Abstract
Sodium-taurocholate cotransporting polypeptide (Ntcp) and bile salt export pump (Bsep) are two key transporters for hepatic bile acid uptake and excretion. Alterations in Ntcp and Bsep expression have been reported in pathophysiological conditions. In the present study, the effects of age, gender, and various chemicals on the regulation of these two transporters were characterized in mice. Ntcp and Bsep mRNA levels in mouse liver were low in the fetus, but increased to its highest expression at parturition. After birth, mouse Ntcp and Bsep mRNA decreased by more than 50%, and then gradually increased to adult levels by day 30. Expression of mouse Ntcp mRNA and protein exhibit higher levels in female than male livers, which is consistent with the trend of human NTCP mRNA expression between men and women. No gender difference exists in BSEP/Bsep expression in human and mouse livers. Hormone replacements conducted in gonadectomized, hypophysectomized, and lit/lit mice indicate that female-predominant Ntcp expression in mouse liver is due to the inhibitory effect of male-pattern GH secretion, but not sex hormones. Ntcp and Bsep expression are in general resistant to induction by a large battery of microsomal enzyme inducers. Administration of cholestyramine increased Ntcp, whereas chenodeoxycholic acid increased Bsep mRNA expression. In silico analysis indicates that female-predominant mouse and human Ntcp/NTCP expression may be due to GH. In conclusion, mouse Ntcp and Bsep are regulated by age, gender, cholestyramine, and bile acid, but resistant to induction by most microsomal enzyme inducers.
Keywords: Ntcp, Bsep, Gender, ontogeny, microsomal enzyme inducer, enterohepatic circulation of bile acids
1. Introduction
Bile acids secreted into bile undergo enterohepatic circulation (97%). Both hepatic uptake and biliary excretion of bile acids are driven by transport proteins. Bile acid uptake from blood into liver is mediated mainly by the Na+-taurocholate cotransporting polypeptide (Ntcp), and efflux from liver into bile by the bile salt export pump (Bsep).
Ntcp (gene symbol Slc10a1) has been cloned in various species [1-4], and localized to the basolateral membrane of hepatocytes. Ntcp transports all physiological bile acids [5-7]. Bsep (gene symbol Abcb11) is responsible for the canalicular excretion of bile acids [8, 9]. Targeted inactivation of the mouse Bsep gene results in mild, non-progressive, but persistent intrahepatic cholestasis [10]. In contrast, a mutation in human BSEP gene is responsible for progressive familial intrahepatic cholestasis subtype 2 (PFIC-2) [11, 12].
Ntcp mRNA and protein expression is uniformly down-regulated in all experimental models of cholestasis and liver disease [13-17]. In contrast, Bsep expression is only modestly impaired during cholestasis even with complete bile-duct obstruction [16, 18].
Bile acids in general are thought to decrease Ntcp expression through farnesoid X receptor (FXR)-small heterodimer partner (Shp) pathway, and increase Bsep expression through direct FXR activation [19-21].
In rats, Ntcp mRNA expression is male-predominant, which is due to the inhibitory effect of female-pattern GH secretion [22]. However, it is not known whether mouse, rat, and human Ntcp/NTCP expression share similar gender predominance and underlying regulatory mechanisms. Except for the influence of bile acids, the regulation of Ntcp and Bsep is not complete. A few studies have been performed in rats, some in humans, but less in mice. Therefore, in the present study, we determined the effects of age, gender, and various chemicals, including microsomal enzyme inducers, cholestyramine, and chenodeoxycholic acid (CDCA) on the regulation of Ntcp and Bsep mRNA expression in mouse livers and evaluate their human relevance.
2. Materials and Methods
2.1. Materials
Sodium chloride, HEPES sodium salt, HEPES free acid, lithium lauryl sulfate, EDTA, and D-(+)-glucose were purchased from Sigma-Aldrich (St. Louis, MO). Micro-O-protect was purchased from Roche Diagnostics (Indianapolis, IN). Formaldehyde, 3-(N-morpholino)propanesulfonic acid, sodium citrate, and NaHCO3 were purchased from Fischer Chemicals (Fairlawn, NJ). Chloroform, agarose, and ethidium bromide were purchased from AMRESCO Inc. (Solon, OH). 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) was a gift from Dr. Karl Rozman (University of Kansas Medical Center, Kansas City, KS), and oltipraz was a gift from Dr. Steven Safe (Texas A&M University, TX). Polychlorinated biphenyl 126 (PCB 126) was obtained from AccuStandard (New Haven, CT). All other chemicals, unless otherwise indicated, were purchased from Sigma-Aldrich Co. (St. Louis, MO). All hormones and their respective vehicles used in the present study were purchased from Innovative Research of America (Sarasota, FL).
2.2. Animals and breeding
Eight-week-old adult male and female C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine), and housed according to the American Animal Association Laboratory Animal Care guidance. For the ontogenic study, mice were bred in the animal facilities at the University of Kansas Medical Center. Livers from male and female C57BL/6 mice were collected at -2, 0, 5, 10, 15, 22, 30, 35, 40, 45, and 56 days of age (n = 5/gender/age). Livers were collected and snap-frozen in liquid nitrogen, and stored at -80°C. Breeding pairs of PXR-null mice in the C57BL/6 background were kindly provided by Dr. Frank J. Gonzalez [23].
For the animal experiments performed in the present study, unless otherwise indicated, mouse tissues were collected between 8:30 am and 11:00 am.
2.3. Human liver tissues
100 individual human liver tissues (from 60 men and 40 women) were purchased from Xenotech (Lenexa, KS). Human livers were cut into pieces and preserved in the TRIZOL® Reagent (Invitrogen, Carlsbad, CA).
2.4. Microsomal enzyme inducer treatments
Groups of five mice were administered one of the following chemicals once daily for 4 days: aryl hydrocarbon receptor (AhR) ligands: TCDD (40 μg/kg, ip in corn oil), β-naphthoflavone (BNF, 200 mg/kg, ip in corn oil), and PCB126 (300 μg/kg, po in corn oil); constitutive androstane receptor (CAR) activators: phenobarbital (PB, 100 mg/kg, ip in saline), 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP, 3 mg/kg, ip in corn oil), and diallyl sulfide (DAS, 200 mg/kg, ip in corn oil); pregnane X receptor (PXR) ligands: pregnenolone-16α-carbonitrile (PCN, 200 mg/kg, ip in corn oil), spironolactone (SPR, 200 mg/kg, ip in corn oil), and dexamethasone (DEX, 50 mg/kg, ip in corn oil); peroxisome proliferator-activated receptor alpha (PPARα) ligands: clofibric acid (CLFB, 500 mg/kg, ip in saline), ciprofibrate (CPFB, 40 mg/kg, ip in saline), and diethylhexylphthalate (DEHP, 1000 mg/kg, po in corn oil); nuclear factor erythroid 2-related factor 2 (Nrf2) activators: butylated hydroxyanisole (BHA, 350 mg/kg, ip in corn oil), ethoxyquin (ETHOXYQ, 250 mg/kg, po in corn oil), and oltipraz (OPZ, 150 mg/kg, po in corn oil). Four different vehicle control groups (corn oil ip, corn oil po, saline po, and saline ip) were used. All injections were administered in a volume of 10 ml/kg. Livers were removed on day 5, 24 hrs after the last treatment, snap-frozen in liquid nitrogen, and stored at -80°C.
Wild-type and PXR-null mice (n=5) were treated once daily for four days with spironolactone (SPR, 200 mg/kg, ip in corn oil) and dexamethasone (DEX, 50 mg/kg, ip in corn oil). Control groups were treated with corn oil. On day 5, liver tissues were collected, snap-frozen in liquid nitrogen, and stored at -80°C.
2.5. Cholesterol/chenodeoxycholic acid/ cholestyramine treatment
These treatments were reported previously [24]. Briefly, the 1% cholesterol diet was formulated from the control diet (Harlan Teklad Rodent Diet, type W) supplemented with 1% w/w cholesterol. After 1 week on experimental diets, mice (n=6/treatment) were euthanized between the 5th and 7th hours of the light period. 1% Chenodeoxycholic acid (CDCA) or 3% cholestyramine (both w/w) were added to powdered control diets (Harlan Teklad Rodent Diet, type W). After 10 days of feeding, mice (n=5-7/treatment) were euthanized between the 5th and 7th hours of the dark period.
2.6. Sex hormone replacement in gonadectomized mice
Mice were castrated or ovariectomized at 37 days of age by Charles River Laboratories (Wilmington, MA). At 54 days of age, vehicle placebo, 5α-dihydroxytestosterone (DHT, 5 mg), or 17β-estradiol (E2, 0.5 mg) in 21-day-release pellets (Innovative Research of America, Sarasota, FL) was subcutaneously implanted in the gonadectomized male and female mice. The mice were separated into six treatment groups (n = 5/gender/treatment): (1) castrated male mice replaced with placebo, (2) castrated male mice replaced with DHT, (3) castrated male mice replaced with E2, (4) ovariectomized female mice replaced with placebo, (5) ovariectomized female mice replaced with DHT, and (6) ovariectomized female mice replaced with E2. Intact, untreated, age-matched mice were used as control. Livers were removed at 64 days of age from gonadectomized and age-matched intact control mice.
