Abstract
Intracerebroventricular administration of the peptide insulin-like growth factor-1 (IGF-1) has been shown to be an effective neuroprotective strategy in the brain of different animal models, a major advantage being the achievement of high concentrations of IGF-1 in the brain without altering serum levels of the peptide. In order to exploit this therapeutic approach further, we used high performance recombinant adenoviral (RAd) vectors expressing their transgene under the control of the potent mouse cytomegalovirus immediate early (mCMV) promoter, to transduce brain ependymal cells with high efficiency and to achieve effective release of transgenic IGF-1 into the cerebrospinal fluid (CSF). We constructed RAd vectors expressing either the chimeric protein (TK/GFP)fus (green fluorescent protein fused to HSV1 thymidine kinase) or the cDNA encoding rat IGF-1, both driven by the mCMV promoter. The vectors were injected into the lateral ventricles of young rats and chimeric GFP expression in brain sections was assessed by fluorescence microscopy. The ependymal cell marker vimentin was detected by immunofluorescence and nuclei were labeled with the DNA dye DAPI. Blood and CSF samples were drawn at different times post vector injection. In all cerebral ventricles, vimentin immunoreactive cells of the ependyma were predominantly transduced by RAd-(TK/GFP)fus, showing nuclear and cytoplasmic expression of the transgene. For tanycytes (TK/GFP)fus expression was evident in their cytoplasmic processes as they penetrated deep into the hypothalamic parenchyma. Intracerebroventricular injection of RAd-IGF-1 induced high levels of IGF-1 in the CSF but not in serum. We conclude that the ependymal route constitutes an effective approach for implementing experimental IGF-1 gene therapy in the brain.
Keywords: ependymal cells, gene delivery, TK/GFPfus, IGF-1, adenoviral vectors, mCMV promoter
Insulin-like growth factor-1 (IGF-1) is a powerful neurotrophic molecule which by itself or associated to other neuroprotective factors such as growth hormone (GH) or estrogens, appears to be part of the physiologic self-repair mechanisms of the adult brain (Sonntag et al., 2005; Carro and Torres-Aleman, 2006; Garcia-Segura et al., 2007). Although some IGF-1 is produced in the central nervous system (CNS), most of the peptide used by the brain comes from the circulation (Carro et al., 2000, 2006). Serum IGF-1 is actively transported through the choroid plexus and translocated to the cerebrospinal fluid (CSF) from where the molecule reaches specific areas of the brain by yet unidentified mechanisms (Guan et al., 1996, Carro et al., 2006). Consequently, the development of methodologies to increase IGF-1 levels in CFS is a logical approach to implement neuroprotective IGF-1-based therapeutic strategies in the adult brain. Furthermore, achieving therapeutic levels of IGF-1 in the brain without altering the concentration of the peptide in the blood, which appears to be associated with neoplastic disease, represents an additional advantage. The therapeutic efficacy of increasing CSF levels of IGF-1 has been documented in several studies (Sonntag et al., 2000; Lichtenwalder et al., 2001; Lynch et al., 2001). However, to our knowledge, the implementation of IGF-1 gene transfer to the ependymal cell layer has not been explored as an alternative to intracerebroventricular (icv) IGF-1 peptide administration via minipumps or chronically implanted cannulae. Here we report that high performance recombinant adenoviral vectors (RAd) expressing their transgene under the control of the mouse cytomegalovirus immediate early (mCMV) promoter, transduce brain ependymal cells with high efficiency and achieve effective release of transgenic IGF-1 into the CSF
EXPERIMENTAL PROCEDURES
Adenoviral vectors
RAd-IGF1
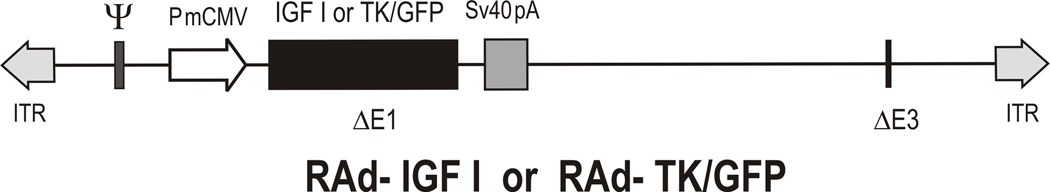
A RAd vector harboring the rat IGF-1 gene (kindly donated by Dr. Peter Rotwein, Oregon Health Sciences University) was constructed in our laboratory by a variant of the two plasmid method (Hitt et al., 1998) as previously described (Hereñu et al., 2007). The cDNA coding for the rat IGF-1 gene obtained from the mRNA for the IGF-1b precursor form (Daughaday and Rotwein, 1989), was placed under the control of the mCMV promoter in order to construct the genome of the desired recombinant adenoviral vector, RAd-IGF-1 (Fig. 1). The newly generated RAd was rescued from HEK293 cell lysates and plaque purified. It was further purified by ultracentrifugation in a CsCl gradient. Final virus stocks were titrated by a serial dilution plaque assay.
