Abstract
Background
Trimethoprim-sulfamethoxazole (TS) prophylaxis is recommended for persons living with human immunodeficiency virus infection and acquired immunodeficiency syndrome in Africa. TS and the antimalarial combination sulfadoxine-pyrimethamine (SP) share mechanisms of action and resistance patterns, and concerns about the impact of TS resistance on SP efficacy have contributed to reluctance to implement TS prophylaxis in Africa.
Methods
To determine whether TS prophylaxis impairs SP efficacy for treatment of uncomplicated falciparum malaria, we conducted a randomized, controlled, open-label study of TS prophylaxis. Two hundred and forty children 5–15 years old were randomized in a 2:1 fashion to receive either thrice-weekly TS for 12 weeks or no prophylaxis and were treated with SP for subsequent episodes of malaria. The incidence of malaria, SP efficacy, and the prevalence of parasite mutations that confer antifolate drug resistance were measured.
Results
TS prophylaxis had a 99.5% protective efficacy against episodes of clinical malaria, with 97% efficacy against infection. Four SP treatment failures occurred in the control group, and none occurred in the TS group. No evidence was seen for selection by TS of antifolate resistance–conferring mutations in parasite dihydrofolate reductase or dihydropteroate synthase during subclinical infections.
Conclusions
In this setting of low antifolate resistance, TS was highly effective in preventing falciparum malaria infection and disease and did not appear to select for SP-resistant parasites.
In 2004, 2.3 million Africans died of HIV/AIDS and 3.1 million acquired HIV infection, bringing the number of persons living with HIV/AIDS in Africa to >25 million [1]. Despite recent progress, antiretroviral therapy remains out of reach for most Africans living with HIV/AIDS. In Cote D’Ivoire, trimethoprim-sulfamethoxazole (TS) prophylaxis reduced morbidity in persons with stage 2 or 3 HIV/AIDS and mortality in persons with both HIV/AIDS and tuberculosis [2, 3], leading UNAIDS to recommend TS prophylaxis for (1) HIV-infected adults and children in Africa who either are symptomatic or are asymptomatic with a CD4 cell count <500/mm3 and (2) infants born to HIV-infected mothers [4]. More-recent studies in Uganda and Zambia found that TS prophylaxis provided benefit [5, 6].
Trimethoprim and pyrimethamine bind and inhibit dihydrofolate reductase (DHFR), and sulfamethoxazole and sulfadoxine target dihydropteroate synthase(DHPS). Cross-resistance exists between trimethoprim and pyrimethamine and between sulfamethoxazole and sulfadoxine [7–12], raising the possibility that use of TS prophylaxis in areas where malaria is endemic would select for Plasmodium falciparum DHFR and DHPS mutations that confer resistance to the antimalarial combination sulfadoxine-pyrimethamine (SP). Prophylaxis with pyrimethamine was strongly and rapidly selective for DHFR mutations in children living in rural areas in Mali [13]. If similar selection occurs with TS prophylaxis, it could accelerate the development of resistance to SP and other antifolate antimalarials in areas where HIV infection and malaria are both highly prevalent.
Although malaria-treatment policies are increasingly recommending newer combination therapies, SP will continue to be used widely in Africa unless and until more-expensive combination therapies are made universally available. SP is also recommended for intermittent preventive treatment during pregnancy and infancy, even in the face of the moderate resistance found in Africa [14, 15]. The widespread use of TS prophylaxis in areas where malaria is highly prevalent might hasten the spread of SP resistance and introduce resistance to other promising antifolate antimalarials that are under development. More importantly, HIV-infected persons receiving TS prophylaxis may be more likely to fail SP treatment when they contract malaria. If so, drugs other than SP will need to be used to treat persons receiving TS prophylaxis who develop malaria. Concerns about the impact of TS resistance on SP efficacy have contributed to reluctance to implement TS prophylaxis in Africa.
PARTICIPANTS, MATERIALS, AND METHODS
Participants
The present study was conducted at the Bandiagara Malaria Project clinical research facility in Bandiagara, Mali, from September through December 2000. Bandiagara is a town of 13,600 persons and is located on a semiarid plateau ~600 km northeast of Bamako, the capital city. Transmission of malaria is seasonal but intense, with peak transmission occurring during the rainy season (July–October); ~2 episodes of clinical malaria occur per child each year [16]. HIV sero-prevalence studies have not been conducted at this site, but the overall prevalence of HIV infection in Mali is reported to be <2%, and our clinical experience at this rural site is consistent with very low rates of HIV infection in children.
