SUMMARY
Schistosome parasites exhibit separate sexes and with the evolution of sex they have developed an intricate relationship between the male and female worms such that signals between the male and female that are initiated at the time of mating, regulate female reproductive development and subsequent egg production. As the egg stage is responsible for pathogenesis and transmission, understanding the molecular mechanisms of female reproductive development may identify novel targets for control of transmission and morbidity of this major world public health problem. Recent data has demonstrated that the pairing process, proliferation and differentiation of vitelline cells, expression of female-specific genes and egg embryogenesis are regulated by the TGFβ pathway and protein tyrosine kinases.
INTRODUCTION
Blood flukes of the genus Schistosoma infect over 200 million people in 76 countries [1]. Schistosomes have a complex lifecycle involving both a snail intermediate and a vertebrate definitive host. Schistosome parasites have co-evolved an intricate relationship with their human and snail hosts as well as a novel interplay between the adult male and female parasites.
Eggs produced by worm pairs are important in transmission of the parasite and responsible for pathogenesis. For S. mansoni, worm pairs produce approximately 300 eggs per day. Approximately half of the deposited eggs reach the outside environment in the excreta, to continue the life cycle. The other 50% are swept into the circulation and filter out in the periportal tracts of the liver eliciting granulomatous inflammatory reactions that can lead to periportal fibrosis, portal hypertension and the serious sequelae of intestinal schistosomiasis such as hepatosplenomegaly and esophageal and gastric varicies [2]. Thus, understanding the molecular basis for male -female interactions that lead to female reproductive development should offer targets to prevent egg production and thus prevent both transmission of the parasite and morbidity due to the eggs. In this review we focus on the biological interplay between the male and female parasite, in particular the recent developments in signal transduction that contribute to reproductive development and subsequent egg production.
Male-female interplay
To develop into a reproductively active female worm and to maintain reproductive activity and egg production, the female worm is dependent on the presence of a mature male worm [3-7] (Figure 1). Female schistosomes from single sex infections are underdeveloped in that they are stunted in size and exhibit an immature reproductive system. In particular, the vitellaria, whose cells produce the eggshell precursors and nutrients for the egg, are not developed. The nature of the stimuli for female growth and for reproductive development are the subject of intense study. At the molecular level, DNA synthesis increases 4-5 fold when females from single-sex infections are paired with male worms indicating mitotic activity associated with the development of the vitellaria [**8]. This coincides with the evidence that the eggshell precursor genes (p14, p48) are expressed only in mature female worms where expression is first detected at the time of worm pairing and increase to a high level with the start of egg production. p14 and p48 mRNA are synthesized and translated in vitelline cells and the gene products are associated with the vitelline droplets (the proteinacious granules that contain the eggshell precursor proteins among other molecules) (Figure 1). Thus the male stimulus is associated with pairing and, in part, results in the regulation of vitelline cell (female-specific) gene expression and mitotic activity. An intimate association between the male and female worm, which is achieved by the female residing within a ventral groove, the gynaecophoric canal of the male, is necessary and sufficient to direct female growth, reproductive development and continuous egg production. The stimulus from the male worm is not only necessary for female worms to complete physical and reproductive development but also for the female to maintain her mature state. The fact that a continuous male stimulus is necessary to maintain female reproductive activity makes this an important target for controlling morbidity and preventing transmission by limiting (preventing) egg production.
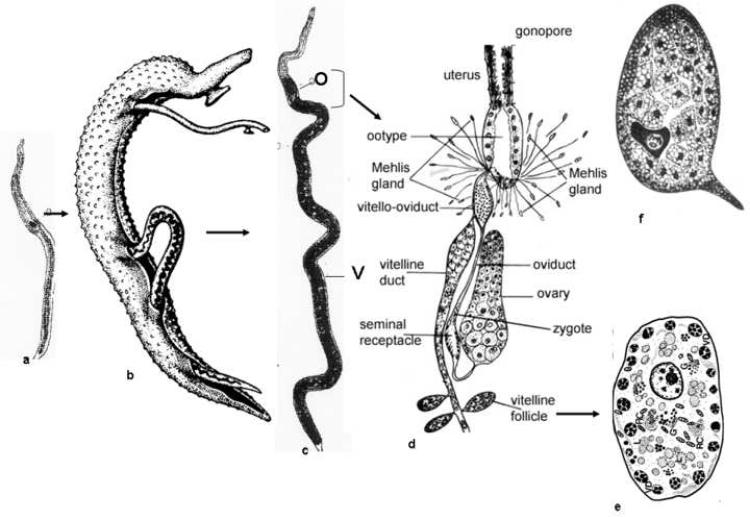
Figure 1.