2.7. Growth hormone replacement in hypophysectomized mice
Mice were hypophysectomized at 30 days of age by Charles River Laboratories (Wilmington, MA). Hypophysectomized mice received water with 5% glucose (w/v) ad libitum. Hypophysectomized mice that gained weight before the start of the study were excluded under the assumption that their surgery was incomplete. The mice were treated with placebo, rat GH in male-pattern (twice daily, intraperitoneal injection, dose at 2.5 mg /kg body weight), or rat GH in female-pattern (continuous infusion via subcutaneously implanted 21-day-release 1mg rGH pellet). Intact, untreated, age-matched mice were used as controls. After 10 days of treatment, livers were removed for total RNA isolation.
2.8. Growth hormone replacement in lit/lit mice
Breeding pairs of GH-releasing hormone receptor mutant heterozygous mice (lit/+) were purchased from Jackson Laboratory (Bar Harbor, MA). After breeding in the University of Kansas Medical Center laboratory animal facilities, lit/lit (dwarf mice with an inactivating mutation of GHRH receptor) were used in this study. Their respective lit/+ and +/+ mice (characterized by normal body size) were used as controls. The mice were treated for one week with vehicle, rat GH in male-pattern (twice daily, intraperitoneal injection, dose of 2.5 mg /kg body weight), or rat GH in female-pattern (continuous infusion via subcutaneously implanted 21-day-release 1mg rGH pellet). After the treatments, livers were removed for total RNA isolation.
2.9. Total RNA isolation
Total RNA was isolated from mouse tissues using RNA Bee reagent (Tel-Test Inc., Friendswood, TX) per the manufacturer's protocol. The concentration of total RNA in each sample was quantified spectrophotometrically at 260 nm. The integrity of each RNA sample was evaluated by formaldehyde-agarose gel electrophoresis before analysis. Total RNA from human livers was isolated according to Invitrogen's protocol (Carlsbad, CA).
2.10. Branched DNA (bDNA) signal amplification assay
The mRNA for mouse Ntcp and Bsep was quantified using the Quantigene® bDNA signal amplification kit (Panomics Inc., Fremont, CA). The gene sequences of mouse Ntcp and Bsep were accessed from GenBank (Table 1). The strategy of multiple oligonucleotide probe set design has been described previously [25]. All probes were synthesized by Operon Technologies (Palo Alto, CA). The luminescence for each gene is reported as relative light units (RLUs) per 8 μg of total RNA.
Table 1. Oligonucleotide probes generated for analysis of mouse Ntcp and Bsep mRNAs expression by Quantigene branched DNA signal amplification assay.
| Ntcp (MMU95131a) | Bsep (NM_021022) | ||
|---|---|---|---|
| CEb | gccttgatcttgctgaactccaTTTTTctcttggaaagaaagt | CE | ggtcgagaaggatatgtaaatttacaTTTTTctcttggaaagaaagt |
| CE | gaggatggccagagcctcaTTTTTctcttggaaagaaagt | CE | atgtttgaacggaggaactgaaTTTTTctcttggaaagaaagt |
| CE | ttgctgtagatgtataagaggagaggTTTTTctcttggaaagaaagt | CE | tttgcggcagctatagctctcTTTTTctcttggaaagaaagt |
| CE | gccttttggacttcaggaagatcTTTTTctcttggaaagaaagt | CE | ccgcgagagagttgagagccTTTTTctcttggaaagaaagt |
| LE | tgtctgtggcccggtggTTTTTaggcataggacccgtgtct | CE | ccctctctggctttgtccagagTTTTTctcttggaaagaaagt |
| LE | agaatgacgctgagcgcagTTTTTaggcataggacccgtgtct | LE | tgcccaggatccacagataccTTTTTaggcataggacccgtgtct |
| LE | catgatgagcagcaacataactaccTTTTTaggcataggacccgtgtct | LE | ccaacgaatgccagcgtcTTTTTaggcataggacccgtgtct |
| LE | tggtgcagccaagcgagagTTTTTaggcataggacccgtgtct | LE | gcttttaccacacccactgctcTTTTTaggcataggacccgtgtct |
| LE | gccactatggcgatgatcaccTTTTTaggcataggacccgtgtct | LE | tccaacagctggatgctggtTTTTTaggcataggacccgtgtct |
| LE | caggagagcagccgcagatTTTTTaggcataggacccgtgtct | LE | tgatcgggatcgtagaaccgtTTTTTaggcataggacccgtgtct |
| LE | aagaggttagacaggttcccccTTTTTaggcataggacccgtgtct | LE | ccatctatcatcaccgttcccTTTTTaggcataggacccgtgtct |
| LE | cacaatgctgaggttcatgtccTTTTTaggcataggacccgtgtct | LE | cattgacttttttgctgtcgtgaTTTTTaggcataggacccgtgtct |
| LE | aagctggagcaggtggtcatTTTTTaggcataggacccgtgtct | LE | gctcctgggagacaatcccaTTTTTaggcataggacccgtgtct |
| LE | ttaagatctccgtcgtagattcctTTTTTaggcataggacccgtgtct | LE | cattatgctacagtcaaataacacggTTTTTaggcataggacccgtgtct |
| LE | cctttgtagggcaccttgtccTTTTTaggcataggacccgtgtct | LE | ttgtccccatacttgatgttgtcTTTTTaggcataggacccgtgtct |
| LE | cctatggcgcaaggaatgaTTTTTaggcataggacccgtgtct | LE | agtcatgcagctgagcctgcTTTTTaggcataggacccgtgtct |
| BL | cctttgggcttccagaagtga | LE | ctggatcccaacattagtttcatatTTTTTaggcataggacccgtgtct |
| BL | ggggcatgataccgtactgg | LE | aatagcaatgcgttgtttctccTTTTTaggcataggacccgtgtct |
| BL | gcccagaaggaaagcactga | LE | tcgtacaatggcccgagcTTTTTaggcataggacccgtgtct |
| BL | atgctggtcagatgaaagacctt | LE | catccagtagcaagattttaggatcTTTTTaggcataggacccgtgtct |
| BL | cccttcatggccagggtg | LE | tctgtgtctaaggcagatgtagcttTTTTTaggcataggacccgtgtct |
| BL | catcatgcccaaggcagtg | LE | caagctgcactgtcttttcacttTTTTTaggcataggacccgtgtct |
| BL | gaaccatgacgagtgataacataatg | LE | agcaatgacaatacaggtccgaTTTTTaggcataggacccgtgtct |
| LE | agttctggatagtagacaagcgatgTTTTTaggcataggacccgtgtct | ||
| LE | gacatgacggcgatgatatctgTTTTTaggcataggacccgtgtct | ||
| BL | gacagtccattcagaacttgtatatca | ||
| BL | tccacggagatctctttggtg | ||
| BL | ttctctgggagtgacatgacga | ||
GenBank accession numbers for each transcript are given in parenthesis after the gene name.
The type of Function of each bDNA oligonucleotide probe. CE, capture extender; LE, label extender; BL, blocker.
NTCP and BSEP mRNA expression in human livers were determined by the QuantiGene Plex assay (Panomics, Inc., Fremont, CA). The data are reported as ratio of mRNA expression of NTCP (or BSEP) to GAPDH mRNA expression per 3 μg of total RNA.
2.11. Membrane protein preparation
Crude plasma membrane samples were prepared from mouse livers according to the method described previously [26]. Briefly, 0.2–0.3 g liver was minced in 10 ml ice-cold homogenizing buffer (0.25 M sucrose, 10 mM Tris–HCl [pH 7.5], containing 25 μg/ml leupeptin, 50 μg/ml aprotinin, 40 μg/ml PMSF, 0.5 μg/ml pepstatin, and 50 μg/ml antipain). The minced tissue was poured into a Dounce homogenizer (Kontes, Vineland, NJ) and homogenized on ice for 10 strokes. The homogenate was filtered through one layer of gauze sponges (Tyco Healthcare Group LP, Mansfield, MA), and then centrifuged at 100,000 × g for 1 hr at 4 °C. The resulting pellet was dissolved in resuspension buffer (0.25 M sucrose, 10 mM HEPES [pH 7.5], and 40 μg/ml PMSF). Protein concentration of each sample was determined with a Bradford protein assay kit from Sigma (St. Louis, MO).
2.12. Western blots
Membrane protein samples mixed with sample loading buffer (75 μg protein/lane) were loaded after heating onto a 10% SDS-polyacrylamide gel. Following electrophoresis, proteins in the gel were electrotransferred to nitrocellulose membrane for 4.5 hrs at 34 volts at 4 °C. Membranes were blocked for 2 hrs at room temperature with 5% non-fat dry milk in Tris–buffered saline containing 0.1% Tween-20 (TBS-T). Blots were then incubated overnight with polyclonal antibody of rat Ntcp and Bsep (kindly provided by Dr. Bruno Stieger, Department of Medicine, University Hospital, Zurich, Switzerland) at room temperature. β-actin antibody (Abcam Inc, Cambridge, MA) was used as a loading control. After thorough washing (three 20-min washes with excess TBS-T), blots were incubated with donkey anti-rabbit IgG horseradish peroxidase-linked secondary antibody (1:5,000 dilution with 5% non-fat milk in TBS-T) for 1 hr. Blots were washed again. Immunoreactive bands were detected with an enhanced chemical luminescence (ECL) kit (GE Healthcare Bio-Sciences Corp, Piscataway, NJ). Ntcp and Bsep proteins were visualized by exposure to Fuji Medical X-Ray film. The protein band intensity on the films was quantified with Gel-Pro 3.1 image analysis software (MediaCybernetics, Silver Spring, MD).
