Abstract
The discovery of the cadmium (Cd)-binding protein from horse kidney in 1957 marked the birth of research on this low-molecular weight, cysteine-rich protein called metallothionein (MT) in Cd toxicology. MT plays minimal roles in the gastrointestinal absorption of Cd, but MT plays important roles in Cd retention in tissues and dramatically decreases billiary excretion of Cd. Cd-bound to MT is responsible for Cd accumulation in tissues and the long biological half-life of Cd in the body. Induction of MT protects against acute Cd-induced lethality, as well as acute toxicity to the liver and lung. Intracellular MT also plays important roles in ameliorating Cd toxicity following prolonged exposures, particularly chronic Cd-induced nephrotoxicity, osteotoxicity, and toxicity to the lung, liver, and immune system. There is an association between human and rodent Cd exposure and prostate cancers, especially in the portions where MT is poorly expressed. MT expression in Cd-induced tumors varies depending on the type and the stage of tumor development. For instance, high levels of MT are detected in Cd-induced sarcomas at the injection site, whereas the sarcoma metastases were devoid of MT. The use of MT-transgenic and MT-null mice has greatly helped define the role of MT in Cd toxicology, with the MT-null mice being hypersensitive and MT-transgenic mice resistant to Cd toxicity. Thus, MT is critical for protecting human health from Cd toxicity. There are large individual variations in MT expression, which might in turn predispose some people to Cd toxicity.
Keywords: Cadmium, Metallothionein, Acute toxicity, Nephrotoxicity, Carcinogenecity, MT Polymorphism
Introduction
Cadmium (Cd) is an environmental pollutant ranked eighth in the Top 20 Hazardous Substances Priority List (http://www.atsdr.cdc.gov, ATSDR, 1999), and human activity has markedly increased the distribution of Cd in the global environment. Food is the major source of Cd exposure for the general population, and cigarette smoking significantly adds to the body burden of Cd (Jarup et al., 1998; Klaassen et al., 1999). Occupational exposures are mainly from Cd fume inhalation, cadmium-nickel battery industry, electroplating, and paint pigments (ATSDR, 1999; Liu et al., 2007).
Cd is toxic to a number of tissues. Acute Cd poisoning causes pulmonary edema, hemorrhage, fulminate hepatitis, testicular injury, and lethality; whereas prolonged exposure to Cd produces nephrotoxicity, osteotoxicity, and immunotoxicity (ATSDR, 1999; Liu et al., 2007). Cd is also classified by IARC as a human carcinogen causing tumors of the lung, prostate, injection site, and other tissues (Waalkes, 2003).
Most of Cd in the body is bound to a small, cysteine-rich, metal-binding protein called metallothionein (MT) (Klaassen et al., 1999; Nordberg, 2004). MT was first discovered in 1957 as a Cd-binding protein in horse kidney (Margoshes and Vallee, 1957), and numerous studies have been conducted thereafter to determine the function of MT in Cd toxicology (Klaassen et al., 1999). MT is easily induced by Cd and various metal ions, as well as by other stimuli (Kagi and Schaffer, 1988). Cd toxicity can be ameliorated by various MT inducers, particular by Zn (Klaassen et al., 1999; Waalkes, 2003). The use of MT-transgenic and MT-null mouse models greatly facilitated research on the role of MT in Cd toxicology (Klaassen and Liu, 1998).
In this mini-review, the role of MT in Cd toxicology is briefly discussed from toxicokinetics to toxicodynamics, and from acute toxicity to carcinogenesis (Table 1). The individual human variations in MT will also be briefly discussed, as people with low expression of MT might be susceptible to Cd toxicity.
Table 1.
A brief summary on the role of metallothionein in cadmium toxicology
| Biological function | Experimental system | Major observation parameters | Role of MT | Representative references |
|---|---|---|---|---|
| Disposition | ||||
| Aborption | Rats and in situ intestinal loop | Dose-dependent Cd absortion | 0 - + | Lehman and Klaassen, 1986; Goon and Klaassen, 1989 |
| MT-TG and MT-null mice | Dose-dependent Cd absortion | 0 - + | Liu and Klaassen, 1996; Liu et al., 2001 | |
| Retention | MT-null mice | Accumulation in kidney, liver | ++++ | Liu et al., 1996; Klaassen et al., 1999 |
| Excretion | Rat bile-duct cannulation | Cd biliary excretion | ++++ | Klaassen, 1978 |
| Conjugation | In vivo and in vitro | Cd-MT, Cd-Cys, Cd-GSH for transport | ++ | Norbderg, 2004; Zalups and Ahmad, 2003 |
| Acute Toxicity | ||||
| Lethality | Rats and MT-null mice | Pretreatment and/or repeated dose | ++++ | Goering and Klaassen, 1983; Park et al., 2001 |
| Hepatotoxicity | Rats and MT-transgenic mice | ALT and histology | ++++ | Goering and Klaassen, 1984; Liu et al., 1995, 1996 |
| Pulmonary toxicity | Intact animals | Histology and biochemistry | +++ | Hart et al., 1995; Pearson et al., 2003 |
