Abstract
Bovine postpartum uterine disease, metritis, affects about 40% of animals and is widely considered to have a bacterial aetiology. Although the gamma herpesvirus BoHV-4 has been isolated from several outbreaks of metritis or abortion, the role of viruses in endometrial pathology and the mechanisms of viral infection of uterine cells are often ignored. The objectives of the present study were to explore the interaction, tropism and outcomes of BoHV-4 challenge of endometrial stromal and epithelial cells. Endometrial stromal and epithelial cells were purified and infected with a recombinant BoHV-4 carrying an EGFP expression cassette to monitor the establishment of infection. BoHV-4 efficiently infected both stromal and epithelial cells, causing a strong non apoptotic CPE, associated with robust viral replication. The crucial step for the BoHV-4 endometriotropism appeared to be after viral entry as there was enhanced transactivation of the BoHV-4 IE2 gene promoter following transiently transfection into the endometrial cells. Infection with BoHV-4 increased COX-2 protein expression and prostaglandin E2 secretion in endometrial stromal cells, but not epithelial cells. Bovine macrophages are persistently infected with BoHV-4 and co-culture with endometrial stromal cells reactivated BoHV-4 replication in the persistently infected macrophages, suggesting a symbiotic relationship between the cells and virus. In conclusion, the present study provides evidence of cellular and molecular mechanisms supporting the concept that BoHV-4 is a pathogen associated with uterine disease.
Introduction
Bovine herpesvirus 4 (BoHV-4) was originally included in the Betaherpesvirinae subfamily and referred to as bovine cytomegalovirus, primarily because its biological properties in tissue culture most closely resembled those of human cytomegaloviruses (Storz et al., 1984). However, molecular evidence has accumulated that indicate BoHV-4 is genetically more closely related to members of the Gammaherpesvirinae subfamily. This evidence includes large blocks of homologous genes arranged in the same order as two other gammaherpesviruses, Epstein-Barr virus and herpesvirus saimiri (Bublot et al. 1992). BoHV-4 replicates in a broad range of cell lines and primary cultures, causing a cytopathic effect (CPE) (Donofrio et al. 2002; Gillet et al. 2004; Peterson & Goyal 1988; Truman et al. 1986). However, like other herpesviruses, BoHV-4 can establish persistent infections in its natural host (Dewals et al. 2005; Dubuisson et al. 1989; Krogman & McAdaragh 1982; Osorio & Reed 1983) and experimental hosts such as the rabbit (Osorio et al. 1982). Although the presence of BoHV-4 has been demonstrated in many tissues, the cells of the monocyte/macrophage lineage are a common site of persistence in natural and experimental hosts (Dubuisson et al. 1988; Dubuisson et al. 1989; Naeem et al. 1993; Osorio & Reed 1983; Osorio et al. 1985). Cell lines persistently infected with gammaherpesviruses such as Epstein–Barr virus, herpesvirus saimiri, human herpesvirus 8, and murine gammaherpesvirus-68 have been established from cells isolated from infected hosts (Ceserman et al. 1995; Jung et al. 1999; Nilsson 1979; Usherwood et al. 1996). This process has likely been greatly facilitated by the growth-transforming ability of these gammaherpesviruses (Flore et al. 1998; Jung et al. 1999; Miller et al. 1997; Moses et al. 1999). In contrast, no evidence for growth-transformation has been obtained for BoHV-4. Each of the genes associated with transformation by other gammaherpesviruses is unique to each virus, and a homologous gene is not found in BoHV-4 (Lomonte et al. 1996).
BoHV-4 was first isolated in Europe from animals with respiratory and ocular diseases by Bartha and colleagues (Bartha et al. 1966) and later in the United States by Mohanty and colleagues (Mohanty et al. 1966). BoHV-4 has been isolated from a variety of samples and cells from healthy cattle and animals with metritis, abortion, pneumonia, diarrhoea, respiratory infection or mammary dermatitis (Bartha et al. 1966; Egyed 1988; Thiry et al. 1988). The pathogenic role of BoHV-4 remains unclear, and correlations between BoHV-4 and disease are unresolved even by experimental infection. Indeed, few investigators have successfully produced experimental disease (Thiry et al. 1988), and direct inoculation of the natural host only occasionally elicited respiratory and genital disease including abortion (Castrucci et al. 1987; Wellemans et al. 1986).
Abortion may follow infection with a variety of alpha-, beta- and gammaherpesvirus, but viral causes of uterine disease are seldom investigated in cattle. Although postpartum metritis affects up to 40% of cattle, causing considerable infertility and economic loss, it has been assumed that most disease is of bacterial origin and virus isolation or serology is rarely considered (Sheldon & Dobson 2004). The first reported isolation of BoHV-4 from a case of bovine metritis was in 1973 (Park & Kendrick 1973). Later several other isolates were obtained from cows with reproductive disorders from different countries, including Italy (Castrucci et al. 1986) and India (Mehrotra et al. 1986). In Belgium, BoHV-4 seroprevalence was associated with postpartum metritis, and chronic infertility of cattle (Czaplicki & Thiry 1998). Postpartum metritis has also been associated with BoHV-4 in the USA (Frazier et al. 2002; Frazier et al. 2001), Spain (Monge et al. 2006) and Serbia (Nikolin et al. 2007). There is a gap in knowledge about the direct correlation between viral infection and uterine pathology. Thus, the objectives of the present study were to determine the interaction, tropism and outcome of BoHV-4 challenge of endometrial epithelial and stromal cells.
Materials and Methods
Endometrial cell isolation and primary cultures
To better understand the interaction of BoHV-4 with the endometrium it was important to use purified populations of epithelial and stromal cells without contamination by leukocytes, because BoHV-4 function can be influenced by macrophages (Donofrio & van Santen 2001). Bovine uteri from post-pubertal non-pregnant BoHV-4 serum negative animals with no evidence of genital disease were collected at a local abattoir immediately after slaughter and kept on ice until further processing in the laboratory. The physiological stage of the reproductive cycle for each genital tract was determined by observation of the ovarian morphology (Ireland et al. 1980). Genital tracts with an ovarian Stage I corpus luteum were selected for endometrial tissue and cell culture, and only the horn ipsilateral to the corpus luteum was used.
The endometrium was cut into strips and placed into serum-free RPMI-1640 (Sigma) supplemented with 50 IU/ml of penicillin, 50 μg/ml of streptomycin and 2.5 μg/ml of Amphotericin B (Sigma), working under sterile conditions. The strips were then chopped into 1 mm3 pieces and placed into HBSS (Sigma) and used as previously described (Asselin et al. 1996; Fortier et al. 1988), with the following modifications. Briefly, tissue was digested in 25 ml sterile filtered digestive solution, which was made by dissolving 50 mg trypsin III (Roche), 50 mg collagenase II (Sigma), 100 mg BSA (Sigma) and 10 mg DNase I (Sigma) in 100ml phenol-red free HBSS. Following a 1.5 h incubation in a shaking water bath at 37°C, the cell suspension was filtered through a 40 μm mesh (Fisher Scientific) to remove undigested material and the filtrate was resuspended in phenol-red free HBSS containing 10% FBS (Sigma) and 3 μg/ml trypsin inhibitor (Sigma)(Washing medium). The suspension was centrifuged at 100 × g for 10 min and following two further washes in washing medium the cells were resuspended in RPMI-1640 containing 10% FBS, 50 IU/ml of penicillin, 50 μg/ml of streptomycin and 2.5 μg/ml of Amphotericin B. The cells were plated at a density of 1 × 105 cells in 2 ml per well using 24-well plates (Nunc). To obtain separate stromal and epithelial cell populations, the cell suspension was removed 18 h after plating, which allowed selective attachment of stromal cells (Fortier et al. 1988). The removed cell suspension was then replated and incubated allowing epithelial cells to adhere (Kim & Fortier 1995). Stromal and epithelial cell populations were distinguished by cell morphology as previously described (Fortier et al. 1996). The absence of immune cells in the uterine cell cultures was confirmed by RT-PCR for the CD45 pan-leukocyte marker as previously described (Herath et al. 2006). The culture media was changed every 48 h until the cells reached confluence. All cultures were maintained at 37°C, 5% CO2 in air, in a humidified incubator.
Cell line cultures
To explore BoHV-4 tropism, the effect of viral challenge of the endometrial cells were compared with other cells, including: Madin–Darby bovine kidney (MDBK, ATCC, CCL-22), and from M. Ferrari (Istituto Zooprofilattico, Brescia, Italy) bovine embryo kidney (BEK), bovine embryo lung (BEL), and bovine turbinates (BT). The cells were maintained as monolayers in DMEM (Cambrex), containing 10% FBS, 2 mM L-glutamine, 100 IU/ml penicillin and 10 μg/ml streptomycin. The cells were incubated at 37°C with 5% CO2 in air, in a humidified incubator and subcultured when growth reached 70–90% confluence every 3 to 5 days.
