Abstract
A large and growing family of over 70 endogenous lipids of the basic structure N-acyl amide has been identified during the last 10 years. Only a few of these lipids have been characterized for biological activity, however, those that have shown a wide range of activity may act at G-protein coupled receptors (GPCRs). Like orphan GPCRs that are identified as being in the genome and expressed in tissue, the majority of these endogenous lipids many produced throughout the body, some predominately in nervous tissue, remain orphaned. Here, we give a brief history of these orphan lipids and highlight the activity of N-arachidonoyl glycine, and farnesyl pyrophosphate at the orphan receptors GPR18 and GPR92 respectively as well as summarizing the biological and pharmacological data for the recently identified N-palmitoyl glycine that suggests activity at a novel GPCR. Working to deorphanize both lipids and GPCRs together provides a unique opportunity for a greater understanding of cellular signaling and a challenge to find them all a home.
Matching endogenous lipid ligands with GPCRs lead to the discovery of orphan lipids
The lipophilic phytocannabinoid, Δ9-tetrahydrocannabinol (THC), was identified from cannabis in 1965 by Raphael Mechoulam [1]. Δ9-THC and other lipids extracted from the cannabis plant [2] where shown to activate G-protein coupled receptors [for rev see 3] leading to the hypothesis that an endogenous ligand, likely a lipid, must be produced. An endogenous analog to Δ9-THC was identified in Mechoulam’s group in 1992 [4] from porcine brain and named anandamide after the transliteration of the Sanskrit word ananda meaning bliss, and the amide bond between the acyl chain, arachidonic acid, and the amine, ethanolamine. This endogenous cannabinoid, N-arachidonoyl ethanolamine (AEA, Figure 1A) shares a molecular structure with the growing family of novel endogenous N-acyl amide signaling molecules with a wide-range of cellular signaling potential [5]. Both Δ9-THC and AEA were shown to activate the G-protein coupled cannabinoid receptor 1 and induce several physiological and behavioral outcomes including hypothermia, analgesia, hypoactivity and catalepsy [1,4]. Additional characteristics of AEA include binding to the GPCR cannabinoid receptor 2 and activating the transient receptor potential vanilloid type-1 channel (TRPV1) [6–7]. A second endogenous cannabinoid lipid, 2-arachidonoyl glycerol (2-AG), which also binds both cannabinoid receptors, was later identified in rat brain and canine gut [8–9]. The field of endogenous cannabinoid research has exploded during the last decade and our understanding of basic neurophysiology, immune function, memory, appetite, and pain [for revs see: 3, 10–14] have grown from these humble beginning. These two endogenous lipids paved the way for the identification of a growing family of lipids often referred to as endocannabinoids/endovanilloids and are primarily N-acyl amides in structure. In this report we will discuss of this family of “orphan” lipids and two of the GPCRs for which a few of them have found a match. Given the number of orphan lipids now identified and the number of still orphan GPCRs the combination of the two systems will likely provide novel and important insights into cell signaling.
Figure 1.
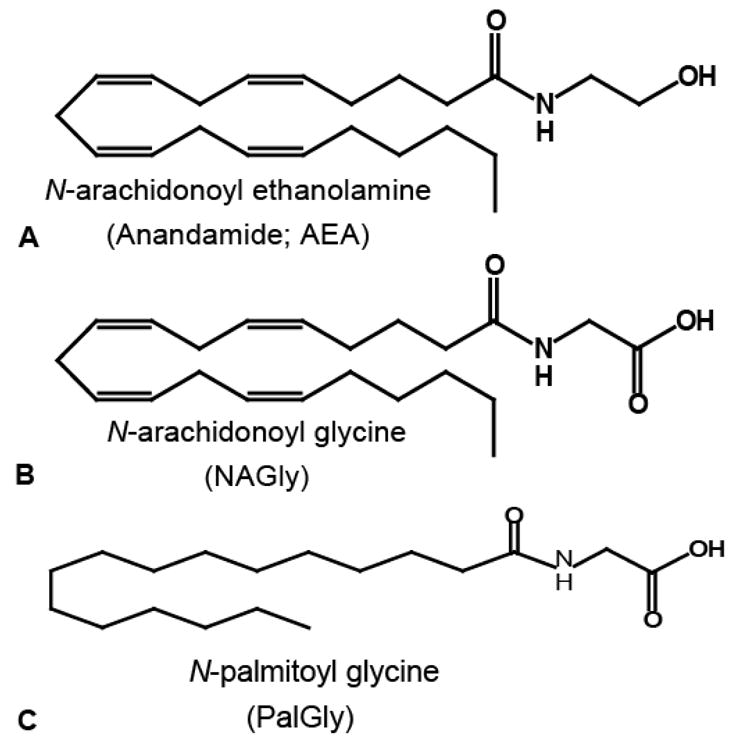
Molecular structure of the endocannabinoid, N-arachidonoyl ethanolamine (AEA), A. B-C) Endogenous structural analogs of AEA, N-arachidonoyl glycine and N-palmitoyl glycine.