Female Schistosoma mansoni, showing (a) an immature female from a single-sex infection that is stunted and reproductively underdeveloped, (b) female lying in the gynecophoric canal of a male, (c) a mature female from a bisexual infection showing that the posterior 2/3rds of the body is comprised of the vitellaria (V) that develops in response to pairing; O, ovary (d) enlargement of the mature female reproductive system. Oocytes produced in the ovary are released into an oviduct. A dilitated region in the oviduct, the seminal receptacle, is the site of fertilization. The fertilized egg moves down the oviduct to a region where it meets the vitelline duct, the vitello-oviduct. Here, each fertilized egg is surrounded by approximately 38 vitelline cells. This bolus of material moves into the ootype which is filled with mucus secretions presumably from the Mehlis' gland. With the contraction of the ootype, which determines the shape of the egg, granules are released from the surrounding vitelline cells. The materials from the granules (vitelline droplets) begin to crosslink, due to the action of a tyrosinase enzyme(s) and the eggshell forms. The egg (f) which contains the fertilized egg and a number of vitelline cells that each contain nutrients for embryogenesis is released into the uterus and one at a time deposited by the female worm through the gonopore, (e) Mature vitelline cell (S4) containing the vitelline droplets (VD) and (f) just formed egg containing the zygote and numerous vitelline cells that will contribute to embryonation. (Modified from [41])
Not surprisingly, the female schistosome has a stimulatory effect on the male as evidenced by changes in levels of glutathione and lipids in the male and expression of new antigens in the gynecophoric canal, all in response to mating with female worms [3, **9].
Role of TGFβ signaling pathway in male-female interactions
Schistosomes have evolved an interesting biological interplay among the male and female parasites such that male schistosomes via an unknown stimulus regulate female-specific gene expression and thus female reproductive development and egg production. Signaling pathways are prime candidates for transduction of the male stimulus to the vitelline cells of the female parasite.
Current data argues for multiple (pleiotropic) roles for the TGFβ signaling pathway throughout the schistosome life cycle, especially in female reproductive development and egg embryogenesis involving the vitelline cells [*10].
The TGFβ signaling pathway comprises a family of structurally related polypeptide growth factors, each capable of regulating an array of cellular processes including cell proliferation, lineage determination, differentiation, and adhesion (e.g. [11], Fig 2). TGFβ members signal through a family of transmembrane protein serine/threonine kinases-type I (TβRI) and type II receptors (TβRII) that directly regulate the intracellular Smad pathway (Figure 2). Upon ligand binding the type II receptor phosphorylates the type I receptor, which subsequently activates receptor-regulated Smads (R-Smads) by phosphorylation. Activated R-Smads bind to Smad4, the common Co-Smad, and the resulting complex enters the nucleus regulating transcription of selected genes in response to ligand. Cooperation between Smads and other transcription regulators permits crosstalk between the TGFβ signal pathway and other known pathways [12]. All essential components of the TGFβ pathways have been identified in schistosomes, and evidence was obtained for their expression in vitelline cells [*10].
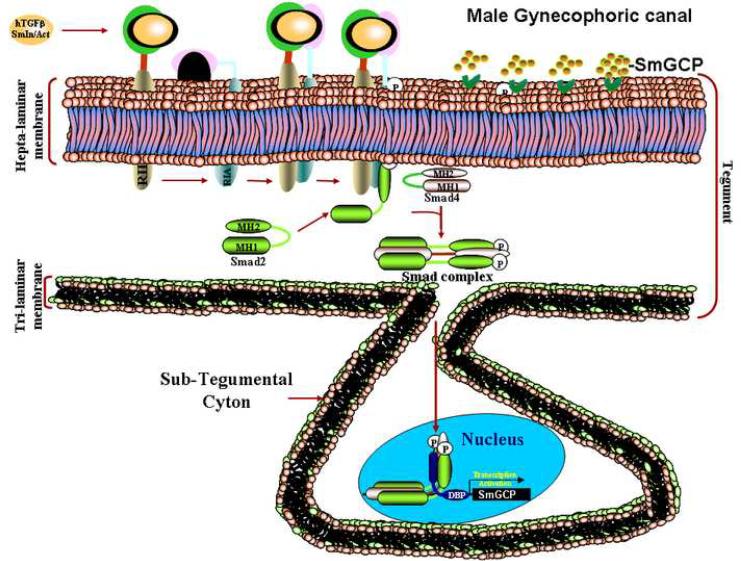
Figure 2.
