SUMMARY
We previously described a mouse model of ulcerative colitis linked to T-bet deficiency in the innate immune system. Here, we report that the majority of T-bet−/− RAG2−/− ulcerative colitis (TRUC) mice spontaneously progress to colonic dysplasia and rectal adenocarcinoma solely as a consequence of MyD88-independent intestinal inflammation. Dendritic cells (DC) are necessary cellular effectors for a pro-inflammatory program that is carcinogenic. While these malignancies arise in the setting of a complex inflammatory environment, restoration of T-bet selectively in DCs was sufficient to reduce colonic inflammation and prevent the development of neoplasia. TRUC colitis-associated CRC resembles the human disease and provides ample opportunity to probe how inflammation drives colorectal cancer development and to test preventative and therapeutic strategies pre-clinically.
SIGNIFICANCE
Inflammatory bowel disease (IBD) is one of the three highest risk factors for colorectal cancer (CRC). The molecular pathogenesis of IBD-associated colorectal cancer (caCRC) differs from that of sporadic CRC. The findings reported here demonstrate that the TRUC model of caCRC is a robust system for studying the human disease. This model establishes the importance of the innate immune system and specifically dendritic cells as key cellular effectors in inflammation that drives neoplasia. Our data also demonstrate that there are MyD88-independent pathways to caCRC. Finally, the observation that over-expression of T-bet in innate immune cells prevents caCRC provides an additional explanation for why colorectal cancer patients with intratumoral T-bet expression may have improved survival and generates interest in immune-based cancer therapeutics.
INTRODUCTION
The three highest risk groups for developing colorectal cancer (CRC) are individuals with ulcerative colitis (UC), familial adenomatous polyposis, and hereditary non-polyposis colon cancer syndrome. Among UC patients, the relative risk of developing CRC correlates with the extent and duration of disease— 18% will have developed CRC after thirty years of disease (Eaden et al., 2001; Xie and Itzkowitz, 2008). The sequence of genetic events in colitis-associated CRC (caCRC) differs from that observed in sporadic colorectal cancer. In caCRC, chromosomal instability and DNA damage can precede and predict the development of dysplasia and alterations in p53 expression occur early in the oncogenic pathway, not as is depicted in the classic adenoma-carcinoma sequence (Cho and Vogelstein, 1992; Clausen et al., 2001; Itzkowitz, 2003; Yoshida et al., 2003). In addition, in contrast to sporadic CRC, alterations in β-catenin localization reflecting APC mutations occur very late in the caCRC transformation process. Sporadic CRC carcinogenesis is typified by the transformation of the adenoma to an adenocarcinoma; however, in caCRC invasive carcinomas frequently arise in flat areas of dysplasia. This feature of caCRC makes clinical surveillance of this at risk population particularly challenging and speaks to the distinct biology of these neoplasias.
Mouse models of intestinal cancer have been instrumental in understanding oncogenesis and have shed light on the role of innate immunity and the commensal microbiota in colon cancer. We recently described a model of commensal-dependent ulcerative colitis termed T-bet−/− RAG2−/− Ulcerative Colitis (TRUC) that results from T-bet deficiency in the innate immune system (Garrett et al., 2007). T-bet is a T-box family transcription factor that controls chemokine, chemokine receptor and cytokine expression; regulates host-commensal homeostasis in the colon; and is expressed only in immune cells (Glimcher, 2007; Ma, 2007). TRUC mice develop a severe and highly penetrant colitis, driven in part by loss of TNF-α regulation in the colon, that can be reversed by antibiotics, TNF-α blockers or transfer of T regulatory cells. Colorectal cancer has been documented in other IBD mouse models including IL-10−/−, TCRα−/−, Gαi2−/−, IL-2−/− ×β2m−/−, and αv−/− mice (Berg et al., 1996; Dianda et al., 1997; Lacy-Hulbert et al., 2007; Rudolph et al., 1995; Shah et al., 1998). This observation coupled with the finding that increased levels of T-bet in human colorectal tumors correlate with increased patient survival spurred us to determine if TRUC mice, whose colitis is mechanistically distinct from other IBD models, would develop caCRC (Pages et al., 2005).
RESULTS
TRUC mice develop dysplasia and carcinoma resembling human IBD-associated colorectal cancer
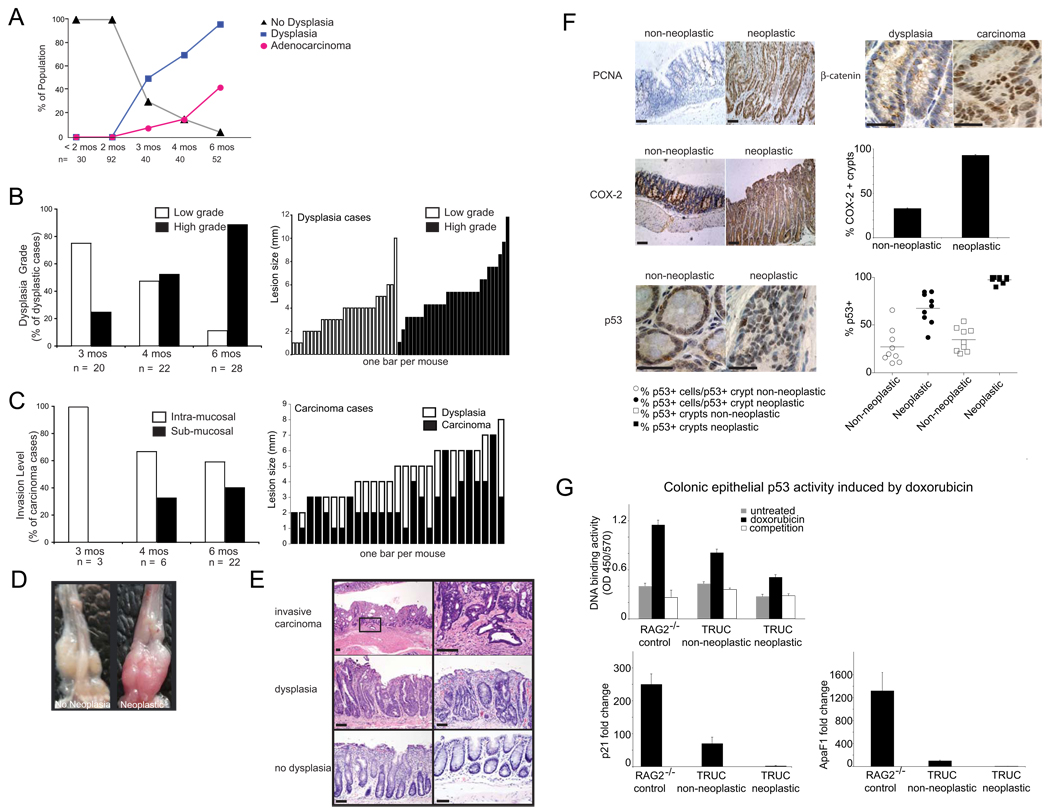
In our colony, TRUC manifest a juvenile colitis that starts rectally, has 100% penetrance, and can result in bacteremia and death as early as 10 weeks of age. We monitored large cohorts (>400 TRUC mice during a three year period) to robustly survey whether dysplasia and carcinoma would develop over time. At 3 months of age, 50% of TRUC mice had dysplastic lesions, and adenocarcinoma was present in 3/40 cases. By 6 months of age, over 96% of mice had dysplastic lesions and 42% had adenocarcinoma (Figure 1A). Low grade dysplasia (LGD) predominated in younger mice (75% of cases), while 6 month olds manifested high grade dysplasia (HGD) (89% of cases) (Figure 1B). In 12/30 of the HGD cases there were adjacent regions of LGD (1–3 mm) (data not shown), suggesting progression to the higher grade. Similarly, adenocarcinomas (ACA) (size range 2–7 mm) were flat and usually arose in the rectum within regions of dysplastic mucosa (25/31 cases), similar to what is observed in UC patients. The number of mice with more advanced sub-mucosal invasive cancers increased over time (Figure 1C). Hence, malignant transformation of intestinal epithelial cells occurs in virtually all TRUC mice by 6 months of age and progresses to frank adenocarcinoma in a significant proportion of animals.
Figure 1. TRUC mice develop colitis-associated colorectal cancer.
