMed] [Google Scholar]
- Toufexis D. Region- and sex-specific modulation of anxiety behaviours in the rat. J Neuroendocrinol. 2007;19:461–473. doi: 10.1111/j.1365-2826.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Bowser ME, Davis M. Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor alpha (ERalpha) and ERbeta. J Neurosci. 2007;27:9729–9735. doi: 10.1523/JNEUROSCI.2529-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol. 2005;488:152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Terasawa E, Ralston HJ., 3rd Estrogen receptor-alpha immunoreactive neurons in the brainstem and spinal cord of the female rhesus monkey: species-specific characteristics. Neuroscience. 2009;158:798–810. doi: 10.1016/j.neuroscience.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderHorst VG, Schasfoort FC, Meijer E, van Leeuwen FW, Holstege G. Estrogen receptor-alpha-immunoreactive neurons in the periaqueductal gray of the adult ovariectomized female cat. Neurosci Lett. 1998;240:13–16. doi: 10.1016/s0304-3940(97)00900-2. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78:523–529. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Warembourg M, Leroy D. Comparative distribution of estrogen receptor alpha and beta immunoreactivities in the forebrain and the midbrain of the female guinea pig. Brain Res. 2004;1002:55–66. doi: 10.1016/j.brainres.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Weiser M, Wu T, Handa R. Estrogen receptor beta (ERbeta) agonist diarylpropionitrile (DPN): biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology . 2009 doi: 10.1210/en.2008-1355. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM. Juvenile-onset major depression includes childhood- and adolescent-onset depression and may be heterogeneous. Arch Gen Psychiatry. 2002;59:223–224. doi: 10.1001/archpsyc.59.3.223. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, Wittchen HU. Sex differences in rates of depression: cross-national perspectives. J Affect Disord. 1993;29:77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- Williams DR, Gonzalez HM, Neighbors H, Nesse R, Abelson JM, Sweetman J, Jackson JS. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305–315. doi: 10.1001/archpsyc.64.3.305. [DOI] [PubMed] [Google Scholar]
- Winokur G. All roads lead to depression: clinically homogeneous, etiologically heterogeneous. J Affect Disord. 1997;45:97–108. doi: 10.1016/s0165-0327(97)00063-3. [DOI] [PubMed] [Google Scholar]
- Zhang X, Beaulieu J, Sotnikova T, Gainetdinov R, Caron M. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gainetdinov R, Beaulieu J, Sotnikova T, Burch L, Williams R, Schwartz D, Krishnan K, Caron M. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zill P, Baghai TC, Zwanzger P, Schule C, Eser D, Rupprecht R, Moller HJ, Bondy B, Ackenheil M. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry. 2004;9:1030–1036. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]