TGFβsignaling pathway in male Schistosoma mansoni showing signaling from the female to the male that contributes to parasite pairing (based on experimental data). The cartoon depicts surface-exposed tegument of the gynecophoric canal. SmTGFβtype II receptor (RII) binds a parasite or a host TGFβ ligand (SmInAct, hTGFβ1). The ligand/RII complex triggers the interaction with a type I receptor (RIA; SmTβRI), phosphorylates and activates it. The activated type I receptor relays the signal down to a R-Smad (RIA to SmSmad2), where the SmSmad2 is activated via phosphorylation. The activated SmSmad2 interacts with the co-Smad (SmSmad4) in the subtegument. The SmSmad complex translocates to the nucleus of the sub-tegumental cell, binds nuclear partners and the assembled transcription complex binds to the Smad binding element in the promoter of the target gene (SmGCP) and regulates target gene transcription. The SmGCP transcript is translated and the protein transported to the surface of the gynecophoric canal where it facilitates worm pairing. See text and [3, 6, *10] for further details.(modified from [6]).
Studies demonstrate that in adult male and female worms, S. mansoni (Sm)TβRII, SmTβRI, SmSmad1B, SmSmad2, and SmSmad4 are localized to the tegument (outer covering of the schistosome) and SmTβRII and SmTβRI are surface exposed suggesting that they can interact with components of the host environment [**9, 13-19]. The fact that the TβRI and RII are surface exposed also opens the possibility of communication between the male and female parasite (see below).
In functional assays, host (human) TGFβ has been shown to bind to SmTβRII resulting in the phosphorylation of SmTβRI [**9, 20] (Figure 2). For example, in the presence of the human ligand, hTGFβ1, SmTβRII is able to interact productively with SmTβRI which results in the phosphorylation of SmSmad2 by the activated SmTβRI and the formation of a complex with SmSmad4 [**9]. This receptor-induced SmSmad2/SmSmad4 heterooligomeric complex formation resulted in the nuclear translocation of the Smad complex and in activation of target genes (by measuring reporter gene expression) [**9, 17, 18, 20, 21]. In fact, it has been demonstrated in in vitro studies and in the schistosome worm itself that hTGβ1 binds to SmTβRII and sends a signal that regulates target gene expression and consequently elicits a specific TGFβ effect [**9]. Thus, there is evidence that a host (human) ligand is capable of transducing a signal through the schistosome TGFβ signaling pathway. Whether this occurs during infection is yet to be demonstrated.
Identification of SmGCP as a S. mansoni TGFβ target gene that is regulated in response to worm pairing
A gynecophoral canal protein (SmGCP) has been demonstrated to be involved in promoting intimate contact of the female with the tegument of the gynecophoric canal of the male [**9, *22]. SmGCP localizes to the surface of the gynecophoric canal of the male (the site of interaction between the mating pair) and to the entire surface of the en copula female but not to non-mated males nor to immature females [23, 24]. SmGCP shows homology to beta-ig-h3 (β-induced gene-Human clone 3) whose expression is induced in response to TGFβ to mediate cell adhesion [25]. SmGCP was shown to be regulated by TGFβ (2-fold increase in SmGCP expression compared to untreated worm controls) and to exhibit an expression peak at 28 days-post infection [**9], which coincides with worm mating. To demonstrate that SmGCP expression induced by hTGFβ1 is a specific effect dependent on direct stimulation of TGFβ signaling pathway, Osman et al. [**9] employed RNAi to knock down the TGF type II receptor expression to block the initial event of TGFβ signaling. RT-PCR data demonstrated that SmTβRII-specific siRNA treatment diminished transcription levels of SmTβRII about 3-4 fold in tested organisms. Not only did SmTβRII-siRNA treatment result in a concomitant reduction in SmGCP levels (there was 2-3 fold reduction in levels of SmGCP compared to levels of untreated samples), but SmGCP also failed to respond to induction demonstrating that the initial step of the TGFβ pathway (hTGFβ binding to the SmTβRII receptor) was prevented. Recently, using siRNA silencing of the S. japonicum GCP, Cheng et al.[*22] demonstrated in vitro that SjGCP gene silencing resulted in an abrogation of worm pairing whereas the systemic delivery (in vivo) to infected mice significantly inhibited early parasite pairing. Taken together the evidence suggests that the female worm stimulates the male worm (presumably by the transfer of SmInAct, see below and Figure 2) to express the SmGCP as part of the pairing process and that the expression of SmGCP is regulated by the TGFβ signal pathway. Whether the human TGFβ ligand is involved in this part of the pathway or whether the female worm produces a schistosome TGFβ ligand (see below) is yet to be determined.