A. % of TRUC mice with and without dysplasia and cancer is shown as a function of mouse age (mos). The number of mice surveyed is displayed along the x-axis. No cancers were observed in age-matched RAG2−/− (data not shown). B. Dysplastic grade is shown as a percentage of dysplastic cases for mice at 3, 4, and 6 months. Size (mm) of low and high grade dysplastic lesions is displayed for each animal. C. Level of invasion (intra-mucosal vs sub-mucosal) is shown as a % of carcinoma cases. Lesion size (mm) of the carcinomas (shaded bars) and continuous areas of dysplasia (open bars) are plotted for individual cases. D. Photographs of TRUC colon with colitis but no dysplasia (left) and TRUC colon with colorectal cancer (right). E. Histology from TRUC mice with invasive carcinoma (upper panel) dysplasia (middle panel, (left: high grade dysplasia, right: low grade dysplasia), no dysplasia (lower panel, (left: colitis, no dysplasia, right: no colitis, no dysplasia), scale bars 100 microns. F. Immunohistochemistry. PCNA: (left upper panel), scale bars 100 microns. β-catenin (right upper panel) staining in dysplastic epithelial crypts (left) and nuclear staining in invasive carcinoma (right), scale bars 25 microns. COX-2: (left middle panel) and quantitation of COX-2+ crypts (right middle panel), scale bards 100 microns. P53 (left lower panel, nuclear staining in crypts) and quantitation of P53 staining (right lower panel), each dot represents data from one mouse, scale bars 25 microns. G. Colonic epithelial p53 activity induced by doxorubicin. (Left upper panel) Consensus oligonucleotide binding activity of p53 in nuclear extracts of colonic epithelial cells from RAG2−/− vs TRUC non-neoplastic vs TRUC neoplastic colons. Gray bars are from untreated samples, black bars are from doxorubicin treated CECs incubated with biotinylated oligonucleotides, and open bars are from doxorubicin treated CECs incubated with biotinylated and 20-fold excess unbiotinylated oligonucleotides. Data represent the mean of two independent experiments with primary epithelial cells pooled from 20 mice per group (RAG2−/−, TRUC non-neoplastic, and TRUC neoplastic), error bars denote +/− SD. (Left lower panel) p21 induction in response to doxorubicin. Real time qPCR was performed on control and doxorubicin treated samples. Fold change between doxorubicin and untreated samples was calculated by dividing their respective 2−ΔCt values. (Right lower panel) APAF1 induction in response to doxorubicin was determined on the same sample set as above. Bars represent the mean of two independent experiments and error bars the +/− SD.
On gross examination, areas of colitis with dysplasia and ACA appeared more vascular and engorged as compared with colons with similar inflammatory scores but no dysplasia or cancer (Figure 1D). We noted the occurrence of fixed anorectal prolapse as it can complicate the diagnosis of cancer and dysplasia (10/21 intramucosal and 2/10 submucosal ACA cases). Photomicrographs are shown from representative cases with carcinoma (Figure 1E (upper panel), dysplasia (middle panel: left, HGD: right, LGD,), and no dysplasia (bottom panel: left, colitis: right, no colitis).
Immunohistochemistry (IHC) for PCNA revealed a high degree of proliferation in mice with ACA and dysplasia consistent with neoplastic transformation (Figure 1F, upper left panel). To determine whether TRUC caCRC phenotypically resembled human caCRC, we assessed the expression of additional markers by IHC. Similar to human caCRC, and in contrast to what is observed in sporadic CRC, β-catenin localization was membranous in non-dysplastic and dysplastic lesions (Figure 1F upper right panel) and nuclear (indicative of APC loss of function) in carcinoma (Figure 1F upper right panel). As is observed in human caCRC, we saw high levels of epithelial cell and immune cell COX-2 expression in inflamed mucosa that was non-neoplastic and neoplastic (Figure 1F, middle panel) (Agoff et al., 2000). Approximately 30% of non-neoplastic crypts were positive for COX-2 and >90% of neoplastic crypts were positive (Figure 1F, middle panel); thus COX-2 epithelial expression is a pre-malignant feature of TRUC transformation. Intense nuclear staining for p53 (CM5 clone: detects both mutant and wild type forms) was detected in the neoplastic epithelium and in non-dysplastic crypts; an observation that is highly suggestive of p53 mutations (Rodrigues et al 1990) (Figure 1F, bottom panel). To assess if this increased p53 expression was a consequence of mutations resulting in loss of p53 function, we performed two complementary experiments examining both the DNA binding capability of p53 and induction of p53 target genes. Specifically, we tested p53 DNA binding activity and expression of the p53 target genes, p21 and APAF1, in colonic epithelial cells (CECs) from control, non-neoplastic TRUC, and neoplastic TRUC in response to treatment with doxorubicin, an anthracycline chemotherapeutic that inhibits topoisomerase II, induces double-stranded DNA breaks, and strongly activates p53 (Ravizza et al 2004). Doxorubicin treatment induced p53 binding to a consensus site oligonucleotide in RAG2−/− (control) CECs in a specific fashion but was reduced in TRUC non-neoplastic CECs and markedly diminished in neoplastic TRUC CECs (Figure 1G, upper panel). p21 expression was induced approximately 250 fold in RAG2−/− CECs treated with doxorubicin, however, this induction was more modest in TRUC non-neoplastic CECs (70.2 fold) and substantially reduced in neoplastic TRUC CECs (2.23 fold) (Figure 1G, lower left panel). APAF1 levels in response to doxorubicin were up-regulated most in RAG2−/− (1321 fold), approximately an order of magnitude less in TRUC non-neoplastic (100.3 fold) and substantially reduced in TRUC neoplastic (1.42 fold) samples (Fig 1G, lower right panel). Thus the TRUC neoplastic process does resemble human caCRC in several aspects of its molecular pathogenesis, specifically in both early loss of function of p53 and increased epithelial COX-2 expression and later APC mutations.
TRUC mice develop colonic epithelial aneuploidy prior to dysplasia and the TRUC mucosa is rich in ROS, colonic epithelial DNA adducts, and cytokines similar to IBD patients who develop dysplasia and cancer
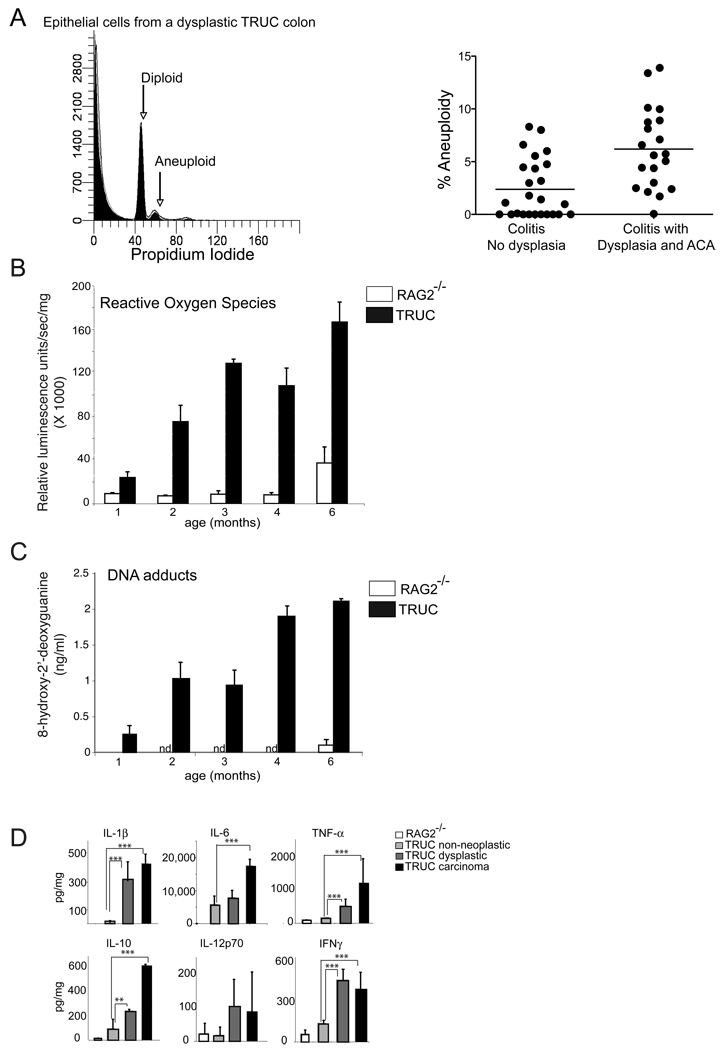
Chromosomal instability (CIN) and its resulting aneuploid DNA content are features of human caCRC. In fact, CIN has been detected in UC patient colonic biopsies that are non-dysplastic, dysplastic, and malignant; and aneuploidy predicts dysplasia (Clausen et al., 2001; Meling et al., 1991a; Meling et al., 1991b; Rubin et al., 1992). To search for CIN and aneuploidy in TRUC mice with and without dysplasia and cancer, we measured DNA content in colonic epithelial cells (CEC) from TRUC colons by flow cytometry. While no aneuploid cell populations were detected in 6 month old RAG2−/− mice (data not shown), aneuploidy was a feature of non-dysplastic and dysplastic TRUC colons and was most pronounced in those with dysplasia and cancer [mean 2.34% (non-neoplastic) vs 6.11% (neoplastic), p = .0005] (Figure 2A).
Figure 2. Chromosomal instability and reactive oxygen induced DNA adducts in TRUC carcinogenesis.
A. Flow cytometric aneuploidy analysis of epithelial cells isolated from mice w/o dysplasia, w/dysplasia, and w/carcinoma. (Left panel) Representative plot from one mouse with diploid and aneuploid populations labeled. (Right panel) % of aneuploid epithelial cells for all mice analyzed. Each dot represents data from one mouse. Horizontal bars represent the mean. B. Reactive oxygen species were measured in TRUC vs RAG2−/− distal colons. Reactive luminescence measured in units/sec/mg colonic tissue X 1000 (y-axis) is plotted as a function of mouse age. Open bars (RAG2−/−), shaded bars (TRUC), 5–8 mice per group, means are graphed, error bars represent +/− SD, p<.001 for all comparison between TRUC 1 mos and TRUC 2–6 mos. C. 8’hydroxy-2’deoxyguanine (ng/ml) levels in colonic epithelial cells. RAG2−/− control mice (age 1 and 6 mos, open bars, n.d. = not performed for RAG2−/− age 2–4 mos) and TRUC mice (age 1–6 months, shaded bars). 6–9 mice were used per time point. Means are graphed; error bars represent +/− SD, p<.001 for all comparison between TRUC 1 mos and TRUC 2–6 mos. D. ELISA based protein determinations for selected cytokines are shown from explant cultures pooled from 5–7 specimens per time point from RAG2−/− vs TRUC colitic (non-neoplastic) vs TRUC dysplastic vs TRUC carcinoma. Bars represent the mean values for three independent sets of pooled samples and error bars represent the +/− SD.