Identification of a large family of endogenous N-acyl amides: Orphan lipids
Sumner Burstein and colleagues suggested that N-arachidonoyl glycine (NAGly; Figure 1B) was a putative endogenous compound in 1997 [15]. The methodologies used in the isolation and measurements of AEA in biological samples (lipid extractions and HPLC/MS/MS) enabled the search for other N-acyl amides of similar structure, which were hypothesized to have comparable function. Thus, Huang and colleagues [16] isolated three novel N-acyl amide molecules in the brain and periphery: NAGly, N-arachidonoyl GABA, and N-arachidonoyl alanine. Subsequent work identified N-acyl dopamines; N-arachidonoyl dopamine, N-palmitoyl dopamine, N-stearoyl dopamine and N-oleoyl dopamine, [17–18]. In addition, other research groups identified and characterized N-arachidonoyl serine [19] and the N-acyl taurines [20]. We continued the identification of the N-acyl glycines by way of N-palmitoyl glycine (PalGly) [21] plus another four N-acyl-glycines from oleoyl, linoleoyl, stearoyl, and docosahexaenoyl acyl groups conjugated to glycine [22]. Tan and colleagues while in the lab of the late J Michael Walker [23–24] further demonstrated structural and chromatographic matches for 54 synthesized N-acyl amino acid standards to endogenous compounds isolated in brain. Like the N-acyl ethanolamine, glycines, dopamines, and taurines that have been identified (Figure 2), these novel endogenous lipids are extended members of the N-acyl amide families in that the acyl chains are typically C16, C18, C20 or C22 with 0 to 6 double-bonds and each conjugated to an amino acid. These compounds represent a collection of orphan signaling molecules whose biological activity remains unknown. N-oleoyl ethanolamine and N-palmitoyl ethanolamine (the oleic and palmitic acid-amide analogs of the endocannabinoid, AEA) were deorphaned by the GPCRs, 119 [25] and GPR55 [26] respectively and this is discussed in another paper in this special edition by Kunos and colleagues. Systematic characterization of each of these orphan lipids at orphan GPCRs will open the door to understanding how these novel lipids work in the brain.
Figure 2.