2.13. In silico analysis of prospective DNA response elements in 6 KB of the promoter/enhancer region of mouse and human Ntcp/NTCP and Bsep/BSEP
6 KB of promoter/enhancer sequences of mouse and human Ntcp/NTCP and Bsep/BSEP were accessed from a genomic DNA database (www.ensembl.org). The DNA sequences were analyzed by Nubiscan (http://www.nubiscan.unibas.ch/) and Alibaba2.1 (http://www.gene-regulation.com/pub/programs/alibaba2/). Putative response element screening of signal transducer and activator of transcription (Stat)5b, a critical transcription factor for growth hormone signaling, was based on the Stat5b consensus DNA-binding sequence (TTCnnGGAA) [27, 28].
2.14. Statistical analysis
Data are presented as mean ± S.E.M. Data were analyzed by one-way ANOVA, followed by Duncan's post-hoc test. Statistical significance was set at p < 0.05. When the difference between genders in a specific tissue was determined, data were analyzed by student's T-test, and statistical significance was considered at p < 0.05.
3. Results
3.1. Constitutive expression of Ntcp and Bsep in adult male and female mouse and human livers
Both Ntcp/NTCP and Bsep/BSEP have been shown to be highly expressed in mouse and human livers [1, 18, 29, 30]. Ntcp protein expression is 60% higher in female than male mouse liver (Fig. 1a), and Ntcp mRNA is 70% higher in female than male mouse livers (Fig. 1b). In human livers, NTCP mRNA is 43% higher in women than men; however, it is not statistically different due to high variation in individual human NTCP expression. In contrast, no gender differences exist in mouse Bsep expression at either the protein or mRNA levels, or in human BSEP mRNA expression (Fig. 1a and 1b).
Fig. 1. mRNA and protein expression of Ntcp/NTCP and Bsep/BSEP in mouse and human livers.
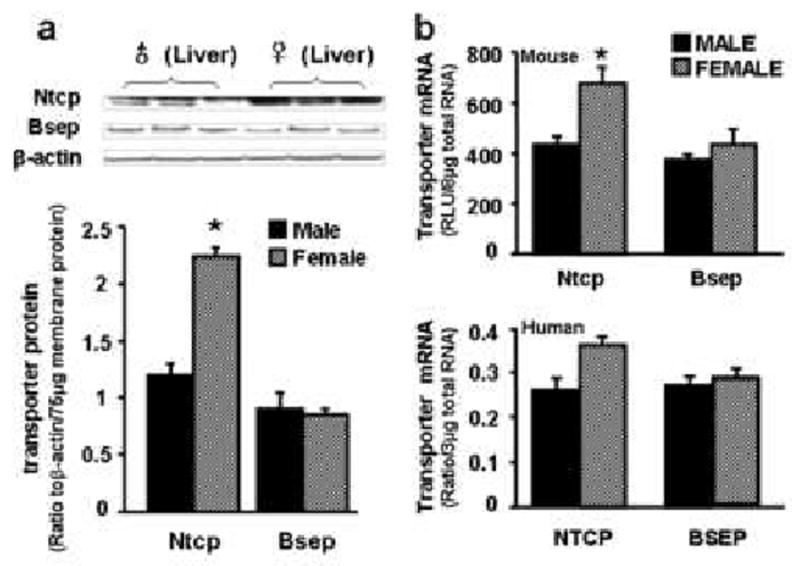
a) Protein levels of Ntcp and Bsep in male and female mouse livers. In the top panel, protein levels of Ntcp and Bsep in liver plasma membranes were analyzed by western blotting. In the bottom panel, protein levels of each transporter in male and female mouse livers are expressed as ratio of Ntcp (or Bsep) to β-actin protein levels per 75μg total membrane protein. Data are presented as mean ± S.E.M. Asterisks indicate statistically significant differences between male and female mice (p<0.05). b) mRNA expression of Ntcp/NTCP and Bsep/BSEP in mouse and human livers. Total RNA from both male and female C57BL/6 mice (n = 10/gender) and human livers (30 male Caucasians and 20 female Caucasians) was analyzed by the bDNA assay for mRNA expression of each transporter. Mouse data (on the top) are reported as relative light unit (RLU) per 10 μg of total RNA. Human data (on the bottom) are reported as ratio of NTCP (or BSEP) to GAPDH mRNA expression per 3 μg of total RNA. Both human and mouse data are presented as mean ± S.E.M. Asterisks indicate statistically significant differences between male and female mice (p<0.05).
3.2. Ontogeny of Ntcp and Bsep in male and female mouse livers
The developmental patterns of Ntcp and Bsep mRNA expression in male and female mouse livers are shown in Fig. 2. Both Ntcp and Bsep mRNA expression are very low before birth. At birth, both Ntcp and Bsep mRNA increased to their highest expression at any age, about 50% higher than adult levels. Ntcp mRNA expression then rapidly returned to low levels after birth, and remained low until about 3 weeks of age, at which time the expression of both bile acid transporters reached adult levels. Gender-dimorphic Ntcp mRNA expression became apparent about 45 days of age, with higher expression in female than male mouse livers (Figs. 1 and 2).
Fig. 2. Ontogenic expression of mouse Ntcp and Bsep mRNA in mouse livers.
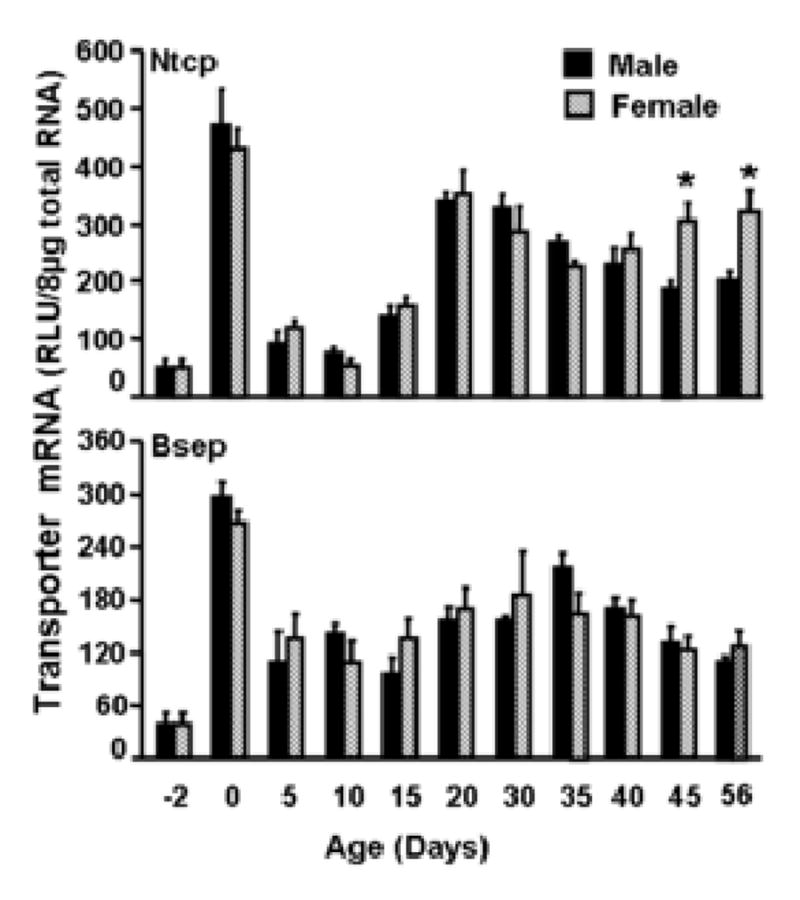
Total RNA from C57BL/6 mice at each age (n = 5/gender) was analyzed by the bDNA assay for expression of Ntcp or Bsep mRNA. Data are presented as mean ± S.E.M. Asterisks indicate statistically significant differences between male and female mice (p<0.05).
3.3. Regulation of Ntcp mRNA by sex hormones and growth hormone
Figs. 1 and 2 illustrate that Ntcp mRNA expression is higher in adult female than male mouse livers. The importance of sex hormones and growth hormone (GH) in the Ntcp female-predominant expression was further determined.
Regulation of Ntcp expression by sex hormones was investigated in gonadectomized mice, as shown in Fig. 3a. Gonadectomy decreased Ntcp mRNA expression in female mouse livers, but not in males. Neither androgen (5α-dihydrotestosterone, DHT) nor estrogen (17β-estradiol, E2) administration altered Ntcp mRNA expression in gonadectomized mice.
Fig. 3. Regulatory mechanism characterization of female-predominant Ntcp expression in mouse liver.