Figure 1. Diagrammatic representation of the construction of RAd-IGF-1 and RAd(TK/GFP)fus.
The vectors were constructed by the two-plasmid method as described in M&M. PmCMV, mouse cytomegalovirus promoter; IGF-1, cDNA for rIGF-1; TK/GFP, hybrid DNA sequence encoding the fusion protein (TK/GFP)fus; ITR, inverted terminal repeat; Δ E1 and Δ E3, deletions in the Ad5 genome; SV40, simian virus 40 polyadenylation signal; ψ, packaging signal; frt, recombination site for the FLP recombinase.
RAd-(TK/GFP)fus
This vector was constructed following the general procedures outlined above (Fig. 1). The vector harbors a hybrid gene encoding the herpes simplex virus type 1 (HSV-1) thymidine kinase fused to the Aequorea victoria enhanced green fluorescent protein (TK/GFP)fus (a kind gift from Dr. Jacques Galipeau, McGill University, Montreal, Canada). The corresponding gene product, fusion protein (TK/GFP)fus, emits green fluorescence with high intensity when excited with 470-nm wideband light (Paquin et al., 2001). This hybrid gene is also driven by the mCMV promoter. The vector was expanded in 293 cells and purified and titrated as indicated above.
Although here we used (TK/GFP)fus as a reporter gene, the fusion protein product possesses TK activity and in the presence of the nucleoside analog ganciclovir, acts as a suicide gene inducing the trasduced cells to undergo apoptosis. This feature of (TK/GFP)fus can be used as a safety component to eliminate transduced cells that undergo uncontrolled proliferation (Paquin et al., 2001).
Animals and surgical procedures
Animals
Young (3–4 mo.) female Sprague-Dawley rats were used. Animals were housed in a temperature-controlled room (22 ± 2°C) on a 12:12 h light/dark cycle. Food and water were available ad libitum. All experiments with animals were performed according to the Animal Welfare Guidelines of NIH (INIBIOLP’s Animal Welfare Assurance No A5647-01).
Stereotaxic injections
Rats were anesthetized with ketamine hydrochloride (40 mg/kg; ip) plus xylazine (8mg/kg; im) and placed in a stereotaxic apparatus. In order to access the lateral ventricles (LV), the tip of a 26G needle fitted to a 50µl syringe was brought to the following coordinates relative to the bregma: 0.8 mm posterior, 3.7 mm ventral and 1.5 mm right and left (Paxinos and Watson, 1998). Animals were injected bilaterally with 25 µl of a suspension containing 1011 plaque forming units (pfu)/ml of the appropriate vector. On experimental days 0 (vector injection), 2, 5, 10, 20 and 30 blood was sampled (0.3–0.4 ml) by puncture of the tail veins whereas CSF samples were taken as described below. Serum and CSF samples were stored at −70°C. The number of animals sampled for blood and CSF ranged from 2 to 6 and is indicated for each time point in Fig. 4.