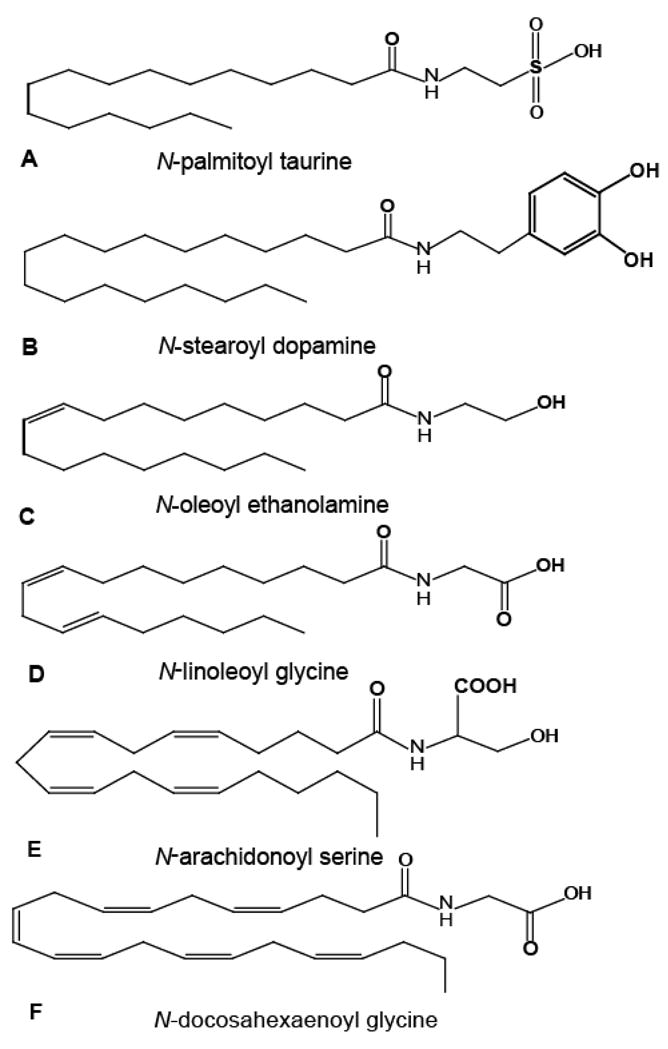
Examples of N-acyl amides that have been identified endogenously and whose targets are still being characterized. Each of these endogenous compounds is a conjugation of an acyl chain ranging from 16–24 carbons in length and contains either 0 or 6 double bonds to a simple amine. A) N-palmitoyl taurine may activate TPRV1 or TRPV4 receptors [20], B) N-stearoyl dopamine is located in mammalian striatum [18], C) N-oleoyl ethanolamine activates GPR119 and plays a role in appetite regulation [38], D) N-linoleoyl glycine is present in most tissues with highest abundance in lung [22], E) N-arachidonoyl serine is present throughout the body and plays a role in vascular function [19], F) N-docosahexaenoyl glycine is present in most tissues with highest abundance in skin [22].
GPR18
The orphan GPCR, GPR18, consists of 331 amino acids and is located in humans, rodents, and canine on chromosome 13 [27]. Studies by Kohno and colleagues [28] found that low concentrations (EC50 ~ 20 nM) of NAGly activate GPR18. NAGly differs from AEA by the oxidation state of the carbon beta to the amido nitrogen (Figure 1B); a modification that drastically reduces its activity at both cannabinoid receptors [29]. Therefore, when NAGly was shown to produce antinociceptive and anti-inflammatory effects in a variety of pain models, it was hypothesized to be through an alternative receptor [16, 30–32]. Consistent with the anti-inflammatory effects of NAGly, GPR18 is highly expressed in peripheral blood leukocytes and several hematopoietic cell lines [28] as well as being highly expressed in the spleen [27]. GPR18 clusters with the Epstien-Barr virus active receptor 2 (EBI2) on chromosome 13 in region q32.3 [33]. EBI2 was also shown to be primary expressed in leukocytes and the spleen [33] and appears to be essential to the immune response to this pathogen. Burstein and colleagues [34] showed that at low concentrations NAGly induces proliferation of T cells, but suppresses production of IL-1β. NAGly also reduces proliferation of the Caco-2, human rectal carcinoma cell line [35]. Additionally, NAGly inhibited the glycine transporter, GLYT2a through direct, non-competitive interactions [36].
The activation of GPR18 in multiple transfected cell lines led to an increase in intracellular calcium and a decrease in the accumulation of cAMP that was abolished by the pre-treatment of PTX [28]. These data support the hypothesis that GPR18 is a Gαi/o coupled receptor. In pancreatic beta cells, NAGly caused intracellular calcium mobilization and insulin release [37]. This effect was blocked by the L-type voltage-gated channel nitredipine (1 μM) or in the absence of extracellular calcium. If the calcium mobilization in the beta cells is through GPR18 activation it would suggest a cooperative mechanism with an ion channel. This type of relationship has been recognized before and was also suspected in the mechanism of action of the endogenous analog to NAGly that was recently identified, N-palmitoyl glycine (PalGly) [21].
