Abstract
The cervical facet joint is implicated as one of the most common sources of chronic neck pain, owing to its rich nociceptive innervation and susceptibility to injurious mechanical loading. Injuries to the facet joint and its ligament can induce inflammation in the joint and spinal cord. Inflammatory molecules which are known to have a role in pain can also stimulate the integrated stress response (ISR). Therefore, we hypothesize that ISR is activated by facet joint injury in a rodent model of pain. To address this hypothesis, we assessed the expression of binding protein (BiP) (also known as growth-related protein 78 (GRP78)), a marker of endoplasmic reticulum stress response, in the dorsal root ganglion (DRG) after painful facet joint injury. In a rodent model of facet joint injury, dynamic distraction of the C6/C7 joint (injury, n = 12) was imposed; sham procedures were performed separately (sham, n = 8). Forepaw mechanical allodynia was assessed postoperatively for 7 days as a quantitative measure of pain symptoms. The C6 DRG was harvested and assessed for BiP expression using triple label immunofluorescent confocal microscopy and immunoblot analyses. BiP was significantly higher (p < 0.001) in the DRG after injury than sham and was expressed predominantly in neurons. Similarly, quantification of BiP by immunoblot demonstrated a significant 2.1-fold increase (p = 0.03) in injury compared to sham at day 7. Findings suggest neuronal stress activation is associated with painful facet joint injury, and that joint loading may directly mediate the behavior of DRG neurons in this class of injury.
Keywords: Facet joint, Strain, Pain, Stress response, BiP, Western blot
Chronic pain from whiplash and neck injuries is among the most common acquired musculoskeletal disorders in the United States, with approximately 30% of Americans suffering chronic pain and an annual cost of $61 billion [25,29]. The cervical facet joint is a candidate for painful injury [1,31] during many neck loading scenarios, and has nociceptive innervation that provides a potential mechanism for pain [4,12]. Recent in vivo studies demonstrate that certain facet joint distractions initiate persistent firing of nociceptive afferents in the facet capsule [4], and induce persistent mechanical allodynia and spinal glial activation [14,35].
Inflammation contributes to persistent pain [5,34]. Cytokines and neurotrophic factors are upregulated in dorsal root ganglion (DRG) neurons in models of facet joint inflammation [20,21]. Lumbar neuropathic pain models exhibit glial cell activation, macrophage reactivity, and cytokine upregulation in the DRG and spinal cord [8,32–34]. Glial activation can alter neuronal signaling and can also induce secretion of several pain mediators, including glutamate, pro-inflammatory cytokines and chemokines [19]. The release of glutamate and cytokines in the inflammatory cascade can also cause neuronal damage [2], and may trigger the integrated stress response (ISR). The integrated stress response is also known as the endoplasmic reticulum (ER) stress response or the unfolded protein response and is a protective cellular response that establishes protein homeostasis in response to a variety of injurious stimuli by increasing transcription and translation of gene products that regulate protein folding, degradation, delivery, and oxidation [16,18]. The ISR binding protein (BiP) is an ER-resident chaperone that acts as a major regulator of the stress response [9,26]. While the mechanisms of activation of the ISR and the specific pathways of the neuronal stress response are receiving attention, their potential role in painful injury remains unexplored.
Facet joint distraction in the rat produces sustained spinal astrocytic activation and persistent mechanical allodynia [14]. Despite the known role of inflammation in pain and the relationship between inflammation and the cellular stress response, there is a paucity of data defining the relationship between the ER stress response and pain. While facet joint injury is common, the cellular mechanisms driving pain remain poorly understood. An in vivo model of painful mechanical injury to the cervical facet joint is used to probe whether painful facet joint loading can induce BiP expression to identify the role of joint loading in modulating BiP responses in afferent neurons of spinal joints.
All procedures were approved by the Institutional Animal Care and Use Committee. Male Holtzman rats (350–430 g) were housed under USDA- and AAALAC-compliant conditions with free access to food and water.