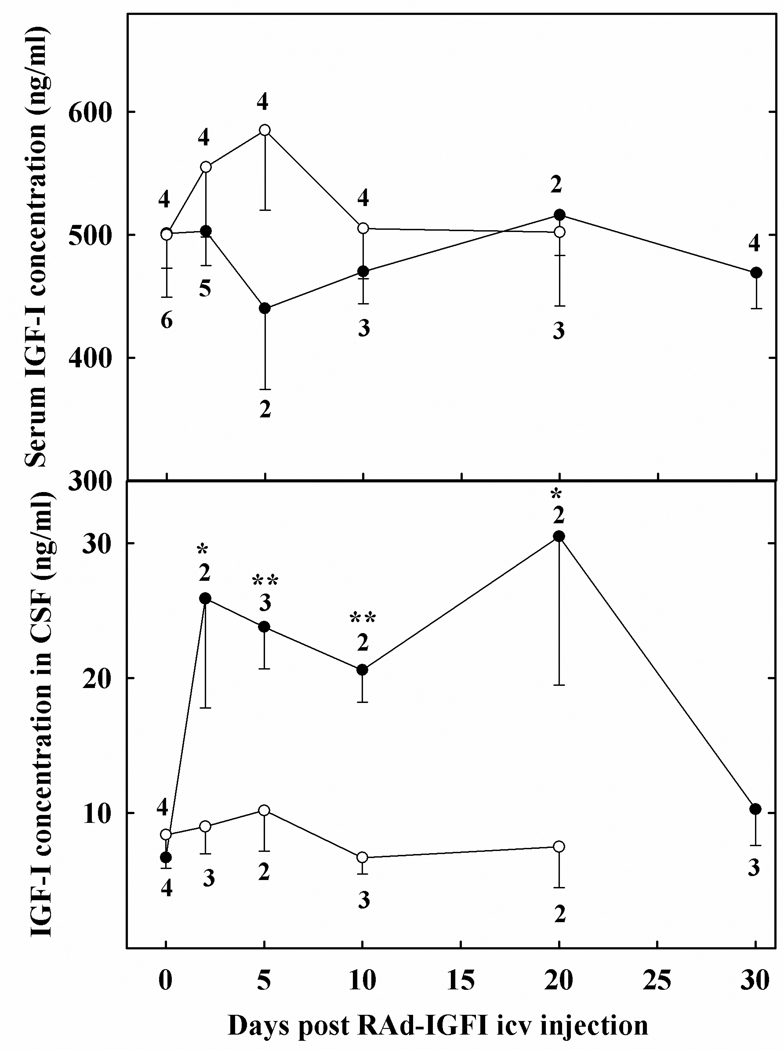
Figure 4. Time course for the effect of RAd-IGF-1 or RAd(TK/GFP)fus injection in the lateral ventricles on CSF and serum levels of IGF-1 in rats.
Blood and CSF was sampled from the same rats at the indicated post-injection times. Solid circles correspond to RAd-IGF-1-injected animals while open circles refer to RAd(TK/GFP)fus–injected rats. Number of samples assayed per time point are as indicated. Error bars indicate SEM values. Asterisks indicate significant difference from time zero counterparts; *, p<0.05; **, p<0.01.
CSF Collection
CSF was obtained from the great cerebral cistern by puncture on the same days indicated for serum sampling. Rats were anesthetized as described for stereotaxic injections and fixed to a 90° head angle in a stereotaxic device. A microsyringe carrying a 26G needle was fixed to the manipulator arm with both, the manipulator and the needle oriented vertically. The hair was removed from the dorsal surface of the skin, the zone between the parietal bone and vertebral column was located by palpation and the middle point marked with a marker. The needle was introduced to a depth between 1.5– 2.0 mm and the piston slowly pulled. This procedure allowed to obtain 50–80 µl CSF. Samples contaminated with blood were discarded in order to avoid artifactually high IGF-1 levels in the CSF. This reduced the original n value per time point and led to uneven sample numbers for the different CSF sampling points. Blood sampling from the tail veins was performed immediately after CSF sampling, with the rats still under ketamine anesthesia. While this procedure avoided pain to the animals it induced some peripheral vasoconstriction making blood sampling by venopuncture of tail veins more difficult, which reduced the final n value per point and led to uneven serum sample numbers for the different time points.
Brain processing and immunofluorescence
On experimental day 30, animals were placed under deep anesthesia and perfused with phosphate buffered formaldehyde 4%, (pH 7.4) fixative. Brains were rapidly removed and serially cut into coronal sections 20 or 40 µm thick on a vibratome. For vimentin immunofluorescence (IF), sections were incubated with a monoclonal mouse anti-vimentin antibody (DakoCytomation), washed twice with PBS, incubated for 45 min with a 1/1,000 Alexa555-conjugated goat anti-mouse IgG (Jackson ImmunoResearch) and counterstained for 15 min with the fluorescent DNA stain 4',6-diamidino-2-phenylindole (DAPI). Images were captured as 16-bit monochrome (Olympus DP71 camera, Japan) and then pseudocolored (ImagePro Plus 6.3 (IPP), Media Cybernetics, USA). Confocal microscopy was performed on some of the brain sections using a Nikon microscope model EZ-C1 2.20 (Nikon, Japan). Serial optical sections were taken every 0.3 µm along the Z-axis and subsequently integrated by means of the IPP 6.3 software in order to obtain 3-D and orthogonal projections.