| Testicular toxicity | Rat and MT-null mice/cells | Histology and biochemistry | 0 - + | Waalkes et al., 1988; Liu et al., 2001; Leslie et al., 2006 |
| Chronic Toxicity | ||||
| Nephrotoxicity | Rats and MT-null mice | Histology and biochemistry | ++++ | Liu et al., 1998a;1998b |
| Renal cells and intact animals | CdMT is less toxic than CdCl2 | ++ | Prozialeck et al., 1993; Groten et al., 1994 | |
| Osteotoxicity | Rats and MT-null mice | Bone mass and calcium | ++++ | Wang et al., 1994; Habeebu et al., 2001 |
| Immunotoxicity | MT-null mice | Histology and biochemistry | ++++ | Liu et al., 1999 |
| Extracellular CdMT | T-cell and antibody production | ++ | Lynes et al., 2006;Growthers et al., 2000 | |
| Liver injury | MT-null mice | Inflammation, apoptosis, proliferation | +++ | Habeebu et al., 2000b |
| Carcinogenecity | ||||
| Prostrate Cancers | Rats and mice | Susceptibility and regional MT | +++ | Waalkes et al., 1989; 2004 |
| Lung cancer | Rats, mice and human | Histology and MT detection | +++ | Tenaka et al., 1993; Hart et al., 1989 |
| Liver Cancer | MT-null mice | Histology | +++ | Waalkes and Liu, 2009 |
Symbols for roles of MT: 0, no effect; +, minimal; ++, mild; +++, significant; ++++, dramatic
MT in Cd disposition
Cd absorption from the gastrointestinal tract is the main route of Cd exposure in humans. In laboratory animal studies, the fraction of Cd that is absorbed from the gastrointestinal tract is low, but increases with dose. For example, Cd absorption rate increased from 0.4% to 1.65% of the dose as the dose increased (Lehman and Klaassen, 1986). Further studies using in situ intestinal loops confirmed these in vivo observations, as the percentage of the Cd dosage absorbed ranged from 0.1% at 0.1 μg Cd/kg to 3.4% at 10,000 μg Cd/kg dosage (Goon and Klaassen, 1989). Intestinal content of MT was increased 25-fold by zinc, and over 90% of Cd in the intestinal cytosol was bound to MT. However, induction of intestinal MT by zinc does not affect intestinal Cd absorption (Goon and Klaassen, 1989). Thus, intestinal MT does not appear to be a major determinant for the dosage-dependent absorption of Cd. The minimal role of MT in Cd absorption from the gastrointestinal tract was further confirmed in studies using MT overexpressing transgenic mice (Liu and Klaassen, 1996) and with MT-null mice (Liu et al., 2001). Whereas Cd accumulation in the duodenium of MT-null mice and rats fed high Cd diets does not appear to be MT-dependent, it is affected by a marginal supply of iron (Park et al., 2002). A diet low in iron increases the expression of the divalent metal transporter 1 (DMT1), which transports iron. Unfortunately, DMT1 also transports Cd.
Although MT plays a limited role in the initial distribution of Cd to various tissues (Liu et al., 1996a; Liu et al., 2001), the retention of Cd in various tissues is MT-dependent. Cd mainly accumulates in kidney and liver, where high MT levels are found. Induction of hepatic MT almost abolishes biliary excretion of Cd (Klaassen, 1978), and renal Cd concentration is proportional to renal MT levels (Liu et al., 1996a; Jarup et al., 1998). The accessibility of Cd to brain is dependent on the age of the animal (Wong et al., 1980; Wong and Klaassen, 1982). The brain of newborn animals is permeable to Cd which decreases with age, probably due to increased MT expression and blood brain barrier maturation (Choudhuri et al., 1996). Nonetheless, MT plays an important role in tissue Cd retention, and is responsible for the long biological half-life of Cd in the body.
Cd is non-biodegradable and redox inert as compared to other transition metals, such as iron or copper. Little is known about Cd biotransformation except its conjugation with sulfhydryl groups, such as MT and glutathione. The Cd-MT complex is mainly formed in the liver, released into the blood, and transported to the kidney (Klaassen et al., 1999; Nordberg, 2004). Molecules other than MT, such as albumin, cysteine, glutathione, and sulfhydryl-rich proteins, can also form associations with Cd. Cd uptake is mediated by transport proteins such as DMT1, metal transport protein 1, calcium channel proteins, and the 8-transmembrane zinc-related iron-related protein (ZIP8) to reach the target tissues. Alteration of transport protein expression can impact cellular Cd uptake and accumulation, and in turn impact Cd toxicity (Zalups and Ahmad, 2003; Dalton et al., 2005; Leslie et al., 2006).
MT in acute Cd poisoning
The most intriguing finding of the role of MT in Cd toxicity came from the observation that pretreatment of animals with a low dose of Cd renders animals highly tolerant to Cd-induced lethality (Goering and Klaassen, 1983). In wild-type and MT-null mice given increasing doses of Cd, the remarkable acquired tolerance to Cd lethality is evident in wild-type mice, with a 7-fold difference in LD50 values, but such tolerance did not happen in the MT-null mice (Park et al., 2001), indicating the critical role for MT as a major protein in protecting acute Cd poisoning.