Surgical procedures were performed under inhalation anesthesia and were modified from previously reported methods [14,15]. Following a skin incision in the back of the neck, muscle and soft tissue were cleared from C5 to T1. The laminae, facet capsules, and spinous processes at the C6/C7 facet joints were exposed bilaterally. The interspinous ligament was transected at C5–T1 to enable attachment of each of the C7 and C6 vertebrae to a loading device via microforceps. The loading device imposed controlled distraction of the C6/C7 facet joint using a stepper motor (Danaher Precision Systems) by displacing the C6 microforceps rostrally. Joint distraction was applied at a rate of 15 mm/s to simulate the injurious loading profile of the C6/C7 facet joint and its capsule during whiplash [22,28,31]. The C6/C7 facet joints underwent either: (1) injury distraction (0.3 mm; n = 12) sufficient to produce sustained mechanical allodynia [6], or (2) sham procedures without any distraction (0 mm; n = 8).
Imaging and biomechanical measurements were acquired during joint distraction to quantify the applied loading and to validate that the same injury severity was applied in all cases. Particles (diameter = 0.17 mm) were affixed to the laminae surrounding the C6/C7 joint on the right side and used to define the overall joint distraction. Additional particles were also placed in a grid covering the right C6/C7 facet capsule, to similarly track the full-field capsule displacements during loading. All particles were tracked using high-speed imaging (Phantom v4.3 CCD camera; Vision Research, Inc.). In order to quantify the severity of loading to the capsule, maximum principal strain was computed using LS-DYNA software (LSTC) [6,15].
Bilateral mechanical allodynia was assessed in the forepaws on days 1, 3, 5, and 7, using 2 strengths of von Frey filaments (2, 4 g; Stoelting Co.) [32]. Procedures for assessing forepaw allodynia were previously reported and validated [11,15]. Briefly, on each day, rats were acclimated to the tester and the environment. Each filament was applied to the plantar surface of each forepaw in three rounds of testing. Each round applied 10 stimulations and was separated by 10 min between rounds. The total number of paw withdrawals was counted as the number of positive responses out of the total 30 stimulations for each forepaw. The responses for the right and left paws were averaged for each group on all testing days. Prior to surgery, baseline measurements were taken as each rat’s control.
The C6 left DRG was harvested on day 7 to analyze and quantify BiP expression. In separate groups of rats, immunohistochemistry identified the cell types expressing BiP, and immunoblot analysis was performed to quantify BiP levels. As such, half of the rats that received injury (n = 6) and half from the sham group (n = 4) were randomly selected and processed for each type of tissue analysis.
For immunofluorescent histochemistry, rats were deeply anesthetized followed by transcardiac perfusion with phosphate-buffered saline (PBS) and paraformaldehyde. Samples were post-fixed in 4% paraformaldehyde and paraffin-embedded. Transverse sections (10 μm) were mounted on APES-coated slides, incubated at 55 °C, deparaffinized and rehydrated as previously described [3]. Sections were blocked using 10% normal goat serum (Chemicon International). Mouse monoclonal antibodies to BiP (clone 40, 1:500,000; BD BioSciences), and the neuronal marker, microtubule associated protein (MAP2; SMI-52, 1:100; Covance Research Products) were used. Tyramide Signal Amplification Technology (New England Biolabs) was used to amplify the BiP signal. The MAP2 primary antibody was visualized by a Cy-3-conjugated, goat anti-mouse antibody (1:200). Confocal microscopy was performed on a Bio-Rad Radiance 2100 equipped with Argon Green He/Ne, Red Diode and Blue Diode lasers to excite the Cy-3 as described previously [30]. Metamorph 6.0 image analysis software (Universal Imaging Inc.) was used to quantify BIP. Total BiP was measured by the integrated pixel intensity for BiP per image and was the total pixel intensity per image multiplied by the area of pixel positive for the signal. The integrated pixel intensity for BiP overlapping positive pixels for MAP2 was used to determine the colocalization of BiP with neurons. Integrated BiP pixel intensity and the colocalization of BiP with neurons were both separately quantified.