Children 5–15 years old who were enrolled in an ongoing cohort study of the incidence of malaria were eligible for inclusion. Exclusion criteria included planned travel outside of the study area, severe acute or chronic illness, pregnancy, allergy to sulfa drugs (including TS or SP), use of SP during the preceding 28 days, and use of chloroquine, TS, or other sulfa drugs during the preceding 7 days.
Sample sizes
Sample sizes of 160 in the TS group and 80 in the control group were chosen, to permit detection of a difference in SP resistance of 5% versus 20% (control group vs. TS group, respectively) with 80% power (evaluated at 2-tailed P =.05 by Fisher’s exact test [EpiInfo 6; Centers for Disease Control and Prevention]) with up to 22.5% losses to follow-up.
Objectives and outcomes
The primary objective was to test the hypothesis that TS prophylaxis decreases the efficacy of SP treatment of P. falciparum infection. The secondary objectives were to test the hypothesis that TS prophylaxis selects for resistance-conferring mutations in P. falciparum DHFR and DHPS and to measure the prophylactic efficacy of TS, calculated using the formula E = 1 − (RTS/RC), where RTS and RC are the rates of episodes of clinical malaria and asymptomatic infection in the TS and control groups, respectively. The primary outcome was the therapeutic efficacy of SP, as defined in the standard World Health Organization (WHO) protocol [17]. The secondary outcomes included the prevalence of resistance-conferring mutations; time to first episode of clinical malaria; incidences of anemia, gastrointestinal illness, and respiratory illness; use of all prescription medicines; use of antibiotics; and change in hemoglobin level.
Treatment allocation and study intervention
Children were randomized in a 2:1 fashion to receive either TS prophylaxis or no prophylaxis. The choice of a prophylaxis:no prophylaxis ratio of 2:1 was based on an estimated TS prophylactic efficacy of 50%, which was, in turn, based on the relatively poor efficacy of TS as a treatment for malaria in other settings [18]. No data on the antimalarial efficacy of TS in Mali were available. In accordance with a standard prophylactic regimen that is recommended by the American Academy of Pediatrics, trimethoprim (150 mg/m2 of body surface area) and sulfamethoxazole (750 mg/m2 of body surface area) were administered as a single dose on 3 consecutive days each week for 12 weeks. Thrice-weekly dosing was chosen to maximize the possibility of detecting the selection of antifolate-resistant P. falciparum. Trained personnel directly observed the administration of TS and monitored each child for 30 min after TS was taken. If a child vomited during the period of observation, a repeat dose of TS was given.
Once a week, a study physician evaluated each child. Any child who had symptoms consistent with malaria, an axillary temperature ≥37.5°C, profound anemia, or jaundice was given a full assessment, including a malaria blood smear and measurement of hemoglobin level. At enrollment and once a month thereafter, a filter-paper blood sample was obtained from each child, and malaria blood smears (thick and thin) were prepared. If symptoms were present, blood smears were read contemporaneously; if no symptoms were present, blood smears were read at the conclusion of the study. Study physicians were continuously available to evaluate any medical complaints. After treatment of malaria with SP, treatment response was assessed on days 1, 2, 3, 7, 14, 21, and 28 after treatment, in accordance with the standard WHO protocol [17].
Definitions, diagnoses, and treatment of malaria and other infectious diseases
Uncomplicated malaria was defined as any level of parasitemia accompanied by signs or symptoms consistent with malaria that either led to the seeking of treatment or were reported during the weekly follow-up visits. These signs and symptoms included fever at the time of evaluation (axillary temperature ≥37.5°C), conjunctival or palmar pallor, jaundice, report of fever during the previous 2 days, lassitude, headache, body aches, cough, diarrhea, and abdominal pain. This broad definition was chosen to reflect actual treatment practices. Severe malaria was defined according to a WHO protocol [19].
Thick blood smears were prepared from 1–2 drops of blood that were obtained by digital puncture. After staining with Field stain, the smears were allowed to air dry. Parasitemia was determined by counting the number of parasites per 300 leukocytes and then multiplying this number by 25, to create an estimate of the parasite count per cubic milliliter on the basis of an assumed leukocyte count of 7500 cells/mm3.