Liver is the major target organ of toxicity following acute Cd poisoning, and Cd hepatotoxicity is the major cause of acute Cd lethality (Goering and Klaassen, 1983). Acquired tolerance to acute Cd hepatotoxicity depends on the presynthesized MT in the liver, which functions to sequestrate Cd in the cytosol, with concomitant reduction of the amount of Cd available for other critical organelles (Goering and Klaassen, 1984). This scenario was further confirmed with many studies with various MT-inducers (Klaassen et al., 1999), and with MT transgenic animals. In all of these models of MT overexpression, mice were protected against acute Cd lethality and hepatotoxicity (Liu et al., 1995), whereas the mice deficient in MT protein shows marked sensitivity to Cd (4.6 mg/kg)-induced liver injury (Figure 1a, Liu et al., 1996b).
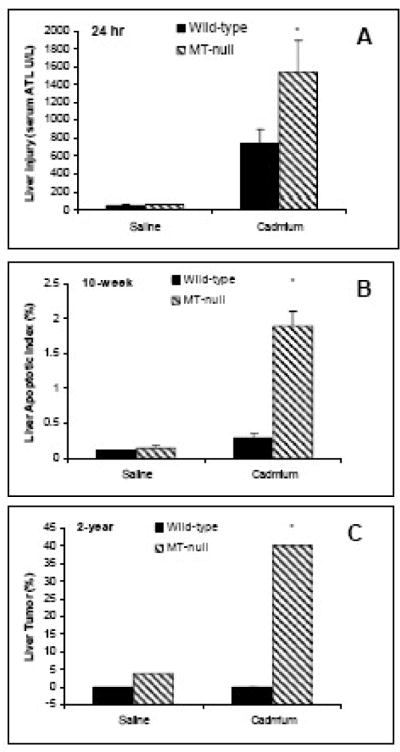
Figure 1.
Susceptibility of MT-null mice to Cd liver injury and hepatocarcinogenesis. A: Wild-type and MT-null mice were given a s.c. injection of CdCl2 at the dose of 25 μmol (4.6 mg) Cd/kg, and liver injury was evidenced 24 hr later by increased serum ALT activity (modified from Liu et al., 1996); B: Wild-type and MT-null mice were given repeated s.c. injections of CdCl2 at the dose of 0.1 mg/kg for 10 weeks, and liver injury was evidenced by increased liver parenchyma cell apoptosis; C: Wild-type and MT-null mice were given a single s.c. injection of CdCl2 at the dose 5 μmol (0.92 mg) Cd/kg, and increased liver tumor incidence was only evident in MT-null mice after 2 years (modified from Waalkes and Liu, 2009).
Acute exposure to Cd fumes or aerosols produces pulmonary edema and hemorrhaging, followed by inflammation, scarring, fibrotic changes and carcinogenesis (ATSDR, 1999; Waalkes, 2003). Pre-exposure of rodents to low doses of Cd aerosols produced a 50-fold increases in MT in the lungs, and protection from subsequent high doses of Cd-induced pulmonary inflammation (Hart et al., 1989). Intratracheal instillation of Cd to mice at a dose known to increase pulmonary MT (Prozialeck, personnel communication), decreased the amount of E-cadherin in the alveoli epithelial cells and VE-cadherin in vascular endothelial cells (Pearson et al., 2003). A high dose of Cd (65 nmoles in 50 μl saline) could saturate endogenous pulmonary MT, and free Cd in turn produces the vascular damage in the lung (Pearson et al., 2003).
Testis is a major target for acute Cd toxicity in experimental animals (ATSDR, 1999). The onset of Cd toxicity in the testes is rapid. The testes first become swollen, followed by congestion and edema, and extensive hemorrhage and necrosis occur within 24 hr after Cd injection. There is a dramatic mouse strain-dependent susceptibility to Cd-induced testicular injury (Taylor et al., 1973). In sensitive strains (e.g., 129/SVIM, AKR/J, DBA/1J, and C57BR/J), a small dose of Cd causes testicular necrosis, even in the absence of overt toxicity to other organs, whereas in resistant strains (e.g., Balb/C, C3H/HeJ, A/J, and C57BL/6J), Cd does not produce visible testicular damage even at the lethal doses (Taylor et al., 1973; Liu et al., 2001). Further studies using microarray analysis of Cd-exposed testes from Cd-sensitive/resistant mouse strains and the MT-null mice indicates that it is the genetic background and not the MT phenotype that dictates sensitivity to cadmium-induced testicular injury (Liu et al., 2001). It is proposed that the expression of the Cd transporter ZIP8, which is encoded by the Slc39a8 gene, is responsible for the strain-difference in Cd-induced toxicity in mouse testis (Dalton et al., 2005; Hei, et al, in this issue). The T-type calcium channel protein has also been suggested in the transport of Cd, because MT-null cells cultured with increasing concentrations of Cd, they also had a decreased Cd uptake and retention in the cells and decreased expression of the calcium channel protein (Leslie et al., 2006). Thus, in contrast to other organs, MT plays a minimal role in Cd testicular injury.