Although the exact mechanisms by which inflammation drives CIN remain controversial, there is more consensus regarding how other types of DNA damage, e.g. DNA adducts, occur in the setting of inflammation. Reactive oxygen species (ROS) and other oxidative stressors drive DNA damage by oxidizing DNA bases and generating DNA adducts at a rate that outpaces DNA repair mechanisms. ROS and DNA adducts have been well characterized in IBD patients and in animal models of intestinal inflammation (D'Inca et al., 2004). We found that the TRUC inflammatory milieu was rich in ROS and that increased ROS levels correlated with TRUC carcinogenesis (Figure 2B). To determine if DNA adducts were an untoward consequence of ROS we measured levels of 8-hydroxy-2-deoxyguanine in TRUC CECs. We found that DNA adducts were detectable in young TRUC and aged RAG2−/− mice; however, the levels of these adducts increased threefold between 1 and 2 months in TRUC mice and were highest in 4 and 6 month old TRUC mice (enriched for dysplasia and carcinoma) [p < .001, for all TRUC comparisons 1 month vs 2–6 months] (Figure 2C). The inflammatory microenvironment was rich in several cytokines (e.g. IL-1β, TNF-α, IL-6, IFNγ, IL-10) many of which increased in colonic explant cultures with dysplasia and carcinoma (Figure 2D). We have examined the expression of a number of cytokines, pro-inflammatory mediators, growth factors, and proteases and found that the vast majority increased in the transition from colitis to dysplasia and colitis to carcinoma (Supplemental Figure Panel A and B). Thus the chronically inflamed TRUC mucosa is a pro-oncogenic environment rich in ROS, DNA adducts, as well as numerous cytokines and growth factors with mitogenic potential.
Unbalanced epithelial cell death and proliferation in TRUC mice
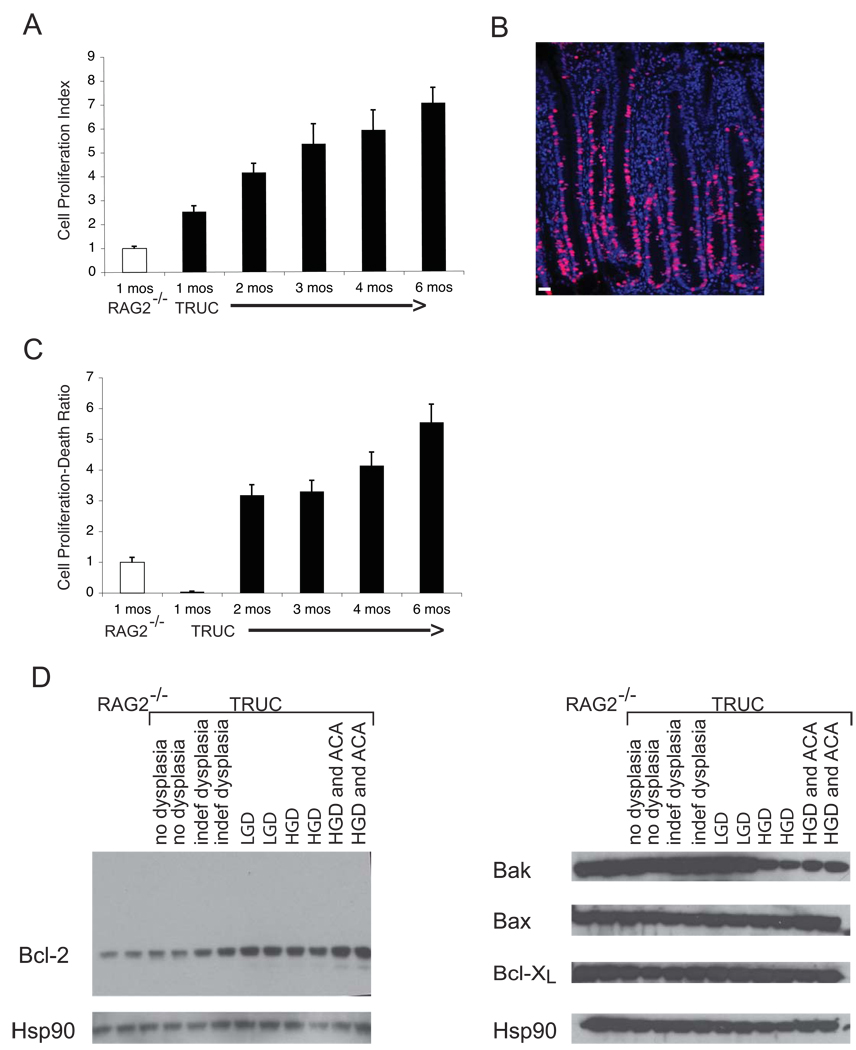
At its core this up-regulation of cytokines, proteases, and growth factors represents a repair response, however, in the setting of ROS and chromosomal instability a pro-oncogenic environment is the result. We investigated the response of the epithelium to this environment by carefully interrogating epithelial cell proliferation and cell death. We found that the TRUC epithelium became increasingly hyper-proliferative with time (Figure 3A, B). Furthermore, quantifying cell death in the epithelium by TUNEL staining and proliferation by BrDU staining revealed marked imbalances between cell death and proliferation in the TRUC epithelium. At four weeks of age, cell death outpaced the proliferative response. However, once initiated, the repair response surpassed a homeostatic level of epithelial maintenance, even considering the increase in regenerative activity secondary to colitis (Figure 3C). It was possible that the TRUC inflammatory milieu also altered the balance of apoptotic and anti-apoptotic proteins in the epithelium as evidenced by imbalances in the expression of proteins regulating apoptosis and hyper-proliferation. Western blot analysis of Bcl-2 family members in CEC extracts from TRUC samples across the neoplastic continuum and from RAG2−/− samples revealed increased anti-apoptotic Bcl-2 in dysplastic samples vs non-dysplastic samples and decreased pro-apoptotic Bak in samples with high grade dysplasia and cancer (Figure 3D). Alterations in Bcl-2 and Bak levels accompany the hyper-proliferation and decreased cell death observed during TRUC neoplastic transformation. The reactive oxygen species of the inflammatory environment may exert selective pressure on the epithelium; and epithelial cells with reduced apoptotic potential, due to increased Bcl-2 and decreased Bak, may have a survival advantage.
Figure 3. Imbalance between cell death and proliferative repair in TRUC mice.
A. The TRUC epithelium is highly proliferative and epithelial proliferation increases with time. Cell proliferation index (BrDU+ cells/(epithelial cells/crypt)) normalized to RAG2−/− levels is plotted along the y-axis. The mean of 6 RAG2−/− mice (age 1 month) and 5–7 TRUC mice per time point (age 1–6 months) is shown, error bars represent +/− SD. B. Representative immunofluorescence micrograph of data quantitated in Panel A showing a hyperproliferative TRUC mucosa with BrDU staining (red) and DAPI-labeled nuclei (blue), scale bar 25 microns. C. Cell proliferation outpaces cell death as TRUC mice age. Sections from the mice in (A) were stained with the TUNEL reagent to label dying epithelial cells. The number of TUNEL+ epithelial cells/(epithelial cells/crypt) was then divided by the Cell Proliferation Index. This ratio normalized to the calculated value observed for the RAG2−/− samples is plotted along the y-axis. The mean value for 6 RAG2−/− mice (age 1 month) and 5–7 TRUC mice per time point (age 1–6 months) is shown, error bars represent +/− SD. D. Epithelial cell lysates were generated from distal colonic epithelial cells from TRUC and RAG2−/−. Each lane represents sample from two mice and two separate groups with the indicated pathology were examined by Western blot for Bcl-2, Bak, Bax, Bcl-XL levels. Hsp 90 was used as a loading control.