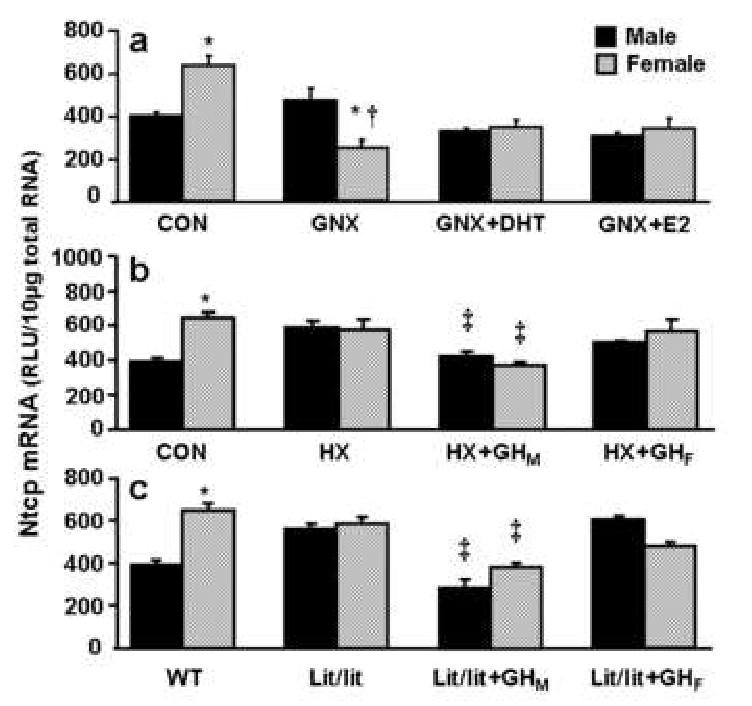
The solid, black bar represents Ntcp mRNA in males; the striated bar depicts Ntcp mRNA in females. Total liver RNA was isolated and analyzed by the bDNA signal amplification assay for Ntcp mRNA content. The data are presented as mean ± SEM (each group, n = 5 mice). a) Effects of gonadectomy and sex hormone replacements on mouse Ntcp mRNA expression in livers from intact and gonadectomized male and female mice. GNX, placebo administered to gonadectomized mice; GNX + DHT, 5α-dihydroxytestosterone administered to gonadectomized mice; and GNX + E2, 17β-estradiol administered to gonadectomized mice. Asterisks (*) represent statistically significant differences (p < 0.05) between males and females in intact mice and the same treated gonadectomized mouse group; single dagger (†) represent statistically significant differences (p < 0.05) between intact mice and the same gender, untreated gonadectomized mice; and double dagger (‡) represents statistically significant differences (p < 0.05) between untreated gonadectomized mice and the same gender, gonadectomized mice administered 5α-dihydroxytestosterone, or 17β-estradiol. b) Effects of hypophysectomy and growth hormone on mouse Ntcp mRNA expression in livers from intact and hypophysectomized male and female mice. HX, placebo administered to hypophysectomized mice; HX+GHM, rat growth hormone twice daily administered by intraperitoneal injection to hypophysectomized mice mimicking male-pattern growth hormone secretion; and HX+GHF, continuous infusion to hypophysectomized mice via subcutaneously implanted 21-day-release 1mg rat GH pellet mimicking female-pattern growth hormone secretion. Asterisks (*) represents statistically significant differences (p < 0.05) between males and females in intact mice and the same treated hypophysectomized mouse group; single dagger (†) represent statistically significant differences (p < 0.05) between intact mice and the same gender, untreated hypophysectomized mice; and double dagger (‡) represents statistically significant differences (p<0.05) between untreated hypophysectomized mice and the same gender, or hypophysectomized mice following hormone replacement treatments. c) Effects of growth hormone on mouse Ntcp mRNA expression in livers from intact and lit/lit male and female mice. Lit/lit, placebo administered to Lit/lit mice; Lit/lit +GHM, rat growth hormone twice daily administered by intraperitoneal injection to Lit/lit mice mimicking male-pattern growth hormone secretion; Lit/lit+GHF, continuous infusion to Lit/lit mice via subcutaneously implanted 21-day-release 1mg rat growth hormone pellet mimicking female-pattern growth hormone secretion.
Two mouse models were used to determine the effects of GH on the regulation of Ntcp expression: hypophysectomized mice and lit/lit mice. In hypophysectomized mice, not only GH, but also other pituitary hormones, such as prolactin, luteinizing hormone, and follicle-stimulating hormone are depleted. In hypophysectomized mice, the gender difference in Ntcp mRNA expression disappeared due to an increase in Ntcp mRNA in the male mice (Fig. 3b). Male-pattern GH administration to hypophysectomized mice decreased Ntcp mRNA expression in both sexes; however female-pattern GH replacement didn't alter Ntcp mRNA expression (Fig. 3b).
In lit/lit male mice, liver Ntcp mRNA expression was higher than that in wild-type male mice, and the gender difference in Ntcp was not observed in the lit/lit mice (Fig. 3c). Male-pattern GH administration to lit/lit mice decreased Ntcp mRNA expression. Female-pattern GH administration in lit/lit mice did not alter Ntcp mRNA expression (Fig. 3c).
3.4. Chemical regulation of mouse Ntcp and Bsep
Male C57BL/6 mice were administered five classes of prototypical drug-metabolizing enzyme inducers. For each class, three chemicals were used that are known to transcriptionally activate a specific signaling pathway. The doses were chosen according to what is commonly cited in the literature to activate specific transcription factors that target specific biotransformation enzymes: cytochrome P450 enzymes (Cyp1a1, Cyp2b10, Cyp3a11, and Cyp4A14) and NAD(P)H:quinone oxidoreductase (Nqo) 1, as reported previously [31]. The regulation of mouse Ntcp and Bsep by drug-metabolizing enzyme inducers is shown in Fig. 4. The AhR, CAR, PXR, PPARα, and Nrf2 activators as a class did not alter Ntcp and Bsep mRNA expression. Individually, the PXR ligands dexamethasone and spironolactone increased mRNA expression of Ntcp and Bsep, respectively.
Fig. 4. mRNA expression of Ntcp and Bsep in adult male C57BL/6 mouse liver after administration of prototypical phase-I and phase-II drug-metabolizing enzyme inducers.
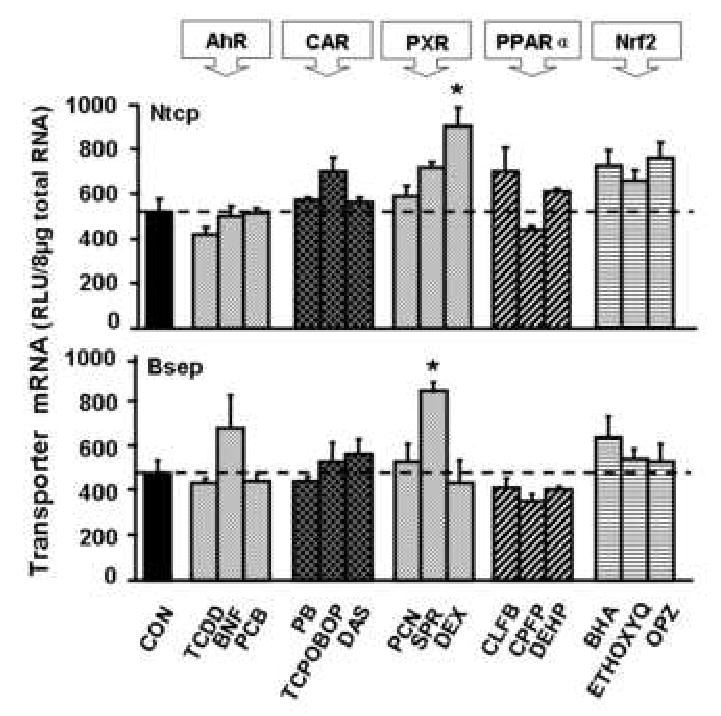
Mice were administrated once daily for 4 days with each of microsomal enzyme inducers (n=5/group) as detailed in Materials and methods. Total RNA from five chemically treated male livers was analyzed by the bDNA assay. All data were expressed as mean ± S.E.M. for five mice in each group, except for control groups, which were combined from the four individual control groups after it was determined that they were not statistically different. Asterisk indicates statistically significant difference between treated and control mice (p < 0.05).
3.5. Regulation of Ntcp by dexamethasone and Bsep by spironolactone
Regulation of mouse Ntcp by DEX in PXR-null mice is depicted in Fig. 5a. In wild-type mice, DEX increased Ntcp mRNA expression (130%). Disruption of PXR function did not alter constitutive expression of Ntcp mRNA, however in PXR-null mice, DEX only increased Ntcp mRNA levels 34%, indicating an attenuation of the up-regulation of Ntcp by DEX compared with treatment of wild-type mice.
Fig. 5. Regulation of mouse Ntcp by dexamethasone and Bsep by spironolactone.