Role of TGFβ signaling in female reproductive development and egg production
Two TGFβ ligands from S. mansoni have been identified; An Inhibin/Activin-like molecule (SmInACT) and a BMP-like molecule (SmBMP) [*10, **26, 27]. As regards a role in female reproductive development and egg production, the S. mansoni TGFβ significant role in female reproduction and egg embryogenesis by demonstrating that in mature (bisexual) female worms, the SmInAct transcript is highly expressed and localizes to the vitellaria and eggs. The protein is absent in immature females and mature female worms recovered from infertile infections in IL-7R -/- mice but very abundant in mature females from normal infections [**26]. Further, the use of RNAi to knock down SmInAct demonstrated that eggs from RNAi treated female worms failed to develop compared to controls. When the eggs themselves were treated with RNAi to knockdown SmInAct expression, only 6.4% of the eggs developed compared to 17.2% for the controls [**26]. Thus, it is clear that SmInAct plays an important role in the embryogenesis of the egg and that the target cell is the vitelline cell. The mature female produces 38 vitelline cells for each egg. The cells which surround the zygote are responsible for eggshell formation and after the egg is laid provide the required nutrients for the embryo during the 5-day period it takes for development of the egg enclosed miracidium in the host tissue (Figure 1). That TβRII and TβRI along with SmSmad1B, SmSmad2, and SmSmad4 are located in the vitelline cells and that SmInAct is expressed in the vitelline cells and acts on egg embryogenesis (vitelline cell development) argues that the TGFβpathway transduces signals between and within cells of the reproductive system to regulate parasite development [**9, 15-18, **26]. That male worms from single-sex infections, and from infections of IL-7R -/- mice lack the SmInAct protein (but not transcripts) suggests that worm pairing plays a role in the post transcriptional regulation of this ligand [**26]. To further support a role for TGFβpathway in female reproductive development, a recent study employing a TβRI kinase inhibitor (TRIKI) demonstrated that female vitelline cell mitotic activity was reduced up to 40% and egg production reduced 30% in TRIKI-treated worms [*28]. In a previous study the same authors demonstrated that Herbimycin A, an inhibitor of protein tyrosine kinases (PTKs), also blocks mitotic activity and egg production of paired females [**8]. The authors suggest that this may indicate a cross talk between PTKs and TGFβ fnfnsignaling pathways that regulates vitelline cell development and thus egg production (see [*28], for discussion). In this regard, it has been shown that the ERK-MAPK pathway can negatively regulate TGFβ signal transmission by phosphorylating SmSmad4, thus preventing the downstream interaction with SmSmad2, blocking the pathway [17, 18].
Role of tyrosine kinases in female reproductive development and egg production
Tyrosine kinases are critical factors in the regulation of female reproductive development and egg production, especially cytoplasmic tyrosine kinases (CTK) of the Src class, SmTK3 and SmTK5 [*28-30].