Virus
To test the susceptibility of cells to BoHV-4 infection, a recombinant BoHV-4 expressing enhanced green fluorescent protein was employed. Recombinant BoHV-4EGFPΔTK was obtained by insertion of the CMV/EGFP expression cassette from the pEGFP-C1 plasmid into the thymidine kynase (TK) locus of the DN 599 BoHV-4 strain (Donofrio et al. 2002). BoHV-4EGFPΔTK and the NADL strain of BVDV, were propagated by infecting confluent monolayers of MDBK at a multiplicity of infection (m.o.i.) of 0.5 tissue cell infectious doses/50 (TCID50) per cell and maintained in MEM with 2% FBS for 2 h. The medium was then removed and replaced by fresh MEM containing 10% FBS. The virus was purified when approximately 90% of the cell monolayer exhibited a CPE, at approximately 72 h post-infection (P.I.). Cell-associated virions were freed by three cycles of freezing the flasks at −80°C and thawing. Cell debris was removed by low-speed centrifugation express as × g, and virions were pelleted through a 3 ml cushion of 30% sucrose in PBS, in a Beckman 70 Ti rotor at 35,000 rpm express as × g for 90 min at 4°C. Viral pellets were resuspended in cold MEM without FBS and TCID50 were determined on MDBK cells by limiting dilution (Vanderplasschen et al. 1995).
Infection of primary cell cultures with BoHV-4
Stromal and epithelial cells were challenged once confluence had been reached with BoHV-4EGFPΔTK at the concentrations indicated in results, or 1 μg/ml O55:B5 lipopolysaccharide (LPS, Sigma) as a positive control. Viral infection was monitored every 12 h by observation of cell fluorescence using a fluorescence microscope (Axiovert S100, Zeiss). The supernatants were harvested and frozen at −20°C until used for prostaglandin radioimmunoassay, and the endometrial cells were collected immediately for RNA isolation and further analysis.
MTT cell survival assay
CPE is the morphological change associated with the detrimental effects of viral replication on host cell homeostasis that ends with cell death. For epithelial cells the CPE induced by BoHV-4 is characterized by swelling, whilst stromal cells shrink and detached from the surface of the culture flask. The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) cell metabolic assay was used to measure the number of live cells. Briefly, 48 h after infection with BoHV-4EGFPΔTK, the cell cultures were incubated for 4 h with 100 μg/well MTT before addition of 100 μl of solubilisation solution (10% SDS in HCl 0.01 M), and further incubation for 16 h at 37 °C. The yellow tetrazolium MTT salt is reduced in metabolically active cells to form insoluble purple formazan crystals, which are solubilized by the addition of a detergent. The optical density was measured at 540 nm, using 690 nm as the reference wavelength in an SLT-Lab microreader (Salzburg, Austria); for each cell type, a linear relationship between cell number and optical density had already been established. Each experiment was done three times and each treatment was performed with eight replicates. Statistical differences among treatments were tested by ANOVA.
Apoptosis assays and viral production
Apoptosis assays were performed because CPE by viruses such as BVDV is mediated by apoptosis. BVDV or BoHV-4EGFPΔTK infected confluent monolayers were gently scraped from 25-cm2 flasks with a sterile scraper in the presence of culture medium and the cells pelleted by centrifugation at 12,000 × g for 1 h at 4 °C. The cell pellet was gently resuspended in 500 μl of extraction buffer (400 mM NaCl, 10 mM Tris–HCl, pH 7.8, 1 mM EDTA, 0.1% NP 40), transferred to a polypropylene microcentrifuge tube and kept on ice for 30 min. The solution was centrifuged at 12,000 × g for 15 min, the supernatant recovered carefully in a fresh microfuge tube, and extracted with phenol. The aqueous phase was transferred to a microfuge tube and a 0.1 volume of 10 M ammonium acetate and 1 volume of isopropanol added, mixed and centrifuged at 12,000 × g for 5 min. The supernatant was removed and the white nucleic acid pellet washed with 500 μl of 70% ethanol and dissolved in 20 μl of TE (10 mM Tris–HCl, 0.1 mM EDTA, pH 7.8) containing 20 μg/ml DNAase-free pancreatic RNAase (Sigma). Nuclear fragments were examined by electrophoresis in a 1.5% agarose gel and visualised by u.v. after staining with ethidium bromide. For propidium iodide staining, cells were washed with PBS, stained with 400 ng/ml propidium iodine for 30 s in the dark and fragmented nuclei visualized by fluorescence microscopy.
Viral production by BT, BEL, MDBK, BEK, endometrial epithelial and stromal cells was tested after infecting cells with 1 m.o.i. of BoHV-4EGFPΔTK. The viral inoculums’ were removed after a 3 h absorption period and replaced with fresh media, and the viral titer quantified 48 h P.I.
Cell culture electroporation and viral reconstitution
To determine which step of the virus life-cycle is important for the expression of the tropic phenotype, a reconstitution viral assay was performed. MDBK, BT and BEK cells from a sub-confluent 25 cm2 flask were electroporated (Equibio apparatus, 270 V, 960 μF) with 2 μg of viral DNA purified as previously described (Gillet et al., 1995) in DMEM without serum and antibiotics. Electroporated cells were returned to new 25 cm2 flasks and fed with DMEM containing 10% FBS, 2 mM L-glutamine, 100 IU/ml penicillin and 10 μg/ml streptomycin. Endometrial stromal, endometrial epithelial and BEL cells from a sub-confluent 25 cm2 flask were electroporated (Equibio apparatus, Opty-Puls, 300 V, 25 μF, 240 V, 1050 μF and 481 R) with 2 μg of viral DNA in DMEM with 10% FBS. Electroporated cells were returned to new 25 cm2 flasks and stromal and epithelia cells were fed with RPMI-1640 containing 10% FBS, 50 IU/ml of penicillin, 50 g/ml of streptomycin and 2.5 g/ml of Amphotericin B and BEL cells with 90% DMEM containing 10% FBS, 2 mM L-glutamine, 100 IU/ml penicillin and 10 μg/ml streptomycin. The time necessary for the formation of viral plaques was monitored every 24 h by fluorescence microscopy
Recombinant IE2 plasmid construction and transfection
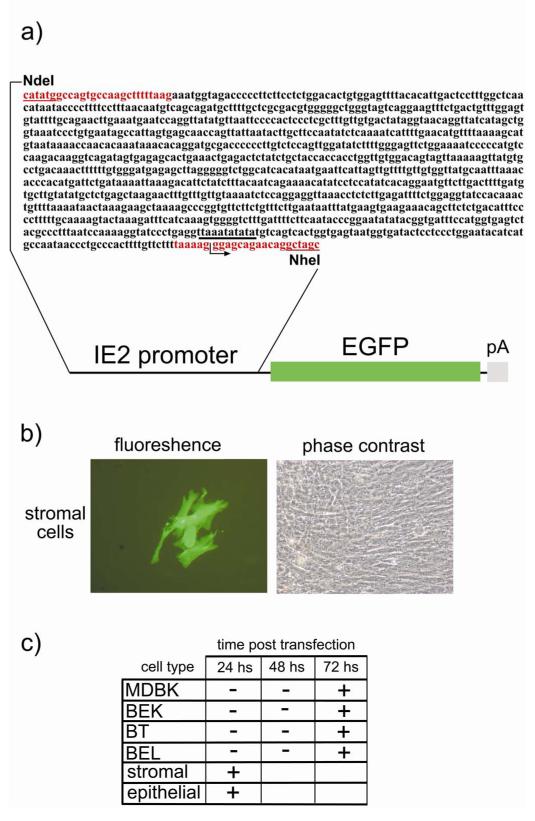
To further investigate the mechanisms associated with endometrial tropism, a molecular switch involving the viral immediate early genes (IE) was investigated. The IE genes are expressed immediately during cell infection, do not require prior viral protein synthesis for their expression, and their expression is mediated by the pool of transcription factors made by the cell, already present at the moment of infection and able to trans-activate at the transcriptional level the IE promoters. BoHV-4 immediate early 2 (IE2) protein (replication and transcription activator homologous, Rta) encoded by open reading frame 50 (ORF 50) is well conserved among gamma herpesviruses (Zimmermann et al. 2002). Rta expression plays a primary role in initiating viral lytic replication, not only during reactivation of latently infected non-permissive cells but also during de novo infection of permissive cells (Donofrio et al. 2004; Song et al. 2002; Sun et al. 1998; van Santen 1993). The capability of endometrial cells to transactivate the BoHV-4 IE2 promoter was investigated by transient transfection of a fluorescent labelled IE2 construct. A region of the BoHV-4 (DN599) genome corresponding to the (from nucleotide 61391 to nucleotide 62534, GeneBank accession number AF318571) (Zimmermann et al. 2002) was generated by PCR using total DNA isolated from BoHV-4 infected MDBK cells as template and a pair of IE2 promoter primers (sense: 5′-gggaattccatatggccagtgccaagctttttaag-3′; antisense: 5′-gggaactagctagcctgttgttctgctccctttta-3′) containing an artificial NdeI site on the 5′ end and a NheI site on the 3′ end, respectively. One microgram sample DNA was amplified over 35 cycles, each cycle consisting of denaturation at 94 °C for 1 min, primer annealing at 55 °C for 1 min, and chain elongation with High Fidelity PCR Enzyme Mix (Fermentas) at 72°C for 2 min. PCR amplification was carried out in a final volume of 50 μl, containing 0.2 mM deoxinucleoside triphosphate and 0.25 μM of each primer. In the first cycle, the samples were denatured at 94°C for 5 min, and in the last cycle the extension step was increased to 7 min. The amplicon was column purified, NdeI, NheI digested and subcloned in pEGFP-C1 vector (Clontech), excised with NdeI and NheI to remove the CMV promoter and generate the pIE2prom.EGFP construct. Transient transfection using 25 μg of the plasmid DNA construct was performed by electroporation, as described above. Endometrial epithelial and stromal cells, BT, BEL, MDBK and BEK cells were electroporated with the reporter construct and monitored every 24 h by fluorescence microscopy for green cells.