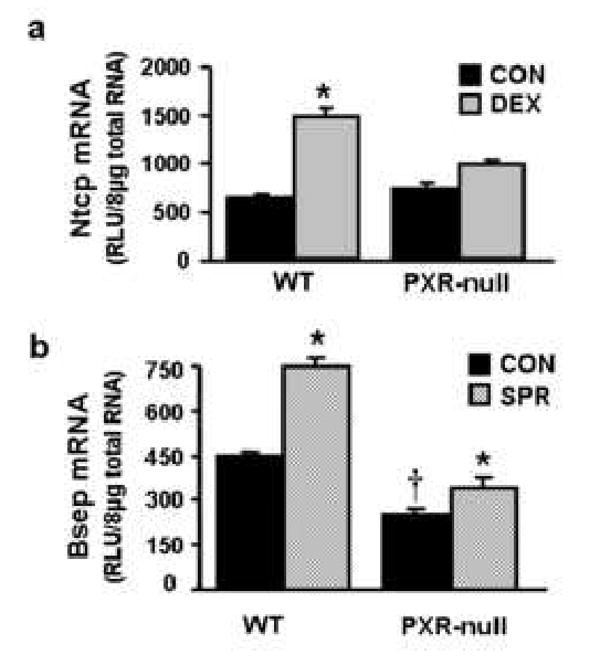
a) Regulation of mouse Ntcp by dexamethasone in wild-type mice and PXR-null mice.Wild-type mice and PXR-null mice (n=4-6/group) were treated once daily for 4 days with 50mg/kg dexamethasone. Control group received corn oil. On day 5, liver tissues were removed and used for total RNA isolation. Individual total RNA sample (n=4-6 mice) were analyzed by bDNA assay. The solid, black bar represents the data from the control group; the gray bar represents the data from mice after treatment with 50 mg/kg dexamethasone. b) Regulation of mouse Bsep by spironolactone in wild-type mice and PXR-null mice. Wild-type and PXR-null mice (n=5/group) were treated once daily for 4 days with corn oil (control) or spironolactone (200 mg/kg, ip in corn oil). On day 5, liver tissues were removed and used for total RNA isolation. Total RNA from five treated male livers was analyzed by the bDNA assay. All data were expressed as mean ± S.E.M. for five mice in each group. Asterisk indicates statistically significant difference between treated and control mice (p < 0.05). Single dragger (†) indicates statistically significant difference between wild-type and PXR-null mice (p < 0.05).
Regulation of Bsep by SPR in PXR-null mice is shown in Fig. 5b. In wild-type mice, SPR treatment increased Bsep mRNA expression 70%. Disruption of PXR protein, as demonstrated in PXR-null mice, decreased expression of Bsep mRNA approximately 60%. In contrast, in PXR-null mice, SPR increased Bsep mRNA expression 35%.
3.6. Regulation of mouse Ntcp and Bsep by cholesterol, chenodeoxycholic acid, and cholestyramine
As depicted in Fig. 6, cholesterol administration didn't alter either Ntcp or Bsep mRNA expression. Cholestyramine induced Ntcp mRNA expression about 50%, but had no effect on Bsep. Administration of CDCA induced Bsep mRNA expression about 160%, but it did not alter Ntcp mRNA expression.
Fig. 6. Regulation of mouse Ntcp and Bsep by feeding with cholesterol, chenodeoxycholic acid, or cholestyramine.
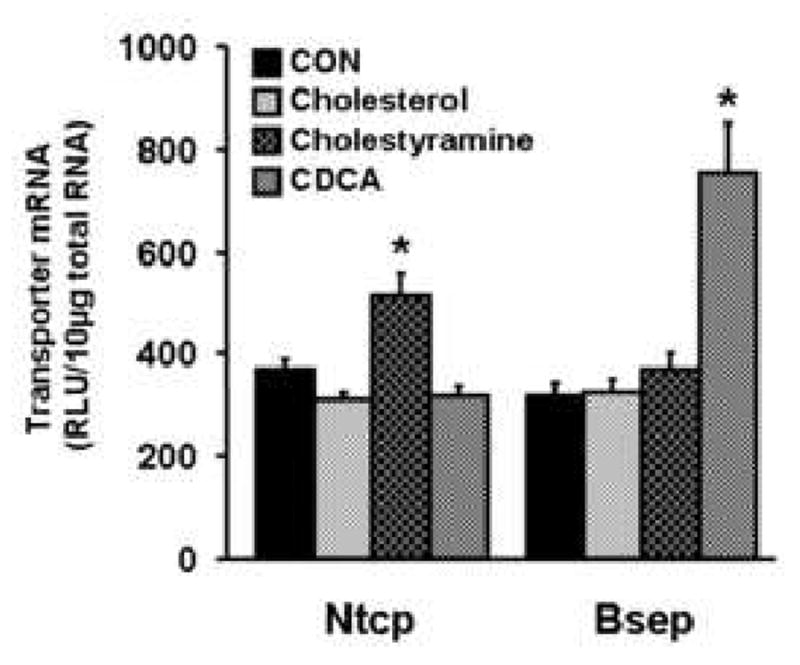
Mice were fed diets containing 1% cholesterol for 7 days, or 1% chenodeoxycholic acid (CDCA) for 10 days, or 3% cholestyramine for 10 days as detailed in Materials and methods, while control mice received the standard diet. All data were expressed as mean ± S.E.M. of six to 10 male mice for each group. Asterisk (*) denotes a statistically significant difference in mRNA levels in treated compared to control mice (p<0.05).
3.7. Putative DNA response elements in 6 KB of the promoter/enhancer region of mouse and human Ntcp/NTCP and Bsep/BSEP
In the mouse Ntcp promoter, as shown in Fig. 7a, three putative GR response elements are found about 20, 500, and 3500 bp upstream from the transcription start site. One putative Stat5b binding site is shown in the Ntcp promoter (-5160 bp; CTCCAGTTCTTGGAATATTGT, consensus Stat5b response element underlined). In the promoter of mouse Bsep (Fig. 7a), there are two putative FXR-binding sites (-360 bp and -3930 bp), two putative GR-binding sites (-1110 bp and -3000 bp), and three putative PXR-binding sites (-1850 bp, -3000 bp, and -4450 bp).
Fig. 7. In silico analysis of prospective DNA response elements of Stat5b, and nuclear receptors including PXR, FXR, and GR in 6 KB of promoter/enhancer of mouse and human Ntcp/NTCP and Bsep/BSEP genes.
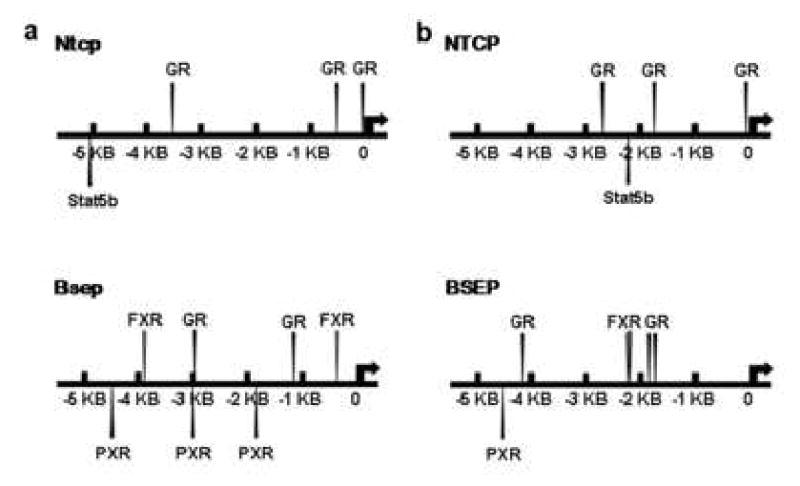
6 KB of promoter/enhancer sequences of mouse and human Ntcp/NTCP and Bsep/BSEP are accessed from genomic DNA database (www.ensembl.org). The DNA sequences were analyzed by Nubiscan (http://www.nubiscan.unibas.ch/) and Alibaba2.1 (http://www.gene-regulation.com/pub/programs/alibaba2/). Response element search of Stat5b is based on their DNA-binding consensus sequence (TTCnnGGAA). a) In silico analysis of promoter/enhancer sequences of mouse Ntcp and Bsep genes. b) In silico analysis of promoter/enhancer sequences of human NTCP and BSEP genes.
In 6 KB of human NTCP promoter, one putative Stat5b binding site (-2270 bp; GTCAGGTTCCTGGAAGAGATT, consensus Stat5b response element underlined), and three putative GR-response elements (-80 bp, -1740 bp, and -2760 bp) are localized (Fig. 7b). In contrast, putative response elements of PXR (one; at -4500 bp), GR (three; at -1730 bp, -1880 bp, and -4370 bp, respectively) and FXR (two; at -2200 bp and -2210 bp) exist in 6 KB of the human BSEP promoter/enhancer (Fig. 7b). There is no Stat5b response element observed in the promoter/enhancer of either mouse or human Bsep/BSEP.
4. Discussion
The goal of the present study was to obtain systematic information on the regulation of Ntcp and Bsep, two critical bile acid transporters in mouse and human livers. There are numerous reports on the regulation of Ntcp [19, 32-34] and Bsep [20, 32, 34] by bile acids. The focus of this study was to characterize the ontogenic and gender-related constitutive expression of these two hepatic bile acid transporters, as well as examine the regulation of Ntcp and Bsep by microsomal enzyme inducers, chenodeoxycholic acid, and cholestyramine.