SmTK3 and SmTK5 are expressed among other places in the vitellaria, ovary and spermatocytes indicating a role in adult reproductive activity. Src kinases among their multiple functions regulate cell proliferation and differentiation in response to growth factor stimulation ([31] for review). The male schistosome by an unknown stimulus, induces a 4-5 fold increase in mitotic activity in the female worm. The mitotic activity begins to disappear if the male stimulus is removed by separating the worms but is regained after repairing. Knobloch et al [**8] were able to show that a Src kinase inhibitor, Herbimicin A was able to block the 4-5 fold increased male-induced mitotic activity in female worms paired with the male. In addition, Herbimicin treatment caused a dose-dependent 40-60% decrease in egg production in mature/paired female worms. The vitellaria of mature female schistosomes consists of a large number of undifferentiated and differentiated cells that occupy about 2/3rds of the entire body volume (Figure 1). The undifferentiated cells (S1) proliferate and differentiate through two stages, S2 and S3 eventually developing into mature (S4) vitelline cells that provide the eggshell precursors for eggshell formation and become part of the egg to provide the yolk (nutrients) for embryonation. The interpretation of the data is that the Src-CTK (SmTK3 and/or SmTK5), that function as transmitter molecules in signal transduction pathways play a central role in mediating the mitosis stimulating effect of the male stimulus on S1 vitelline cells. Inhibition of a third SmCTK, SmTK4 which is found in oocytes but not vitellaria, has no effect on mitosis or egg production indicating specificity of the Src-CTKs [*28, 32].
Interestingly, Herbimicin A treatment caused a 2.5 fold up-regulation of p14 transcription and translation in paired and unpaired female schistosomes. This indicates that the up-regulation in response to the inhibitor is pairing (male stimulus) independent and suggests that the p14 gene expression is tightly controlled in immature female worms by a repressor that is inactivated by the male stimulus as opposed to an activator that turns on p14 gene expression [*28]. Cumulative data suggest that the p14 gene expression is regulated by nuclear receptors ([3, 6] for reviews). In this context, two schistosome retinoid acid receptors, SmRXR1 and SmRXR2 localize among other tissues to the vitelline cells of female S. mansoni. Furthermore SmRXR1, SmRXR2 and SmRXR1/SmNR1 have been shown to bind to cis-elements in the 5' upstream region of p14 and SmRXR1 and SmRXR2 have been shown to drive p14 gene expression [33-35].
Conclusions
A model is emerging in which signals that originate from worm pairing may involve a male-derived stimulus or an initiating signal from a female residing in the gynecophoric canal that would induce the male to send a signal, in either case, with the end result reproductive development and egg production (see [3] for discussion). This could be a single signal that that has a number of effects such as with the TGFβ pathway in which a single signal is converted into programs of gene regulation and developmental outcomes. In this regard it is obvious that the signaling pathways like the TGFβ pathway depend on extensive communication with other signaling pathways leading to programmed biological outcomes (e.g. [12]). Alternatively there could be several male signals that each has a directed outcome. In any case a male-derived ligand binds a receptor on the female surface and transduces a signal via one or more pathways from the tegument to the target vitelline cell receptor which is located on the vitelline cell membrane and then through intercellular substrates to the vitelline cell nucleus where vitelline cell gene expression and proliferation is activated. The activation of proliferation for the immature stage 1 vitelline cell to undergo development to a mature stage 4 cell that will participate in egg production involves Src-CTK (SmTK3/SmTK5?) as a central component [**8]. The mitotic inducing signal is male-derived [*28].
As vitelline cells undergo differentiation, the male-derived signal affects SmInAct, a TGFβ pathway ligand, to transduce a signal within the vitelline cell that results in regulation of embryogenesis of the schistosome eggs. Worm pairing is responsible for the source of the signal as the SmInAct ligand is not present in males or females from single sex infections [**26]. Previous studies have identified a number of female-specific genes (e.g. p14 and p48) that were expressed in vitelline cells in response to worm pairing ([3] for review). The studies that show inhibition of Src-CTK activity block proliferation also demonstrated a concomitant increase in p14 eggshell precursor gene expression in immature and paired (mature) female worms. These data now indicate that the male-derived signal did not activate the p14 gene as previously postulated but instead expression seems to be tightly controlled through the action of a suppressor molecule that is displaced by the male-derived signal.
The role of SmGCP in facilitating mating seems to be the result of the female sending a signal (SmInAct?) that is transduced through the male TGFβ pathway and results in the expression of SmGCP in the gynecophoric canal of the male schistosome [**9, *22]. It is clear from the studies presented that schistosome pairing results in a number of interrelated outcomes, such as proliferation of S1 vitelline cells to differentiate into S4 vitelline cells, regulation of p14 gene expression and egg embryogenesis.