Prostaglandin radioimmunoassay
Prostaglandins have a central role in many reproductive functions in mammals (Poyser 1995). Indeed, the duration of ovarian cycles and pregnancy depends on the presence of an active corpus luteum in the ovary, and the life span of the corpus luteum is regulated by the secretion of prostaglandin F2α (PGF) and prostaglandin E2 (PGE) from the uterine endometrium. Under physiological conditions, the uterine epithelial cells predominantly secrete PGF when stimulated with oxytocin, whereas the stromal cells produce PGE (Asselin 1996). However, when epithelial or stromal cells are challenged with bacterial lipopolysaccharide (LPS), prostaglandin secretion is also stimulated (Herath et al. 2006). As BoHV-4 is isolated from animals with uterine disease, the effect on endometrial cell function of 24 h challenge with different amounts of BoHV-4 (10, 1 and 0.1 m.o.i. of virus) or a positive control of 1 ug/ml LPS, was determined by measuring prostaglandin E2 (PGE) and prostaglandin F2α (PGF) in culture supernatants by RIA, as previously described (Cheng et al. 2001; Leung et al. 2001). Samples were diluted in 0.05 M Tris buffer containing 0.1% gelatine and 0.01% sodium azide. Standards and tritiated tracers for the PGs were purchased from Sigma and Amersham International PLC (Amersham, UK), respectively. The antisera were a generous gift from Professor N.L. Poyser (University of Edinburgh, UK) and their cross-reactivity was: PGF2α antiserum, 34% with PGF1α, 25% with PGF3α and 0.54% with PGE2; PGE2 antiserum, 23% with PGE1, 15% with PGE3 and 0.47% with PGF2α (Poyser 1987). The limit of detection for PGE2 and PGF2α was 2 pg/tube and 1 pg/tube, respectively. The intra-assay and inter-assay coefficients of variation were 4.4 and 7.8% for PGE2, and 5.1 and 9.7% for 2 PGF2α respectively.
Western blotting
PGF and PGE are synthesized from arachidonic acid (AA) under the regulation of cyclooxygenase 2 (COX2) isoenzymes in the endometrium (Arosh et al. 2002; Smith et al. 1996). Stromal cells in 6-well culture plates were serum starved overnight; infected with 5 m.o.i. of BoHV-4 in the absence of serum; and, the cells collected 2, 4, 6 and 6 h P.I. to measure COX2 protein expression by Western immunoblotting, with a positive control established by treating stromal cells with medium containing 20% FBS for 1 h. Cell extracts were prepared by adding 100 μl/well of cell extraction buffer (50 mM Tris-HCl, 150 mM NaCl and 1% NP-40; pH 8). Cell extracts containing 50 μg of total protein were electrophoresed through 12% SDS-polyacrylamide gels and transferred to nylon membranes by electro blotting. Membranes were incubated with rabbit anti-COX2 polyclonal antibody (AB5118; Chemicon International), probed with horseradish peroxidase-labeled goat anti-rabbit immunoglobulin G1 (IgG1) antibody (A0545, Sigma), and visualized by enhanced chemiluminescence (ECL Kit; Pierce, Rockford, IL).
The effect of stromal cells on a macrophage cell line persistently infected with BoHV-4
As BoHV-4 stimulated stromal cell PGE secretion, a scenario whereby PGE produced by stromal cells could activate BoHV-4 lytic replication in persistently infected macrophages was envisioned: (ì) Macrophages are the site of persistency of BoHV-4 (Donofrio & van Santen 2001; Lopez et al. 1996; Osorio et al. 1985) (ìì) persistently infected macrophages can easily reach the endometrium through the blood stream (ììì) the small amount of BoHV-4 viral particles could infect stromal cells, which will release PGE and reactivate BoHV-4 in persistently infected macrophages. To test the above scenario, stromal cells were co-cultivated with a bovine macrophage cell line persistently infected with BoHV-4EGFPΔTK producing small amount of infectious viral particles (Donofrio et al. 2005). The persistently infected macrophage cell line (BOMAC/BoHV-4EGFPΔTK) was established as previously described (Donofrio & van Santen 2001) by infecting BOMAC cells, a cell line established from peritoneal macrophages by transformation with simian virus 40 DNA (Stabel & Stabel 1995). Confluent monolayers of BOMAC cells were inoculated (multiplicity of infection (m.o.i.) of 1 TCID50/cell) with recombinant BoHV-4 (BoHV-4EGFPΔTK), by the third day after inoculation, more than 95% of cells had detached from the monolayer, leaving behind a small number of cells that did not exhibit cytopathic effect (CPE). Confluent monolayers established from surviving cells showed 100% infection, as indicated by the strong fluorescent signal, but without apparent signs of CPE. Also consistent with our previous observations (Donofrio & van Santen 2001), the persistently infected macrophages produced infectious BoHV-4; medium recovered from BOMAC/BoHV-4EGFPΔTK cells inoculated onto BEK cells produced green plaques typical of BoHV-4EGFPΔTK. BOMAC/BoHV-4EGFPΔTK cells were sub cultured at a dilution of 1:2 every 3 days and their growth medium was stored at −80°C for viral titration. The yield of virus in the culture medium on the day the cells were sub cultured remained in the range of 4 × 102 TCID50/ml throughout the first 20 passages. The macrophages were co-cultured with uninfected stromal cells or control BEK cells, using a transwell approach. After 48 h of co-culture, the stromal cells or BEK cells in the lower well were analyzed under fluorescence microscope for EGFP expression. In addition, the viral titre was measured in the medium from the upper well, where the persistently infected macrophages were located.
Results
Isolation of pure populations of bovine endometrial stromal and epithelial cells
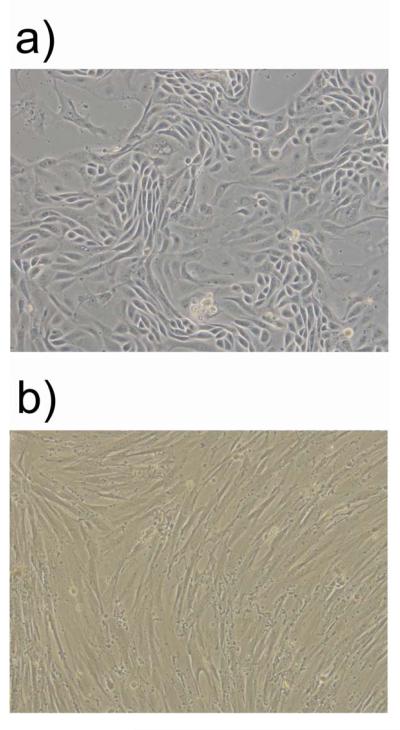
The bovine endometrium principally comprises of epithelial cells and stromal cells but there are also sporadic leukocytes. To study the interaction of BoHV-4 with the endometrium it was important to use purified populations of epithelial and stromal cells without leukocytes, which can influence BoHV-4 function (Donofrio & van Santen 2001). Endometrial cells cultures were established by the fractional enzyme dissociation method described in Materials and Methods, and there was no mRNA expression of the CD45 pan-leukocyte marker in epithelial or stromal cells (data not shown) as previously described (Herath et al. 2006). The epithelial and stromal cell purity was greater than 95% as determined by microscopy (Fig. 1a and b) and the preferential secretion of prostaglandin F2α and E2, respectively, as described previously (Asselin et al. 1996; Herath et al. 2006).
Fig. 1.
Representative phase contrast images (10X) of pure population of bovine endometrial epithelial (a) and stromal cells (b).
Bovine herpesvirus 4 infects bovine endometrial cells and induces cell death
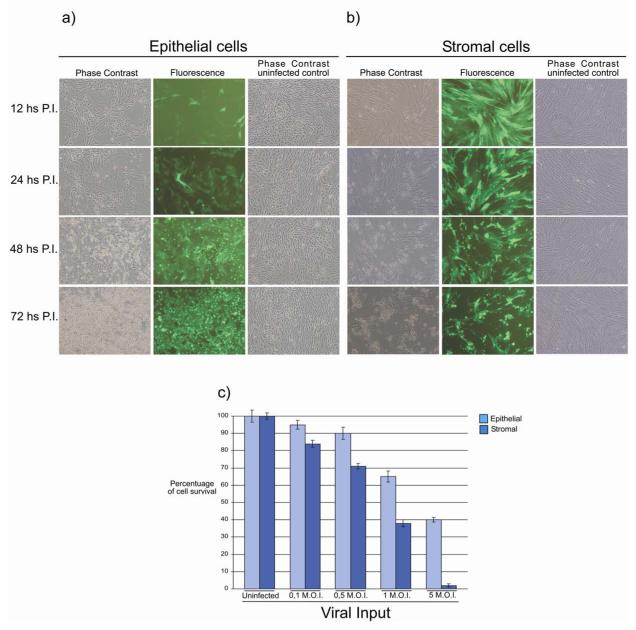
To test the susceptibility of bovine endometrial cells to BoHV-4 infection, epithelia and stromal cells were infected with 1 m.o.i. of a recombinant BoHV-4 (BoHV-4EGFPΔTK) expressing enhanced green fluorescent protein (Donofrio et al. 2002). A few epithelial cells showed fluorescence 12 h P.I. (Fig. 2a) and this became generalized by 24 h, with the CPE starting 48 h P.I. and complete by 72 h. In contrast, most stromal cells were infected by 12 h P.I. (Fig. 2b), and the CPE started at 24 h P.I. and was complete by 48 h. To quantify the cytopathic effect of BoHV-4 infection, cell survival after challenge with different viral doses was measured using a MTT assay. Epithelial and stromal cells were killed in a dose dependent manner 48 h after infection with BoHV-4 and the CPE was greater for stromal than epithelial cells (Fig. 2c). Thus, it appears that BoHV-4 infects both epithelial and stromal cells leading to a CPE, but the stromal cell infection seems to be more efficient.