MT in chronic Cd toxicity
Kidney is a major target following chronic Cd exposure (Jarup et al., 1998). It has been long thought that Cd-induced nephrotoxicity is mediated by the Cd-MT complex. The induction of MT by Cd and the subsequent sequestration of Cd by MT protects tissues from Cd toxicity. However, the Cd-MT complex is acutely nephrotoxic after i.v. injection to experimental animals (Nordberg et al., 1975). In this scenario, Cd-MT is formed in the liver in response to Cd exposure, and is released into the blood stream from damaged hepatocytes. Cd-MT complex in the blood is then filtered by the kidney and taken up into proximal tubule cells, where it is degraded, releasing locally high levels of “free” Cd to produce tubular injury. This scenario was held for three decades, until it was recently challenged by two lines of experimental data: first, MT-null mice, which are unable to produce MT and thus to form the Cd-MT complex, are hypersensitive to chronic Cd nephropathy (Liu et al., 1998a), even though the accumulation of Cd in the kidneys was only 7% of that in wild-type mice (10 vs 140 μg/g); second, kidney pathology from a single injection of Cd-MT differs greatly from that induced by chronic oral Cd ingestion (Liu et al., 1998b). Indeed, Cd salts are taken into the kidney from the basolateral membrane, not only from the luminal side (Zalpus, 2000; Zalups and Ahmad, 2003) in the absence of Cd-induced hepatotoxicity. Thus, Cd-induced nephrotoxicity is not necessarily mediated through the Cd-MT complex. In cultured renal cells, cytotoxicity of Cd-MT is much less than CdCl2, corresponding to less Cd uptake and accumulation from Cd-MT than from CdCl2 (Prozialeck et al., 1993; Liu et al., 1994). In animals chronically exposed to CdCl2 or CdMT for 10 months, CdMT showed less nephrotoxicity than CdCl2, with less renal Cd accumulation in kidneys (Groten et al., 1994). Thus, Cd nephrotoxicity appears to have a similar mechanism of action as Cd hepatotoxicity, that is, it is due to accumulation of inorganic Cd, rather than CdMT.
Itai-Itai disease was caused by long-term exposure to Cd in the Toyama Prefecture in Japan. Patients with Itai-Itai disease showed various symptoms, including nephropathy, osteomalacia, anemia, and severe pain (ATSDR, 1999; Bhattachayya, this issue). Bone demineralization begins soon after Cd exposure, well before the onset of kidney injury (Wang et al., 1994). Cd exposure in conjunction with calcium deficiency, pregnancy, and lactation are key etiologic factors for Itai-Itai disease (Wang et al., 1994). MT is induced in rat bones soon after Cd exposure, mainly in osteocytes (Oda et al., 2001; Regunathan et al., 2003). MT-null mice are hypersensitive to Cd-induced osteotoxicity, including bone mass loss, reduction in bone calcium content, decreases in bone density, increased osteoid seams, and expansion of hyperplastic bone marrow into metaphyseal cortical bone (Habbebu et al., 2000a; Regunathan et al., 2003). Thus, MT plays a protective role in Cd osteotoxicity, as it does in other tissues.
Anemia and immune dysfunction seen in the Itai-Itai patients and are major toxic manifestations following long-term exposure to Cd in humans and laboratory animals (ATSDR, 1999). Liver, spleen, and bone are important hematopietic organs, which are also targets of Cd exposure (Klaassen et al., 1999). Thus, it is not surprising that mice deficient in MT are more sensitive than wild-type mice to Cd-induced anemia and immunotoxicity (Liu et al., 1999). On the other hand, blood MT is non-protective, and may act as a “danger signal” to cellular damage (Lynes et al., 2006). For example, blood MT has effects both on the severity of autoimmune disease, and on the development of adaptive immune functions (Lynes et al., 2006). Mice deficient in MT displayed a significantly higher humoral response to challenge with ovalbumin compared to wild-type controls. Overall circulatory immunoglobulin levels are also substantially higher in MT-null mice than in wild-type mice (Crowthers et al., 2000).
Liver is a major target organ of Cd toxicity following acute exposure (Figure 1a), but also a target of chronic Cd toxicity following chronic CdCl2 (0.1 mg/kg s.c. for 10 weeks, Figure 1b, Habbebu et al., 2000b). In wild-type mice, hepatic Cd concentrations increase in a dose- and time-dependent manner, reaching 400 μg Cd/g liver by 10 weeks of exposure to inorganic Cd, along with a 150-fold increase in hepatic MT concentrations, whereas in MT-null mice, hepatic Cd concentrations were less than 10 μg Cd/g liver. Despite the lower accumulation of Cd in livers of MT-null mice, the maximum tolerated dose of Cd was only 1/8 of that for wild-type mice, and liver injury was more pronounced. Repeated administration of CdCl2 to the MT-null mice produced nonspecific chronic inflammation in the parenchyma and portal tracts, and higher doses produced granulomatous inflammation and preneoplastic proliferative lesions in MT-null mice (Figure 2, Habeebu et al., 2000b). Apoptosis and mitosis occurred concomitantly in liver following repeated Cd exposure, and more apoptosis is seen in MT-null mice at the dose of 0.1 mg Cd/kg for 10 weeks than wild-type mice that received the same dose of Cd (Figure 1b, Habbebu et al., 2000b). Thus, intracellular MT is an important protein protecting against chronic Cd-induced liver injury.