Human, rat, and mouse NTCP/Ntcp and BSEP/Bsep share similar postnatal developmental patterns, with lower levels in newborns and then gradually increasing to adult levels with age. Both NTCP and BSEP mRNA expression in human livers is detectable in fetus at mid-gestational age (14-20 weeks) by real-time PCR. Human NTCP and BSEP mRNA levels are about 50-fold and 3-fold higher in adult than fetal livers, respectively [35]. Rat Ntcp mRNA has been detected at approximately 20 days of gestation, and gradually increases to adult levels after postnatal day 28 [36-38]. Hardikar et al further showed that mRNA levels of rat Ntcp prior to birth were less than 20% of adult values, increased to 35% on the first postnatal day, and reached adult values by 1 week of age [39]. Rat Bsep was first detected at embryonic day 20 [37], and then gradually increases to adult levels. Bsep mRNA in adult rats is approximately twice that in newborn rats [37, 40]. Hardikar et al further showed that mRNA levels of rat Bsep were 12% of adult values prior to birth and showed a 2-fold increase by the first day after birth, with adult levels being reached at 4 weeks of age [39]. Mouse Ntcp and Bsep mRNA expression in liver is minimal before birth, and then rapidly increases to the highest expression at birth (Fig. 2). After birth, mouse Ntcp mRNA expression decreases, and then gradually increases to adult levels at approximately 3 weeks of age (Fig. 2). In contrast, mouse Bsep mRNA decreased at 5 days after birth to half that at birth, and remained at this level thereafter (Fig. 2). Therefore, postnatal NTCP/Ntcp and BSEP/Bsep mRNA expression in humans, rats, and mice are low in newborns, and then increases to adult levels with age.
There is a species difference in the gender-divergent expression of Ntcp between rats, mice, and humans. In rats, Ntcp mRNA is male-predominant, with twice the amount in male as in female livers [22]. In contrast, mouse Ntcp mRNA expression is female-predominant with 70% higher expression in female than male livers. In addition, mouse Ntcp protein levels are 60% higher in female than male liver (Fig. 1a). NTCP mRNA expression in human livers was not statistically different between men and women, but mRNA levels were 43% higher in women than men (Fig. 1b). In rats, the gender differences in Ntcp mRNA levels were reported to be due to inhibitory effects of female-pattern GH secretion [22]. However, the present study indicates that in mice, female-predominant Ntcp mRNA expression is due to inhibitory effects of male-pattern GH secretion (Fig. 3b and 3c), but not sex hormones (Fig. 3a). In humans, GH gender-dimorphic secretion patterns are similar to that observed in rodents [41]. Therefore, mouse and human Ntcp/NTCP gender-dimorphic expression may share similar regulatory effects by GH. Recent microarray studies performed by Clodfelter et al [42, 43] showed that stat5a and stat5b play important roles in growth hormone-initiated gender-divergent liver gene expression. However, in both studies, regulation of mouse Ntcp by growth hormone was not included.
In silico analysis of 6 KB of the promoter/enhancer sequence of mouse Ntcp and Bsep showed that putative DNA binding sites of Stat5b exist in the promoter of mouse Ntcp, but not in mouse Bsep (Fig. 7). Stat5b is a down-stream target of the GH signaling pathway by which gender-specific liver gene expression occurs [27, 42, 44]. The existence of putative Stat5b response elements may explain why gender differences exist in the expression of mouse Ntcp, but not in mouse Bsep. In the human NTCP promoter, there is one putative DNA binding site of Stat5b (-2270 bp), but it does not exist in the human BSEP promoter (Fig.7b). Therefore, female-predominant human NTCP expression may be also due to gender-related GH secretion pattern.
Numerous chemicals, which are known to activate various transcription factors and induce phase-I and –II drug-metabolizing enzymes, regulate some hepatic transporters such as organic anion transporting polypeptides (Oatps) and multidrug resistance-associated proteins (Mrps) [31, 45, 46]. It was therefore of interest to determine whether these chemicals might also alter Ntcp and/or Bsep expression. In human primary hepatocytes, TCDD (AhR ligand), rifampicin (a PXR activator), phenobarbital (a CAR activator), and oltipraz (a Nrf2 activator) treatment down-regulate Ntcp and Bsep mRNA expression [47]. In contrast, the present study indicates that mouse Ntcp or Bsep mRNA expression are relatively resistant to alteration by most microsomal enzyme inducers, but induced by a couple of these chemicals (Fig. 4). Variation in induction of Oatps, Mrps, xenobiotic enzymes, Ntcp, and Bsep by microsomal enzyme inducers is logical in relation to their functions. Both Oatps and Mrps are xenobiotic transporters, which are regulated by the challenge of drugs and other xenobiotics. Xenobiotics are detected by the nuclear receptors PXR and CAR [48-50] and consequently up-regulate various transporters and xenobiotic enzyme systems. However, Ntcp and Bsep are bile acid transporters, and not thought to be as important for xenobiotic transport, and as shown here, are not readily induced by xenobiotics [51].
Even through mouse Ntcp and Bsep mRNA expression are relatively resistant to alteration by most microsomal enzyme inducers, a couple of these chemicals alter their expression (Fig. 4). Specifically, dexamethasone induced Ntcp mRNA expression, whereas spironolactone induced Bsep mRNA expression.
Dexamethasone is a well established inducer of CYP3A by activating the glucocorticoid receptor (GR) and PXR [52, 53]. Rat Ntcp mRNA was shown to be up-regulated by dexamethasone in hypophysectomized rats and in isolated rat hepatocytes [22], but down-regulated in sandwich-cultured rat hepatocytes [54]. Human NTCP promoter can also be activated by dexamethasone via the GR [55]. In mouse liver, DEX increased Ntcp mRNA expression in wild-type mice, but not in PXR-null mice (Fig. 5a). However, there is no apparent PXR response element exists in the mouse Ntcp promoter (Fig. 7a). In addition, PCN, the prototypical PXR ligand did not increase mouse Ntcp (Fig. 4). Dexamethasone induced mouse Ntcp mRNA expression may be mediated by GR, which is suggested by the presence of GR-response elements in the mouse Ntcp promoter (Fig. 7).
Spironolactone increases bile flow in experimental animals [56, 57]. Spironolactone is a ligand for PXR, GR, and the mineralocorticoid receptor. PXR was shown to be required for the up-regulation of mouse Bsep by PCN and RU486 during inflammation [58]. However, in the present study, PCN did not increase Bsep expression (Fig. 4). SPR increased Bsep mRNA expression 70% in wild-type mice (Figs. 4 and 5b), and 35% in PXR-null mice (Fig. 5b), suggesting partial involvement of PXR, which is supported by the presence of PXR response elements in the mouse Bsep promoter (Fig. 7a). However, PCN, the prototypical PXR agonist did not increase mouse Bsep (Fig. 7). Therefore, mechanisms other than PXR are involved in Bsep induction by spironolactone. For example, Ghanem et al [59] showed that in rats, spironolactone treatment increased p-glycoprotein expression and thus decreased intestinal absorption and liver content of digoxin. Therefore, spironolactone may be capable of altering disposition of some exogenous or endogenous compounds, such as bile acids, which might be responsible for regulation of Bsep.
Bile acids have been shown to either increase or decrease mouse Ntcp expression, but uniformly increase mouse Bsep expression. Ursodeoxycholic acid has no effect on mouse Ntcp expression, whereas it increased mouse Bsep mRNA and protein [32]. Taurocholate and/or cholic acid down-regulated mouse Ntcp via induction of the nuclear repressor short heterodimer partner (SHP) [33, 34, 60], whereas they up-regulated mouse Bsep expression [23, 32, 34]. Therefore, bile acids down-regulated Ntcp expression in some studies and had no effect in other studies, but consistently increased Bsep expression. In the present study, we further showed that mice treated with chenodeoxycholic acid (CDCA) increased Bsep mRNA expression, but did not alter Ntcp expression (Fig. 6).
Cholestyramine, which is a bile-acid sequestrant in the intestine, has been shown to increase bile-acid biosynthesis via Cyp7a1 induction in liver [61, 62]. In the present study, cholestyramine treatment increased mouse Ntcp mRNA expression 40% (Fig. 6). In contrast, Wolters et al [34] stated that cholestyramine did not alter mouse Ntcp expression; however, the figures in their publication depicted that cholestyramine slightly increased mouse Ntcp expression at both the protein and mRNA levels. The mechanism by which cholestyramine induces mouse Ntcp expression is not known. However, transcription factors, such as hepatic nuclear factor (Hnf) 4α and Stat5b might be involved. Cholestyramine-induced human CYP7A1 expression is mediated by increased Hnf4α expression [62, 63]. In addition, Hnf4α strongly inhibits Stat5b transcriptional activity via the inhibitory effects of Hnf4α on JAK2 phosphorylation [44]. In Figure 3, male-pattern GH secretion decreased hepatic Ntcp mRNA expression in hypophysectomized and lit/lit mice probably via activation of Stat5b [27, 42]. Therefore, cholestyramine may increase Hnf4α levels, which inhibits Stat5b transcriptional activity, and consequently increases Ntcp mRNA expression. Another potential mechanism is due to attenuation of the FXR-Shp pathway. By binding bile acids in the intestine, cholestyramine disrupts the enterohepatic circulation of bile acids. Less absorption of bile acids might lead to less activation of FXR, and as a consequence, attenuate the repressive effect of the FXR-Shp pathway on Ntcp expression. In rats, cholestyramine has been shown to decrease Shp mRNA levels [64]. However, a recent paper showed that cholestyramine administration to rats did not alter Shp expression [65]. The conflicting data on the regulation of Shp by cholestyramine, merits further studies.