Fig. 2.
Representative fluorescence (green) and phase contrast images (10X) of bovine endometrial epithelial (a) and stromal (b) cells at different time (12, 24, 48, 72 h) post infection (P.I.) with 1 m.o.i. of BoHV-4 EGFPΔTK and the respective phase contrast images of uninfected control. Spreading of the infection can be observed by the green colour invading the field during the time and the CPE is morphologically appreciable by the change of the cell shape, where the cells tend to shrink, becoming roundest and detaching the flask surface. The experiment was repeated three times giving the same result. c) Assessment of cell survival measured by the reduction of 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) at 48 h post infection. Hundred percent of survival was considered the uninfected control. Values are the mean ± S.D. of three independent experiments (n= 8 per treatment, P≤0,009).
CPE induced by BoHV-4 in bovine endometrial epithelial and stromal cells is not mediated by apoptosis but is associated with a productive infection
BoHV-4 replicates in cell lines or primary cell cultures from a broad spectrum of host species. The infection in some permissive cells leads to viral progeny and a CPE; in other cells, there is a CPE even though no viral progeny are produced; whereas in some non-permissive cells, there is persistent BoHV-4 infection with no effect on cell survival (Donofrio et al. 2000). As BoHV-4 infection induced a CPE in endometrial cells, the nature of the cell death and virus production was investigated.
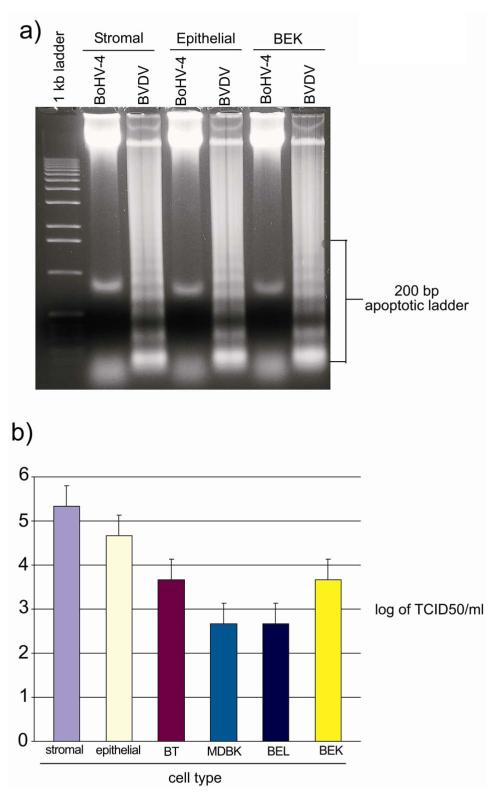
Endometrial epithelial and stromal cells were infected with 5 m.o.i. of BoHV-4 and compared with 5 m.o.i. of BVDV, which is an established apoptotic virus (Schweizer & Peterhans 2001). The CPE induced by BoHV-4 was not mediated by cell apoptosis as determined by intranucleosomal DNA fragmentation (Fig. 3a) and propidium iodine staining (data not shown), in contrast to the cells infected with BVDV where there was clear DNA fragmentation (Fig. 3a).
Fig. 3.
a) 1, 5 % agarose gel electrophoresis of DNA extracts from bovine endometrial epithelial, stromal and control BEK cells, infected with 5 m.o.i. of BoHV-4 and BVDV control virus, at 72 h post infection. b) Titers of BoHV-4 particles released by different cell types at 48 h post infection. Values are the mean ± S.D. of three independent experiments (n= 3 per treatment, P≤0, 05).
Endometrial epithelial and stromal cell viral production was compared with other fully permissive cell types (BT, BEL, MDBK and BEK cells) infected with 1 m.o.i. of BoHV-4EGFPΔTK. The viral titres 48 h P.I. were ~ one log higher for epithelial and two logs higher for stromal cells compared with the other cell types (Fig. 3b).
Post viral entry is a determinant step for BoHV-4 replication in endometrial cells
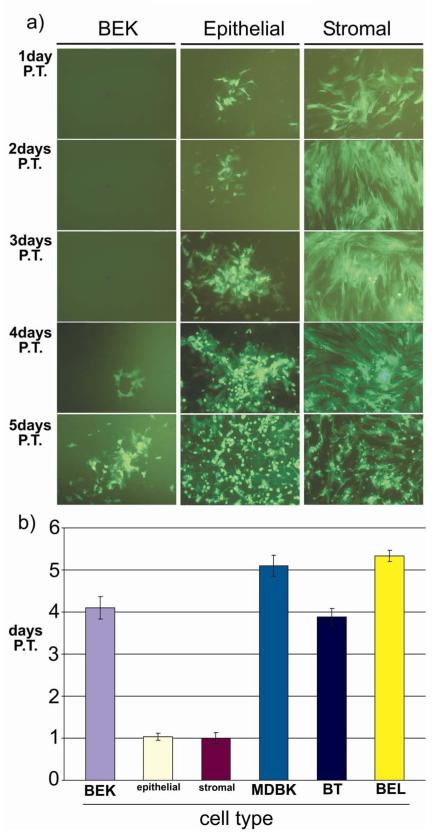
From the above data it appears that BoHV-4 has a striking tropism for endometrial cells or that endometrial cells are highly susceptible to BoHV-4 replication. However, viral attachment and penetration do not appear to be critical because many cell types were infected by BoHV-4. To determine which step of the virus life-cycle is important for the expression of the tropic phenotype, a reconstitution viral assay was performed. Endometrial epithelial and stromal cells, BT, BEL, MDBK and BEK cells were electroporated with purified BoHV-4EGFPΔTK genomic DNA and the time necessary for the formation of viral plaques was monitored by fluorescence microscopy. Green plaques started to appear by 24 h after electroporation in epithelial and stromal cells, in contrast to the other cell types where visible green plaques formed between 3 and 5 days after electroporation (Fig. 4a and b).
Fig. 4.
a) BoHV-4 plaques obtained at different time (1, 2, 3, 4, 5 days) post electroporation (P.E.) of nude BoHV-4EGFPΔTK DNA in different cell types. Images are representative for bovine epithelial, stromal and BEK cells. b) Graph bars, summarizing the time necessary for plaques formation in different cell types (endometrial epithelial cells, endometrial stromal cells, BT, BEL, MDBK and BEK cells). Values are the mean ± S.D. of three independent experiments (n= 3 per treatment, P≤0, 05).
BoHV-4 Immediate Early 2 gene promoter is strongly trans-activated in endometrial cells
To further investigate the mechanisms associated with endometrial tropism, a molecular switch involving the viral immediate early genes (IE) was investigated using an EGFP labelled construct containing the IE2 gene (Fig. 5a) electroporated into endometrial epithelial and stromal cells, BT, BEL, MDBK and BEK cells. EGFP started to accumulate robustly as soon as 24 h after electroporation in the cytoplasm of stromal cells (Fig. 5b) and epithelial cells, in contrast to the other cell types where weak visible green cells appeared not before than 3 days post electroporation (Fig. 5c).
Fig. 5.
a) Diagram showing the 1,143 bp IE2 promoter sequence containing the putative TATA box (underlined in black) and the first 15 non coding nucleotides of the first exon. Sense and antisense primers used for the PCR amplification are in red and contain the NdeI and NheI restriction sites (underlined in red) respectively for sub-cloning of the amplicon in front of the EGFP ORF (green box) followed by the bovine growth hormone polyadenylation signal (pA (grey box)). b) Representative fluorescence and phase contrast images of transfected endometrial stromal cells with the above described construct and expressing EGFP 24 hours post transfection. c) Summarising schema of the time necessary for EGFP accumulation into the different cell types (endometrial epithelial cells, endometrial stromal cells, BT, BEL, MDBK and BEK cells), following transfection with the above described construct. Values are the mean of three independent experiments (n= 3 and P≤0, 05).