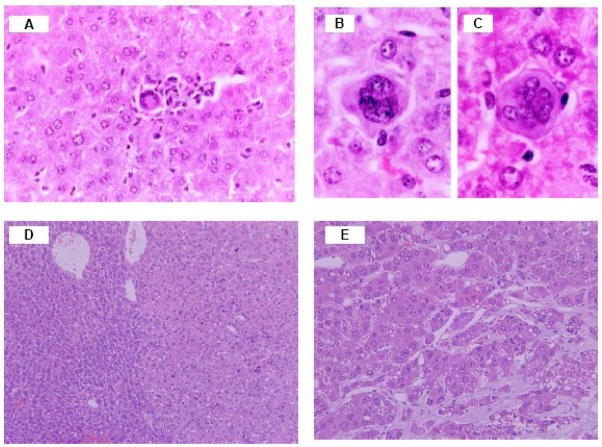
Figure 2.
Representative photographs of Cd-induced preneoplastic and neoplastic lesions in MT-null mice. The granuloma (A) and bizarre multinucleate giant cells (B–C) from the liver of the MT-null mice received null mice were given repeated s.c. injections of CdCl2 at the dose of 0.1 mg/kg for 10 weeks (Modified from Habbebu et al., 2000); A hepatocellular adenoma(D), right-light color and normal liver on left -dark color) and a trabecular hepatocellular carcinoma (E) in MT-null mice received a single s.c. injection of CdCl2 at the dose 5 μmol (0.92 mg) Cd/kg for 2 years.
MT in Cd carcinogenesis
Cd is classified as a human carcinogen, causing tumors of the lung, prostate, testes, and the injection site (Waalkes, 2003). Cd-induced lung cancer in rats was first reported two decades ago (Tanaka et al., 1983), however, the role of MT in Cd-induced lung cancer is not known. Inhalation of an atmosphere containing 1.6 mg Cd/m3 for 4 weeks (3 hr/day, 5 days/week) exhibited pulmonary tolerance when challenged with a single 3-hr acute exposure to 8.4 mg Cd/m3 (Hart et al., 1989). This may be a consequence of increased thiol-containing antioxidants including MT and glutathione and these Cd-adapted cells were less efficient at repairing oxidative DNA damage (Hart et al., 2001). Using lesion-specific enzymes in the comet assay (formamidopyrimidine DNA glycosylase (Fpg) for 8-oxoguanine repair and endonuclease III for repairing oxidized pyrimidines), adaptation to Cd results in impaired repair of both Fpg- and endonuclease III-sensitive lesions, as compared with non-adapted lung cells in response to hydrogen peroxide exposure. Both enzyme activities in whole cell extracts were also inhibited by Cd (Hart et al., 2001). Cd-adapted cells may escape apoptosis and proliferate with DNA lesions, potentially leading to carcinogenesis (Waalkes et al., 2003).
An association between human and rodent Cd exposure and prostate cancer has been suspected (Waalkes, 2003). There are indications that MT is poorly expressed in the specific lobe of the rat prostate in which Cd induces tumors, potentially indicating a basis for regional sensitivity (Waalkes et al., 1989). Repository injections of Cd also induces local sarcomas, and repeated Cd injections enhance the malignant progression of ensuring sarcomas in rats (Waalkes et al., 2000). MT expression in Cd-induced tumors varies depending on the type and the stage of tumor development. For instance, high levels of MT are detected in Cd-induced sarcomas at the injection site, whereas the sarcoma metastasis was devoid of MT (Waalkes et al., 2000). Thus, poor expression of MT clearly enhances the carcinogenic potential of Cd.
Deficiency in MT predisposes the MT-null mice to Cd hepatotoxicity following chronic exposures, including increased preneoplastic lesions in MT-null mice (Habeebu et al., 2000b). Indeed, MT-null mice were more sensitive than wild-type mice to Cd-induced hepatocarcinogenesis at 2 years. The liver tumor incidences are increased from 4.5% (control), to 20% (1 μmol Cd/kg) and 40% (5 μmol or 0.92 mg Cd/kg), after a single s.c. injection (Waalkes and Liu, 2009), indicating a generalized protective role of MT in Cd carcinogenesis. Figure 2 illustrates representative liver tumors produced by Cd in MT-null mice, including hepatocellular adenoma (D), and a trabecular hepatocellular carcinoma (F) in MT-null mice 2 years after receiving a single s.c. injection of Cd.
Conclusions and perspectives
The protective role of MT against Cd toxicity has been unequivocally established, not only for acute Cd poisoning, but also for chronic Cd toxicity, and Cd carcinogenesis. The only exception is Cd-induced testicular injury, where the genetic factors involved in Cd transport appear to dictate the testicular sensitivity to the metal.
MT functions in Cd detoxication primarily through the high affinity binding of the metal to MT, thus sequestration of Cd away from critical macromolecules. Other proposed functions of MT, such as maintaining essential metal (zinc) homeostasis, scavenging reactive oxygen species, regulating gene expression and tissue regeneration (Cherian and Kang, 2006), could all contribute to MT protection against Cd.