Uncomplicated malaria was treated with SP (one-fourth of a tablet/5 kg of weight for children ≤12 years old, and 3 tablets for children >12 years old [1 tablet equaled 25 mg of pyrimethamine and 500 mg of sulfadoxine]). After administration, children were monitored for 60 min for adverse reactions and vomiting. If vomiting occurred within 30 min, the full dose of SP was repeated; if vomiting occurred between 30 and 60 min, one-half of the dose was repeated. When SP treatment was administered to a child receiving TS prophylaxis, the prophylaxis was stopped for 1 week after SP treatment. Severe malaria was treated with intravenous quinine and parenteral SP [19]. SP treatment outcomes were defined as adequate clinical and parasitological response, late parasitological failure, late clinical failure, and early treatment failure, in accordance with the standard WHO protocol [17]. Children with persistent symptoms of malaria and/or parasitemia after SP treatment were promptly evaluated by a study physician, and children who experienced treatment failure were treated with chloroquine. At the time of the study, chloroquine was the official first-line drug for uncomplicated falciparum malaria in Mali and retained good efficacy [20].
Gastrointestinal and respiratory illnesses were diagnosed on the basis of the presence of clinical signs and symptoms supporting these syndromes and the absence of P. falciparum infection as determined by thick blood smear. For gastrointestinal illness, signs and symptoms included diarrhea, bloody diarrhea, vomiting, and abdominal pain; for respiratory illness, signs and symptoms included cough, rhinitis, tachypnea, and an auscultatory finding suggestive of bronchitis or pneumonia. Further gastrointestinal or respiratory signs and symptoms occurring over the next 7 days of follow-up were considered to be part of the initial illness. If malaria was detected during follow-up, the presenting symptoms were attributed to malaria and not gastrointestinal or respiratory illness. Children who received a diagnosis of a bacterial infection were treated with an appropriate nonsulfa antibiotic.
Data management and statistical methods
Data were double-entered into a FileMaker Pro (version 5.0) database. Duplicate entries were reconciled, and the data were then exported to SPSS (version 10.1.0; SPSS) for analysis. Means, SDs, and medians were calculated for continuous variables. Student’s t test was used to compare means for continuous variables, and either the χ2 test or Fisher’s exact test was used to compare proportions for categorical variables. All statistical tests were 2-tailed; P < .05 was considered to be statistically significant.
We calculated the incidence of episodes of clinical malaria in the TS and control groups as the number of episodes per person-week of follow-up and expressed these results as a rate ratio (RR). Other RRs (for gastrointestinal illness, respiratory illness, use of all prescription medicines, and use of antibiotics) were calculated similarly. Children who received SP for malaria were considered to not be at risk for P. falciparum reinfection for 28 days after SP treatment [21]. The mean change in hemoglobin level was calculated by subtracting the initial hemoglobin level from the final hemoglobin level; means were compared by the paired-samples Student’s t test. Time to first episode of clinical malaria was estimated by Kaplan-Meier analysis, with a log-rank test to compare the distribution of malaria-free time.
Molecular analyses
All molecular analyses were performed at the Malaria Research and Training Center in Mali. For quality control, a subset of samples were reanalyzed at the University of Maryland. Parasite DNA was extracted from filter-paper blood samples, and the prevalence of SP resistance–conferring DHFR and DHPS point mutations was determined by nested polymerase chain reaction (PCR), as described elsewhere [22, 23]. DHFR serine→asparagine 108, asparagine→isoleucine 51, and cysteine→arginine 59 mutations and DHPS alanine→glycine 437 and lysine→glutamic acid 540 mutations were determined. Because malarial parasites may be present at levels that are undetectable by light microscopy, microscopy negative as well as positive samples were analyzed by PCR. The prevalences of point mutations were compared between pre– and post–TS prophylaxis samples.
Ethics review and informed consent
The study protocol was reviewed and approved by the institutional review boards of the Faculty of Medicine, University of Mali, and the University of Maryland, Baltimore. Written, informed consent was obtained from the parents or guardians of the study participants.
RESULTS
Participants
The characteristics of the study participants at enrollment are shown in table 1. Enrollment took place in September 2000, and follow-up was from late September through December 2000. Fifty-six percent of the TS group and 50% of the control group were female. The mean age in both groups was 10 years. At enrollment, there were no significant differences in weight, height, axillary temperature, and hemoglobin level between the 2 groups. Twenty percent of the TS group and 16% of the control group had blood smears that were positive for P. falciparum at enrollment. Approximately 80% of the children in both groups had used such protective measures as impregnated bed nets or mosquito coils during the previous week. Of the 160 children assigned to receive TS and the 80 assigned to receive no prophylaxis, 157 and 77, respectively, completed follow-up and were analyzed on a per-protocol basis for the primary outcome.
Table 1.