TRUC colonic neoplasia is dependent upon an inflammatory response to commensal bacteria that is MyD88-independent and can be blunted by early but not late TNF-α neutralization
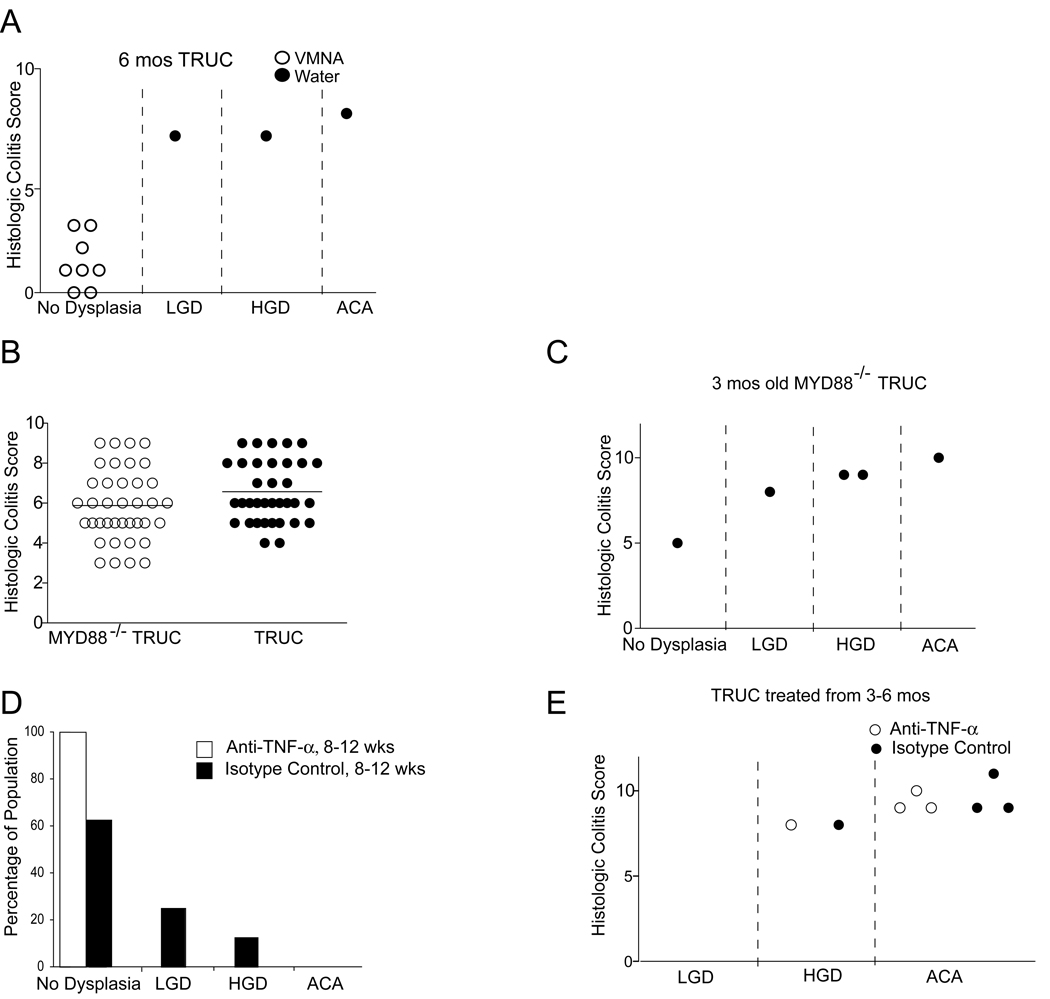
The profound effects on proliferation and apoptosis observed in TRUC epithelium amidst the complexity of the TRUC inflammatory milieu suggested that treatment of the underlying inflammation might alter the development of dysplasia and cancer. We had previously shown that broad-spectrum antibiotic treatment ameliorated TRUC colitis by changing but not eliminating the commensal microflora. To determine if altering the commensal microflora would reduce the incidence of TRUC neoplasia, we undertook a long-term course (6 months) of continuous antibiotic treatment over the time period during which TRUC mice develop dysplasia and cancer. None of the mice in the antibiotic treatment group developed colitis (Figure 4A, left panel), but 100% of the mice in the control group had very severe colitis by six months of age. In contrast to the control group, which showed HGD (40% of cases) and ACA (60% of cases) none of the antibiotic treated mice had neoplasia (Figure 4A, right panel). These data suggest that neoplasia in TRUC mice is dependent on an inflammatory response which in turn requires the presence of the full complement of the TRUC commensal microflora.
Figure 4. TRUC colonic neoplasia is dependent upon an inflammatory response to commensal bacteria that is MyD88-independent and can be blunted by early but not late TNF-α neutralization.
A. Six month TRUC mice treated with continuous broad spectrum antibiotics do not develop neoplasia. Histologic colitis score (y-axis) and neoplastic classification (x-axis) [low grade dysplasia (LGD), high grade dysplasia (HGD), and adenocarcinoma (ACA)]. Vancomycin, metronidazole, neomycin, and ampicillin treated (open circles) and water control (shaded circles). B. MyD88−/− TRUC develop colitis and neoplasia. (Left panel) Histologic colitis scores for 8 week old MyD88−/− TRUC (open circles) and TRUC (shaded circles). (Right panel) Histologic colitis scores (y-axis) and distribution of the degree of neoplasia (x-axis) for 12 week old MyD88−/− TRUC. C. Mice treated with anti-TNF-α from 8–12 weeks do not develop neoplasia while isotype control 8–12 week group mice do. % of the population with no dysplasia, LGD, HGD, and ACA is shown for each group (n= 8 per group). D. Treatment with anti-TNF-α does not ameliorate colitis or prevent the development of neoplasia in mice treated from 3–6 months, histologic colitis score (y-axis) and distribution of the degree of neoplasia (x-axis) (open circles, anti-TNF-α treated; shaded circles, isotype control treated). Each circle represents an individual mouse).
Typically, the innate immune system senses microbes via microbial pattern-recognition receptors and the Toll-like receptors (TLRs) are one major family of these. TLRs have emerged as negative regulators of cancer and TLR stimulation is now recognized as a driver of tumorigenesis (Rakoff-Nahoum and Medzhitov, 2009). Therefore, we tested whether loss of MyD88, a crucial TLR signaling molecule, would affect TRUC colitis and caCRC as has been shown in the IL-10−/− colitis model (Rakoff-Nahoum et al., 2006) and in a carcinogen-treated IL-10−/− caCRC model (Uronis et al 2009). Remarkably, MyD88−/− TRUC mice developed colitis of similar severity to TRUC (Figure 4B). Although these mice breed poorly and are severely immuno-compromised, we generated 5 MyD88−/− TRUC mice that survived until 3 months of age. One of the five mice had sub-mucosal ACA and 3/5 had dysplasia (Figure 4C). Thus, both inflammation and neoplasia in TRUC appear independent of MyD88 suggesting that classical Toll-like receptor pathways are not necessary for TRUC caCRC.
We had previously shown that treating TRUC mice from 4–8 wks or 8–12 wks of age with anti-TNF-α neutralizing antibody cured them of their microbe-driven inflammatory colitis. When mice were treated from 4–8 wks with anti-TNF-α or isotype control antibody, no dysplasia was observed in either group, consistent with the limited exposure of the epithelium to an inflammatory milieu. However, 2/8 mice from the isotype control treated 8–12 week group had low-grade dysplasia and (1/8) mice had high grade dysplasia, similar to the prevalence of neoplastic lesions we observed above (Figure 1). In contrast, none of the 8 mice treated with anti-TNF-α antibody from 8–12 weeks developed dysplasia (Figure 4D). In recognition of published results that anti-TNF-α prevented dysplasia and carcinoma in the dextran sulfate sodium/azoxymethane (DSS/AOM) caCRC model even when administered at late time points well after adenocarcinoma has developed, we attempted to treat TRUC mice from 3–6 months of age (Popivanova et al., 2008). We had high mortality (60–75%) in both control treated and anti-TNF-α treated cohorts and the four mice displayed for each group represent survivors of three independent experiments. Treatment with anti-TNF-α from three to six months of age had no effect on colitis and both the treatment and control groups had the same number of mice with HGD and ACA (Figure 4E). While this small number of animals precludes a robust interrogation into the differences of the neoplastic microenvironment in the setting of this intervention, we did observe modest differences in several cytokines (IL-1β, IL-6, and IL-18) between anti-TNF and control treated mice (Supplemental Figure Panel C). Thus the commensal microflora and TNF-α are central initiators of caCRC, but delayed TNF-α neutralization is ineffective in halting neoplastic progression because it is unable to extinguish inflammation at this later stage.