Low dose BoHV-4 infection stimulates PGE production in stromal cells
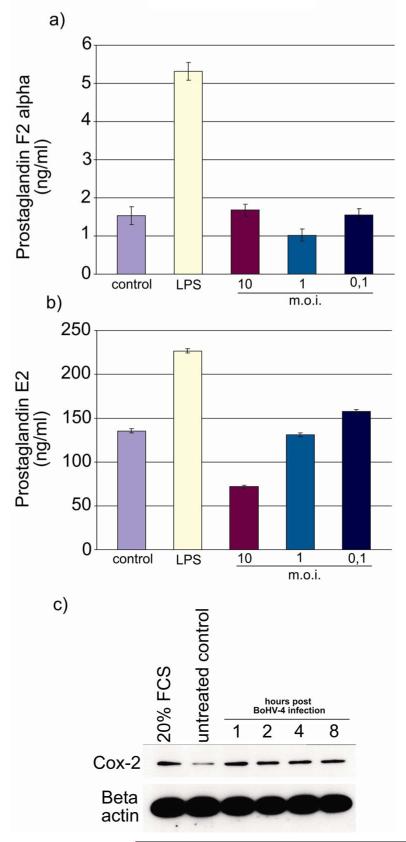
Under physiological conditions, the uterine epithelial cells predominantly secrete PGF when stimulated with oxytocin, whereas the stromal cells produce PGE (Asselin 1996). The capacity of the endometrial cells to produce PGs in response to BoHV-4 was investigated as BoHV-4 is frequently isolated from the uterus. The cells were capable of responding to pathophysiological challenge as LPS stimulated PGF (Fig. 6a) and PGE (Fig. 6b) secretion from the epithelial and stromal cells, respectively, as reported previously (Herath et al. 2006). Epithelial cells did not produce PGF in response to BoHV-4 (Fig. 6a). However, stromal cells challenged with 0.1 m.o.i. produced more PGE than untreated controls (Fig. 6b). In contrast higher viral doses suppressed PGE production, which is likely a consequence of the greater CPE induced by the virus replication. The rate limiting enzyme for PGE synthesis in the endometrium is COX-2 (Arosh et al. 2002; Smith et al. 1996). So, as BoHV-4 stimulated PGE secretion by endometrial stromal cells the induction of COX-2 by BoHV-4 was examined by Western blotting and COX-2 was up-regulated as early as 1 h P.I. (Fig. 6c).
Fig.6.
Prostaglandin production by (a) epithelial and (b) stromal cells after challenge with LPS (1 μg/ml) or BoHV-4 at the different m.o.i. indicated. After 24 h in culture, supernatants were harvested and prostaglandin production was measured by RIA. Values are presented as the mean ± S.D. of three independent experiments. Differences were statistically different at P < 0.05 compared with a media control. c) Western immunoblotting of endometrial stromal cells extracts and infected with BoHV-4 EGFPΔTK. Positive control was established treating cells with medium containing 20% of FBS. Beta actin as the loading control. The same experiment was repeated three times, giving the same results.
Endometrial stromal cells enhance BoHV-4 replication in persistently infected macrophages
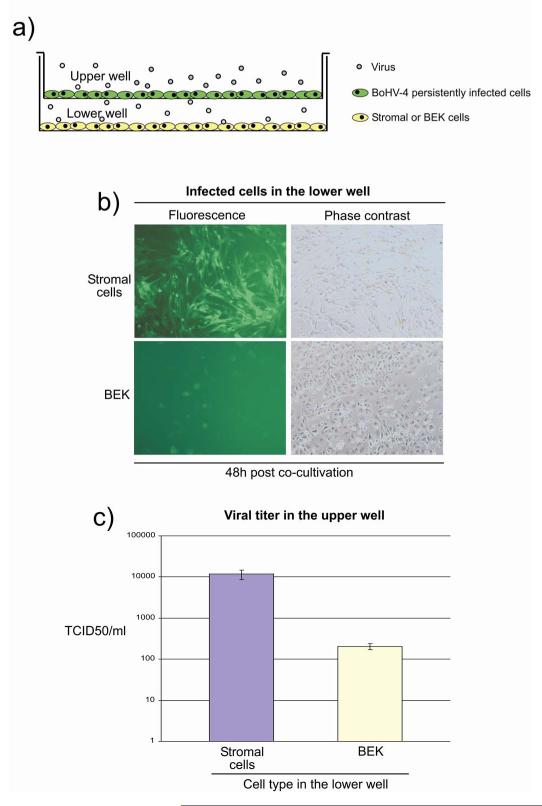
As BoHV-4 stimulated stromal cell PGE secretion, the effect of stromal cells on BoHV-4 replication in persistently infected macrophages was tested using a transwell approach (Fig. 7a). After 48 h of co-culture, the stromal cells had a higher level of infection and a higher viral titer in the upper well, than the BEK cells (Fig. 7a and b). These data support the concept of a symbiotic relationship between the stromal cells and persistently infected macrophages.
Fig. 7.
a) Schematic illustration of the transwell model used for co-culturing BoHV-4EGFPΔTK persistently infected bovine macrophage cell line (in green in the upper well) producing low amount of infectious virus (grey dots) with bovine endometrial stromal or control BEK cells (in yellow) seated in the lower well. The transwell consists of a permeable membrane and allows diffusion of the viral particles. b) Fluorescence and phase contrast images of bovine endometrial stromal and control BEK cells seated in the lower well at 48 hours (48h) post co-cultivation. c) Graph bar of the viral titer in the upper well. Values are the mean ± S.D. of three independent experiments (n= 3 and P≤0,05).
Discussion
Bos taurus are particularly prone to uterine infection and metritis after parturition, which causes considerable infertility and economic loss (Sheldon & Dobson 2004). The most commonly recognized uterine pathogens are Arcanobacterium pyogenes and Escherichia coli (Bonnet et al. 1993; Griffin et al. 1974; Olson et al. 1984; Ruder et al. 1981; Sheldon & Dobson 2004). Although viral isolation or serology is not routinely performed in animals with uterine disease, BoHV-4 has been implicated in cases of bovine metritis (Czaplicki & Thiry 1998; Fortier et al. 1988; Frazier et al. 2002; Monge et al. 2006; Nilsson 1979; Park & Kendrick 1973). The present study demonstrated a viral tropism by BoHV-4 for the endometrium. The high efficiency of BoHV-4 replication in endometrial cells was associated with strong transactivation of viral IE genes. Furthermore, BoHV-4 increased stromal cell COX-2 protein and stimulated prostaglandin E2 secretion. This viral induced stromal cell PGE secretion may be a mechanism by which viral replication is stimulated in macrophages, which are the usual repository for persistent bovine infections.
Viruses are restricted to using the metabolic and biosynthetic pathways of the cells that they infect. These pathways vary between cell types, lineage, stage of differentiation, and with the state of cell activation. There are many examples of viruses that replicate in specific cells and at particular stages of cell growth, differentiation, or activation. This includes the reactivation of cytomegalovirus when host cells differentiate into macrophages; initiation of papillomavirus replication by keratinocytes; and, replication of minute virus in testicular cells. The key mechanism mediating these effects is the regulation of viral gene expression at the transcriptional level by host cells factors. The present study identified a striking tropism of BoHV-4 for endometrial cells. BoHV-4 efficiently infected purified populations of bovine endometrial stromal and epithelial cells, leading to a non-apoptotic cell death and de novo viral production, which could be an important mechanism underlying the metritis associated with BoHV-4 infection in cattle. The lack of BoHV-4 apoptogenicity found in endometrial cells where BoHV-4 infection was highly productive, well fit with the BoHV-4 pro-apoptotic behaviors found in some cell types (Gillet et al. 2005) where, BoHV-4 infection is completely unproductive. In a complex multicellular organism, rapid apoptotic cell suicide on virus infection can be seen as an altruist response (Allops et al. 2000). If it occurs before complete virus replication, and assembly, it will be highly effective in limiting viral production. Many, and possibly most, large virus like herpesvirus that, due to their size and complexity, have relatively long replication time, encode antiapoptotic genes, as is the case for BoHV-4 (Gillet et al. 2004).
For many viruses, tropism and successful replication is determined by specific cellular receptors that must be engaged for virus binding and entry. However, BoHV-4 can enter many cell types from different species (Donofrio et al. 2002). Furthermore, successful replication of BoHV-4 seems to be governed primarily by post-entry events as shown by the fast viral reconstitution following the electroporation of nude viral DNA into the cells. The open reading frame (ORF) 50 gene product, also known as the replication and transcription activator (Rta), is an immediate-early gene which is well conserved among all gamma-2 herpesviruses. BoHV-4 IE2 RNA is the less abundant, spliced, 1.8 kb RNA, which is transcribed from the left to the right on the restriction map of the BoHV-4 genome and contained in the 8.3 kb Hind III fragment F (van Santen 1993). The predicted amino acid sequence, of the protein encoded by IE2 RNA, reveals that it could encode a 61 kDa protein with amino acid sequence homology to the Epstein-Barr virus transactivator R and its homolog including, herpesvirus saimiri, equine herpesvirus 2, murine herpesvirus 68 and Kaposi’s sarcoma-associated herpesvirus. Transactivation studies have shown that BoHV-4 IE2 protein specifically transactivates expression of a reporter gene linked to the promoter regulatory region of the BoHV-4 early (E) gene encoding the BoHV-4 homologue of herpes simplex virus type 1 major DNA binding protein (van Santen 2003). The BoHV-4 ORF 50 homologues have been shown to play a pivotal role in regulating the lytic switch in a non-permissive cell line following his over expression (Donofrio et al. 2004). Indeed, in the present study IE2 promoter transactivation in endometrial cells triggered the BoHV-4 phenotype in those cells, as shown by the fast up-regulation of EGFP reporter gene expression driven by IE2 promoter. Interestingly, a preliminary analysis of the BoHV-4 IE2 promoter by Tfsitescan/dynamicPlus server, reveals 5 positive regulatory elements that are also found in the rabbit uteroglobin promoter and apparently specific for endometrial cells. The most surprising similarity was found for the responsive element close to the TATA box, which may indicate the existence of an endometrial-specific TATA box, (Misseyanni et al. 1991). A comparison with promoters of two other endometrium specific genes, the rat homologous to rabbit uteroglobin (Hagen 1990) and the pig uteroferrin gene (Simmen 1989) indicates that this TATA box element is well conserved. Further studies are needed to identify the nature of the factors in bovine endometrial cells and how they interact with BoHV-4 IE2 promoter.