In humans, for reasons that are not fully understood, there are large individual variations in MT expression (Onosaka et al., 1986; Allan et al., 2000; Wu et al., 2000; Liu et al., 2007). For example, MT protein levels in human liver without any pathology varied from 1 to 104 μg/g tissue, and a 100-fold difference in the mRNA levels has been reported (Wu et al., 2000; Liu et al., 2007). Polymorphisms in the human MT-2A gene can limit MT expression (Kita et al., 2006). Low MT expression theoretically would predispose people to Cd toxicity (Nordberg, 2004). The susceptible population of Guizhou, China to arsenic exposure were shown to have reduced MT expression (Liu et al., 2007). Thus, inter-individual differences in MT could alter the susceptibility of humans to the toxicity of Cd. More research is required to test this important hypothesis.
Acknowledgments
The authors thank Drs. Michael P. Waalkes, Wei Qu and Larry Keefer for their critical review of this manuscript. This review was supported by the NIEHS grants ES-013714, ES-09716, ES-09649 for Dr. Klaassen, and in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and the federal funds from the National Cancer Institute, National Institutes of Health, under contract No. NO1-CO-12400.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan AK, Hawksworth GM, Woodhouse LR, Sutherland B, King JC, Beattie JH. Lymphocyte metallothionein mRNA responds to marginal zinc intake in human volunteers. Br J Nutr. 2000;84:747–756. [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Cadmium(update) Atlanta, Georgia: Agency for Toxic Substances and Disease Registry; 1999. pp. 1–397. [Google Scholar]
- Cherian MG, Kang YJ. Metallothionein and liver cell regeneration. Exp Biol Med. 2006;231:138–144. doi: 10.1177/153537020623100203. [DOI] [PubMed] [Google Scholar]
- Choudhuri S, Liu WL, Berman NE, Klaassen CD. Cadmium accumulation and metallothionein expression in brain of mice at different stages of development. Toxicol Lett. 1996;84:127–133. doi: 10.1016/0378-4274(95)03444-7. [DOI] [PubMed] [Google Scholar]
- Crowthers KC, Kline V, Giardina C, Lynes MA. Augmented humoral immune function in metallothionein-null mice. Toxicol Appl Pharmacol. 2000;166:161–172. doi: 10.1006/taap.2000.8961. [DOI] [PubMed] [Google Scholar]
- Dalton TP, He L, Wang B, Miller ML, Jin L, Stringer KF, Chang X, Baxter CS, Nebert DW. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc Natl Acad Sci U S A. 2005;102:3401–3406. doi: 10.1073/pnas.0406085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering PL, Klaassen CD. Altered subcellular distribution of cadmium following cadmium pretreatment: possible mechanism of tolerance to cadmium-induced lethality. Toxicol Appl Pharmacol. 1983;70:195–203. doi: 10.1016/0041-008x(83)90095-9. [DOI] [PubMed] [Google Scholar]
- Goering PL, Klaassen CD. Tolerance to cadmium-induced toxicity depends on presynthesized metallothionein in liver. J Toxicol Environ Health. 1984;14:803–812. doi: 10.1080/15287398409530628. [DOI] [PubMed] [Google Scholar]
- Goon D, Klaassen CD. Dosage-dependent absorption of cadmium in the rat intestine measured in situ. Toxicol Appl Pharmacol. 1989;100:41–50. doi: 10.1016/0041-008x(89)90090-2. [DOI] [PubMed] [Google Scholar]
- Groten JP, Koeman JH, van Nesselrooij JH, Luten JB, Fentener van Vlissingen JM, Stenhuis WS, van Bladeren PJ. Comparison of renal toxicity after long-term oral administration of cadmium chloride and cadmium-metallothionein in rats. Fundam Appl Toxicol. 1994;23:544–552. doi: 10.1006/faat.1994.1139. [DOI] [PubMed] [Google Scholar]
- Habeebu SS, Liu J, Liu Y, Klaassen CD. Metallothionein-null mice are more susceptible than wild-type mice to chronic CdCl(2)-induced bone injury. Toxicol Sci. 2000a;56:211–219. doi: 10.1093/toxsci/56.1.211. [DOI] [PubMed] [Google Scholar]
- Habeebu SS, Liu J, Liu Y, Klaassen CD. Metallothionein-null mice are more sensitive than wild-type mice to liver injury induced by repeated exposure to cadmium. Toxicol Sci. 2000b;55:223–232. doi: 10.1093/toxsci/55.1.223. [DOI] [PubMed] [Google Scholar]
- Hart BA, Voss GW, Willean CL. Pulmonary tolerance to cadmium following cadmium aerosol pretreatment. Toxicol Appl Pharmacol. 1989;101:447–460. doi: 10.1016/0041-008x(89)90193-2. [DOI] [PubMed] [Google Scholar]
- Hart BA, Potts RJ, Watkin RD. Cadmium adaptation in the lung - a double-edged sword? Toxicology. 2001;160:65–70. doi: 10.1016/s0300-483x(00)00436-4. [DOI] [PubMed] [Google Scholar]
- Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure--a review of the literature and a risk estimate. Scand J Work Environ Health. 1998;24(Suppl 1):1–51. [PubMed] [Google Scholar]