TRUC and TRUC associated CRC is driven by DCs and can be prevented by targeted re-expression of T-bet in DCs
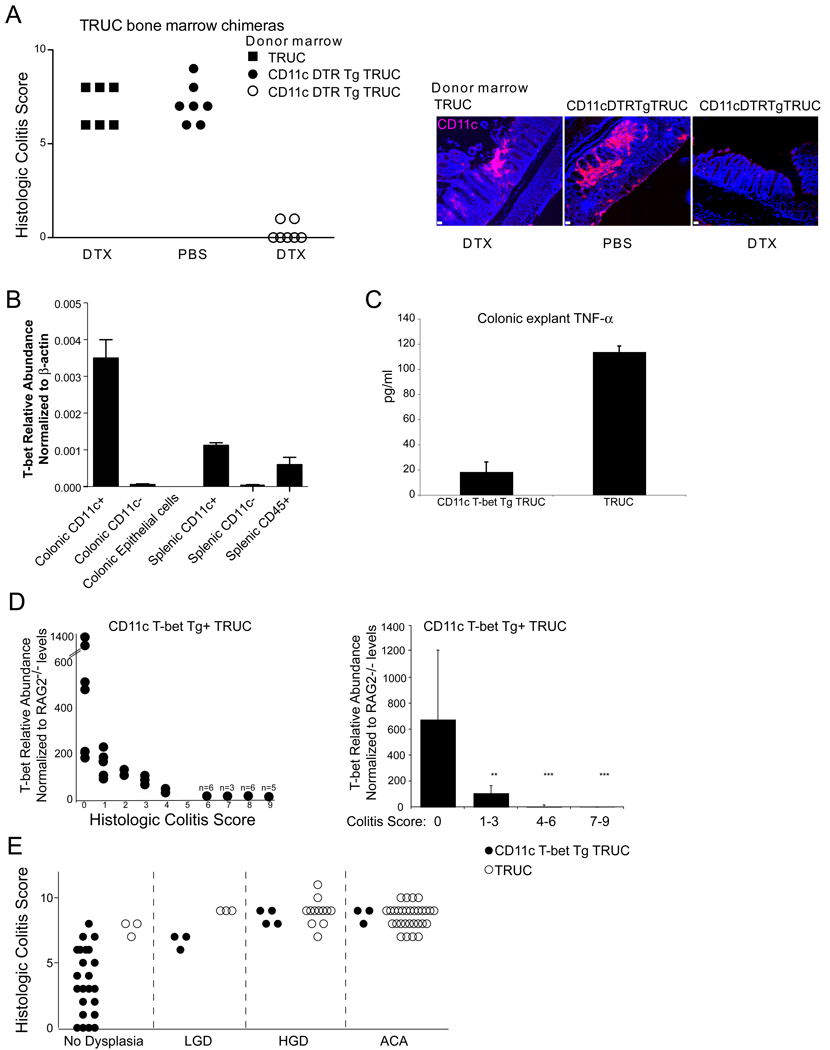
Innate immune cells play a key role in chronic inflammatory responses and much attention has been focused on macrophages and mast cells as inciters of the neoplastic environment (Bui and Schreiber, 2007; de Visser et al., 2006; Johansson et al., 2008). T-bet is not expressed, however, in macrophages or mast cells (Glimcher unpublished observation and Alcaide et al., 2007). Our previous work on the TRUC model suggested that T-bet functioned as a repressor of TNF-α in DCs, but did not establish that DCs were necessary and sufficient for TRUC colitis. Two approaches were taken to determine the function of T-bet expressing DCs. In the first approach, we used the CD11c diphtheria toxin receptor transgene to delete DCs in TRUC mice. TRUC mice expressing the CD11c DTR transgene were generated and then served as donors in bone marrow chimera experiments with TRUC mice as the recipients. After engraftment (8wks), we treated TRUC mice with diphtheria toxin every other day for eight weeks to delete their DCs. Although the diphtheria treatment was toxic (~ 40% of the treatment cohort died), DC-depleted mice that did survive the treatment showed no evidence of colitis while controls (TRUC into TRUC chimeras and PBS treated CD11c DTR TRUC chimeras) had colitis (Figure 5A, left panel). As expected, diphtheria toxin treatment of transgenic bone marrow chimeric mice resulted in diminished colonic CD11c+ populations documented by indirect immunofluorescence microscopy (Figure 5A, right panel). Thus TRUC colitis appears dependent upon DCs.
Figure 5. TRUC colitis is dependent upon DCs and T-bet over-expression in DCs reduces the prevalence of TRUC colitis-associated dysplasia and colorectal cancer.
A. TRUC colitis is dependent upon DCs. CD11c-T-bet-DTR-GFP tg TRUC mice were generated and used as donors in bone marrow chimera experiments with TRUC mice as recipients, TRUC bone marrow was engrafted into irradiated TRUC recipients as a control. After engraftment (8 wks), CD11c Tbet tg TRUC mice were treated every other day with PBS (shaded circles) or DTX (open circles) for 8 wks (left panel). Control TRUC bone marrow chimeras were DTX treated for 8 weeks (shaded squares) (left panel). Each dot represents one mouse. 40% of the original DTX-treated cohort group died during the 8 wk course of treatment. Deletion of CD11c+ DC subsets was confirmed by indirect immunofluorescence microscopy for CD11c on paraffin embedded sections from representative mice from each group. A selected image from the distal colon is shown for each group CD11c (pink), Hoescht (blue), scale bars 50 microns. B. T-bet expression in CD11c T-bet Tg TRUC mice. T-bet expression was measured in purified myeloid colonic cell populations, in colonic epithelial cell populations, and in splenic cell populations pooled from 15 sex-matched CD11c T-bet Tg TRUC mice without evidence of colitis, using real time qPCR. Bars represent the mean values across three replicate qPCR reactions on the samples and error bars +/− SD. C. Restoration of T-bet expression using a CD11c transgenic cassette reduces colonic explant TNF-α levels in transgene expressing TRUC mice. TNF-α ELISA-based protein determinations from colonic explants from CD11c T-bet Tg TRUC and TRUC, age 4 weeks. Bars represent the mean value across 3 sets of explant samples (5 individual mice per group) and error bars +/− SD. D. T-bet over-expression inversely correlates with colitis score. qPCR for T-bet was performed on cDNA generated from RNA isolated from paraffin embedded CD11c T-bet IRES GFP tg TRUC colons and RAG2−/− controls. T-bet abundance relative to β-actin and then normalized to RAG2−/− levels (y-axis) versus colitis score (x-axis). (Left panel) individual mice depicted by dots (when overlapping, the number of mice is shown above the dot. (Right panel) Bars represent the mean values for mice grouped by colitis score: (0), (1–3), (4–6), (7–9) and error bars represent +/− SD. E. CD11c T-bet tg TRUC mice have reduced prevalence of dysplasia and carcinoma. Cohorts were aged to six months and evaluated for colitis (y-axis) and neoplasia (grouped by histopathology along the x-axis), CD11c T-bet Tg TRUC (shaded circles, n= 35) and control TRUC (open circles, n= 48).
A second complementary approach addressed whether restoration of T-bet expression in DCs would ameliorate colitis and hence prevent neoplasia. We generated TRUC mice that over-expressed T-bet under the control of the CD11c promoter and verified expression in CD11c subsets from the colon and spleen of the transgenic TRUC mice (Figure 5B). Our previous published work, using primarily in vitro methodology, suggested that T-bet functioned as a repressor of TNF-α in DCs. The CD11c Tg TRUC had markedly reduced TNF-α levels in colonic explant cultures performed on mice at four weeks of age suggesting that T-bet functions as a repressor in DCs in vivo (Figure 5C). Expression of T-bet did vary across the transgenic founders generated and we found that there was an inverse correlation between T-bet over-expression and colitis score in the CD11c T-bet Tg TRUC adult progeny of these founders (Figure 5 D). We set aside a cohort of mice for 6 mos to observe the effects of T-bet over-expression in DCs on the development of inflammation and neoplasia. As shown in Figure 5E, (25/36) transgenics had no dysplasia vs (3/48) controls, (4/36) transgenics had HGD vs (12/48) controls, and (3/35) transgenics had ACA vs (30/48) controls. The difference between the number of non-neoplastic and neoplastic cases observed was statistically significant (p<.0001) between the CD11c T-bet transgenic TRUC and control TRUC. These data establish that DCs are critical for chronic inflammation in TRUC mice and that over-expression of T-bet in DCs rescues TRUC from dysplasia and cancer.
DISCUSSION
Inflammation is a key contributor to carcinogenesis and chronic inflammatory diseases, like IBD, initiate complex pathways to neoplasia. Here we report on a model of inflammation-driven colonic neoplasia dependent upon the transcription factor T-bet and explore its similarity to human caCRC. TRUC mice provide unique opportunities to understand the role of innate immunity-driven inflammation and DCs in caCRC. Despite the intricacy of the inflammatory environment; selectively targeting one cell type, the dendritic cell, ablated inflammation in TRUC colons. Notably, selective, targeted over-expression of T-bet to DCs also substantially reduced neoplasia. Although initially discovered as the key regulator for CD4+ T helper type 1 T cell differentiation, T-bet has subsequently been found to have important functions in immune cell types spanning both the adaptive and innate immune system (Glimcher, 2007). Our group has previously examined the role of T-bet in the TRAMP prostate cancer and B16 melanoma models, and defined a role for T-bet in limiting metastasis in TRAMP mice and more specifically mediating NK cell control of metastasis in the B16 model (Peng et al., 2004; Werneck et al., 2008). T-bet expression in colorectal cancer correlates with increased patient survival and decreased lymphovascular invasion within the tumor itself (Pages et al., 2005). T-bet expression in patient CRC samples is protective and while expression has understandably been attributed to admixed T cells, our data that T-bet over-expression in DCs blunts colonic inflammation and reduces colonic neoplasia raise interest in the idea that T-bet expression in DCs may also play a protective role in patients as well. Transcription factor targeting has proven elusive as a cancer therapeutic, however, small molecule microarray-based assays and integrin-antibody targeted nanoparticles have made engineering therapeutics for altering T-bet expression in colonic DCs an achievable therapy for patients (Peer et al., 2008, Vegas et al 2008).