Specific enhancers and transcriptional activators produced by host cells are important to initiate and maintain viral replication. In persistently infected macrophages, BoHV-4 replication is stimulated by the addition of exogenous PGE (Donofrio et al. 2005). Bovine endometrial cells induce COX2 and PGE production in response to a number of cell activators and inflammatory signals, such as lipopolysaccharide (Herath et al. 2006). Taken together these observations support a scenario where viral particles infecting the endometrium, could stimulate PGE production and establish a positive-feedback loop between PGE production and viral replication (Donofrio et al. 2005). That model of BoHV-4 induced metritis seems to be supported in the present ex-vivo study, by the observations that bovine endometrial stromal cells produced PGE following BoHV-4 infection and cells persistently infected with BoHV-4 were reactivated after co cultivation with endometrial stromal cells using a transwell approach. Infections by several viruses, including many herpesviruses, such as Hepes Simplex Virus, human cytomegalovirus, Epstein-Barr virus and murine gammaherpesvirus 68 have been reported to alter COX-2 expression (Reynolds & Enquist 2006). In fact, rhesus cytomegalovirus even encodes a COX-2 homolog in its genome, emphasizing the importance of this enzyme (Hansen et al. 2003). In addition, many studies have examined the regulation of COX-2 expression and PGE2 production during viral infection as well as the effect of PGE2 production on viral replication and virulence (Steer & Corbett 2003). Prostaglandins are potent mediators of many critical physiological and inflammatory responses, and they modulate the host defence against various pathogens. They suppress some innate immune factors, including nitric oxide (NO) production, and have effects on the acquired immune response, specifically by suppressing the Th1 response. For instance, PGE2 can inhibit the production of gamma interferon by activated human T cells in vitro (Snijdewint et al. 1993) and that of Th1 cytokines such as interleukin-12 in vivo (Kuroda et al. 2000; Newberry et al. 1999). In addition to inhibiting the production of Th1 cytokines, PGE2 switches the immune response toward a Th2 response, which is less effective in mounting an antiviral response (Betz & Fox 1991; Kuroda et al. 2000). PGE is one of the most potent and abundant PGs present during inflammatory reactions (Appleton et al. 1996). The very early host responses to viral infections are usually non specific and include the induction of cytokines such as interferons and tumour necrosis factor alpha. Nitric oxide synthase (NOS) is an interferon-inducible protein that is activated during innate immune responses (Reiss & Comatsu 1998). When present at high concentrations after expression of the inducible isoform of NOS (iNOS), NO functions as a cytotoxic molecule, reacting with proteins or H2O2 to form a highly toxic compound called peroxynitrite (ONOO−) (Reiss & Comatsu 1998). Nitric oxide is also thought to participate in the antiviral response to infection by attenuating the replication of both DNA and RNA viruses (Reiss & Comatsu 1998). The products of COX and NOS enzymes, PGs and NO, have been shown to share an antagonistic relationship with one another. The inhibition of COX activity in vesicular stomatitis virus (VSV) infected cells causes a reduction in viral propagation and a concordant increase in extracellular NO levels. Treatment with an iNOS inhibitor, L-NAME, or exogenous PGE2 in the presence of COX inhibitors can restore VSV growth and decrease NO production, underscoring a role for PGs in counteracting the antiviral effects of NO (Chen et al. 2000). Besides their role in immunomodulation and counteraction of the antiviral effects of NO, PGs have been shown to be involved in modulating transcription from viral promoters. The human immunodeficiency virus type 1 (HIV-1) long terminal repeat (LTR) contains sequences that are important for DNA integration, as well as signals, such as an internal polymerase II promoter, that are necessary for transcription of the integrated retroviral DNA. PGE2 can increase transcription driven by the HIV-1 LTR in T lymphocytes (Dumais et al. 1998). Transcription of one of the immediate-early genes (IE2) of HCMV was reduced in cells that were treated with COX2 inhibitors. Therefore, a potential role for COX induction in the context of a virus infection is the activation of transcription from viral promoters via PGs.
In the case of BoHV-4 persistently infected animals, stromal cell PGE secretion may reactivate viral replication, which in turn would lead to endometrial inflammation. Bacterially induced metritis in cattle persistently infected with BoHV-4 could possible be exacerbated or become chronic following the recruitment from the blood stream to the site of inflammation of macrophages persistently infected with BoHV-4. This theory could explain the fact that BoHV-4 can be isolated from healthy animal too, where in the absence of inflammation the pathogenic potential of BoHV-4 is ameliorated.
In conclusion, the present study indicates that BoHV-4 is a likely pathogen associated with uterine disease. The virus is trophic for endometrial epithelial and stromal cells, causing a rapid CPE. It appears that the endometrial cells rapidly induce IE gene expression and viral replication. Furthermore, there was a symbiotic relationship between stromal cell PGE secretion in response to BoHV-4 and reactivation of viral replication in macrophages. These data provide evidence of cellular and molecular mechanisms underlying the observation of uterine disease in animals with BoHV-4.
Acknowledgements
We thank the Italian Minister of Science (Italian National Grant MIUR, PRIN 2005,2005078885) and BBSRC (Grant 48/S19795) for financial support.
References
- Allsopp TE, Fazakerley JK. Altruistic cell suicide and the specialized case of the virus-infected nervous system. Trends in Neuroscience. 2000;23:284–90. doi: 10.1016/s0166-2236(00)01591-5. [DOI] [PubMed] [Google Scholar]
- Appleton I, Tomlinson A, Willoughby DA. Induction of cyclo-oxygenase and nitric oxide synthase in inflammation. Advances in Pharmacology. 1996;35:27–78. doi: 10.1016/s1054-3589(08)60274-4. [DOI] [PubMed] [Google Scholar]
- Arosh JA, Parent J, Chapdelaine P, Sirois J, Fortier MA. Expression of cyclooxygenases 1 and 2 and prostaglandin E synthase in bovine endometrial tissue during the estrous cycle. Biology of Reproduction. 2002;67:161–169. doi: 10.1095/biolreprod67.1.161. [DOI] [PubMed] [Google Scholar]
- Asselin E, Goff AK, Bergeron H, Fortier MA. Influence of sex steroids on the production of prostaglandins F2 alpha and E2 and response to oxytocin in cultured epithelial and stromal cells of the bovine endometrium. Biology of Reproduction. 1996;54:371–379. doi: 10.1095/biolreprod54.2.371. [DOI] [PubMed] [Google Scholar]
- Bartha A, Juhasz M, Liebermann H. Isolation of a bovine herpesvirus from calves with respiratory disease and keratoconjuntivitis. Acta Veterinaria Academiae Scientiarum Hungaricae. 1966;16:357–358. [PubMed] [Google Scholar]
- Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. Journal of Immunology. 1991;146:108–113. [PubMed] [Google Scholar]
- Bonnett BN, Martin SW, Meek AH. Associations of clinical findings, bacteriological and histological results of endometrial biopsy with reproductive performance of postpartum dairy cows. Preventive Veterinary Medicine. 1993;15:205–220. [Google Scholar]
- Bublot M, Lomonte P, Lequarre AS, Albrecht JC, Nicholas J, Fleckenstein B, Pastoret PP, Thiry E. Genetic relationships between bovine herpesvirus 4 and the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. Virology. 1992;190:654–655. doi: 10.1016/0042-6822(92)90903-3. [DOI] [PubMed] [Google Scholar]
- Castrucci G, Frigeri F, Ferrari M, Ranucci S, Aldrovandi V, Cilli V, Rampichini L, Gatti R. Experimental infection of calves with strain of bovid herpesvirus-4. Comparative Immunology Microbiology and Infectious Disease. 1987;10:41–49. doi: 10.1016/0147-9571(87)90039-7. [DOI] [PubMed] [Google Scholar]
- Castrucci G, Frigeri F, Cilli V, Donelli G, Ferrari M, Chicchinri U, Bordoni E. A study of herpesvirus isolated from dairy cattle with a history of reproductive disorders. Comparative Immunology Microbiology and Infectious Disease. 1986;9:13–21. doi: 10.1016/0147-9571(86)90070-6. [DOI] [PubMed] [Google Scholar]
- Ceserman E, Moore PS, Rao PH, Inghirami G, Knowles DM, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- Chen N, Warner JL, Reiss CS. NSAID treatment suppresses VSV propagation in mouse CNS. Virology. 2000;276:44–51. doi: 10.1006/viro.2000.0562. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Robinson RS, Pushpakumara PG, Mansbridge RJ, Wathes DC. Effect of dietary polyunsaturated fatty acids on uterine prostaglandin synthesis in the cow. Journal of Endocrinology. 2001;171:463–473. doi: 10.1677/joe.0.1710463. [DOI] [PubMed] [Google Scholar]