Colocalization analysis
In RAd-(TK/GFP)fus-injected rats, colocalization analysis of vimentin, GFP and DAPI labeling was performed in images of the lateral ventricles. Briefly, monochrome images were pseudocolored using the dye list function of the image analysis program. Using the Color Composite function of the Process menu, images were coupled in pairs of dyes. For quantifying the colocalization indexes of the 3 used labels, the Colocalization function of the IPP software was used and the Mander overlap coefficient (R) calculated which is 0.0 for no colocalization and 1.0 for 100% colocalization of two intensity patterns (Agnati et al., 2005).
IGF-1 assay
IGF-1 was extracted from serum and CSF samples (20 µl) by acid-ethanol cryoprecipitation and was radioimmunoassayed as previously described (Hereñu et al., 2007) using antibody UB2-495 distributed by AF. Parlow, NHPP, NIDDK. Recombinant human IGF-1 (Cell Sciences Inc., Canton, MA) was used as tracer and unlabeled ligand.
Statistical Analysis
One way analysis of variance (ANOVA) was used to evaluate group variance. Tukey’s method was chosen as a post hoc test.
RESULTS
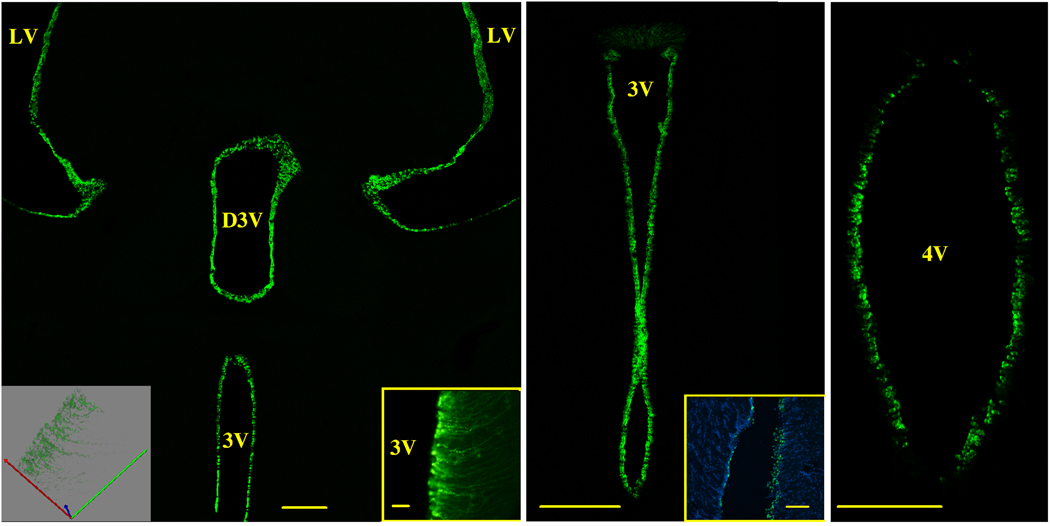
Low magnification brain sections from animals injected with RAd(TK/GFP)fus in the LV revealed a predominant expression of GFP in the ependymal cell layer of the cerebral ventricles (Fig. 2). Flourescence intensity was distributed evenly in the ependymal cell layer of the LV, third and fourth ventricles (3V and 4V, respectively) and remained at maximal levels in the transduced cells for up to five days post vector injection. As expected, GFP expression became progressively weaker with time. On day 30, GFP expression was no longer detectable by fluorescence microscopy. In the ventral third ventricle, transduced tanycytes showed strong green fluorescence with their long cytoplasmic processes expressing GFP as they penetrated deep in the hypothalamic parenchyma (Fig. 2, insets in left panel).
Figure 2. GFP expression in the ependymal cell layer of brain ventricles.
Animals received bilateral stereotaxic injections of RAd(TK/GFP)fus in the lateral ventricles, were perfused with fixative 2 days later and brain sections observed for green fluorescence. The left panel shows a low magnification confocal view of GFP expression in the ependymal cell layer of the lateral ventricles (LV), and third ventricle, dorsal (D3V) and main (3V) portions. The center panel is a confocal image showing GFP expression in the ependymal cell layer of the 3V at a higher magnification. The right panel is a confocal image showing strong GFP expression in the ependymal cell layer of the fourth ventricle (4V). Note that no GFP expression is visually detectable in the brain parenchyma.
Right inset in left panel.- Transduced tanycytes in the ventral portion of the 3V. Notice GFP expression in the ependymal cell processes penetrating the hypothalamic parenchyma. Standard fluorescence. Scale bar, 20 µm.
Left inset in left panel.- Confocal 3-D projection of transduced tanycytes in the ventral portion of the 3V. An alternative view of GFP expression in the ependymal cell processes.