Inflammatory bowel disease driven CRC represents one extreme of how chronic inflammation shapes a pro-neoplastic environment. Pro-inflammatory cytokines, such as TNF-α, orchestrate effective defensive mechanisms against pathogens that can become inherently self-destructive in the acute setting of sepsis, pathogenic when triggered in response to the commensal microbiota or cross-reactive self-antigens in IBD and rheumatoid arthritis, or pro-metastatic in the sub-clinical inflammation present in numerous solid tumors (Smith OM 2009). TNF was originally characterized by Lloyd Old in 1975 as a factor secreted by macrophages that killed the L-929 fibrosarcoma cell line (Old, 1988). Recombinant TNF-α is currently in clinical trials for ovarian and head and neck cancer as well as a number of advanced solid tumors (www.cancer.gov/clinicialtrials). However, TNF-α also may be a culprit in driving metastasis as recently suggested by the observations that TNF-α stabilizes Snail via NF-κB activation of the COP9 signalosome2 and that knock-down of Snail inhibited inflammation-driven breast cancer metastasis (Wu et al 2009). Clearly, the relationship between TNF-α and cancer is complex as highlighted here. We have previously demonstrated that T-bet functions as a repressor of TNF-α in DCs and that TNF-α neutralization can both ameliorate and block colitis at its inception and cure established colitis. Nevertheless, there appear to be therapeutic limits to TNF-α blockade in preventing neoplasia in TRUC, as neutralization from 3–6 months of age did not prevent high grade dysplasia and adenocarcinoma. This observation is in contrast to recent findings in the DSS-AOM model of caCRC where late stage CRC was successfully treated with TNF blockade (Popivanova et al., 2008). We have previously shown that TRUC colonic epithelial cells cultured ex vivo demonstrate an increased sensitivity to TNF-α induced cell death. However, the cell death and proliferation experiments in conjunction with the TNF-α colon explant ELISA data suggest that TRUC colonic epithelial cells may re-program this sensitivity to TNF-α induced cell death. Our studies of TRUC mice, a spontaneous model of caCRC, suggest that TNF-α in a chronically inflamed mucosa may initiate a cascade of events that engender dysplasia and cancer. However, once this process is unleashed neither blockade of TNF-α nor excess TNF-α is effective in halting neoplastic progression.
While TNF is a key effector in both IBD and inflammation-driven cancer, other pro-inflammatory cytokines play central roles as well. Pleiotropic pro-tumorigenic effects have been ascribed to IL-6 in a number of epithelial cancers (colon, breast, lung, liver) (Lin and Karin 2007). More recently, IL-6 has been shown to be critical for the development of caCRC via its anti-apoptotic effects and induction of hypoxia genes via the IL-6 downstream effector STAT3 in the colonic epithelium (Grivennikov et al., 2009). Furthermore, carcinoma produced factors are adept at eliciting high levels of host myeloid IL-6 and TNF-α (Kim et al 2009). IL-1β, like TNF-α, can initiate pro-inflammatory cascades driving the production of pro-inflammatory mediators (COX-2 and iNOS), cytokines (IL-6), growth factors, adhesion molecules, and matrix metalloproteinases. Numerous cytokines trigger mediators aimed at the resolution phase of injury and these growth factors and proteases may be construed as healing. However, in a chronically inflamed mucosa the epithelium is altered and compromised in appropriate signal processing. In our experiments, we show clear evidence of aneuploidy, reactive oxygen species, and DNA adducts prior to the histologic diagnosis of dysplasia or cancer. The importance of DNA repair mechanisms in response to reactive oxygen and nitrogen species-induced DNA damage was elegantly demonstrated in the setting of of alkyladenine DNA glycosylase, a major DNA repair enzyme, deficiency in inducible mouse models of gastritis and colon cancer (Meira et al., 2008). A model put forth in that study emphasizes both the importance of pro-inflammatory effectors and epithelial cells with DNA alterations for inflammatory-associated neoplasia. While cytokines, inflammatory mediators, and DNA damage may all be essential for caCRC, the inciting event rests in the host’s response to the commensal microbiota.
There is a growing appreciation for host-commensal inter-relationships in colon cancer. Genetic deletion of Toll-like receptor 4, which signals through MyD88, protects mice from CaCRC induced by DSS and AOM, several patients with CaCRC have increased TLR4 epithelial expression, and AOM induced CRC in IL-10−/− mice is commensal and MyD88 dependent (Fukata et al., 2007; Uronis et al 2009). The innate immune adaptor molecule, MyD88, controls the expression of many epithelial genes influencing tumor development in the APC/min- mouse model and in carcinogen (AOM)-induced colon cancer (Rakoff-Nahoum and Medzhitov, 2007). However, our results that TRUC colitis and caCRC are MyD88-independent suggest that not all caCRC is dependent upon MyD88. Commensal microbial signals may act via Toll-like receptor pathways that signal to NF-κB through TRIF or non-Toll-like receptor pathways, driven by glycan-based PAMPs that bind C-type lectin receptors and activate Syk resulting in NF-κB activation via Card9, as is the case for Dectin-1 (Ruland 2008) or via intracellular bacterial derived products that activate the NOD1/2 pathway. Identification of the microbes driving inflammation in TRUC will hopefully inform our understanding of the pathogen recognition receptor pathways they trigger.
Our experiments provide insight into the role of T-bet and the innate immune system in colon cancer. We show that a complex and pro-neoplastic inflammatory environment can be ablated by deletion of DCs. Furthermore, we demonstrate that over-expression of the transcription factor T-bet rescues colitic mice from caCRC. In conclusion, we present a robust model of caCRC that provides insight into both disease pathogenesis and therapeutic approaches and also furnishes ample opportunities for basic scientific discovery and pre-clinical testing.
EXPERIMENTAL PROCEDURES
Generation of T-bet−/− × RAG2−/− (TRUC) mice and CD11c T-bet IRES eGFP Tg T-bet−/− × RAG2−/− mice
Mice were housed in micro-isolator cages in the barrier facility of the Harvard School of Public Health. Animal studies and experiments were approved and carried out according to Harvard University’s Standing Committee on Animals and NIH guidelines for animal use and care. Mice in the colony are specific pathogen free and are negative for Helicobacter hepaticus, bilis, and muridarum.
Mice with deletion of T-bet and RAG2 and their genotyping have been described (Lugo-Villarino et al., 2005).
The CD11c expression cassette was generated in the laboratory of Dan Littman and a T-bet IRES GFP construct was subcloned into it. CD11c T-bet IRES GFP transgenic mice were generated by injection of this linearized construct into T-bet−/− RAG2−/− deficient embryos and implanted into pseudo-pregnant TRUC mice to preserve the TRUC microbiota. Mice were genotyped for transgene expression using a modified protocol from Jackson Laboratory, primer sets: (oIMR0042) CTAGGCCACAGAATTGAAAGATCT; (oIMR0043) GTAGGTGGAAATTCTAGCATCATCC); (oIMR0872) AAGTTCATCTGCACCACCG; and (oIMR1416) TCCTGAAGAAGATGGTGCG (GFP and endogenous IL-2).
Founder mice were screened for expression using flow cytometry for GFP fluorescence of CD11c+ subsets from blood, spleen, and colon. Mice were subsequently screened by real time qPCR for T-bet expression from RNA isolated from spleen and colon cell subsets (generated using MACS selection) using the primers: CAACAACCCCTTTGCCAAAG and TCCCCCAAGCAGTTGACAGT.
T-bet−/− RAG2−/− were bred to the MyD88 deficient mice (obtained from the lab of Daniel Goldstein with permission from S. Akira).
Broad-spectrum antibiotic treatment of TRUC colitis
Mice were treated for six months with ampicillin (1 g/L; Roche), vancomycin (500mg/L; Henry Schein, Inc. (Hospira, Inc.)), neomycin sulfate (1 g/L; Sigma (Teva Pharmaceuticals)), and metronidazole (1 g/L; Sigma, solubilized with 15ml of 0.1N acetic acid/L) dissolved in their autoclaved drinking water acid and fluid intake monitored.
Generation of CD11c DTR GRP TRUC mice
CD11c DTR GFP tg mice from Jackson labs were crossed to T-bet−/− RAG2−/− mice and genotyped for presence of the transgene as per Jackson lab protocol. High expressing mice (by analysis of CD11c+ GFP+ population from peripheral blood) were bred and selected as donors in bone marrow chimera experiments.
TRUC mice were irradiated with 800 rads and received CD11c DTR GFP tg+ TRUC or TRUC bone marrow two hours after irradiation. Following engraftment (8 wks), transgene expression was tested by peripheral blood flow cytometry and mice were treated with PBS or 10 ng/gm mouse weight of diphtheria toxin (Sigma) every other day for 8 wks.
Histology
Colons were removed from mice post-termination and dissected free from the anus to distal to the cecum. Colonic contents were removed and colons cleaned with phosphate buffer saline prior to fixation in 4% PFA or 10% neutral buffered formalin (NBF) followed by routine paraffin embedding. After paraffin embedding, 0.5 micron sections were cut and stained with hematoxylin and eosin or as noted.
Sections were examined and colitis scored in a blinded fashion (with respect to genotype and experimental protocol) by one of the authors (J.N.G.). Each of four histologic parameters were scored as absent (0), mild (1), moderate (2), or severe (3): mononuclear cell infiltration, polymorphonuclear cell infiltration, epithelial hyperplasia, and epithelial injury, similar to previous studies (Neurath et al., 2002). For cancer incidence studies, JNG examined all tissues for dysplasia and colorectal cancer in a blinded fashion on two independent occasions. Assessment for dysplasia and cancer was based on the criteria set forth in the Mouse Models of Intestinal Cancers consensus report: location, presence/absence of prolapse/herniation, size (in mm), dysplasia grade, invasion level, presence of desmoplasia, presence of irregular architecture (gland crowding, solid nests of cells vs single cells, irregularity or cribiforming, presence of lateral spread, and loss of acini) (Boivin et al., 2003).
Immunohistochemistry
Paraffin-embedded colon sections were deparaffinized, re-hydrated, and pre-treated with hydrogen peroxidase in PBS buffer. Heat-induced antigen retrieval was performed.