Characteristics of the study participants at enrollment.
| Characteristic | TS group (n = 160) |
Control group (n = 80) |
P |
|---|---|---|---|
| Female | 90 (56) | 40 (50) | .36 |
| Age, mean ± SD, years | 10 ± 2.9 | 10 ±2.8 | .69 |
| Weight, mean ± SD, kg | 26 ± 9 | 25 ±7 | .74 |
| Height, mean ± SD, cm | 130 ± 15 | 130 ±16 | .84 |
| Axillary temperature, mean ± SD, °C | 36.4 ± 0.5 | 36.4 ±0.4 | .35 |
| Hemoglobin level, mean ± SD, g/dL | 11.9 ± 1.3 | 12.1 ±1.3 | .30 |
| Microscopy positive for Plasmodium falciparum | 32 (20) | 13 (16) | .46 |
| Parasitemia, mean ± SD, parasites/mm3 | 127 ± 303 | 73 ± 56 | .53 |
| Use of protective measures during previous weeka | 128 (80) | 65 (81) | .82 |
NOTE. Data are no. (%) of children, unless otherwise noted. TS, trimethoprim-sulfamethoxazole.
Such as impregnated bed nets or mosquito coils.
SP efficacy
The single episode of clinical malaria in the TS group occurred in a child who had an adequate clinical and parasitological response to SP treatment. In the control group, 5 episodes of clinical malaria occurred in children who had a parasitemia of >100,000 parasites/mm3 and were treated with SP and parenteral quinine, and 1 episode occurred in a child who was infected with multiple parasite species, including P. falciparum and P. malariae. Among the remaining 66 episodes, 3 instances of SP failure occurred (4.5%): 1 instance each of early treatment failure and late parasitological failure after 2 separate episodes in the same child and 1 instance of late clinical failure. With just 1 episode of clinical malaria occurring in the TS group, meaningful comparison of SP efficacy, the primary outcome, between the TS and control groups was not possible.
Prophylactic efficacy
The mean follow-up times were 11.8 weeks in the TS group and 11.7 weeks in the control group (table 2). Three children in each group were lost to follow-up. Among the remaining 234 children, there was just 1 episode of clinical malaria during 1890 person-weeks of follow-up in the TS group, and there were 72 episodes during 681 person-weeks of follow-up in the control group. The 72 episodes in the control group consisted of a single episode in 43 children, 2 episodes in 13 children, and 3 episodes in 1 child. The prophylactic efficacy of TS against uncomplicated malaria was 99.5% (95% confidence interval [CI], 96%–100%) (P < .001). Figure 1 shows the Kaplan-Meier curves for time to the first episode of clinical malaria in the TS and control groups. The time to the first episode was shorter in the control group (P < .001) than in the TS group. Asymptomatic P. falciparum infections were found for 3 of 466 blood smears in the TS group and for 43 of 231 blood smears in the control group, an efficacy of 97% (95% CI, 89%–99%) (P < .001).
Table 2.
Health indicators in the study participants receiving trimethoprim-sulfamethoxazole (TS) prophylaxis or no prophylaxis (the control group).
| Indicator | TS group (n = 157) |
Control group (n = 77) |
RR (95% CI) | P |
|---|---|---|---|---|
| Episodes of clinical malaria | 1/1890 (0.1) | 72/681 (10.6) | 0.005 (0.00–0.04) | <.001 |
| Asymptomatic parasitemia during monthly surveysa | 3/466 (0.6) | 43/231 (18.6) | 0.03 (0.01–0.11) | <.001 |
| Gastrointestinal illness | 68/1894 (3.6) | 49/933 (5.3) | 0.68 (0.47–0.99) | .04 |
| Respiratory illness | 78/1894 (4.1) | 37/933 (4.0) | 1.04 (0.70–1.54) | .77 |
| Use of all prescription medicines | 125/1894 (6.6) | 88/933 (9.4) | 0.70 (0.53–0.92) | .01 |
| Use of antibiotics | 11/1894 (0.6) | 8/933 (0.9) | 0.68 (0.27–1.69) | .41 |
| Increase in hemoglobin level, g/dL | 0.97 | 0.42 | … | .004 |
| Anemia during monthly surveysa | 20/471 (4.2) | 18/224 (8.0) | 0.53 (0.29–0.98) | .04 |
NOTE. Data are no. (%) of events/person-weeks of follow-up, unless otherwise noted. CI, confidence interval; RR, rate ratio.
Defined as a hemoglobin level <10 g/dL; includes results from surveys conducted 1, 2, and 3 months after the start of TS prophylaxis.
Figure 1.