For immunoblotting, rats were anesthetized and perfused with PBS. The DRG was removed and homogenized in lysis buffer and cocktails of protease and phosphatase inhibitors. Homogenized tissue was centrifuged at 25,000 rpm for 30 min and the supernatants were collected from the lysates. Total protein concentration of the lysates was determined using a BCA protein assay reagent kit (Pierce Biotechnology). Protein (10 μg) was loaded into each lane of a 4–12% Bis–Tris gradient gel (Invitrogen Corp.) for separation. A broad-range molecular weight ladder was run on each gel. Subsequent to separation, proteins were transferred onto a PVDF membrane and blocked in TBS with 1% Tween-20 (TBS-T) and 5% non-fat milk for 1 h at room temperature. The membrane was incubated at 4 °C overnight with mouse monoclonal antibody to BiP (1:1000; BD BioSciences) in TBS-T with 5% bovine serum albumin. The membrane was washed and incubated with HRP-conjugated secondary antibody (1:1000, Pierce Biotechnology) in blocking buffer for 30 min at room temperature, and developed using SuperSignal West Dura Extended Duration Substrate (Pierce Biotechnology) and exposed to Amersham Hyperfilm (GE Healthcare). The membrane was stripped with stripping buffer (Pierce Biotechnology) and re-probed for actin (goat polyclonal, 1:1000; Santa Cruz Biotechnology) to serve as a loading control. Autographs were scanned and band size and pixel intensity were quantified by NIH ImageJ. Target bands were normalized to an actin loading control to account for protein degradation and loading discrepancies.
Significant differences in allodynia were determined between groups over time using a repeated measures ANOVA with Bonferroni correction. Tissue results were analyzed by one-way ANOVA followed by the Newman Keuls post hoc test. Significance was defined at p < 0.05 for all statistical analyses.
The mean applied joint distraction for the injury group was 0.23 ± 0.19 mm, with a corresponding mean maximum principal strain of 21.5 ± 12.3%. There was no significant difference in either joint distraction or maximum principal strain in the capsule between the groups of rats with tissues prepared for immunofluorescence (distraction = 0.30 ± 0.21 mm; strain = 18.1 ± 4.9%) and those for immunoblotting (distraction = 0.16 ± 0.15 mm; strain = 24.9 ± 16.8%). Similarly, there was no significant difference in the mechanical allodynia measured between either of the groups used for the two types of tissue assays.
Mechanical allodynia was immediately elevated above baseline unoperated responses after a facet joint distraction injury, for both filaments (Fig. 1) and remained significantly (p < 0.001) elevated on each day. In contrast, sham procedures did not produce allodynia at any time point. The mechanical allodynia produced by a facet joint distraction injury was significantly (p < 0.001) elevated over sham responses for all time points.
Fig. 1.
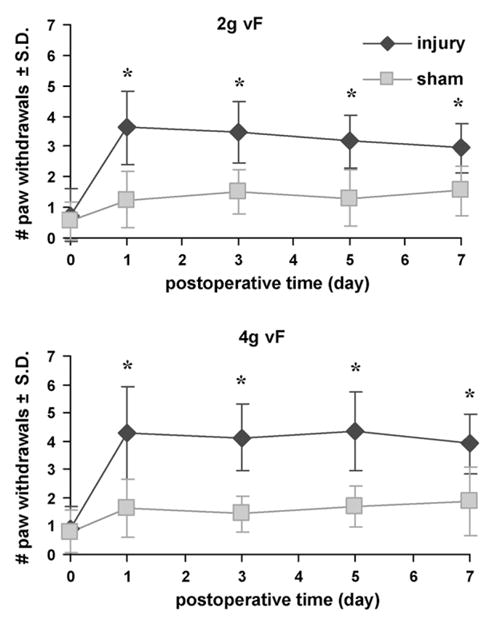
Average forepaw mechanical allodynia for injury and sham. Allodynia following injury was significantly elevated and remained appreciably higher than sham for both von Frey filaments (p < 0.001). Results are similar for both filaments. Data are depicted as average ±standard deviation. Asterisk (*) indicates significant increases over sham (p < 0.001).