- Czaplicki G, Thiry E. An association exists between bovine herpesvirus-4 seropositivity and abortion in cows. Preventive Veterinary Medicine. 1998;33:235–240. doi: 10.1016/s0167-5877(97)00036-6. [DOI] [PubMed] [Google Scholar]
- Dewals B, Gillet L, Gerdes T, Taracha EL, Thiry E, Vanderplasschen A. Antibodies against bovine herpesvirus 4 are highly prevalent in wild African buffaloes throughout eastern and southern Africa. Veterinary Microbiology. 2005;110:209–220. doi: 10.1016/j.vetmic.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Donofrio G, van Santen VL. A bovine macrophage cell line supports bovine herpesvirus 4 persistent infection. Journal of General Virology. 2001;82:1181–1185. doi: 10.1099/0022-1317-82-5-1181. [DOI] [PubMed] [Google Scholar]
- Donofrio G, Cavirani S, van Santen VL, Flammini CF. Potential secondary pathogenic role for bovine herpesvirus 4. Journal of Clinical Microbiology. 2005;43:3421–3426. doi: 10.1128/JCM.43.7.3421-3426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donofrio G, Cavirani S, van Santen VL. Establishment of a cell line persistently infected with bovine herpesvirus 4 using a recombinant virus. Journal of General Virology. 2000;81:1807–1814. doi: 10.1099/0022-1317-81-7-1807. [DOI] [PubMed] [Google Scholar]
- Donofrio G, Cavirani S, Taddei S, Flammini CF. Activation of bovine herpesvirus 4 lytic replication in a non-permissive cell line by over expression of BoHV-4 immediate early (IE) 2 gene. Journal of Virological Methods. 2004;116:203–7. doi: 10.1016/j.jviromet.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Donofrio G, Cavirani S, Taddei S, van Santen VL. Bovine herpesvirus 4 as a gene delivery vector. Journal of Virology Methods. 2002;101:49–61. doi: 10.1016/s0166-0934(01)00419-0. [DOI] [PubMed] [Google Scholar]
- Dubuisson J, Thiry E, Thalasso F, Bublot M, Pastoret PP. Biological and biochemical comparison of bovid herpesvirus-4 strains. Veterinary Microbiology. 1988;16:339–349. doi: 10.1016/0378-1135(88)90015-6. [DOI] [PubMed] [Google Scholar]
- Dubuisson J, Thiry E, Bublot M, Thomas I, van Bressem MF, Coignoul F, Pastoret PP. Experimental infection of bulls with a genital isolate of bovine herpesvirus-4 and reactivation of latent virus with dexamethasone. Veterinary Microbiology. 1989;21:97–114. doi: 10.1016/0378-1135(89)90022-9. [DOI] [PubMed] [Google Scholar]
- Dumais N, Barbeau B, Olivier M, Tremblay MJ. Prostaglandin E2 up-regulates HIV-1 long terminal repeat-driven gene activity in T cells via NF-kappaB-dependent and -independent signaling pathways. Journal of Biological Chemistry. 1998;273:27306–27314. doi: 10.1074/jbc.273.42.27306. [DOI] [PubMed] [Google Scholar]
- Egyed L. Replication of bovine herpesvirus type 4 in human cells in vitro. Journal of Clinical Microbiology. 1998;36:2109–2111. doi: 10.1128/jcm.36.7.2109-2111.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flore O, Rafii S, Ely S, O’Leary JJ, Hyjek EM, Cesarman E. Transformation of primary human endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Nature. 1998;394:588–592. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- Fortier MA, Guilbault LA, Grasso F. Specific properties of epithelial and stromal cells from the endometrium of cows. Journal of Reproduction and Fertility. 1988;83:239–248. doi: 10.1530/jrf.0.0830239. [DOI] [PubMed] [Google Scholar]
- Frazier KS, Baldwin CA, Pence M, West J, Bernard J, Liggett A, Miller D, Hines ME. Seroprevalence and comparison of isolates of endometriotropic bovine herpesvirus-4. Journal of Veterinary Diagnostic Investigation. 2002;14:457–462. doi: 10.1177/104063870201400602. [DOI] [PubMed] [Google Scholar]
- Frazier K, Pence M, Mauel MJ, Liggett A, Hines ME, Sangster L, Lehmkuhl HD, Miller D, Styer E, West J, Baldwin CA. Endometritis in postparturient cattle associated with bovine herpesvirus-4 infection: 15 cases. Journal of Veterinary Diagnostic Investigation. 2001;13:502–508. doi: 10.1177/104063870101300608. [DOI] [PubMed] [Google Scholar]
- Gillet L, Dewals B, Farnir F, de Leval L, Vanderplasschen A. Bovine herpesvirus 4 induces apoptosis of human carcinoma cell lines in vitro and in vivo. Cancer Research. 2005;65:9463–72. doi: 10.1158/0008-5472.CAN-05-1076. [DOI] [PubMed] [Google Scholar]
- Gillet L, Minner F, Detry B, Farnir F, Willems L, Lambot M, Thiry E, Pastoret PP, Schynts F, Vanderplasschen A. Investigation of the susceptibility of human cell lines to bovine herpesvirus 4 infection: demonstration that human cells can support a nonpermissive persistent infection which protects them against tumor necrosis factor α-induced apoptosis. Journal of Virology. 2004;78:2336–47. doi: 10.1128/JVI.78.5.2336-2347.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JF, Hartigan PJ, Nunn WR. Non-specific uterine infection and bovine fertility. I. Infection patterns and endometritis during the first seven weeks post-partum. Theriogenology. 1974;1:91–106. doi: 10.1016/0093-691x(74)90052-1. [DOI] [PubMed] [Google Scholar]
- Hagen G, Wolf M, Katyal SL, Singh G, Beato M, Suske G. Tissue-specific expression, hormonal regulation and 5′-flanking gene region of the rat Clara cell 10 kDa protein: comparison to rabbit uteroglobi. Nucleic Acids Research. 1990;18:2939–2946. doi: 10.1093/nar/18.10.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Strelow LI, Franchi DC, Anders DG, Wong SW. Complete sequence and genomic analysis of rhesus cytomegalovirus. Journal of Virology. 2003;77:6620–6636. doi: 10.1128/JVI.77.12.6620-6636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath S, Fischer DF, Werling D, Williams EJ, Lilly ST, Dobson H, Bryant CE, Sheldon IM. Expression and function of toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology. 2006;147:562–570. doi: 10.1210/en.2005-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland JJ, Murphee RL, Coulson PB. Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum. Journal of Dairy Science. 1980;63:155–160. doi: 10.3168/jds.S0022-0302(80)82901-8. [DOI] [PubMed] [Google Scholar]
- Jung JU, Choi JK, Ensser A, Biesinger B. Herpesvirus saimiri as a model for gammaherpesvirus oncogenesis. Seminar in Cancer Biology. 1999;9:231–239. doi: 10.1006/scbi.1998.0115. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fortier MA. Cell type specificity and protein kinase C dependency on the stimulation of prostaglandin E2 and prostaglandin F2 alpha production by oxytocin and platelet-activating factor in bovine endometrial cells. Journal of Reproduction and Fertility. 1995;103:239–247. doi: 10.1530/jrf.0.1030239. [DOI] [PubMed] [Google Scholar]
- Krogman LA, McAdaragh JP. Recrudescence of bovine herpesvirus-5 in experimentally infected calves. American Journal of Veterinary Research. 1982;43:336–338. [PubMed] [Google Scholar]
- Kuroda E, Sugiura T, Zeki K, Yoshida Y, Yamashita U. Sensitivity difference to the suppressive effect of prostaglandin E2 among mouse strains: a possible mechanism to polarize Th2 type response in BALB/c mice. Journal of Immunology. 2000;164:2386–2395. doi: 10.4049/jimmunol.164.5.2386. [DOI] [PubMed] [Google Scholar]
- Leung ST, Cheng Z, Sheldrick EL, Derecka K, Flint AP, Wathes DC. The effects of lipopolysaccharide and interleukins-1alpha, -2 and -6 on oxytocin receptor expression and prostaglandin production in bovine endometrium. Journal of Endocrinology. 2001;168:497–508. doi: 10.1677/joe.0.1680497. [DOI] [PubMed] [Google Scholar]
- Lomonte P, Bublot M, vanSanten VL, Keil G, Pastoret PP, Thiry E. Bovine herpesvirus 4: genomic organization and relationship with two other gammaherpesviruses, Epstein–Barr virus and herpesvirus saimiri. Veterinary Microbiology. 1996;53:79–89. doi: 10.1016/s0378-1135(96)01236-9. [DOI] [PubMed] [Google Scholar]
- Lopez OJ, Galeotta J, Osorio FA. Bovine herpesvirus type-4 (BHV-4) persistently infects cells of the marginal zone of spleen in cattle. Microbial Pathogeneis. 1996;21:47–58. doi: 10.1006/mpat.1996.0041. [DOI] [PubMed] [Google Scholar]
- Mehrotra ML, Shucla DC, Srivastava NC. Isolation of a new herpesvirus from cases of reproductive disorders in cow. Indian Journal of Animal Science. 1986;56:1196–1199. [Google Scholar]
- Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov VM, Grossberg S, Chang Y. Selective switch between latency and lytic replication of Kaposi’s sarcoma herpesvirus and Epstein–Barr virus in dually infected body cavity lymphoma cells. Journal of Virology. 1997;71:314–324. doi: 10.1128/jvi.71.1.314-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misseyanni A, Klug J, Suske G, Beato M. Novel upstream elements and the TATA-box region mediate preferential transcription from the uteroglobin promoter in endometrial cells. Nucleic Acids Research. 1991;19:2849–59. doi: 10.1093/nar/19.11.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty SB, Hammond RC, Lillie MG. A new bovine herpesvirus and its effect on experimentally infected calves. Arch Gesamte Virusforsch. 1971;34:394–395. doi: 10.1007/BF01254696. [DOI] [PubMed] [Google Scholar]