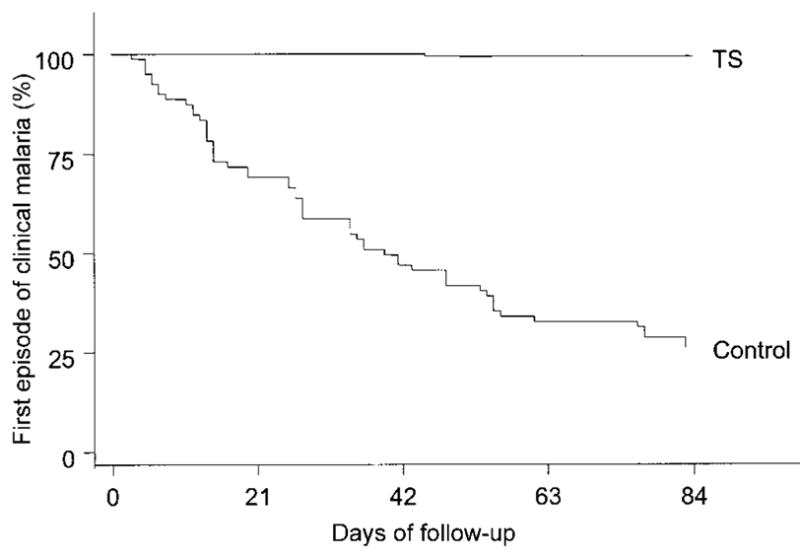
Kaplan-Meier curves showing time to first episode of clinical malaria. TS, trimethoprim-sulfamethoxazole.
The TS group experienced fewer gastrointestinal illnesses (RR, 0.68) and used fewer prescription medicines (RR, 0.70) than did the control group. No difference in the incidence of respiratory illnesses or use of antibiotics was found. The TS group had a mean increase in hemoglobin level of 0.97 g/dL from enrollment to study conclusion, whereas the control group had a mean increase of 0.42 g/dL (P < .01). As determined by monthly surveys, fewer children in the TS group had anemia (hemoglobin level <10 g/dL), compared with the control group (RR, 0.53). During the malaria season of the following year, there was no difference in the incidence of episodes of clinical malaria between the TS and control groups (data not shown).
Adverse events
One serious adverse event occurred—a 6-year-old boy developed acute hepatitis 3 days after starting TS prophylaxis. Serological analysis showed evidence of past infection with hepatitis A virus and equivocal evidence of past or recent infection with hepatitis B virus. TS prophylaxis was stopped, and the acute hepatitis resolved without sequelae.
Resistance mutations
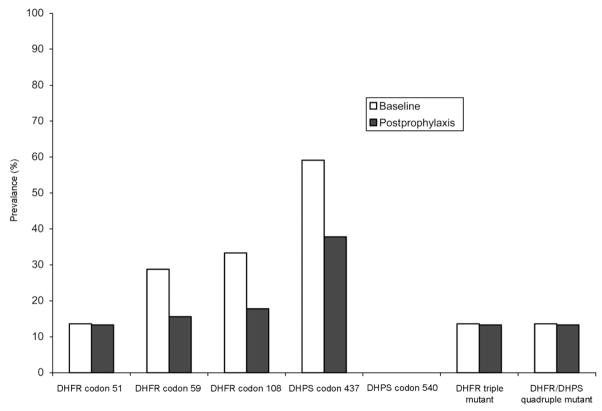
For infections detected by microscopy at the time of enrollment, the baseline prevalences of DHFR mutations were 13.6% (n = 66), 28.8% (n = 66), and 33.3% (n = 66) for codons 51, 59, and 108, respectively, and the baseline prevalences of DHPS mutations were 59.1% (n = 66) and 0% (n = 66) for codons 437 and 540, respectively (figure 2). The prevalences of the DHFR triple mutant and the DHFR/DHPS quadruple mutant were both 13.6% (n = 66), because all DHFR triple mutants also carried the DHPS 437 mutation.
Figure 2.
Prevalences of dihydrofolate reductase (DHFR) and dihydropteroate synthase (DHPS) mutations in Plasmodium falciparum parasites infecting the children in the trimethoprim-sulfamethoxazole (TS) group at baseline (microscopy-positive asymptomatic infections) and after 1 month of TS prophylaxis (microscopy-negative, polymerase chain reaction–positive asymptomatic infections).