N-palmitoyl glycine biological activity
Rimmerman and colleagues [21] showed that the NAGly analog, PalGly (Figure 1C) is produced throughout the body and plays a role in sensory neuronal signaling. The authors found that PalGly is produced following cellular stimulation and occurs in high levels in rat skin and spinal cord. PalGly was upregulated in fatty acid amide hydrolase (FAAH) knockout (KO) mice suggesting a pathway for enzymatic regulation. PalGly potently inhibited heat-evoked firing of nociceptive neurons in rat dorsal horn. In addition, PalGly induced transient calcium influx in native adult DRG cells and a DRG-like cell line (F-11). The effect of PalGly on the latter was characterized by strict structural requirements, PTX-sensitivity and dependence on the presence of extracellular calcium, which suggests actions through a novel GPCR. PalGly-induced calcium influx was blocked by the non-selective calcium channel blockers ruthenium red, SKF96365 and La3+. Furthermore, PalGly contributed to the production of nitric oxide (NO) through calcium-sensitive nitric oxide synthase (NOS) enzymes present in F-11 cells, and was inhibited by the nitric oxide synthase inhibitor 7-NI. Together these data point to a signaling cascade that involves an unidentified GPCR working in conjunction with a calcium channel. An analogous pathway involving the neuropeptide head activator (HA), which drives cells into mitosis by its actions at GPR37, has been previously characterized [38]. GPR37 activates a PTX-sensitive pathway regulating a TRPV-like calcium channel (the growth-factor-regulated calcium-permeable cation channel) that could be inhibited by SK&F 96365 [39].
GPR92
GPCR92 was identified as coupling with a lysophosphatidic acid (LPA) receptor, which when activated increases intracellular cAMP and calcium [40]. These authors demonstrated that GPR92 has ~35% amino acid identity with LPA4 (also known as GPR23) [40]. Expression of GPR92 mRNA was shown at low levels in embryonic brain, moderate levels in skin, spleen, stomach, thymus, lung, and liver and comparatively high levels in small intestine. Another area of relatively high expression of GPCR92 was in the dorsal root ganglion, suggesting that this receptor may contribute to sensory processing [40].
Originally targeted as another LPA receptor, recent evidence supports farnesyl pyrophosphate (FPP; Figure 3), and NAGly as more potent activators of this GPCR [41].
Figure 3.
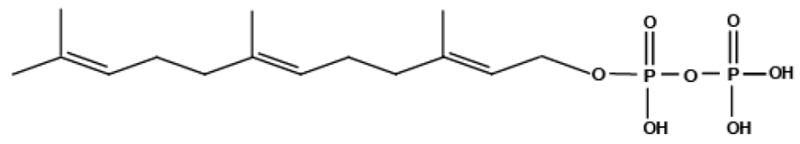
Farnesyl pyrophosphate (FPP). This endogenous bioactive lipid at GPR92 [41] has a similar structure to N-acyl amides in that it is an acyl chain with multiple double bonds conjugated to a more polar head group and is in the middle of the molecular weight of those N-acyl amides identified to date.
FPP is a key intermediate in the biosynthesis of steroids including cholesterol as well as the donor of the farnesyl group for isoprenylation of many proteins, including the βγ subunit of some G proteins and GTPases, Ras and Rho [42]. FPP is synthesized from farnesol via two successive phosphorylation reactions mediated by farnesol kinase and farnesyl phosphate kinase, transitioning first through farnesyl monophosphate (FMP) then to FPP [43]. FPP has a molecular weight of 433.42 amu, which puts it in the amu category of most of the other endogenous lipid molecules recently identified [24] and of the endogenous cannabinoids. At the GPR92 receptor, FPP increases IP production, cAMP levels, and Ca2+ levels in dose-dependent manner. Optimal activation of FPP and NAGly occurred in the presence of LPA, though the FPP/LPA combination had the highest efficacy [41]. Oh and colleagues further demonstrated that FPP and LPA are able to activate Gq/11 and Gs-mediate signaling pathways, while NAGly activated only the Gq/11 pathway suggesting a fine-tuning of this response that is phenotypically distinct depending on the combination of lipids activating the receptors and which G-proteins are available.