- Monge A, Elvira L, Gonzalez JV, Astiz S, Wellenberg GJ. Bovine herpesvirus 4-associated postpartum metritis in a Spanish dairy herd. Research in Veterinary Science. 2006;80:120–5. doi: 10.1016/j.rvsc.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Moses AV, Fish KN, Ruhl R, Smith PP, Strussenberg JG, Zhu L, Chandran B, Nelson JA. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. Journal of Virology. 1999:736892–6902. doi: 10.1128/jvi.73.8.6892-6902.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem K, Caywood DD, Goyal SM, Werdin RE, Murtaugh MP. Persistence of bovid herpesvirus-4 in experimentally inoculated pregnant rabbits. New Microbiologica. 1993;16:87–93. [PubMed] [Google Scholar]
- Newberry RD, Stenson WF, Lorenz RG. Cyclooxygenase-2-dependent arachidonic acid metabolites are essential modulators of the intestinal immune response to dietary antigen. Nature Medicine. 1999;5:900–906. doi: 10.1038/11341. [DOI] [PubMed] [Google Scholar]
- Nikolin V, Donofrio G, Milošević B, Taddei S, Radosavljević V, Milićević V. First Serbian isolates of bovine herpesvirus 4 (BoHV-4) from a herd with history of postpartum metritis. New Micribiologica. 2007 in press. [PubMed] [Google Scholar]
- Nilsson K. The nature of lymphoid cell lines and their relationship to the virus. In: Epstein MA, Achong BG, editors. The Epstein–Barr Virus. Springer-Verlag; Berlin: 1979. pp. 225–281. [Google Scholar]
- Olson JD, Ball L, Mortimer RG, Farin PW, Adney WS, Huffman EM. Aspects of bacteriology and endocrinology of cows with pyometra and retained foetal membranes. American Journal of Veterinary Research. 1984;45:2251–2255. [PubMed] [Google Scholar]
- Osorio FA, Reed DE. Experimental inoculation of cattle with bovine herpesvirus-4: evidence for a lymphoid-associated persistent infection. American Journal of Veterinary Research. 1983;44:975–980. [PubMed] [Google Scholar]
- Osorio FA, Reed DE, Rock DL. Experimental infection of rabbits with bovine herpesvirus-4: acute and persistent infection. Veterinary Microbiology. 1982;7:503–513. doi: 10.1016/0378-1135(82)90045-1. [DOI] [PubMed] [Google Scholar]
- Osorio FA, Reed DE, Van Der Maaten MJ, Metz CA. Comparison of the herpesviruses of cattle by restriction endonuclease analysis and serologic analysis. American Journal of Veteterinary Ressearch. 1985;46:2104–2109. [PubMed] [Google Scholar]
- Osorio FA, Rock DL, Reed DE. Studies on the pathogenesis of a bovine cytomegalo-like virus in an experimental host. Journal of General Virology. 1985;66:1941–1951. doi: 10.1099/0022-1317-66-9-1941. [DOI] [PubMed] [Google Scholar]
- Pagnini U, Montanaro S, Pacelli F, De Martino L, Florio S, Rocco D, Iovane G, Pacilio M, Gabellini C, Marsili S, Giordano A. The involvement of oxidative stress in bovine herpesvirus type 4-mediated apoptosis. Frontiers in Bioscience. 2004;9:2106–14. doi: 10.2741/1320. [DOI] [PubMed] [Google Scholar]
- Park JB, Kendrick JW. The isolation and partila characterization of a herpesvirus from a case of bovine metritis. Arch Gesamte Virusfrsch. 1973;41:211–215. doi: 10.1007/BF01252768. [DOI] [PubMed] [Google Scholar]
- Peterson RB, Goyal SM. Propagation and quantitation of animal herpesviruses in eight cell culture systems. Comparative Immunology Microbiology and Infectious Disease. 1988;11:93–98. doi: 10.1016/0147-9571(88)90023-9. [DOI] [PubMed] [Google Scholar]
- Poyser NL. Effects of various factors on prostaglandin synthesis by the guinea-pig uterus. Journal of Reproduction and Fertility. 1987;81:269–276. doi: 10.1530/jrf.0.0810269. [DOI] [PubMed] [Google Scholar]
- Poyser NL. The control of prostaglandin production by the endometrium in relation to luteolysis and menstruation. Prostaglandins Leukotriens and Essential Fatty Acids. 1995;53:147–195. doi: 10.1016/0952-3278(95)90115-9. [DOI] [PubMed] [Google Scholar]
- Reiss CS, Komatsu T. Does nitric oxide play a critical role in viral infections? Journal of Virology. 1998;72:4547–4551. doi: 10.1128/jvi.72.6.4547-4551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Enquist LW. Biological interactions between herpesviruses and cyclooxygenase enzymes. Review in Medical Virology. 2006;16:393–403. doi: 10.1002/rmv.519. [DOI] [PubMed] [Google Scholar]
- Ruder CA, Sasser RG, Williams RJ, Ely JK, Bull RC, Butler JE. Uterine infections in the postpartum cow. II, Possible synergistic effect of Fusobacterium necrophorum and Corynebacterium pyogenes. Theriogenology. 1981;15:573–580. [Google Scholar]
- Schweizer M, Peterhans E. Noncytopathic bovine viral diarrhea virus inhibits double-stranded RNA-induced apoptosis and interferon synthesis. Journal of Virology. 2001;75:4692–4698. doi: 10.1128/JVI.75.10.4692-4698.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciortino MT, Perri D, Medici MA, Foti M, Orlandella BM, Mastino A. The γ-2-herpesvirus bovine herpesvirus 4 causes apoptotic infection in permissive cell lines. Virology. 2000;277:27–39. doi: 10.1006/viro.2000.0575. [DOI] [PubMed] [Google Scholar]
- Sheldon IM, Dobson H. Postpartum uterine health in cattle Animal Reproduction. Science. 2004;82-83:295–306. doi: 10.1016/j.anireprosci.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Simmen RC, Srinivas V, Roberts RM. cDNA sequence, gene organization, and progesterone induction of mRNA for uteroferrin, a porcine uterine iron transport protein. DNA. 1989;8:543–554. doi: 10.1089/dna.1989.8.543. [DOI] [PubMed] [Google Scholar]
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. Journal of Biological Chemistry. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. Journal of Immunology. 1993;150:5321–5329. [PubMed] [Google Scholar]
- Song MJ, Li X, Brown HJ, Sun R. Characterization of interactions between, R.T.A., and the promoter of polyadenylated nuclear RNA in Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8. Journal of Virology. 2002;76:5000–5013. doi: 10.1128/JVI.76.10.5000-5013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabel JR, Stabel TJ. Immortalization and characterization of bovine peritoneal macrophages transfected with SV40 plasmid DNA. Veterinary Immunology and Immunopathology. 1995;45:211–220. doi: 10.1016/0165-2427(94)05348-v. [DOI] [PubMed] [Google Scholar]
- Steer SA, Corbett JA. The role and regulation of COX-2 during viral infection. Viral Immunology. 2003;16:447–460. doi: 10.1089/088282403771926283. [DOI] [PubMed] [Google Scholar]
- Storz J, Ehlers B, Todd VJ, Ludwig H. Bovine cytomegaloviruses: identification and differential properties. Journal of General Virology. 1984;65:697–706. doi: 10.1099/0022-1317-65-4-697. [DOI] [PubMed] [Google Scholar]
- Sun R, Lin SF, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi’s sarcoma-associated herpesvirus. Proceeding of the National Academy of Sciience of USA. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiry E, Meersschaert C, Pastoret PP. Epizootiology of herpesvirus infections in wild ruminants. I. Infectious bovine rhinotracheitis virus and antigenically related viruses. Revue d’élevage et de médecine vétérinaire des pays tropicaux. 1988;41:113–20. [PubMed] [Google Scholar]
- Truman D, Ludwig H, Storz J. Bovine herpesvirus type 4: studies on the biology and spread in cattle herds and in insemination bulls. Journal of Veteterinary Medicine (Series B) 1986;33:485–501. [PubMed] [Google Scholar]
- Usherwood EJ, Stewart JP, Nash AA. Characterization of tumor cell lines derived from murine gammaherpesvirus-68-infected mice. Journal of Virology. 1996;70:6516–6518. doi: 10.1128/jvi.70.9.6516-6518.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Santen VL. Characterization of the bovine herpesvirus 4 major immediate-early transcript. Journal of Virology. 1991;65:5211–5224. doi: 10.1128/jvi.65.10.5211-5224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Santen VL. Characterization of a bovine herpesvirus 4 immediate-early RNA encoding a homolog of the Epstein-Barr virus R transactivator. Journal of Virology. 1993;67:773–784. doi: 10.1128/jvi.67.2.773-784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderplasschen A, Goltz M, Lyaku J, Benarafa C, Buhk HJ, Thiry E, Pastoret PP. The replication in vitro of the gammaherpesvirus bovine herpesvirus 4 is restricted by its DNA synthesis dependence on the S phase of the cell cycle. Virology. 1995;213:328–340. doi: 10.1006/viro.1995.0006. [DOI] [PubMed] [Google Scholar]
- Wellemans G, Van Opdenbosh E, Mammerickx M. Inoculation expérimental du virus LVR 140 (herpes bovin IV) à des vaches gestantes et non-gestantes. Annales de Rrecherches Rétérinaires. 1986;17:89–94. [PubMed] [Google Scholar]
- Zimmermann W, Broll H, Ehlers B, Buhk HJ, Rosenthal A, Goltz M. Genome sequence of bovine herpesvirus 4, a bovine Rhadinovirus, and identification of an origin of DNA replication. Journal of Virology. 2001;75:1186–1194. doi: 10.1128/JVI.75.3.1186-1194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]