The data available to date support the notion that Src-CTK and TGFβ signaling pathway both effect vitelline cell proliferation, differentiation, gene expression and subsequent egg reproduction. This work brings us closer to understanding the reproductive biology of the schistosome parasites. Although these recent studies have focused on the role of the male and female schistosome interaction, the surface of the female worm is in direct contact with the host environment as well as the male parasite. In this regard, there is solid evidence that host signals also play a role in schistosome reproductive development, such interactions need to be considered in studies of reproductive development (eg [**9, 36-40]).
As the egg stage is responsible for the pathogenesis in schistosomiasis, understanding the molecular basis for the intricate relationship between the sexes as presented above will identify useful targets for control of parasite development and /or parasite caused morbidity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chitsulo L, Loverde PT, Engels D. Schistosomiasis. Nat Rev Microbiol. 2004;2:12–13. doi: 10.1038/nrmicro801. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann KF, Wynn TA, Dunne DA. Cytokine-mediated host responses during schistosome infections; walking the fine line between immunological control and immunopathology. Adv Parasitol. 2002;52:265–307. doi: 10.1016/s0065-308x(02)52014-5. [DOI] [PubMed] [Google Scholar]
- 3.LoVerde PT, Niles EG, Osman A, Wu W. Schistosoma mansoni male-female interactions. Can J Zool. 2004;82:357–374. [Google Scholar]
- 4.LoVerde PT, Chen L. Schistosome female reproductive development. Parasitol Today. 1991;7:303–308. doi: 10.1016/0169-4758(91)90263-n. [DOI] [PubMed] [Google Scholar]
- 5.Kunz W. Schistosome male-female interaction: induction of germ-cell differentiation. Trends Parasitol. 2001;17:227–231. doi: 10.1016/s1471-4922(01)01893-1. [DOI] [PubMed] [Google Scholar]
- 6.LoVerde P. Sex and Schistosomes: An interesting biological interplay with control implications. J Parasitol. 2002;88:3–13. doi: 10.1645/0022-3395(2002)088[0003:PASASA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann KF. An historical and genomic view of schistosome conjugal biology with emphasis on sex-specific gene expression. Parasitology. 2004;128(Suppl 1):S11–22. doi: 10.1017/S0031182004006213. [DOI] [PubMed] [Google Scholar]
- **8.Knobloch J, Kunz W, Grevelding CG. Herbimycin A suppresses mitotic activity and egg production of female Schistosoma mansoni. Int J Parasitol. 2006;36:1261–1272. doi: 10.1016/j.ijpara.2006.06.004. [DOI] [PubMed] [Google Scholar]; Using a specific inhibitor, the authors demonstrate that Src tyrosine kinases play a central role in the male-derived mitogenic stimulus of female reproductive cells, eggshell precursor gene expression and subsequent egg production
- **9.Osman A, Niles EG, Verjovski-Almeida S, LoVerde PT. Schistosoma mansoni TGF-beta receptor II: role in host ligand-induced regulation of a schistosome target gene. PLoS Pathog. 2006;2:e54. doi: 10.1371/journal.ppat.0020054. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate that a host (human) TGFβ ligand is able to bind to a schistosome TGFβ receptor, tranduce a signal to regulate the expression of a schistosome gene that encodes a gynecophoric canal protein (SmGCP) that is postulated to play a role in worm pairing
- *10.LoVerde PT, Osman A, Hinck AP. Schistosoma mansoni: TGF-β Signaling Pathways. Exp. Parasitol. 2007;117:304–317. doi: 10.1016/j.exppara.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; A detailed review of the TGFb signaling pathway in host-parasite and male-female interactions
- 11.Massagué J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;22:580, 2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies SJ, Pearce EJ. Surface-associated serine-threonine kinase in Schistosoma mansoni. Mol Biochem Parasitol. 1995;70:33–44. doi: 10.1016/0166-6851(95)00002-i. [DOI] [PubMed] [Google Scholar]