After blocking with the appropriate anti-sera in blocking buffer, sections were incubated with anti-PCNA (1:100), anti-β-catenin (BD Pharmingen clone:14/β-catenin 1:900 dilution), anti-p53 (clone CM5(1:400)), or anti-COX-2 (Santa Cruz clone c-20. 1:50 dilution) for 1 hr at room temperature. After incubation with HRP-conjugated secondary antibody and tyramide amplification followed by streptavidin-HRP, positive signals were visualized by DAB kit and counter-stained with hematoxylin.
Quantitation
COX-2: Total crypts and COX-2+ crypts were scored from three TRUC mice with and three without neoplasia. Four lower power (obj 10x), contiguous fields spanning the distal colons were scored.
P53: Total crypt #, # of crypts with p53 staining, and # of positive cells per crypt were quantitated across two low power (obj 10x) fields spanning the distal colon for 9 TRUC mice with and 9 without carcinoma and dysplasia.
Isolation of RNA from paraffin embedded tissues
RNA was isolated from microdissected paraffin-embedded tissues using the RecoverAll Total Nucleic Acid Isolation Kit according to manufacturer’s instructions.
Generation of cDNA from RNA
cDNA was generated from isolated RNA using the Invitrogen iScript kit.
Real time qPCR
Real time qPCR was carried out using ABI SyBR reagent on a ABI 7700 or Stratagene Mx3005p using SYBR or POWER SYBR green master mix from ABI. HPRT unless otherwise noted was the housekeeping gene used.
Primer Design
Unless otherwise noted, primers used were from validated sets designed by Primer Bank: http://pga.mgh.harvard.edu/primerbank/index.html
BrDU staining
Mice were injected with 200 microliters of BrDU i.p. and sacrificed after two hours. Colons were subsequently fixed for 2hrs in Neutral Buffered Formalin, placed in 70% EtOH overnight, embedded, and stained for BrDU using the BrDU detection kit from BD. Sections were counter-stained with DAPI and for assessment of cell death in conjunction with cell proliferation; sections were stained using the Roche TUNEL in situ death fluorescence kit.
Colonic Crypt Isolation
Briefly, cells were isolated from cleaned minced colons using HBBS with 1mM EDTA/1mM EGTA as previously described (Garrett et al., 2007).
P53 activity assays
Colonic crypts were isolated as above from the distal colon of RAG2−/− mice or colitic or neoplastic segments of TRUC colons. CECs were treated with doxorubicin at 0.5 micrograms/ml for 8 hours or vehicle alone. 20 segments were pooled per group to generate sufficient number of cells for RNA isolation or nuclear extracts. Two separate isolations and treatments of the CECs were performed and data represent the mean +/− SD across these groups. RNA was isolated and cDNA generated as described. β-actin was the house keeping gene employed for relative abundance calculations and a fold change was calculated for p21 and APAF1 induction relative to detection levels in control treated samples. For DNA binding activity, nuclear extracts were generated as per manufacturer’s instructions for the Duoset IC intracellular human/mouse p53 activity assay with the exception that lysis Buffer A and B were prepared with Roche protease and PhosSTOP inhibitor cocktail tablets and 20 micrograms of extract was used per well.
Aneuploidy Analysis
Colonic crypt cells were isolated, cells were then fixed in ice-cold ethanol at a final concentration of 70% using vigorous vortexing, RNase treated, stained with anti-CD45-APC antibody to exclude any myeloid contaminating subsets, and stained with propidium iodide. Data were acquired using a BD LSRII flow cytometer and the flow cytometer was calibrated for DNA ploidy anaylsis using the BD DNA QC particle kit. The HT-29 cell line was used as a positive control for aneuploid populations. Data were analysed using ModFIT software (Clausen et al., 2001).
Measurement of reactive oxygen species
Colons were removed after sacrifice and cleaned distal colons were divided into three segments and placed in a buffer of 1mM CaCl2 and 5mM glucose. Colonic segments were weighed and then placed into luminol assay buffer (.045 gm luminol in PBS). After a three minute incubation, luminescence was measured using a luminometer. The average across all segments constituted the value for each mouse. After weight correction, values were averaged for 6–9 mice per time point (Millar et al., 1996).
Western Blots
CECs were isolated and lysates generated using RIPA buffer in the presence of protease inhibitors. Protein lysates were resolved using SDS PAGE and transferred to PVDF membrane using a Biorad Wet transfer apparatus. Blots were probed with antibodies directed against Bcl-2, Bax, Bak, Bcl-xL, and Hsp90 all from Santa Cruz. After incubation with the appropriate HRP-conjugated secondary antibody, we employed ECL to develop the blots.
Measurement of DNA adducts
DNA was extracted from CECs from the distal colon using the Qiagen genomic DNA extraction kit as per manufacturer’s protocol. Samples were processed as per the manufacturer’s instructions for the high sensitivity 8-OHdG ELISA kit K0G-HS10E with the following exceptions. Nuclease P1 and alkaline phosphatase digestions were performed in an An02 hood. After processing, DNA digested samples from 6–9 mice per time point were pooled and subjected to quantitation by ELISA in triplicate.
Anti-Cytokine Therapy
Anti-TNF-α (clone TN3-19.12), a hamster anti-mouse TNF-α neutralizing IgG1, and isotype control were a kind gift of Robert Schreiber and purchased from Leinco Technologies, Inc. 15 microgram/gm mouse weight of Ab was injected i.p. every seven days for durations as noted. Mice were sacrificed one week following the last injection.
Colon Explant Culture
Explant cultures were carried out following a modification of previously described procedures (Rakoff-Nahoum et al., 2004). 1 cm segments of the distal colon were washed in HBBS containing penicillin, streptomycin, and gentamicin. The segments were cultured in 48 well flat bottom plates with complete RPMI media supplemented with penicillin, streptomycin, and gentamicin. Media was collected after 4 hrs and centrifuged to remove debris. After centrifugation, the supernatant was aliquoted and stored at −80C.
Measurement of cytokines
Cytokines were measured in culture supernatants utilizing SearchLight high dynamic range (HDR) imaging and analysis unless otherwise indicated. For IL-10 and TNF-α, the mouse BD OptEIA ELISA kit was used as per the manufacturer’s instructions.
Statistical Analysis
Statistical analyses were performed using the Mann Whitney U non-parametric test for ordinal data. Error bars represent +/− SD unless otherwise noted.
Supplementary Material
A. Summary of selected gene expression patterns of colitis and colitis-associated dysplasia or CRC TRUC samples. B. Real time qPCR analysis of selected genes. RAG2−/− (open bars), inflamed TRUC colorectal mucosa without dysplasia (light gray bars), dysplastic TRUC colorectal mucosa (dark gray bars), and carcinomatous TRUC colorectal mucosa (black bars), 5–8 regionally matched samples were evaluated per histologic type, bars represent the mean of samples tested, and error bars represent standard deviations, all data normalized to RAG2−/− levels, *** p<.=0001; ** p=.02. C. Real time qPCR analysis of selected genes on anti-TNF-α and isotype control treated samples receiving treatment from 3–6 mos of age. One mouse per group for the HGD samples, two mice per group for the IMACA samples, and one mouse per group for the SMACA samples. For the HGD and SMACA samples, bars are the mean values and error bars represent +/− SD of replicates. For the IMACA samples, bars represent the mean values and error bars represent +/− SD for the two samples in each group.