Inset in center panel.- Nuclear staining and GFP expression in the ependymal cell layer of the LV. Nuclei were stained with DAPI (blue fluorescence) while transduced ependymal cells express green fluorescence. Standard fluorescence. Scale bars 100 µm.
Scale bars in left and center main panels, 200 µm; scale bar in right main panel, 150 µm;
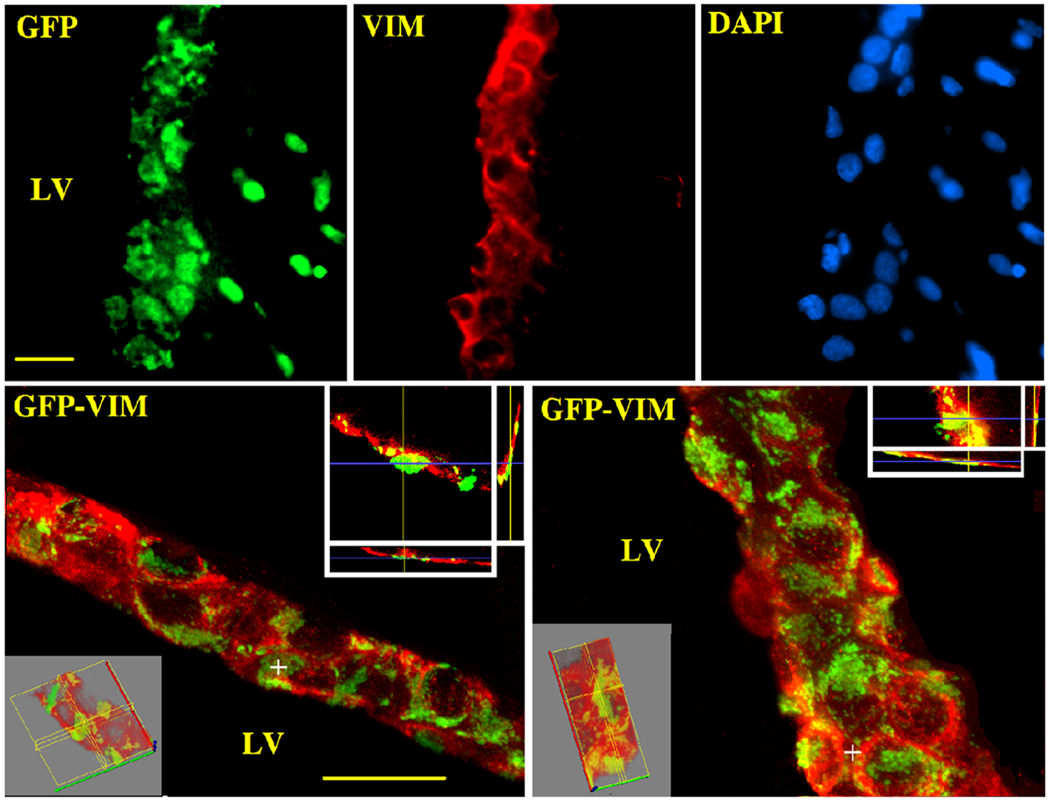
In RAd(TK/GFP)fus-injected animals, analysis of brain sections treated with the nuclear marker dye DAPI and immunolabeled for vimentin (an intermediate filament protein expressed by ependymocytes), revealed a high efficiency of the vector for transducing ependymal cells with only few vimentin-negative cells expressing GFP (Fig. 3, upper panels). Additionally, confocal microscopy confirmed the presence of GFP in vimentin-immunoreactive ependymal cells (Fig. 3, lower panels). Quantitative colocalization analysis (the R coefficient calculated in 6 sections of the LV; ×̄ ± SEM ) indicated a 70 ± 7 % colocalization for GFP fluorescence with red fluorescence (vimentin), while there was a 75 ± 3% colocalization of DAPI (blue fluorescence) and GFP fluorescence. Additionally, confocal microscopy confirmed the presence of GFP in vimentin-immunoreactive ependymal cells (Fig. 3, lower panels).
Figure 3. Preferential expression of adenovirally-delivered GFP in brain ependymal cells.
Sections from the lateral ventricle (20 µm thick) were submitted to IF and confocal microscopy with a primary mAb against vimentin and a secondary goat anti-mouse IgG serum conjugated to Alexa 555 (red); DAPI was also used in order to induce blue fluorescence in cell nuclei. The upper panels show single color standard fluorescence while the lower panels show confocal 3-D and orthogonal projections of two sections passing through the LV of RAd(TK/GFP)fus–injected rats. Red fluorescence corresponds to vimentin immunolabeling. Scale bars, 20 µm
Intracerebroventricular injection of RAd-IGF-1, but not RAd(TK/GFP)fus, induced a 3–4 fold increase in IGF-1 levels in the CSF. The CSF levels of the transgenic peptide remained high for approximately 20 days, falling to near basal levels on day 30 post vector injection (Fig 4, lower panel). Serum IGF-1 levels were not significantly affected by icv administration of either vector (Fig. 4, upper panel).