Taken together, this study systemically characterized the regulation of Ntcp and Bsep mRNA expression in mouse livers. Liver at birth has the highest mRNA expression of both Ntcp and Bsep. Ntcp expression is female-predominant in mouse and human liver, which is due to the inhibitory effect of male-pattern GH secretion, and not sex hormones. Ntcp and Bsep expression in general are not regulated by microsomal enzyme inducers. Cholestyramine increased Ntcp and chenodeoxycholic acid increased Bsep mRNA expression in mouse livers. In silico analysis indicates that human and mouse NTCP/ Ntcp and BSEP/Bsep genes may share similar regulatory mechanisms, and thus regulation of mouse Ntcp and Bsep may extrapolate to humans.
Acknowledgments
This work was supported by NIH grants RR021940, ES09649, and ES09716.
Abbreviations
- bDNA
branched DNA signal amplification assay
- Bsep
bile salt export pump
- DHT
5α-dihydrotestosterone
- E2
17β-estadiol
- GHRH
growth hormone releasing hormone
- GNX
gonadectomy
- HX
hypophysectomy
- Ntcp
sodium-taurocholate cotransporting polypeptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cattori V, Eckhardt U, Hagenbuch B. Molecular cloning and functional characterization of two alternatively spliced Ntcp isoforms from mouse liver. Biochim Biophys Acta. 1999;1445:154–9. doi: 10.1016/s0167-4781(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 2.Hagenbuch B, Stieger B, Foguet M, Lubbert H, Meier PJ. Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc Natl Acad Sci U S A. 1991;88:10629–33. doi: 10.1073/pnas.88.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagenbuch B, Meier PJ. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Invest. 1994;93:1326–31. doi: 10.1172/JCI117091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer W, Stengelin S, Baringhaus KH, Enhsen A, Heuer H, Becker W, et al. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J Lipid Res. 1999;40:1604–17. [PubMed] [Google Scholar]
- 5.Meier PJ, Stieger B. Bile salt transporters. Annu Rev Physiol. 2002;64:635–61. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- 6.Meier PJ, Eckhardt U, Schroeder A, Hagenbuch B, Stieger B. Substrate specificity of sinusoidal bile acid and organic anion uptake systems in rat and human liver. Hepatology. 1997;26:1667–77. doi: 10.1002/hep.510260641. [DOI] [PubMed] [Google Scholar]
- 7.Kullak-Ublick GA, Stieger B, Hagenbuch B, Meier PJ. Hepatic transport of bile salts. Semin Liver Dis. 2000;20:273–92. doi: 10.1055/s-2000-9426. [DOI] [PubMed] [Google Scholar]
- 8.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–71. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 9.Childs S, Yeh RL, Georges E, Ling V. Identification of a sister gene to P-glycoprotein. Cancer Res. 1995;55:2029–34. [PubMed] [Google Scholar]
- 10.Wang R, Salem M, Yousef IM, Tuchweber B, Lam P, Childs SJ, et al. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci U S A. 2001;98:2011–6. doi: 10.1073/pnas.031465498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen PL, Strautnieks SS, Jacquemin E, Hadchouel M, Sokal EM, Hooiveld GJ, et al. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology. 1999;117:1370–9. doi: 10.1016/s0016-5085(99)70287-8. [DOI] [PubMed] [Google Scholar]
- 12.Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233–8. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 13.Zollner G, Fickert P, Zenz R, Fuchsbichler A, Stumptner C, Kenner L, et al. Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases. Hepatology. 2001;33:633–46. doi: 10.1053/jhep.2001.22646. [DOI] [PubMed] [Google Scholar]
- 14.Trauner M, Arrese M, Lee H, Boyer JL, Karpen SJ. Endotoxin downregulates rat hepatic ntcp gene expression via decreased activity of critical transcription factors. J Clin Invest. 1998;101:2092–100. doi: 10.1172/JCI1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slitt AL, Allen K, Morrone J, Aleksunes LM, Chen C, Maher JM, et al. Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim Biophys Acta. 2007;1768:637–47. doi: 10.1016/j.bbamem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Lee JM, Trauner M, Soroka CJ, Stieger B, Meier PJ, Boyer JL. Expression of the bile salt export pump is maintained after chronic cholestasis in the rat. Gastroenterology. 2000;118:163–72. doi: 10.1016/s0016-5085(00)70425-2. [DOI] [PubMed] [Google Scholar]
- 17.Gartung C, Ananthanarayanan M, Rahman MA, Schuele S, Nundy S, Soroka CJ, et al. Down-regulation of expression and function of the rat liver Na+/bile acid cotransporter in extrahepatic cholestasis. Gastroenterology. 1996;110:199–209. doi: 10.1053/gast.1996.v110.pm8536857. [DOI] [PubMed] [Google Scholar]
- 18.Green RM, Hoda F, Ward KL. Molecular cloning and characterization of the murine bile salt export pump. Gene. 2000;241:117–23. doi: 10.1016/s0378-1119(99)00460-6. [DOI] [PubMed] [Google Scholar]
- 19.Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, et al. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121:140–7. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- 20.Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276:28857–65. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- 21.Plass JR, Mol O, Heegsma J, Geuken M, Faber KN, Jansen PL, et al. Farnesoid X receptor and bile salts are involved in transcriptional regulation of the gene encoding the human bile salt export pump. Hepatology. 2002;35:589–96. doi: 10.1053/jhep.2002.31724. [DOI] [PubMed] [Google Scholar]
- 22.Simon FR, Fortune J, Iwahashi M, Qadri I, Sutherland E. Multihormonal regulation of hepatic sinusoidal Ntcp gene expression. Am J Physiol Gastrointest Liver Physiol. 2004;287:G782–94. doi: 10.1152/ajpgi.00379.2003. [DOI] [PubMed] [Google Scholar]
- 23.Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB, Jr, Kliewer SA, et al. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem. 2003;278:45062–71. doi: 10.1074/jbc.M307145200. [DOI] [PubMed] [Google Scholar]
- 24.Dieter MZ, Maher JM, Cheng X, Klaassen CD. Expression and regulation of the sterol half-transporter genes ABCG5 and ABCG8 in rats. Comp Biochem Physiol C Toxicol Pharmacol. 2004;139:209–18. doi: 10.1016/j.cca.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Cheng X, Maher J, Chen C, Klaassen CD. Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps) Drug Metab Dispos. 2005;33:1062–73. doi: 10.1124/dmd.105.003640. [DOI] [PubMed] [Google Scholar]
- 26.Johnson DR, Guo GL, Klaassen CD. Expression of rat Multidrug Resistance Protein 2 (Mrp2) in male and female rats during normal and pregnenolone-16alpha-carbonitrile (PCN)-induced postnatal ontogeny. Toxicology. 2002;178:209–19. doi: 10.1016/s0300-483x(02)00231-7. [DOI] [PubMed] [Google Scholar]
- 27.Waxman DJ, O'Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol. 2006;20:2613–29. doi: 10.1210/me.2006-0007. [DOI] [PubMed] [Google Scholar]
- 28.Cao J, Gowri PM, Ganguly TC, Wood M, Hyde JF, Talamantes F, et al. PRL, placental lactogen, and GH induce NA(+)/taurocholate-cotransporting polypeptide gene expression by activating signal transducer and activator of transcription-5 in liver cells. Endocrinology. 2001;142:4212–22. doi: 10.1210/endo.142.10.8456. [DOI] [PubMed] [Google Scholar]
- 29.Hagenbuch B, Dawson P. The sodium bile salt cotransport family SLC10. Pflugers Arch. 2004;447:566–70. doi: 10.1007/s00424-003-1130-z. [DOI] [PubMed] [Google Scholar]
- 30.Arrese M, Ananthanarayanan M. The bile salt export pump: molecular properties, function and regulation. Pflugers Arch. 2004;449:123–31. doi: 10.1007/s00424-004-1311-4. [DOI] [PubMed] [Google Scholar]
- 31.Cheng X, Maher J, Dieter MZ, Klaassen CD. Regulation of mouse organic anion-transporting polypeptides (oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos. 2005;33:1276–82. doi: 10.1124/dmd.105.003988. [DOI] [PubMed] [Google Scholar]
- 32.Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Pojer C, Zenz R, et al. Effects of ursodeoxycholic and cholic acid feeding on hepatocellular transporter expression in mouse liver. Gastroenterology. 2001;121:170–83. doi: 10.1053/gast.2001.25542. [DOI] [PubMed] [Google Scholar]