Facet joint injury also increased BiP expression in the DRG at day 7. Regardless of treatment, BiP staining was predominantly cytoplasmic in neurons (Fig. 2A), a localization consistent with its role as a chaperone protein in the ER. However, the overall level of BiP immunostaining in the DRG was significantly greater (p < 0.05) after injury than sham (Fig. 2B). A significant increase in neuronal BiP immunostaining on day 7 was observed after painful injury relative to sham (p < 0.001) (Fig. 2C). Similar increases in BiP protein in the DRG were also confirmed by immunoblot analysis. Quantification of BiP by normalizing to actin expression showed a 2.1-fold significant (p = 0.03) increase in injury compared to sham (Fig. 3).
Fig. 2.
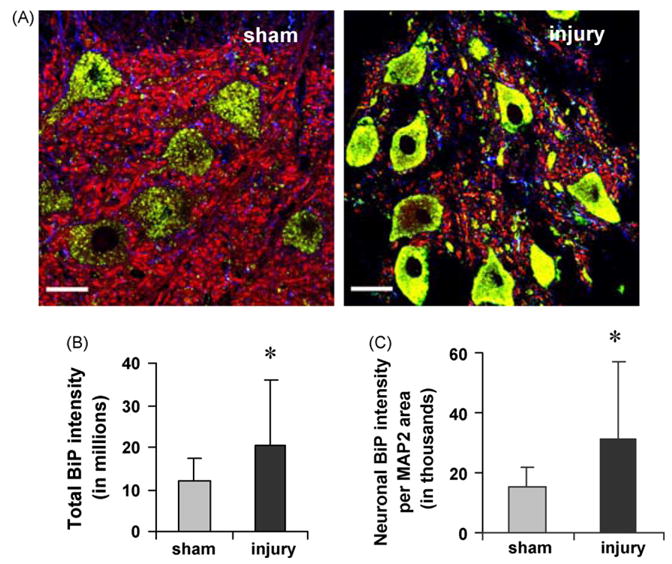
BiP immunostaining increases in DRG neurons after injury (A). Using triple label immunosfluorescent confocal microscopy, BiP staining (green) is more prominent in injured DRG neurons (MAP2; red). Nuclei are shown in blue. Red and green co-localize to yellow. Scale is 30 μm for both images. Quantification of total BiP-integrated pixel intensity (B), and integrated pixel intensity of BiP colocalizing with MAP2, normalized to MAP2 area (C) show increased BiP expression. Data are presented as average ±standard deviation, with significant increases (*) over sham.
Fig. 3.
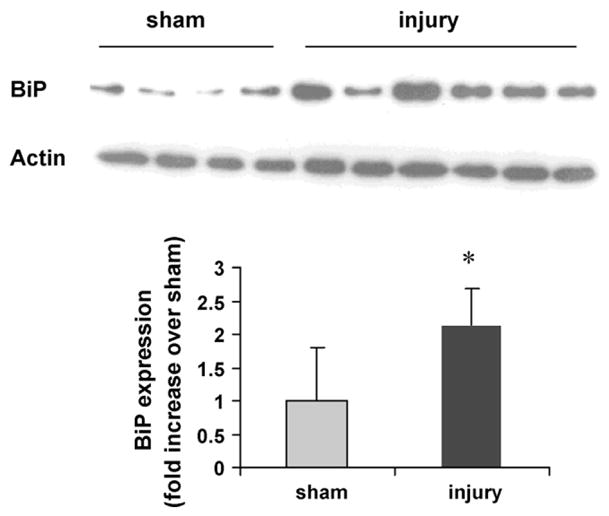
BiP protein increases at day 7 after an injury. A single band of protein was detected by immunoblot analysis for either BiP or actin. Quantification of BiP normalized by actin shows a 2.1-fold increase in injury over sham (*p = 0.03). Data are presented as average ±standard deviation.