DISCUSSION
The present results constitute the first demonstration that the preference of adenoviral vectors for ependymocytes can be exploited to implement minimally invasive IGF-1 gene therapy in the brain. Previous studies indicate that there appears to be substantial therapeutic benefit to increasing CSF levels of IGF-1 in different animal models of brain aging or injury. For example, icv administration of IGF-1 to 29 months old rats using osmotic minipumps ameliorated the age-related decline in hippocampal neurogenesis (Lichtenwalder et al., 2001), increased the rates of local cerebral glucose utilization (Lynch et al., 2001), and hippocampal NMDAR2A and R2B subunit expression (Sonntag et al., 2000). Furthermore, 28-day icv infusion of IGF-1, using osmotic minipumps, attenuated age-related deficits in working and reference memory assessed in the Morris water maze and object recognition tasks (Markowska et al., 1998). A single dose of IGF-1 (50 µg) delivered icv to rats, via a prefixed cannula, 2 h after hypoxic–ischemic injury reduced both somatosensory deficits and neuronal loss 20 days post-injury (Guan et al., 2001). Although the therapeutic efficacy of increasing CSF levels of IGF-1 by means of minipumps or cannulas has been demonstrated, these techniques are likely to result in substantial and continuous damage to brain structures. Our results demonstrate that increased IGF-1 levels in CSF can be achieved through a single injection of adenoviral vectors that preferentially transduce ependymal cells. Comparison between the levels of IGF-1 in CSF achieved by our approach and those using icv infusion by minipumps or cannulas cannot be made due to the fact that in the IGF-1 infusion studies the dose of IGF-1 administered was indicated but the concentration of peptide achieved in the CSF was not reported (Sonntag et al., 2000; Lichtenwalder et al., 2001; Lynch et al., 2001; Guan et al., 2001). Nevertheless, it should be mentioned that the average concentration of IGF-1 in CSF achieved in our rats (about 25 ng/ml) was almost twice the tissue concentration achieved in the mediobasal hypothalamus (13 ng/ml) of rats receiving an intrahypothalamic injection of RAd-IGF-1 (Hereñu et al., 2007). Since this concentration of IGF-1 in the hypothalamus was effective to restore dopaminergic (DA) neuron function and reduce the chronic hyperprolactinemia of senile female rats, it could be assumed that the CSF levels of IGF-1 achieved in the present study should be therapeutically effective. In the above study, 17 days of IGF-1 overexpression in the hypothalamus of senile rats was sufficient to obtain a restorative effect on DA neuron function (Hereñu et al., 2007). In the perfusion studies mentioned above the length of IGF-1 administration ranged from 5 to 28 days and in all cases a therapeutic effect was demonstrated. Therefore, the 20-day period of overexpression of RAd-IGF-1 in the ependymal cells observed in the present study should allow the assessment of the effectiveness of the ependymal route for IGF-1 gene therapy on different rat models of CNS dysfunction.
Previous studies in rats demonstrated that icv administration of Ad.RSVβgal, a RAd expressing E. coli β-galactosidase (β-gal) under the control of the Rous Sarcoma Virus (RSV) promoter, led to widespread expression of the transgene in the ependymal cells of the lateral, third and fourth ventricles as well as in the leptomeninges (Bajocchi et al., 1993). Similarly, icv administration of AxCAHBG, a RAd expressing human β-glucuronidase under the control of the cytomegalovirus-enhancer-chicken β-actin hybrid (CAG) promoter, to β-glucuronidase deficient mice, induced transgenic β-glucuronidase activity in ependymal cells and choroid of these mutants although some transgene expression was also observed in the brain parenchyma (Ohashi et al., 1997). In nonhuman primates, injection into the ventricles, suboccipitally (within the cisterna magna) or by lumbar puncture of a RAd expressing β-gal under the control of the human CMV promoter, resulted in the transduction of ependymal cells surrounding the CSF space (Driesse et al., 1999).