Parasite DHFR and DHPS were successfully amplified from 45 of 90 microscopy-negative samples obtained from children in the TS group during active surveillance 1 month after the start of TS prophylaxis. The prevalences of DHFR mutations in these parasites detected in children receiving TS were 13.3%, 15.6%, and 17.8% for codons 51, 59, and 108, respectively, and the prevalences of DHPS mutations were 37.8% and 0% for codons 437 and 540, respectively. The prevalences of both the DHFR triple mutant and DHFR/DHPS quadruple mutant were again identical (13.3%; n = 45). The prevalences of DHFR mutations at codons 51, 59, and 108 were not significantly different between baseline and after 1 month of TS prophylaxis. However, the prevalence of the DHPS mutation at codon 437 was lower after 1 month of TS prophylaxis (P = .027). PCR amplification was unsuccessful for nearly all filter-paper blood samples collected during the surveys conducted after 2, 3, and 4 months of TS prophylaxis, despite successful amplification of positive controls.
DISCUSSION
In the present study, TS was found to be a highly effective prophylactic agent against falciparum malaria infection and disease. A single dose of TS given for 3 consecutive days each week for 12 weeks reduced the incidence of uncomplicated falciparum malaria by 99.5% and reduced the monthly prevalence of asymptomatic microscopy-confirmed P. falciparum infection by 97%. Such high prophylactic efficacy of TS was unexpected. Antifolate-resistant P. falciparum was more common in most other African settings than in Mali in 2000 [23–25], and TS should not be relied on for antimalarial prophylaxis in nonimmune travelers or others.
Our study was conducted in healthy children in an area with very low rates of HIV infection. Although the antimalarialefficacy of TS prophylaxis might have been lower in HIV-infected children, studies of TS prophylaxis in people living with HIV/AIDS in areas with low [3] and moderate [5] levels of SP resistance have reported good efficacy against malaria, although not as high as we saw in the present study. In Lusaka, Zambia, where the incidence of malaria is low and the level of SP resistance is relatively high, TS prophylaxis did not prevent malaria [6].
The ability to detect an impact of TS prophylaxis on SP efficacy may have been limited by the duration of our study, in that antifolate-resistant parasites might have been selected for under more-prolonged drug pressure. However, studies of pyrimethamine prophylaxis have shown that antifolate-resistant parasites emerge within days to weeks of starting prophylaxis [23, 26]. The most highly antifolate-resistant forms of the parasite have recently been shown to share common ancestry in several East African countries as well as in Southeast Asia and South America, supporting the idea that highly antifolate-resistant DHFR and DHPS genotypes of P. falciparum do not arise de novo in response to drug pressure but rather are introduced from the outside [27, 28]. Nonetheless, this would not explain the lack of selection for the DHFR triple mutant or the DHFR/DHPS quadruple mutant under TS prophylaxis—these were already present in the study population but did not increase in frequency after the start of TS prophylaxis.
Most of the antifolate resistance–conferring mutations were less frequent in microscopy-negative samples after the start of TS prophylaxis then they were at baseline. This result should be interpreted with caution, both because of the lack of statistical significance and because the post–TS prophylaxis samples, in contrast to the baseline samples, corresponded to asymptomatic children with no P. falciparum infection detectable by microscopy. Appropriate controls were run and assays were repeated by separate workers, to rule out PCR contamination. Similar rates of successful PCR amplification of different target sequences from microscopy-negative samples have been observed in subsequent studies at this site (authors’ unpublished data).
The failure to detect selection for DHFR and DHPS mutations does not imply different mechanisms of resistance for TS and SP. Although we have previously shown that pyrimethamine prophylaxis [13] and SP treatment [23] select for DHFR mutations in Mali, it is possible that trimethoprim, with its much shorter half-life and frequent dosing, does not provide as much selection pressure over the short term as is seen with the much longer acting pyrimethamine when it is administered as a single or weekly dose.
TS prophylaxis reduced the incidence of gastrointestinal illness by >30%. This may reflect decreased infection with enteric bacteria, such as nontyphoid Salmonella species, and/or parasites that are susceptible to TS, such as Isospora species [29]. Children receiving TS prophylaxis had a slightly greater increase in hemoglobin level than did those not receiving TS, although anemia was uncommon in both groups. This likely reflects the decreased incidence of falciparum malaria infection and disease in the TS group. Although the preventive efficacy against malaria, anemia, and gastrointestinal disease do not warrant consideration of routine TS prophylaxis for healthy children, these benefits mitigate concerns about TS use in HIV-exposed children whose HIV status is not yet known.