This study is the first to demonstrate that facet joint loading sufficient to produce behavioral sensitivity also increases a marker of integrated stress response activation (Figs. 2 and 3). The increased BiP-immunoreactivity observed predominantly in neurons of the DRG after painful injury supports activation of the integrated stress response in association with facet-mediated pain. These findings suggest that the neurons innervating the facet joint and/or its capsule may act as ‘sensors’ of the capsule’s injury. This finding is consistent with electrophysiological reports of altered signaling and persistent after-discharges in afferents of the facet joint during its injurious loading [4]. Taken with the literature, our data further implicate cellular responses of neuronal afferents in the facet capsule as having an essential role in chronic pain [4,14]. This study does not directly define relationships between the magnitude of capsule loading and BiP responses or allodynia; studies are needed to define BiP in specific neuron populations and evaluate if such responses are sensitive to mechanical inputs and/or relate directly to resulting allodynia.
The current study demonstrates that ISR activation, indicated by increased BiP, occurs in neurons of the DRG after painful facet distraction. Given the presence of glial activation in the spinal cord at the associated injury level [14,35], activation of the ISR in the DRG is not surprising. Activation of the ISR alters translational initiation by directly phosphorylating, and subsequently inactivating, the initiating factor required for charging the initiator methionine tRNA (eukaryotic initiation factor 2 alpha) [10,27], reducing translation of non-stress-associated mRNAs that are localized to stress granules to await establishment of cellular homeostasis [7,13]. Diversion of the translation machinery and sequestration of cellular mRNA may have a dramatic effect on function of a metabolically active cell, such as the neuron. Thus, activation of the ISR in DRG neurons as seen in our study may alter, or even hinder, neuronal function leading to increased pain perception. Although BiP expression was increased in neurons of all sizes suggesting a ubiquitous neuronal increase in response to facet loading, certain neuronal cell populations are specifically responsible for nociceptive signaling. Thus, it will be important to define the timing and extent of ISR activation by neuronal sub-type, as well as to determine whether inhibiting specific arms of the ISR pathway may attenuate pain.
This in vivo model of painful injury simulates a real-world mechanical neck injury. Loading was applied at a rate (500%/s) matching those experienced by the lower cervical spine and its facet joint during whiplash (150–1000%/s) [22,28,31] and the range of maximum principal strains (18–25%) is comparable to those reported in whiplash (16–40%) [23,24]. An electrophysiological study in the goat determined a strain-threshold of 47% to activate nociceptors for similar loading [17]. Since the current study demonstrates persistent allodynia and BiP expression at lower strains, it may be possible that the threshold for initiating nociceptive cascades in this injury paradigm may actually be lower than previously thought.
Behavioral hypersensitivity and some spinal glial responses are modulated by the magnitude of facet joint distortion [6,14]. Specifically, strains between 11 and 42% reliably induce allodynia and spinal astrocytic activation, while strains below 10% do not. Joint loading at higher rates elicits a behavioral response for capsule strains as low as 11% that is also sensitive to strain magnitude with greater allodynia for higher strains (~26%) [6]. The present study defined responses for a single injury severity and it does not address whether BiP expression is differentially activated by the amount of strain in the capsule or if it is expressed in other cell types over different time courses. Nonetheless, the relationship among tissue loading, neuronal BiP levels in the DRG, and pain suggests BiP may be an important factor in regulating the production and/or maintenance of pain.
This study demonstrates activation of the ISR in a pain model. BiP expression in DRG neurons increases after painful facet joint distraction, which implies those neurons may be injured. While this study focused on neuronal behavior, glial cells are also involved in pain, and also in this injury model [5,14,35]. These results suggest an association between the neuronal stress response and pain, and the potential for mechanics to directly mediate behavior of affected neurons. Continued investigations will advance the understanding of the cellular mechanisms by which mechanical trauma-induced neuroinflammation contributes to pain, and shed light on the development of effective therapeutic interventions.