The ependymal route has been successfully used to implement cytokine-gene therapy in the CNS. In this case, injection into the CSF of a RAd expressing human IL-10 ameliorated disease symptoms in mice with active experimental autoimmune encephalomyelitis (EAE) Cua et al., 2001). Furthermore, it is well-established that the delivery of genes encoding IL-10, IL-4, TGF-β, IFN-β, p55TNFR-Ig and p75TNFR-Ig into the CNS, is superior to intravenous administration of the same anti-inflammatory cytokines in the treatment of murine EAE (Triantaphyllopoulos et al., 1998; Croxford et al., 1998, 2000;). Although the specific mechanisms that favor adenoviral expression in ependymal cells are unknown, this route of gene delivery has numerous advantages including the ability to increase the levels of a transgenic therapeutic protein throughout the CNS.
Since the promoter is a key factor in determining both the intensity and specificity of transgene expression, we used the mCMV promoter which in rodents is known to be stronger than the promoters used in the studies cited previously (Addison et al., 1997; Sallenave et al., 1998). Our results show that the mCMV promoter efficiently drove the expression of (TK/GFP)fus and IGF-1 in rat ependymal cells.
As mentioned above, it has been shown that short-term intrahypothalamic IGF-1 gene therapy in senile female rats effectively restored their reduced dopaminergic function and reversed their chronic hyperprolactinemia (Hereñu et al., 2007). In that study, IGF-1 gene delivery to a specific area of the hypothalamus was adequate to achieve the therapeutic results sought. However, in IGF-1-based therapeutic approaches aimed at restoring cognitive or other complex brain functions in aged or lesioned animals, simultaneous delivery of IGF-1 to multiple brain regions would be required. The present results suggest that in such situations, IGF-1 gene therapy via the ependymal route would allow the transgenic peptide to be effectively distributed from the CSF to its physiologic targets in the brain.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to Joaquin Piris, Cajal Institute, for advice on CSF sampling. This study was supported in part by grants # PICT13588 to RGG and PICT25265 to ELP from the Argentine Agency for the Promotion of Science and Technology (ANPCYT), grant R01AG029798-2 from the Fogarty International Center and the National Institute on Aging (NIA) to RGG and NIA Grant AG11370 to WES.
Abbreviations
- DA
dopaminergic
- mo
months
- RAd
recombinant adenoiviral vector
- HSV-1
herpes simplex virus type 1
- TK
HSV-1 thymidine kinase
- IF
immunofluorescence
- GFP
green fluorescent protein
- LV
lateral ventricle
- 3V
3rd ventricle
- 4V
4th ventricle
- CSF
cerebrospinal fluid
- HEK293
human embryo kidney 293 cells
- IGF-1
Insulin-like growth factor -1
- mCMV
mouse cytomegalovirus immediate early promoter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Addison CL, Hitt M, Kunskel D, Graham FL. Comparison of the human versus murine cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vector. J Gen Virol. 1997;78:1653–1661. doi: 10.1099/0022-1317-78-7-1653. [DOI] [PubMed] [Google Scholar]
- Agnati LF, Fuxe K, Torvinen M, Genedani S, Franco R, Watson S, Nussdorfer GG, Leo G, Guidolin D. New methods to evaluate colocalization of fluorophores in immunocytochemical preparations as exemplified by a study on A2 and D2 receptors in Chinese Hamster Ovary cells. J Histochem Cytochem. 2005;53:941–953. doi: 10.1369/jhc.4A6355.2005. [DOI] [PubMed] [Google Scholar]
- Bajocchi G, Feldman SH, Crystal RG, Mastrangeli A. Direct in vivo gene transfer to ependymal cells in the central nervous system using recombinant adenovirus vectors. Nat Genet. 1993;3:229–234. doi: 10.1038/ng0393-229. [DOI] [PubMed] [Google Scholar]
- Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Torres-Aleman I. Serum insulin-like growth factor I in brain function. Keio J Med. 2006;55:59–63. doi: 10.2302/kjm.55.59. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Spuch C, Bohl D, Heard JM, Torres-Aleman I. Blockade of the insulin-like growth factor I receptor in the choroid plexus originates Alzheimer’s-like neuropathology in rodents: New cues into the human disease? Neurobiol.. Aging. 2006;27:1618–1631. doi: 10.1016/j.neurobiolaging.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Triantaphyllopoulos KA, Neve RM, Feldmann M, Chernajovsky Y, Baker D. Gene therapy for chronic relapsing experimental allergic encephalomyelitis using cells expressing a novel soluble p75 dimeric TNF receptor. J Immunol. 2000;164:2776–2781. doi: 10.4049/jimmunol.164.5.2776. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Triantaphyllopoulos K, Podhajcer OL, Feldmann M, Baker D, Chernajovsky Y. Cytokine gene therapy in experimental allergic encephalomyelitis by injection of plasmid DNA–cationic liposome complex into the central nervous system. J Immunol. 1998;160:5181–5187. [PubMed] [Google Scholar]