The present study was not able to assess the impact of TS prophylaxis on SP efficacy for treatment of falciparum malaria, because of the unexpected lack of prophylaxis failures in the TS group. Despite its compromised efficacy, SP continues to be commonly used for treatment of falciparum malaria and for intermittent preventive treatment of pregnant women and infants, and it is a component of combination therapies being considered or implemented in some countries. Although policies have changed from recommending SP to recommending newer combination therapies, obstacles to universal access remain [30], and SP will continue to be relied on to some degree for the foreseeable future, particularly in the most resource-poor settings. Studies of SP efficacy in persons receiving TS prophylaxis are needed in areas with moderate levels of antifolate resistance. Until such studies are conducted, SP should be used to treat persons receiving TS prophylaxis who develop malaria only with caution, vigilance for high failure rates should be maintained, and alternative treatments, such as newer combination therapies [31], should be used if they are available. The potential for adverse reactions, in particular to sulfa drugs, also favors avoiding the use of SP in persons receiving TS prophylaxis. In light of the results of the present study and the clear evidence that TS prophylaxis prevents death in persons living with HIV/AIDS in a variety of African settings, it is evident that concerns about the spread of SP resistance do not justify further delays in the implementation of TS prophylaxis.
Acknowledgments
We thank the participants, their families, and the people of Bandiagara, Mali, for their participation in this project; the chief physician of the Bandiagara District Health Center and his staff; and the Bandiagara Traditional Healers Association.
Financial support: National Institute of Allergy and Infectious Diseases, National Institutes of Health (contract N01-AI-85346); Fogarty International Center (grant D43-TW-001589).
Footnotes
Presented in part: 50th annual meeting of the American Society of Tropical Medicine and Hygiene, Atlanta, 13 November 2001 (abstract 361).
Potential conflicts of interest: none reported.
References
- 1.UNAIDS. [Accessed 6 October 2005];AIDS epidemic update. 2004 December; Available at: http://www.unaids.org/wad2004/report.html.
- 2.Wiktor SZ, Sassan-Morokro M, Grant AD, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d’Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–75. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 3.Anglaret X, Chene G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d’Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353:1463–8. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS. [Accessed 6 October 2005];Provisional WHO/UNAIDS secretariat recommendations on the use of cotrimoxazole prophylaxis in adults and children living with HIV/AIDS in Africa. Available at: http://www.unaids.org/html/pub/publications/irc-pub04/recommendation_en_pdf.htm. [PMC free article] [PubMed]
- 5.Mermin J, Lule J, Ekwaru JP, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–34. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 6.Chintu C, Bhat GJ, Walker AS, et al. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet. 2004;364:1865–71. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- 7.Petersen E. In vitro susceptibility of Plasmodium falciparum malaria to pyrimethamine, sulfadoxine, trimethoprim and sulfamethoxazole, singly and in combination. Trans R Soc Trop Med Hyg. 1987;81:238–41. doi: 10.1016/0035-9203(87)90226-4. [DOI] [PubMed] [Google Scholar]
- 8.Wang P, Read M, Sims PF, Hyde JE. Sulfadoxine resistance in the human malaria parasite Plasmodium falciparum is determined by mutations in dihydropteroate synthetase and an additional factor associated with folate utilization. Mol Microbiol. 1997;23:979–86. doi: 10.1046/j.1365-2958.1997.2821646.x. [DOI] [PubMed] [Google Scholar]
- 9.Triglia T, Menting JGT, Wilson C, Cowman AF. Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc Natl Acad Sci USA. 1997;94:13944–9. doi: 10.1073/pnas.94.25.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin DC, Arnold JD. Trimethoprim in therapy of acute attacks of malaria. J Clin Pharmacol J New Drugs. 1967;7:336–41. doi: 10.1002/j.1552-4604.1967.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 11.Feikin DR, Dowell SF, Nwanyanwu OC, et al. Increased carriage of trimethoprim/sulfamethoxazole-resistant Streptococcus pneumoniae in Malawian children after treatment for malaria with sulfadoxine/pyrimethamine. J Infect Dis. 2000;181:1501–5. doi: 10.1086/315382. [DOI] [PubMed] [Google Scholar]