Acknowledgments
This work was funded in part by grants from the NHTSA/SCIB (DTNH-22-04-H-01423), the CDC/NCIPC (R49-CE000689), the NIH/NINDS (NS056885), and support from a departmental GAANN fellowship.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit:
References
- 1.April C, Bogduk N. The prevalence of cervical zygapophyseal joint pain. Spine. 1992;17:744–747. doi: 10.1097/00007632-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bezzi P, Domercq M, Brambilla L, Calli R, Schols D, Clercq ED, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR-4-activated astrocyte glutamate release via TNF alpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 3.Chalovich EM, Koike MA, Aras MA, Murphey-Corb M, Wiley CA, Jordan-Sciutto KL. Pocket proteins p107 and p130 exhibit increased expression in macrophages during SIV encephalitis. Neuropathology. 2005;25:315–325. doi: 10.1111/j.1440-1789.2005.00645.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen CY, Lu Y, Kallakuri S, Patwardhan A, Cavanaugh JM. Distribution of A-δ and C-fiber receptors in the cervical facet joint capsule and their response to stretch. J Bone Joint Surg Am. 2006;88:1807–1816. doi: 10.2106/JBJS.E.00880. [DOI] [PubMed] [Google Scholar]
- 5.DeLeo JA, Tawfik VL, Lacroix-Fralish ML. The tetrapartite synapse: path to CNS sensitization and chronic pain. Pain. 2006;122:17–21. doi: 10.1016/j.pain.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 6.Dong L, Lee KE, Winkelstein BA. Dynamic Distraction of the Cervical Facet 295 Joint Produces Higher Mechanical Allodynia than Quasistatic Distraction: 296 Implications of Displacement Thresholds for Pain in Whiplash Loading. ASME-SBC Keystone, Co; 2007. p. #176587. [Google Scholar]
- 7.Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999;339:135–141. [PMC free article] [PubMed] [Google Scholar]
- 8.Fenzi F, Benedetti MD, Moretto G, Rizzuto N. Glial cell and macrophage reactions in rat spinal ganglion after peripheral nerve lesions: an immunocytochemical and morphometric study. Arch Ital Biol. 2001;139:357–365. [PubMed] [Google Scholar]
- 9.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 10.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 11.Hubbard RD, Winkelstein BA. Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and apinal glial activation: mechanical factors in painful neck injuries. Spine. 2005;301:1924–1932. doi: 10.1097/01.brs.0000176239.72928.00. [DOI] [PubMed] [Google Scholar]
- 12.Kallakuri S, Singh A, Chen C, Cavanaugh JM. Demonstration of substance P, calcitonin-gene-related peptide, and protein gene product 9.5 containing nerve fibers in human cervical facet joint capsules. Spine. 2004;29:1182–1186. doi: 10.1097/00007632-200406010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Kimball SR, Horetsky RL, Ron D, Jefferson LS, Harding HP. Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am J Physiol Cell Physiol. 2003;284:C273–C284. doi: 10.1152/ajpcell.00314.2002. [DOI] [PubMed] [Google Scholar]
- 14.Lee KE, Davis MB, Mejilla RM, Winkelstein BA. In vivo cervical facet capsule distraction: mechanical implications for whiplash and neck pain. Stapp Car Crash J. 2004;48:373–393. doi: 10.4271/2004-22-0016. [DOI] [PubMed] [Google Scholar]
- 15.Lee KE, Thinnes JH, Gokhin DS, Winkelstein BA. A novel rodent neck pain model of facet-mediated behavioral hypersensitivity: implications for persistent pain and whiplash injury. J Neurosci Methods. 2004;137:151–159. doi: 10.1016/j.jneumeth.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Lindl KA, Akay C, Wang Y, White MG, Jordan-Sciutto KL. Expression of the endoplasmic reticulum stress response marker, BiP, in the central nervous system of HIV-positive individuals. Neuropathol Appl Neurobiol. 2007;33:369–658. doi: 10.1111/j.1365-2990.2007.00866.x. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y, Chen C, Kallakuri S, Patwardhan A, Cavanaugh JM. Neural response of cervical facet joint capsule to stretch: a study of whiplash pain mechanism. Stapp Car Crash J. 2005;49:49–65. doi: 10.4271/2005-22-0003. [DOI] [PubMed] [Google Scholar]