- Kagi JH, Schaffer A. Biochemistry of metallothionein. Biochemistry. 1988;27:8509– 8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- Klaassen CD. Effect of metallothionein on hepatic disposition of metals. Am J Physiol. 1978;234:E47–53. doi: 10.1152/ajpendo.1978.234.1.E47. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Liu J. Metallothionein transgenic and knock-out mouse models in the study of cadmium toxicity. J Toxicol Sci. 1998;23(Suppl 2):97–102. doi: 10.2131/jts.23.supplementii_97. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol. 1999;39:267–294. doi: 10.1146/annurev.pharmtox.39.1.267. [DOI] [PubMed] [Google Scholar]
- Lehman LD, Klaassen CD. Dosage-dependent disposition of cadmium administered orally to rats. Toxicol Appl Pharmacol. 1986;84:159–167. doi: 10.1016/0041-008x(86)90423-0. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Liu J, Klaassen CD, Waalkes MP. Acquired cadmium resistance in metallothionein-I/II(−/−) knockout cells: role of the T-type calcium channel Cacnalpha1G in cadmium uptake. Mol Pharmacol. 2006;69:629–639. doi: 10.1124/mol.105.014241. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Klaassen CD. Nephrotoxicity of CdCl2 and Cd-metallothionein in cultured rat kidney proximal tubules and LLC-PK1 cells. Toxicol Appl Pharmacol. 1994;128:264–270. doi: 10.1006/taap.1994.1206. [DOI] [PubMed] [Google Scholar]
- Liu J, Klaassen CD. Absorption and distribution of cadmium in metallothionein-I transgenic mice. Fundam Appl Toxicol. 1996;29:294–300. doi: 10.1006/faat.1996.0034. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Michalska AE, Choo KH, Klaassen CD. Distribution and retention of cadmium in metallothionein I and II null mice. Toxicol Appl Pharmacol. 1996a;136:260–268. doi: 10.1006/taap.1996.0033. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Michalska AE, Choo KH, Klaassen CD. Metallothionein plays less of a protective role in cadmium-metallothionein-induced nephrotoxicity than in cadmium chloride-induced hepatotoxicity. J Pharmacol Exp Ther. 1996b;276:1216–1223. [PubMed] [Google Scholar]
- Liu J, Liu Y, Habeebu SS, Klaassen CD. Susceptibility of MT-null mice to chronic CdCl2-induced nephrotoxicity indicates that renal injury is not mediated by the CdMT complex. Toxicol Sci. 1998a;46:97–203. doi: 10.1006/toxs.1998.2541. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Habeebu SS, Klaassen CD. Acute CdMT injection is not a good model to study chronic Cd nephropathy: comparison of chronic CdCl2 and CdMT exposure with acute CdMT injection in rats. Toxicol Appl Pharmacol. 1998b;153:48–58. doi: 10.1006/taap.1998.8506. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Habeebu SS, Klaassen CD. Metallothionein-null mice are highly susceptible to the hematotoxic and immunotoxic effects of chronic CdCl2 exposure. Toxicol Appl Pharmacol. 1999;159:98–108. doi: 10.1006/taap.1999.8718. [DOI] [PubMed] [Google Scholar]
- Liu J, Corton C, Dix DJ, Liu Y, Waalkes MP, Klaassen CD. Genetic background but not metallothionein phenotype dictates sensitivity to cadmium-induced testicular injury in mice. Toxicol Appl Pharmacol. 2001;176:1–9. doi: 10.1006/taap.2001.9262. [DOI] [PubMed] [Google Scholar]
- Liu J, Goyer R, Waalkes MP. Toxic effects of metals. In: Klaassen CD, editor. Casarett and Doull’s Toxicology - The Basic Science of Poisons. 7. McGraw Hill; 2007. pp. 931–979. [Google Scholar]
- Liu J, Cheng ML, Yang Q, Shan KR, Shen J, Zhou Y, Zhang X, Dill AL, Waalkes MP. Blood metallothionein transcript as a biomarker for metal sensitivity: low blood metallothionein transcripts in arsenicosis patients from Guizhou, China. Environ Health Perspect. 2007;115:1101–1106. doi: 10.1289/ehp.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu J, Iszard MB, Andrews GK, Palmiter RD, Klaassen CD. Transgenic mice that overexpress metallothionein-I are protected from cadmium lethality and hepatotoxicity. Toxicol Appl Pharmacol. 1995;135:222–228. doi: 10.1006/taap.1995.1227. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu J, Klaassen CD. Metallothionein-null and wild-type mice show similar cadmium absorption and tissue distribution following oral cadmium administration. Toxicol Appl Pharmacol. 2001;175:253–259. doi: 10.1006/taap.2001.9244. [DOI] [PubMed] [Google Scholar]
- Lynes MA, Zaffuto K, Unfricht DW, Marusov G, Samson JS, Yin X. The physiological roles of extracellular metallothionein. Exp Biol Med. 2006;231:1548–1554. doi: 10.1177/153537020623100915. [DOI] [PubMed] [Google Scholar]
- Margoshes M, Vallee BL. A cadmium protein from equine kidney cortex. J Am Chem Soc. 1957;79:1813–14. [Google Scholar]