- 12.Iyer JK, Milhous WK, Cortese JF, Kublin JG, Plowe CV. Plasmodium falciparum cross-resistance between trimethoprim and pyrimethamine. Lancet. 2001;358:1066–7. doi: 10.1016/S0140-6736(01)06201-8. [DOI] [PubMed] [Google Scholar]
- 13.Doumbo OK, Kayentao K, Djimde A, et al. Rapid selection of Plasmodium falciparum dihydrofolate reductase mutants by pyrimethamine prophylaxis. J Infect Dis. 2000;182:993–6. doi: 10.1086/315787. [DOI] [PubMed] [Google Scholar]
- 14.Shulman CE, Dorman EK, Cutts F, et al. Intermittent sulphadoxine-pyrimethamine to prevent severe anaemia secondary to malaria in pregnancy: a randomised placebo-controlled trial. Lancet. 1999;353:632–6. doi: 10.1016/s0140-6736(98)07318-8. [DOI] [PubMed] [Google Scholar]
- 15.Schellenberg D, Menendez C, Kahigwa E, et al. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: a randomised, placebo-controlled trial. Lancet. 2001;357:1471–7. doi: 10.1016/S0140-6736(00)04643-2. [DOI] [PubMed] [Google Scholar]
- 16.Coulibaly D, Diallo DA, Thera MA, et al. Impact of preseason treatment on incidence of falciparum malaria and parasite density at a site for testing malaria vaccines in Bandiagara, Mali. Am J Trop Med Hyg. 2002;67:604–10. doi: 10.4269/ajtmh.2002.67.604. [DOI] [PubMed] [Google Scholar]
- 17.Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria (WHO/HTM/RBM/2003.50) Geneva: World Health Organization; 2003. [Google Scholar]
- 18.Kilian AH, Jelinek T, Prislin I, et al. Resistance in vivo of Plasmodium falciparum to co-trimoxazole in western Uganda. Trans R Soc Trop Med Hyg. 1998;92:197–200. doi: 10.1016/s0035-9203(98)90748-9. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization, Communicable Diseases Cluster. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 20.Plowe CV, Doumbo OK, Djimde A, et al. Chloroquine treatment of uncomplicated Plasmodium falciparum malaria in Mali: parasitologic resistance versus therapeutic efficacy. Am J Trop Med Hyg. 2001;64:242–6. doi: 10.4269/ajtmh.2001.64.242. [DOI] [PubMed] [Google Scholar]
- 21.Watkins WM, Mosobo M. Treatment of Plasmodium falciparum malaria with pyrimethamine-sulfadoxine: selective pressure for resistance is a function of long elimination half-life. Trans R Soc Trop Med Hyg. 1993;87:75–8. doi: 10.1016/0035-9203(93)90431-o. [DOI] [PubMed] [Google Scholar]
- 22.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg. 1995;52:565–8. doi: 10.4269/ajtmh.1995.52.565. [DOI] [PubMed] [Google Scholar]
- 23.Diourte Y, Djimde A, Doumbo OK, et al. Pyrimethamine-sulfadoxine efficacy and selection for mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase in Mali. Am J Trop Med Hyg. 1999;60:475–8. doi: 10.4269/ajtmh.1999.60.475. [DOI] [PubMed] [Google Scholar]
- 24.Plowe CV, Kublin JG, Dzinjalamala FK, et al. Sustained clinical efficacy of sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Malawi after 10 years as first line treatment: five year prospective study. BMJ. 2004;328:545. doi: 10.1136/bmj.37977.653750.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasasira AF, Dorsey G, Nzarubara B, et al. Comparative efficacy of aminoquinoline-antifolate combinations for the treatment of uncomplicated falciparum malaria in Kampala, Uganda. Am J Trop Med Hyg. 2003;68:127–32. [PubMed] [Google Scholar]
- 26.Clyde DF, Shute GT. Resistance of Plasmodium falciparum in Tanganyika to pyrimethamine administered at weekly intervals. Trans R Soc Trop Med Hyg. 1957;51:505–13. doi: 10.1016/0035-9203(57)90039-1. [DOI] [PubMed] [Google Scholar]
- 27.Roper C, Pearce R, Bredenkamp B, et al. Antifolate antimalarial resistance in southeast Africa: a population-based analysis. Lancet. 2003;361:1174–81. doi: 10.1016/S0140-6736(03)12951-0. [DOI] [PubMed] [Google Scholar]
- 28.Roper C, Pearce R, Nair S, Sharp B, Nosten F, Anderson T. Intercontinental spread of pyrimethamine-resistant malaria. Science. 2004;305:1124. doi: 10.1126/science.1098876. [DOI] [PubMed] [Google Scholar]
- 29.Asrat D, Amanuel YW. Prevalence and antibiotic susceptibility pattern of bacterial isolates from blood culture in Tikur Anbassa Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2001;39:97–104. [PubMed] [Google Scholar]
- 30.Mutabingwa TK. Artemisinin-based combination therapies (ACTs): best hope for malaria treatment but inaccessible to the needy! Acta Trop. 2005;95:305–15. doi: 10.1016/j.actatropica.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 31.White NJ, Nosten F, Looareesuwan S, et al. Averting a malaria disaster. Lancet. 1999;353:1965–7. doi: 10.1016/s0140-6736(98)07367-x. [DOI] [PubMed] [Google Scholar]