- 33.Torchia EC, Cheema SK, Agellon LB. Coordinate regulation of bile acid biosynthetic and recovery pathways. Biochem Biophys Res Commun. 1996;225:128–33. doi: 10.1006/bbrc.1996.1141. [DOI] [PubMed] [Google Scholar]
- 34.Wolters H, Elzinga BM, Baller JF, Boverhof R, Schwarz M, Stieger B, et al. Effects of bile salt flux variations on the expression of hepatic bile salt transporters in vivo in mice. J Hepatol. 2002;37:556–63. doi: 10.1016/s0168-8278(02)00247-7. [DOI] [PubMed] [Google Scholar]
- 35.Chen HL, Chen HL, Liu YJ, Feng CH, Wu CY, Shyu MK, et al. Developmental expression of canalicular transporter genes in human liver. J Hepatol. 2005;43:472–7. doi: 10.1016/j.jhep.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 36.Boyer JL, Hagenbuch B, Ananthanarayanan M, Suchy F, Stieger B, Meier PJ. Phylogenic and ontogenic expression of hepatocellular bile acid transport. Proc Natl Acad Sci U S A. 1993;90:435–8. doi: 10.1073/pnas.90.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomer G, Ananthanarayanan M, Weymann A, Balasubramanian N, Suchy FJ. Differential developmental regulation of rat liver canalicular membrane transporters Bsep and Mrp2. Pediatr Res. 2003;53:288–94. doi: 10.1203/01.PDR.0000047509.54253.01. [DOI] [PubMed] [Google Scholar]
- 38.Ananthanarayanan M, Bucuvalas JC, Shneider BL, Sippel CJ, Suchy FJ. An ontogenically regulated 48-kDa protein is a component of the Na(+)-bile acid cotransporter of rat liver. Am J Physiol. 1991;261:G810–7. doi: 10.1152/ajpgi.1991.261.5.G810. [DOI] [PubMed] [Google Scholar]
- 39.Hardikar W, Ananthanarayanan M, Suchy FJ. Differential ontogenic regulation of basolateral and canalicular bile acid transport proteins in rat liver. J Biol Chem. 1995;270:20841–6. doi: 10.1074/jbc.270.35.20841. [DOI] [PubMed] [Google Scholar]
- 40.Zinchuk VS, Okada T, Akimaru K, Seguchi H. Asynchronous expression and colocalization of Bsep and Mrp2 during development of rat liver. Am J Physiol Gastrointest Liver Physiol. 2002;282:G540–8. doi: 10.1152/ajpgi.00405.2001. [DOI] [PubMed] [Google Scholar]
- 41.Jaffe CA, Ocampo-Lim B, Guo W, Krueger K, Sugahara I, DeMott-Friberg R, et al. Regulatory mechanisms of growth hormone secretion are sexually dimorphic. J Clin Invest. 1998;102:153–64. doi: 10.1172/JCI2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol. 2006;20:1333–51. doi: 10.1210/me.2005-0489. [DOI] [PubMed] [Google Scholar]
- 43.Clodfelter KH, Miles GD, Wauthier V, Holloway MG, Zhang X, Hodor P, et al. Role of STAT5a in Regulation of Sex-specific Gene Expression in Female but not Male Mouse Liver Revealed by Microarray Analysis. Physiol Genomics. 2007 doi: 10.1152/physiolgenomics.00055.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park SH, Wiwi CA, Waxman DJ. Signalling cross-talk between hepatocyte nuclear factor 4alpha and growth-hormone-activated STAT5b. Biochem J. 2006;397:159–68. doi: 10.1042/BJ20060332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD. Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab Dispos. 2005;33:956–62. doi: 10.1124/dmd.105.003798. [DOI] [PubMed] [Google Scholar]
- 46.Guo GL, Choudhuri S, Klaassen CD. Induction profile of rat organic anion transporting polypeptide 2 (oatp2) by prototypical drug-metabolizing enzyme inducers that activate gene expression through ligand-activated transcription factor pathways. J Pharmacol Exp Ther. 2002;300:206–12. doi: 10.1124/jpet.300.1.206. [DOI] [PubMed] [Google Scholar]
- 47.Jigorel E, Le Vee M, Boursier-Neyret C, Parmentier Y, Fardel O. Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab Dispos. 2006;34:1756–63. doi: 10.1124/dmd.106.010033. [DOI] [PubMed] [Google Scholar]
- 48.Guo GL, Staudinger J, Ogura K, Klaassen CD. Induction of rat organic anion transporting polypeptide 2 by pregnenolone-16alpha-carbonitrile is via interaction with pregnane X receptor. Mol Pharmacol. 2002;61:832–9. doi: 10.1124/mol.61.4.832. [DOI] [PubMed] [Google Scholar]
- 49.Slitt AL, Cherrington NJ, Dieter MZ, Aleksunes LM, Scheffer GL, Huang W, et al. trans-Stilbene oxide induces expression of genes involved in metabolism and transport in mouse liver via CAR and Nrf2 transcription factors. Mol Pharmacol. 2006;69:1554–63. doi: 10.1124/mol.105.014571. [DOI] [PubMed] [Google Scholar]
- 50.Klaassen CD, Slitt AL. Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab. 2005;6:309–28. doi: 10.2174/1389200054633826. [DOI] [PubMed] [Google Scholar]
- 51.Chandra P, Brouwer KL. The complexities of hepatic drug transport: current knowledge and emerging concepts. Pharm Res. 2004;21:719–35. doi: 10.1023/b:pham.0000026420.79421.8f. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Faucette SR, Gilbert D, Jolley SL, Sueyoshi T, Negishi M, et al. Glucocorticoid receptor enhancement of pregnane X receptor-mediated CYP2B6 regulation in primary human hepatocytes. Drug Metab Dispos. 2003;31:620–30. doi: 10.1124/dmd.31.5.620. [DOI] [PubMed] [Google Scholar]
- 53.Huss JM, Kasper CB. Two-stage glucocorticoid induction of CYP3A23 through both the glucocorticoid and pregnane X receptors. Mol Pharmacol. 2000;58:48–57. doi: 10.1124/mol.58.1.48. [DOI] [PubMed] [Google Scholar]
- 54.Turncliff RZ, Meier PJ, Brouwer KL. Effect of dexamethasone treatment on the expression and function of transport proteins in sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2004;32:834–9. doi: 10.1124/dmd.32.8.834. [DOI] [PubMed] [Google Scholar]
- 55.Eloranta JJ, Jung D, Kullak-Ublick GA. The human Na+-taurocholate cotransporting polypeptide gene is activated by glucocorticoid receptor and peroxisome proliferator-activated receptor-gamma coactivator-1alpha, and suppressed by bile acids via a small heterodimer partner-dependent mechanism. Mol Endocrinol. 2006;20:65–79. doi: 10.1210/me.2005-0159. [DOI] [PubMed] [Google Scholar]
- 56.von Bergmann K, Schwarz HP, Paumgartner G. Effect of phenobarbital, spironolactone and pregnenolone-16 alpha-carbonitrile on bile formation in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1975;287:33–45. doi: 10.1007/BF00632636. [DOI] [PubMed] [Google Scholar]
- 57.Klaassen CD. Effect of microsomal enzyme inducers on the biliary excretion of cardiac glycosides. J Pharmacol Exp Ther. 1974;191:201–11. [PubMed] [Google Scholar]
- 58.Teng S, Piquette-Miller M. The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. J Pharmacol Exp Ther. 2005;312:841–8. doi: 10.1124/jpet.104.076141. [DOI] [PubMed] [Google Scholar]
- 59.Ghanem CI, Gomez PC, Arana MC, Perassolo M, Delli Carpini G, Luquita MG, et al. Induction of rat intestinal P-glycoprotein by spironolactone and its effect on absorption of orally administered digoxin. J Pharmacol Exp Ther. 2006;318:1146–52. doi: 10.1124/jpet.106.105668. [DOI] [PubMed] [Google Scholar]
- 60.Zollner G, Fickert P, Silbert D, Fuchsbichler A, Stumptner C, Zatloukal K, et al. Induction of short heterodimer partner 1 precedes downregulation of Ntcp in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G184–91. doi: 10.1152/ajpgi.00215.2001. [DOI] [PubMed] [Google Scholar]
- 61.Nakamura T, Matsuzawa Y. Drug treatment of hyperlipoproteinemia: bile acid-binding resins. Nippon Rinsho. 1994;52:3266–70. [PubMed] [Google Scholar]
- 62.Abrahamsson A, Gustafsson U, Ellis E, Nilsson LM, Sahlin S, Bjorkhem I, et al. Feedback regulation of bile acid synthesis in human liver: importance of HNF-4alpha for regulation of CYP7A1. Biochem Biophys Res Commun. 2005;330:395–9. doi: 10.1016/j.bbrc.2005.02.170. [DOI] [PubMed] [Google Scholar]
- 63.Crestani M, Sadeghpour A, Stroup D, Galli G, Chiang JY. Transcriptional activation of the cholesterol 7alpha-hydroxylase gene (CYP7A) by nuclear hormone receptors. J Lipid Res. 1998;39:2192–200. [PubMed] [Google Scholar]
- 64.Gupta S, Pandak WM, Hylemon PB. LXR alpha is the dominant regulator of CYP7A1 transcription. Biochem Biophys Res Commun. 2002;293:338–43. doi: 10.1016/S0006-291X(02)00229-2. [DOI] [PubMed] [Google Scholar]
- 65.Shibata S, Hayakawa K, Egashira Y, Sanada H. Roles of nuclear receptors in the up-regulation of hepatic cholesterol 7alpha-hydroxylase by cholestyramine in rats. Life Sci. 2007;80:546–53. doi: 10.1016/j.lfs.2006.10.003. [DOI] [PubMed] [Google Scholar]