- 14.Davies SJ, Shoemaker CB, Pearce EJ. A divergent member of the transforming growth factor beta receptor family from Schistosoma mansoni is expressed on the parasite surface membrane. J Biol Chem. 1998;273:11234–11240. doi: 10.1074/jbc.273.18.11234. [DOI] [PubMed] [Google Scholar]
- 15.Forrester SG, Warfel PW, Pearce EJ. Tegumental expression of a novel type II receptor serine/threonine kinase (SmRK2) in Schistosoma mansoni. Mol Biochem Parasitol. 2004;136:149–156. doi: 10.1016/j.molbiopara.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Knobloch J, Rossi A, Osman A, LoVerde PT, Klinkert MQ, Grevelding CG. Cytological and biochemical evidence for a gonad-preferential interplay of SmFKBP12 and SmTbetaR-I in Schistosoma mansoni. Mol Biochem Parasitol. 2004;138:227–236. doi: 10.1016/j.molbiopara.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Osman A, Niles EG, LoVerde PT. Identification and characterization of a Smad2 homologue from Schistosoma mansoni, a transforming growth factor-beta signal transducer. J Biol Chem. 2001;276:10072–10082. doi: 10.1074/jbc.M005933200. [DOI] [PubMed] [Google Scholar]
- 18.Osman A, Niles EG, LoVerde PT. Expression of functional Schistosoma mansoni Smad4: role in Erk-mediated transforming growth factor beta (TGF-beta) down-regulation. J Biol Chem. 2004;279:6474–6486. doi: 10.1074/jbc.M310949200. [DOI] [PubMed] [Google Scholar]
- 19.Carlo JM, Osman A, Niles EG, Wu W, Fantappie MR, Oliveira FM, Lo Verde PT. Identification and Characterization of a R-Smad orthologue (SmSmad1B) from Schistosoma mansoni. FEBS J. 2007;274:4075–4093. doi: 10.1111/j.1742-4658.2007.05930.x. [DOI] [PubMed] [Google Scholar]
- 20.Beall MJ, Pearce EJ. Human transforming growth factor-beta activates a receptor serine/threonine kinase from the intravascular parasite Schistosoma mansoni. J Biol Chem. 2001;276:31613–31619. doi: 10.1074/jbc.M104685200. [DOI] [PubMed] [Google Scholar]
- 21.Beall J, McGonigle S, Pearce E. Functional conservation of Schistosoma mansoni Smads in TGF beta signaling. Mol Biochem Parasitol. 2000;111:131–142. doi: 10.1016/s0166-6851(00)00307-8. [DOI] [PubMed] [Google Scholar]
- *22.Cheng G, Fu Z, Lin J, Shi Y, Zhou Y, Jin Y, Cai Y. In vitro and in vivo evaluation of small interference RNA-mediated gynaecophoric canal protein silencing in Schistosoma japonicum. J Gene Med. 2009;11:412–421. doi: 10.1002/jgm.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrated using RNAi that knockdown of the S.mansoni gynecophoric protein gene resulted in a reduction in pairing
- 23.Bostic JR, Strand M. Molecular cloning of a Schistosoma mansoni protein expressed in the gynecophoral canal of male worms. Mol Biochem Parasitol. 1996;79:79–89. doi: 10.1016/0166-6851(96)02640-0. [DOI] [PubMed] [Google Scholar]
- 24.Aronstein WS, Strand M. A glycoprotein antigen of Schistosoma mansoni expressed on the gynecophoral canal of mature male worms. Am J Trop Med Hyg. 1985;34:508–512. doi: 10.4269/ajtmh.1985.34.508. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson JW, Mikesh MF, Wheeler EF, LeBaron RG. Developmental expression patterns of Beta-ig (betaIG-H3) and its function as a cell adhesion protein. Mech Dev. 2003;120:851–864. doi: 10.1016/s0925-4773(03)00165-5. [DOI] [PubMed] [Google Scholar]
- **26.Freitas TC, Jung E, Pearce EJ. TGF-beta Signaling Controls Embryo Development in the Parasitic Flatworm Schistosoma mansoni. PLoS Pathog. 2007;3:e52. doi: 10.1371/journal.ppat.0030052. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identified a S. mansoni TGFβligand, SmInAct and demonstrated that it is expressed in vitelline cells among other cell types and plays a central role in egg embryogenesis