Sheardown and colleagues including AH Dickenson who has extensive work in pain mechanisms reported that GPR92 knockout mice did not develop neuropathic pain measured by decreased pain sensitivity subsequent to peripheral nerve injury during the annual Society for Neuroscience meeting [44]. However, this report has not yet been followed by a publication, so these data are still only speculative. Supporting evidence for a role in pain comes from the fact that GPR92 is highly expressed in and largely co-localized with TRPV1 in mouse and human dorsal root ganglion [40], suggesting that this receptor may contribute to processing of noxious sensory stimuli. Again, the likelihood that a GPCR and an ion channel are working together to special
Deorphanizing endogenous lipids
Evolutionary processes have driven the specialization in the expansion and modification of GPCRs. We are at an exciting cross-road to have a myriad of molecular tools to control expression of orphan GPCRs in order to characterize the signaling properties of this diverse group of proteins. Likewise, we are at the beginning of the path to discovery of large families of orphan endogenous lipids. It is interesting to note that while we speak in terms of percent homology of GPCRs across species; endogenous lipids are in essence, 100% homologous among species. N-arachidonoyl ethanolamine is an arachidonic acid conjugated to an ethanolamine in a snail [45] and a human. Therefore, the study of functional relevance of orphan lipids can be done in any species possessing that lipid and there is no need for species-specific reagents to do so. As in the cases of Δ9-THC from cannabis driving the identification of the two cannabinoid GPCRs and how the wide-range of biological effects of NAGly outlined in this review drove the identification of its activity at both GPR18 and GPR92, a greater understanding of functional relevance of endogenous lipids can drive the search for their receptors. The examples presented in this and other reports in the special edition highlight the importance of characterizing the interactions of lipids with GPCRs to understand cellular signaling and ultimately to regulate pathophysiology. Working to deorphanize both lipids and GPCRs provides a unique opportunity and challenge to find them all a home.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mechoulam R, Gaoni Y. A Total Synthesis of Dl-Delta-1-Tetrahydrocannabinol, the Active Constituent of Hashish. J Am Chem Soc. 1965;87:3273–3275. doi: 10.1021/ja01092a065. [DOI] [PubMed] [Google Scholar]
- 2.Mechoulam R, Gaoni Y. Hashish. IV. The isolation and structure of cannabinolic cannabidiolic and cannabigerolic acids. Tetrahedron. 1965;21(5):1223–1229. doi: 10.1016/0040-4020(65)80064-3. [DOI] [PubMed] [Google Scholar]
- 3.Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20 (Suppl 1):10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 4.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 5.Bradshaw HB, Walker JM. The expanding field of cannabimimetic and related lipid mediators. Br J Pharmacol. 2005;144(4):459–465. doi: 10.1038/sj.bjp.0706093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140(5):790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48(3):443–450. [PubMed] [Google Scholar]
- 8.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215(1):89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 9.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 10.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008;7(5):438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- 12.Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 2008;153(2):319–334. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunos G, Osei-Hyiaman D. Endocannabinoids and liver disease. IV. Endocannabinoid involvement in obesity and hepatic steatosis. Am J Physiol Gastrointest Liver Physiol. 2008;294(5):G1101–1104. doi: 10.1152/ajpgi.00057.2008. [DOI] [PubMed] [Google Scholar]