- 18.Markowitz AJB, White MG, Kolson DL, Jordan-Scuitto KL. Cellular interplay between neurons and glia: towards a comprehensive mechanism for excitotoxic neuronal loss in neurodegeneration. Cell Sci Rev. 2007;4 [PMC free article] [PubMed] [Google Scholar]
- 19.McMahon SB, Cafferty WBJ, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Miyagi M, Ohtori S, Ishikawa T, Aoki Y, Ozawa T, Doya H, Saito T, Moriya H, Takahashi K. Up-regulation of TNFα in DRG satellite cells following lumbar facet joint injury in rats. Eur Spine J. 2006;15:953–958. doi: 10.1007/s00586-005-1031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtori S, Takahashi K, Moriya H. Inflammatory pain mediated by a phenotypic switch in brain-derived neurotrophic factor-immunoreactive dorsal root ganglion neurons innervating the lumbar facet joints in rats. Neurosci Lett. 2002;323:129–132. doi: 10.1016/s0304-3940(02)00120-9. [DOI] [PubMed] [Google Scholar]
- 22.Panjabi MM, Cholewicki J, Nibu K, Babat LB, Dvorak J. Simulation of whiplash trauma using whole cervical spine specimens. Spine. 1998;23:17–24. doi: 10.1097/00007632-199801010-00005. [DOI] [PubMed] [Google Scholar]
- 23.Panjabi MM, Pearson AM, Ito S, Ivancic PC, Gimenez E, Tominaga Y. Cervical spine ligament injury during simulated frontal impact. Spine. 2004;29:2395–2403. doi: 10.1097/01.brs.0000143173.92241.ab. [DOI] [PubMed] [Google Scholar]
- 24.Pearson AM, Ivancic PC, Ito S, Panjabi MM. Facet joint kinematics and injury mechanisms during simulated whiplash. Spine. 2004;29:390–397. doi: 10.1097/01.brs.0000090836.50508.f7. [DOI] [PubMed] [Google Scholar]
- 25.Quinlan KP, Annest JL, Myers B, Ryan G, Hill H. Neck strains and sprains among motor vehicle occupants—United States, 2000. Accident Anal Prev. 2004;36:21–27. doi: 10.1016/s0001-4575(02)00110-0. [DOI] [PubMed] [Google Scholar]
- 26.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stemper BD, Yoganandan N, Pintar FA. Effects of abnormal posture on capsular ligament elongations in a computational model subjected to whiplash loading. J Biomech. 2005;38:1313–1323. doi: 10.1016/j.jbiomech.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 30.Strachan GD, Kopp AS, Koike MA, Morgan KL, Jordan-Sciutto KL. Chemokine- and neurotrophic factor-induced changes in E2F1 localization and phosphorylation of the retinoblastoma susceptibility gene product (pRb) occur by distinct mechanisms in murine cortical cultures. Exp Neurol. 2005;193:455–468. doi: 10.1016/j.expneurol.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 31.Sundararajan S, Prasad P, Demetropoulos CK, Tashman S, Begeman PC, Yang KH, King AI. Effect of head–neck position on cervical facet stretch of post mortem human subjects during low speed rear end impacts. Stapp Car Crash J. 2004;48:331–372. doi: 10.4271/2004-22-0015. [DOI] [PubMed] [Google Scholar]
- 32.Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Res. 1999;829:209–221. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- 33.Uceyler N, Tscharke A, Sommer C. Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav Immunol. 2007;21:553–560. doi: 10.1016/j.bbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 35.Winkelstein BA, Santos DG. Facet capsular ligament tension is required to produce pain due to cervical facet joint loading. Spine. 2008;33:856–862. doi: 10.1097/BRS.0b013e31816b4710. [DOI] [PubMed] [Google Scholar]