- 27.Freitas TC, Jung E, Pearce EJ. A bone morphogenetic protein homologue in the parasitic flatworm, Schistosoma mansoni. Int J Parasitol. 2009;39:281–287. doi: 10.1016/j.ijpara.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *28.Knobloch J, Beckmann S, Burmeister C, Quack T, Grevelding CG. Tyrosine kinase signaling in the reproductive organs of Schistosoma mansoni. Exp Parasitol. 2007;117:318–336. doi: 10.1016/j.exppara.2007.04.006. [DOI] [PubMed] [Google Scholar]; Review of protein tyrosine kinases in the regulation of female reproductive development. The authors propose two models, one for the regulation of development and another for the regulation of p14 (eggshell precursor) gene expression
- 29.Kapp K, Schussler P, Kunz W, Grevelding CG. Identification,isolation and characterisation of a Fyn-like tyrosine kinase from Schistosoma mansoni. Parasitology. 2001;122:317–327. doi: 10.1017/s0031182001007430. [DOI] [PubMed] [Google Scholar]
- 30.Kapp K, Knobloch J, Schussler P, Sroka S, Lammers R, Kunz W, Grevelding CG. The Schistosoma mansoni Src kinase TK3 is expressed in the gonads and likely involved in cytoskeletal organization. Mol Biochem Parasitol. 138:171–182. doi: 10.1016/j.molbiopara.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Ann Rev Biochem. 2000;69:373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- 32.Knobloch J, Winnen R, Quack M, Kunz W, Grevelding CG. A novel Syk-family tyrosine kinase from Schistosoma mansoni which is preferentially transcribed in reproductive organs. Gene. 2002;294:87–97. doi: 10.1016/s0378-1119(02)00760-6. [DOI] [PubMed] [Google Scholar]
- 33.Fantappié MR, Freebern WJ, Osman A, LaDuca J, Niles EG, LoVerde PT. Evaluation of Schistosoma mansoni retinoid X receptor (SmRXR1 and SmRXR2) activity and tissue distribution. Mol Biochem Parasitol. 2001;115:87–99. doi: 10.1016/s0166-6851(01)00274-2. [DOI] [PubMed] [Google Scholar]
- 34.Fantappié MR, Furtado DR, Rumjanek FD, LoVerde PT. A unique nuclear receptor direct repeat 17 (DR17) is present within the upstream region of Schistosoma mansoni female-specific p14 gene. Biochem Biophys Res Commun. 2008;371:689–693. doi: 10.1016/j.bbrc.2008.04.125. [DOI] [PubMed] [Google Scholar]
- 35.Freebern WJ, Osman A, Niles EG, Christen L, LoVerde PT. Identification of a cDNA encoding a retinoid X receptor homologue from Schistosoma mansoni. Evidence for a role in female-specific gene expression. J Biol Chem. 1999;274:4577–4585. doi: 10.1074/jbc.274.8.4577. [DOI] [PubMed] [Google Scholar]
- 36.Amiri P, Locksley RM, Parslow TG, Sadick M, Rector E, Ritter D, McKerrow JH. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992;356:604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- 37.Davies SJ, Grogan JL, Blank RB, Lim KC, Locksley RM, McKerrow JH. Modulation of blood fluke development in the liver by hepatic CD4+ lymphocytes. Science (Wash.,D.C.) 2001;294:1358–1361. doi: 10.1126/science.1064462. [DOI] [PubMed] [Google Scholar]
- 38.Saule P, Adriaenssens E, Delacre M, Chassande O, Bossu M, Auriault C, Wolowczuk I. Early variations of host thyroxine and interleukin-7 favor Schistosoma mansoni development. J Parasitol. 2002;88:849–855. doi: 10.1645/0022-3395(2002)088[0849:EVOHTA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 39.Khayath N, Vicogne J, Ahier A, Benyounes A, Konrad C, Trolet J, Viscogliosi E, Brehm K, Dissous C. Diversification of the insulin receptor family in the helminth parasite Schistosoma mansoni. FEBS J. 2007;274:659–676. doi: 10.1111/j.1742-4658.2006.05610.x. [DOI] [PubMed] [Google Scholar]
- 40.Vicogne J, Cailliau K, Tulasne D, Browaeys E, Yan YT, Fafeur V, Vilain JP, Legrand D, Trolet J, Dissous C. Conservation of epidermal growth factor receptor function in the human parasitic helminth Schistosoma mansoni. J Biol Chem. 2004;279 doi: 10.1074/jbc.M313738200. [DOI] [PubMed] [Google Scholar]
- 41.LoVerde PT, Niles EG, Osman A, Wu W. Gender-specific biology of Schistosoma mansoni: Male/female interactions. In: Secor W. Evan, Colley D., editors. World Class Parasites. volume 10. Kluwer publishing group; 2005. [Google Scholar]