Acknowledgements
We thank Jacobo Ramirez and Diana Pascual for outstanding care of our mice; Landy Kangaloo and members of the Glimcher laboratory for helpful discussion; Drs. Vanja Lazarevic, Marc Wein, Fabio Martinon, and Tracy Staton for critical review of the manuscript; and Drs. Tyler Jacks, Ji-Hye Paik, and Alfred Zullo for advice. This work was supported by grants from the NIH (CA112663) and an Ellison Scholar Award to LHG. WSG is a recipient of a Damon Runyon Cancer Research Foundation fellowship, a Burroughs Wellcome Career in Medical Sciences Award, and funding from the V Foundation, DF/HCC GI SPORE 1P50CA127003-02, Irving Janock Fellowship, and HDDC Pilot Award. Dedicated in memory of R.B., L.K., and N.H. whose battles with CRC were an inspiration for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agoff SN, Brentnall TA, Crispin DA, Taylor SL, Raaka S, Haggitt RC, Reed MW, Afonina IA, Rabinovitch PS, Stevens AC, et al. The role of cyclooxygenase 2 in ulcerative colitis-associated neoplasia. Am J Pathol. 2000;157:737–745. doi: 10.1016/S0002-9440(10)64587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaide P, Jones TG, Lord GM, Glimcher LH, Hallgren J, Arinobu Y, Akashi K, Paterson AM, Gurish MA, Luscinskas FW. Dendritic cell expression of the transcription factor T-bet regulates mast cell progenitor homing to mucosal tissue. J Exp Med. 2007;204:431–439. doi: 10.1084/jem.20060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Cho KR, Vogelstein B. Genetic alterations in the adenoma--carcinoma sequence. Cancer. 1992;70:1727–1731. doi: 10.1002/1097-0142(19920915)70:4+<1727::aid-cncr2820701613>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Clausen OP, Andersen SN, Stroomkjaer H, Nielsen V, Rognum TO, Bolund L, Koolvraa S. A strategy combining flow sorting and comparative genomic hybridization for studying genetic aberrations at different stages of colorectal tumorigenesis in ulcerative colitis. Cytometry. 2001;43:46–54. doi: 10.1002/1097-0320(20010101)43:1<46::aid-cyto1018>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- D'Inca R, Cardin R, Benazzato L, Angriman I, Martines D, Sturniolo GC. Oxidative DNA damage in the mucosa of ulcerative colitis increases with disease duration and dysplasia. Inflamm Bowel Dis. 2004;10:23–27. doi: 10.1097/00054725-200401000-00003. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Dianda L, Hanby AM, Wright NA, Sebesteny A, Hayday AC, Owen MJ. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91–97. [PMC free article] [PubMed] [Google Scholar]
- Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RA, Wang K, Davis JS, Birnbaumer L. Role for epithelial dysregulation in early-onset colitis-associated colon cancer in Gi2-alpha−/− mice. Inflamm Bowel Dis. 2008;14:898–907. doi: 10.1002/ibd.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galamb O, Gyorffy B, Sipos F, Spisak S, Nemeth AM, Miheller P, Tulassay Z, Dinya E, Molnar B. Inflammation, adenoma and cancer: objective classification of colon biopsy specimens with gene expression signature. Dis Markers. 2008;25:1–16. doi: 10.1155/2008/586721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher LH. Trawling for treasure: tales of T-bet. Nat Immunol. 2007;8:448–450. doi: 10.1038/ni0507-448. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- Itzkowitz S. Colon carcinogenesis in inflammatory bowel disease: applying molecular genetics to clinical practice. J Clin Gastroenterol. 2003;36:S70–S74. doi: 10.1097/00004836-200305001-00012. discussion S94-76. [DOI] [PubMed] [Google Scholar]
- Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M. Trefoil factors and human gastric cancer (review) Int J Mol Med. 2003;12:3–9. [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lind WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo-Villarino G, Ito S, Klinman DM, Glimcher LH. The adjuvant activity of CpG DNA requires T-bet expression in dendritic cells. Proc Natl Acad Sci U S A. 2005;102:13248–13253. doi: 10.1073/pnas.0506638102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A. Loss of T-bet sends host-microbe mutualism awry. Cell. 2007;131:15–17. doi: 10.1016/j.cell.2007.09.030. [DOI] [PubMed] [Google Scholar]
- Mannick EE, Cote RL, Schurr JR, Krowicka HS, Sloop GD, Zapata-Velandia A, Correa H, Ruiz B, Horswell R, Lentz JJ, et al. Altered phenotype of dextran sulfate sodium colitis in interferon regulatory factor-1 knock-out mice. J Gastroenterol Hepatol. 2005;20:371–380. doi: 10.1111/j.1440-1746.2005.03573.x. [DOI] [PubMed] [Google Scholar]
- Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meling GI, Clausen OP, Bergan A, Schjolberg A, Rognum TO. Flow cytometric DNA ploidy pattern in dysplastic mucosa, and in primary and metastatic carcinomas in patients with longstanding ulcerative colitis. Br J Cancer. 1991a;64:339–344. doi: 10.1038/bjc.1991.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meling GI, Rognum TO, Clausen OP, Chen Y, Lunde OC, Schlichting E, Wiig JN, Hognestad J, Bakka A, Havig O, et al. Association between DNA ploidy pattern and cellular atypia in colorectal carcinomas. A new clinical application of DNA flow cytometric study? Cancer. 1991b;67:1642–1649. doi: 10.1002/1097-0142(19910315)67:6<1642::aid-cncr2820670628>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Millar AD, Rampton DS, Chander CL, Claxson AW, Blades S, Coumbe A, Panetta J, Morris CJ, Blake DR. Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut. 1996;39:407–415. doi: 10.1136/gut.39.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J Exp Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut. 1996;39:87–92. doi: 10.1136/gut.39.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old LJ. Tumor necrosis factor. Sci Am. 1988;258:59–60. 69–75. doi: 10.1038/scientificamerican0588-59. [DOI] [PubMed] [Google Scholar]
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SL, Townsend MJ, Hecht JL, White IA, Glimcher LH. T-bet regulates metastasis rate in a murine model of primary prostate cancer. Cancer Res. 2004;64:452–455. doi: 10.1158/0008-5472.can-03-3401. [DOI] [PubMed] [Google Scholar]
- Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Ravizza R, Gariboldi M, Passarelli L, Monti E. Role of the p53/p21 system in the response of human colon carcinoma cells to Doxorubicin. BMC Cancer. 2004;4:92. doi: 10.1186/1471-2407-4-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues NR, Rowan A, Smith MEF, Kerr IB, Bodmer WF, Gannon JV, Lane DP. p53 mutations in colorectal cancer. Proc Natl Acad Sci USA. 1990;87:7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin CE, Haggitt RC, Burmer GC, Brentnall TA, Stevens AC, Levine DS, Dean PJ, Kimmey M, Perera DR, Rabinovitch PS. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology. 1992;103:1611–1620. doi: 10.1016/0016-5085(92)91185-7. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Bradley A, Birnbaumer L. Gi2 alpha protein deficiency: a model of inflammatory bowel disease. J Clin Immunol. 1995;15:101S–105S. doi: 10.1007/BF01540899. [DOI] [PubMed] [Google Scholar]
- Ruland J. CARD9 signaling in the innate immune response. Ann N Y Acad Sci. 2008;1143:35–44. doi: 10.1196/annals.1443.024. [DOI] [PubMed] [Google Scholar]
- Saaf AM, Halbleib JM, Chen X, Yuen ST, Leung SY, Nelson WJ, Brown PO. Parallels between global transcriptional programs of polarizing Caco-2 intestinal epithelial cells in vitro and gene expression programs in normal colon and colon cancer. Mol Biol Cell. 2007;18:4245–4260. doi: 10.1091/mbc.E07-04-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SA, Simpson SJ, Brown LF, Comiskey M, de Jong YP, Allen D, Terhorst C. Development of colonic adenocarcinomas in a mouse model of ulcerative colitis. Inflamm Bowel Dis. 1998;4:196–202. doi: 10.1097/00054725-199808000-00004. [DOI] [PubMed] [Google Scholar]
- Smith OM. Leading Edge Immunology Select. Cell. 2009;137:591–593. [Google Scholar]
- Sohn KJ, Shah SA, Reid S, Choi M, Carrier J, Comiskey M, Terhorst C, Kim YI. Molecular genetics of ulcerative colitis-associated colon cancer in the interleukin 2- and beta(2)-microglobulin-deficient mouse. Cancer Res. 2001;61:6912–6917. [PubMed] [Google Scholar]
- Sturlan S, Oberhuber G, Beinhauer BG, Tichy B, Kappel S, Wang J, Rogy MA. Interleukin-10-deficient mice and inflammatory bowel disease associated cancer development. Carcinogenesis. 2001;22:665–671. doi: 10.1093/carcin/22.4.665. [DOI] [PubMed] [Google Scholar]
- Toiyama Y, Mizoguchi A, Kimura K, Araki T, Yoshiyama S, Sakaguchi K, Miki C, Kusunoki M. Persistence of gene expression changes in noninflamed and inflamed colonic mucosa in ulcerative colitis and their presence in colonic carcinoma. World J Gastroenterol. 2005;11:5151–5155. doi: 10.3748/wjg.v11.i33.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uronis JM, Muhlbauer M, Herfarth HN, Rubinas TC, Jones GS, Jobin C. Modulation of Intestinal Microbiota Alters Colitis-Associated Colorectal Susceptibility. PLOS One. 2009;4:e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegas AJ, Fuller JH, Koehler AN. Small-molecule microarrays as tools in ligand discovery. Chem Soc Rev. 2008;37:1385–1394. doi: 10.1039/b703568n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Kobunai T, Toda E, Kanazawa T, Kazama Y, Tanaka J, Tanaka T, Yamamoto Y, Hata K, Kojima T, et al. Gene expression signature and the prediction of ulcerative colitis-associated colorectal cancer by DNA microarray. Clin Cancer Res. 2007;13:415–420. doi: 10.1158/1078-0432.CCR-06-0753. [DOI] [PubMed] [Google Scholar]
- Werneck MB, Lugo-Villarino G, Hwang ES, Cantor H, Glimcher LH. T-bet plays a key role in NK-mediated control of melanoma metastatic disease. J Immunol. 2008;180:8004–8010. [Google Scholar]
- Wu Y, Deng J, Rychahou PG, Oiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378–389. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Mikami T, Mitomi H, Okayasu I. Diverse p53 alterations in ulcerative colitis-associated low-grade dysplasia: full-length gene sequencing in microdissected single crypts. J Pathol. 2003;199:166–175. doi: 10.1002/path.1264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Summary of selected gene expression patterns of colitis and colitis-associated dysplasia or CRC TRUC samples. B. Real time qPCR analysis of selected genes. RAG2−/− (open bars), inflamed TRUC colorectal mucosa without dysplasia (light gray bars), dysplastic TRUC colorectal mucosa (dark gray bars), and carcinomatous TRUC colorectal mucosa (black bars), 5–8 regionally matched samples were evaluated per histologic type, bars represent the mean of samples tested, and error bars represent standard deviations, all data normalized to RAG2−/− levels, *** p<.=0001; ** p=.02. C. Real time qPCR analysis of selected genes on anti-TNF-α and isotype control treated samples receiving treatment from 3–6 mos of age. One mouse per group for the HGD samples, two mice per group for the IMACA samples, and one mouse per group for the SMACA samples. For the HGD and SMACA samples, bars are the mean values and error bars represent +/− SD of replicates. For the IMACA samples, bars represent the mean values and error bars represent +/− SD for the two samples in each group.