- 14.Pattij T, Wiskerke J, Schoffelmeer AN. Cannabinoid modulation of executive functions. Eur J Pharmacol. 2008;585(2–3):458–463. doi: 10.1016/j.ejphar.2008.02.099. [DOI] [PubMed] [Google Scholar]
- 15.Burstein SMA, Pearson W, Rooney T, Yagen B, Zipkin R, Zurier A. Studies with analogs of anandamide and indomethacin., Symposium on Cannabinoids. International Cannabinoid Research Society; Burlington, VT: 1997. p. 31. [Google Scholar]
- 16.Huang SM, Bisogno T, Petros TJ, Chang SY, Zavitsanos PA, Zipkin RE, Sivakumar R, Coop A, Maeda DY, De Petrocellis L, et al. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J Biol Chem. 2001;276(46):42639–42644. doi: 10.1074/jbc.M107351200. [DOI] [PubMed] [Google Scholar]
- 17.Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci U S A. 2002;99(12):8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JD, Zipkin RE, Daddario N, Appendino G, Di Marzo V, et al. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem. 2003;278(16):13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- 19.Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Batkai S, Mo FM, Offertaler L, Pacher P, Kunos G, et al. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci U S A. 2006;103(7):2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saghatelian A, McKinney MK, Bandell M, Patapoutian A, Cravatt BF. A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry. 2006;45 (30):9007–9015. doi: 10.1021/bi0608008. [DOI] [PubMed] [Google Scholar]
- 21.Rimmerman N, Bradshaw HB, Hughes HV, Chen JS, Hu SS, McHugh D, Vefring E, Jahnsen JA, Thompson EL, Masuda K, et al. N-palmitoyl glycine, a novel endogenous lipid that acts as a modulator of calcium influx and nitric oxide production in sensory neurons. Mol Pharmacol. 2008;74(1):213–224. doi: 10.1124/mol.108.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradshaw HB, Rimmerman N, Hu SS-J, Burstien S, Walker JM. Novel endogenous N-acyl glycines: Identification and Characterization. Vitamins and Hormones. 2009;81 doi: 10.1016/S0083-6729(09)81008-X. (in press) [DOI] [PubMed] [Google Scholar]
- 23.Tan B, Bradshaw HB, Rimmerman N, Srinivasan H, Yu YW, Krey JF, Monn MF, Chen JS, Hu SS, Pickens SR, et al. Targeted lipidomics: discovery of new fatty acyl amides. AAPS J. 2006;8(3):E461–465. doi: 10.1208/aapsj080354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan B, Yu YW, Monn FM, Hughes HV, O’Dell DK, Walker JM. Targeted lipidomics approach for endogenous N-acyl amino acids in rat brain tissue. Journal of Chromatography B. 2009 doi: 10.1016/j.jchromb.2009.01.002. (in press) [DOI] [PubMed] [Google Scholar]
- 25.Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3(3):167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Borrelli F, Izzo AA. Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance. Best Pract Res Clin Endocrinol Metab. 2009;23(1):33–49. doi: 10.1016/j.beem.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Gantz I, Muraoka A, Yang YK, Samuelson LC, Zimmerman EM, Cook H, Yamada T. Cloning and chromosomal localization of a gene (GPR18) encoding a novel seven transmembrane receptor highly expressed in spleen and testis. Genomics. 1997;42 (3):462–466. doi: 10.1006/geno.1997.4752. [DOI] [PubMed] [Google Scholar]
- 28.Kohno M, Hasegawa H, Inoue A, Muraoka M, Miyazaki T, Oka K, Yasukawa M. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun. 2006;347(3):827–832. doi: 10.1016/j.bbrc.2006.06.175. [DOI] [PubMed] [Google Scholar]
- 29.Sheskin T, Hanus L, Slager J, Vogel Z, Mechoulam R. Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J Med Chem. 1997;40 (5):659–667. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- 30.Succar R, Mitchell VA, Vaughan CW. Actions of N-arachidonyl-glycine in a rat inflammatory pain model. Mol Pain. 2007;3:24. doi: 10.1186/1744-8069-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuong LA, Mitchell VA, Vaughan CW. Actions of N-arachidonyl-glycine in a rat neuropathic pain model. Neuropharmacology. 2008;54(1):189–193. doi: 10.1016/j.neuropharm.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Burstein SH, Huang SM, Petros TJ, Rossetti RG, Walker JM, Zurier RB. Regulation of anandamide tissue levels by N-arachidonylglycine. Biochem Pharmacol. 2002;64 (7):1147–1150. doi: 10.1016/s0006-2952(02)01301-1. [DOI] [PubMed] [Google Scholar]