- Cua DJ, Hutchins B, LaFace DM, Stohlman SA, Coffman RL. Central nervous system expression of IL-10 inhibits autoimmune encephalomyelitis. J Immunol. 2001;166:602–608. doi: 10.4049/jimmunol.166.1.602. [DOI] [PubMed] [Google Scholar]
- Daughaday WH, Rotwein P. Insulin-like growth factors I and II: peptide messenger ribonucleic acid and gene structures, serum and tissue concentrations. Endocr Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- Driesse MJ, Kros JM, Avezaat CJ, Valerio D, Vecht CJ, Bout A, Smitt PA. Distribution of recombinant adenovirus in the cerebrospinal fluid of nonhuman primates. Hum Gene Ther. 1999;10:2347–2354. doi: 10.1089/10430349950016997. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Diz-Chaves Y, Perez-Martin M, Darnaudéry M. Estradiol, insulin-like growth factor-I and brain aging. Psychoneuroendocrinology. 2007;32 Suppl 1:S57–S61. doi: 10.1016/j.psyneuen.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Guan J, Miller OT, Waugh KM, McCarthy DC, Gluckman PD. Insulin-like growth factor-1 improves somatosensory function and reduces the extent of cortical infarction and ongoing neuronal loss after hypoxia–ischemia in rats. Neuroscience. 2001;105:299–306. doi: 10.1016/s0306-4522(01)00145-2. [DOI] [PubMed] [Google Scholar]
- Guan J, Skinner SJ, Beilharz EJ, Hua KM, Hodgkinson S, Gluckman PD, Williams CD. The movement of IGF-1 into the brain parenchyma after hypoxic–ischaemic injury. Neuroreport. 1996;7:632–636. doi: 10.1097/00001756-199601310-00061. [DOI] [PubMed] [Google Scholar]
- Hereñu CB, Cristina C, Rimoldi OJ, Becu-Villalobos D, Cambiaggi V, Portiansky EL, Goya RG. Restorative effect of Insulin-like Growth Factor-I gene therapy in the hypothalamus of senile rats with dopaminergic dysfunction. Gene Ther. 2007;14:237–245. doi: 10.1038/sj.gt.3302870. [DOI] [PubMed] [Google Scholar]
- Hitt M, Bett A, Prevec L, Graham FL. Cell Biology: A Laboratory Handbook. San Diego: Academic Press; 1998. Construction and propagation of human adenovirus vectors; pp. 1500–1512. [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lynch CD, Lyons D, Khan A, Bennett SA, Sonntag WE. Insulin-like growth factor-1 selectively increases glucose utilization in brains of aged animals. Endocrinology. 2001;142:506–509. doi: 10.1210/endo.142.1.8053. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Ohashi T, Watabe K, Uehara K, Slyq WS, Vogler C, Eto Y. Adenovirus-mediated gene transfer and expression of human β-glucuronidase gene in the liver, spleen and central nervous system in mucopolysaccharidosis type VII mice. Proc natl Acad Sci USA. 1997;94:1287–1292. doi: 10.1073/pnas.94.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin A, Jaalouk De, Galipeau J. Retrovector encoding a Green Fluorescent Protein–Herpes Simplex Virus Thymidine Kinase fusion protein Serves as a Versatile Suicide/Reporter for Cell and Gene Therapy Applications. Hum Gene Ther. 2001;12:13–23. doi: 10.1089/104303401450924. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Sallenave JM, Xing Z, Simpson AJ, Graham FL, Gauldie J. Adenovirus-mediated expresison of the elastase-specific inhibitor (elafin): a comparison of different promoters. Gene Ther. 1998;5:352–360. doi: 10.1038/sj.gt.3300610. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Bennett SA, Khan AS, Thornton PL, Xu X, Ingram RL, Brunso-Bechtold JK. Age and insulin-like growth factor-1 modulate N-methyl-D-aspartate receptor subtype expression in rats. Brain Res Bull. 2000;51:331–338. doi: 10.1016/s0361-9230(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Ramsey M, Carter C. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Age Res Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Triantaphyllopoulos K, Croxford J, Baker D, Chernajovsky Y. Cloning and expression of murine IFN-β and a TNF antagonist for gene therapy of experimental allergic encephalomyelitis. Gene Ther. 1998;5:253–263. doi: 10.1038/sj.gt.3300570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.