- Nordberg GF. Cadmium and health in the 21st century--historical remarks and trends for the future. Biometals. 2004;17:485–489. doi: 10.1023/b:biom.0000045726.75367.85. [DOI] [PubMed] [Google Scholar]
- Nordberg GF, Goyer R, Nordberg M. Comparative toxicity of cadmium-metallothionein and cadmium chloride on mouse kidney. Arch Pathol. 1975;99:192–197. [PubMed] [Google Scholar]
- Oda N, Sogawa CA, Sogawa N, Onodera K, Furuta H, Yamamoto T. Metallothionein expression and localization in rat bone tissue after cadmium injection. Toxicol Lett. 2001;123:143–150. doi: 10.1016/s0378-4274(01)00387-3. [DOI] [PubMed] [Google Scholar]
- Onosaka S, Min KS, Fukuhara C, Tanaka K, Tashiro S, Shimizu I, Furuta M, Yasutomi T, Kobashi K, Yamamoto K. Concentrations of metallothionein and metals in malignant and non-malignant tissues in human liver. Toxicology. 1986;38:261–268. doi: 10.1016/0300-483x(86)90142-3. [DOI] [PubMed] [Google Scholar]
- Park JD, Liu Y, Klaassen CD. Protective effect of metallothionein against the toxicity of cadmium and other metals. Toxicology. 2001;163:93–100. doi: 10.1016/s0300-483x(01)00375-4. [DOI] [PubMed] [Google Scholar]
- Park JD, Cherrington NJ, Klaassen CD. Intestinal absorption of cadmium is associated with divalent metal transporter 1 in rats. Toxicol Sci. 2002;68:288–294. doi: 10.1093/toxsci/68.2.288. [DOI] [PubMed] [Google Scholar]
- Pearson CA, Lamar PC, Prozialeck WC. Effects of cadmium on E-cadherin and VE-cadherin in mouse lung. Life Sci. 2003;72:1303–1320. doi: 10.1016/s0024-3205(02)02379-2. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Wellington DR, Lamar PC. Comparison of the cytotoxic effects of cadmium chloride and cadmium-metallothionein in LLC-PK1 cells. Life Sci. 1993;53:337–342. doi: 10.1016/0024-3205(93)90567-m. [DOI] [PubMed] [Google Scholar]
- Regunathan A, Glesne DA, Wilson AK, Song J, Nicolae D, Flores T, Bhattacharyya MH. Microarray analysis of changes in bone cell gene expression early after cadmium gavage in mice. Toxicol Appl Pharmacol. 2003;191:272–93. doi: 10.1016/s0041-008x(03)00163-7. [DOI] [PubMed] [Google Scholar]
- Takenaka S, Oldiges H, König H, Hochrainer D, Oberdörster G. Carcinogenicity of cadmium chloride aerosols in W rats. J Natl Cancer Inst. 1983;70:367–373. [PubMed] [Google Scholar]
- Taylor BA, Heiniger HJ, Meier H. Genetic analysis of resistance to cadmium-induced testicular damage in mice. Proc Soc Exp Biol Med. 1973;143:629–633. doi: 10.3181/00379727-143-37380. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Perantoni A, Rehm S. Tissue susceptibility factors in cadmium carcinogenesis. Correlation between cadmium-induction of prostatic tumors in rats and an apparent deficiency of metallothionein. Biol Trace Elem Res. 1989;21:483–490. doi: 10.1007/BF02917292. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Rehm S, Cherian MG. Repeated cadmium exposures enhance the malignant progression of ensuing tumors in rats. Toxicol Sci. 2000;54:110–120. doi: 10.1093/toxsci/54.1.110. [DOI] [PubMed] [Google Scholar]
- Waalkes MP. Cadmium carcinogenesis. Mutat Res. 2003;533:107–120. doi: 10.1016/j.mrfmmm.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J. Metallothionein in inorganic carcinogenesis. In: Sigel A, Sigel H, Sigel RKO, editors. Met Ions Life Sci. Vol. 5. 2009. pp. 399–412. [Google Scholar]
- Wang C, Brown S, Bhattacharyya MH. Effect of cadmium on bone calcium and 45Ca in mouse dams on a calcium-deficient diet: evidence of Itai-Itai-like syndrome. Toxicol Appl Pharmacol. 1994;127:320–330. doi: 10.1006/taap.1994.1168. [DOI] [PubMed] [Google Scholar]
- Wong KL, Cachia R, Klaassen CD. Comparison of the toxicity and tissue distribution of cadmium in newborn and adult rats after repeated administration. Toxicol Appl Pharmacol. 1980;56:317–325. doi: 10.1016/0041-008x(80)90064-2. [DOI] [PubMed] [Google Scholar]
- Wong KL, Klaassen CD. Neurotoxic effects of cadmium in young rats. Toxicol Appl Pharmacol. 1982;63:330–337. doi: 10.1016/0041-008x(82)90261-7. [DOI] [PubMed] [Google Scholar]
- Wu MT, Demple B, Bennett RA, Christiani DC, Fan R, Hu H. Individual variability in the zinc inducibility of metallothionein-IIA mRNA in human lymphocytes. J Toxicol Environ Health. 2000;61:553–567. doi: 10.1080/00984100050194081. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Evidence for basolateral uptake of cadmium in the kidneys of rats. Toxicol Appl Pharmacol. 2000;164:15–23. doi: 10.1006/taap.1999.8854. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Molecular handling of cadmium in transporting epithelia. Toxicol Appl Pharmacol. 2003;186:163–188. doi: 10.1016/s0041-008x(02)00021-2. [DOI] [PubMed] [Google Scholar]