- 33.Rosenkilde MM, Benned-Jensen T, Andersen H, Holst PJ, Kledal TN, Luttichau HR, Larsen JK, Christensen JP, Schwartz TW. Molecular pharmacological phenotyping of EBI2. An orphan seven-transmembrane receptor with constitutive activity. J Biol Chem. 2006;281(19):13199–13208. doi: 10.1074/jbc.M602245200. [DOI] [PubMed] [Google Scholar]
- 34.Burstein SH, Rossetti RG, Yagen B, Zurier RB. Oxidative metabolism of anandamide. Prostaglandins Other Lipid Mediat. 2000;61(1–2):29–41. doi: 10.1016/s0090-6980(00)00053-8. [DOI] [PubMed] [Google Scholar]
- 35.Gustafsson SB, Lindgren T, Jonsson M, Jacobsson SO. Cannabinoid receptor-independent cytotoxic effects of cannabinoids in human colorectal carcinoma cells: synergism with 5-fluorouracil. Cancer Chemother Pharmacol. 2009;63(4):691–701. doi: 10.1007/s00280-008-0788-5. [DOI] [PubMed] [Google Scholar]
- 36.Wiles AL, Pearlman RJ, Rosvall M, Aubrey KR, Vandenberg RJ. N-Arachidonyl-glycine inhibits the glycine transporter, GLYT2a. J Neurochem. 2006;99(3):781–786. doi: 10.1111/j.1471-4159.2006.04107.x. [DOI] [PubMed] [Google Scholar]
- 37.Ikeda Y, Iguchi H, Nakata M, Ioka RX, Tanaka T, Iwasaki S, Magoori K, Takayasu S, Yamamoto TT, Kodama T, et al. Identification of N-arachidonylglycine, U18666A, and 4-androstene-3,17-dione as novel insulin Secretagogues. Biochem Biophys Res Commun. 2005;333(3):778–786. doi: 10.1016/j.bbrc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Rezgaoui M, Susens U, Ignatov A, Gelderblom M, Glassmeier G, Franke I, Urny J, Imai Y, Takahashi R, Schaller HC. The neuropeptide head activator is a high-affinity ligand for the orphan G-protein-coupled receptor GPR37. J Cell Sci. 2006;119(Pt 3):542–549. doi: 10.1242/jcs.02766. [DOI] [PubMed] [Google Scholar]
- 39.Boels K, Glassmeier G, Herrmann D, Riedel IB, Hampe W, Kojima I, Schwarz JR, Schaller HC. The neuropeptide head activator induces activation and translocation of the growth-factor-regulated Ca(2+)-permeable channel GRC. J Cell Sci. 2001;114(Pt 20):3599–3606. doi: 10.1242/jcs.114.20.3599. [DOI] [PubMed] [Google Scholar]
- 40.Lee CW, Rivera R, Dubin AE, Chun J. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J Biol Chem. 2007;282(7):4310–4317. doi: 10.1074/jbc.M610826200. [DOI] [PubMed] [Google Scholar]
- 41.Oh DY, Yoon JM, Moon MJ, Hwang J-I, Choe H, Lee JY, Kim JI, Kim S, Rhim H, O’Dell DK, et al. Identification of Farnesyl Pyrophosphate and N-Arachidonylglycine as Endogenous Ligands for GPR92. J Biol Chem. 2008;283(30):21054–21064. doi: 10.1074/jbc.M708908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liliom K, Tsukahara T, Tsukahara R, Zelman-Femiak M, Swiezewska E, Tigyi G. Farnesyl phosphates are endogenous ligands of lysophosphatidic acid receptors: inhibition of LPA GPCR and activation of PPARs. Biochim Biophys Acta. 2006;1761(12):1506–1514. doi: 10.1016/j.bbalip.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westfall D, Aboushadi N, Shackelford JE, Krisans SK. Metabolism of farnesol: phosphorylation of farnesol by rat liver microsomal and peroxisomal fractions. Biochem Biophys Res Commun. 1997;230(3):562–568. doi: 10.1006/bbrc.1996.6014. [DOI] [PubMed] [Google Scholar]
- 44.Sheardown M, Messager S, Mathews E, Dickenson AH, Aparicio S, Brice NL. Knockout of GPR92 reveals key role in neuropathic pain. Soc for Neurosci Abstracts. 2004;64.16 [Google Scholar]
- 45.Lemak MS, Bravarenko NI, Bobrov MY, Bezuglov VV, Ierusalimsky VN, Storozhuk MV, Malyshev AY, Balaban PM. Cannabinoid regulation in identified synapse of terrestrial snail. Eur J Neurosci. 2007;26(11):3207–3214. doi: 10.1111/j.1460-9568.2007.05945.x. [DOI] [PubMed] [Google Scholar]


