Abstract
Embryonic stem cells (ESC) have the ability of indefinite self-renewal and multilineage differentiation, and carry great potential in cell based therapies. The rhesus macaque is the most relevant preclinical model for assessing the benefit, safety and efficacy of ESC based transplantations in the treatment of neurodegenerative diseases. In the case of neural cell grafting, tracing both the neurons and their axonal projections in vivo is essential for studying the integration of the grafted cells in the host brain. Tau-green fluorescent protein (tau-GFP) is a powerful viable lineage tracer, allowing to visualize cell bodies, dendrites and axons in exquisite details. Here, we report the first rhesus monkey ESC line that ubiquituously and stably expresses tau-GFP. First, we derived a new line of rhesus monkey ESC (LYON-ES1), that show marker expression and cell cycle characteristics typical of primate ES cells. LYON-ES1 cells are pluripotent, giving rise to derivatives of the three germ layers in vitro and in vivo through teratoma formation. They retain all their undifferentiated characteristics and a normal karyotype after prolonged culture. Using lentiviral infection, we then generated a monkey ES cell line stably expressing tau-GFP that retains all the characteristics of the parental wild type line and is clonogenic. We show that neural precursors derived from the tau-GFP ESC line are multipotent and that their fate can precisely be mapped in vivo after grafting in the adult rat brain.
Keywords: Alkaline Phosphatase; metabolism; Animals; Blastocyst; cytology; Cell Differentiation; Cell Line; Embryonic Stem Cells; cytology; enzymology; physiology; virology; Genes, Reporter; Green Fluorescent Proteins; genetics; Lentivirus; Macaca mulatta; Reverse Transcriptase Polymerase Chain Reaction; Stem Cell Transplantation; methods; Teratoma; genetics; pathology; Transfection; Zona Pellucida; physiology; tau Proteins; genetics
Introduction
Embryonic stem cells (ESC) are capable of indefinite self-renewal and multilineage differentiation. One of the most important potential application of human ESC is cell-based therapy. Before the clinical application of human ESC transplantation can be attempted, extensive studies assessing the benefit, safety and efficacy of ES-derived cell transplantation in preclinical non human primate models will be necessary, particularly in the case of neurodegenerative diseases 1.
ESC lines established in non human primates (Rhesus, cynomolgus and marmoset monkeys) 2–8 have characteristics similar to those of human ESC, proving to be an invaluable preclinical research tool. Like their human counterparts, monkey ESC are able to differentiate into many clinically relevant cell types including hematopoietic cells 9, hepatocytes 10, insulin-producing cells 11, cardiomyocytes 12 and neural derived cells 7, 9, 13–18. Following transplantation, non-human primates ESC and their derivatives have been shown to survive, differentiate and integrate into the host tissue of various animal models (rodents and monkeys), particularly the brain 19–23.
Tracing the cells in vivo is essential for studying the fate and the interaction of the grafted cells with their host environment. In the case of neural cells grafting, this makes it necessary to be able to reliably label both the parent neurons and their axonal projections in the recipient brain. Such a label is possible via tau-green fluorescent protein (GFP) fusion protein expression. By binding the GFP to microtubules, tau-GFP tagging reveals the detailed morphology of cell bodies, dendrites and axons 24, 25. Here, we report the generation and characterization of the first Rhesus monkey ESC line that ubiquituously and stably expresses a tau-GFP fusion protein. This cell line retains all the characteristics of the parental wild type line and is clonogenic. We show that neural precursors derived from the tau-GFP ESC line are multipotent, and that their integration can be monitored in vivo after grafting in the adult rat brain.
MATERIALS AND METHODS
ES cells derivation and culture
Zonae pellucidae of blastocysts were removed by brief exposure (45–60 sec) to 0.5% pronase in TH3 medium. Expanded blastocysts, possessing large and distinct inner cell masses (ICMs), were subjected to the immunosurgical method. Zona-free blastocysts were exposed to anti-monkey antiserum (Sigma; St. Louis, http://www.sigmaaldrich.com) for 30 min at 37°C. After washing in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen; Carlsbad, CA, http://www.invitrogen.com) supplemented with 20% FBS (Hyclone; Perbio; http://www.perbio.com), embryos were incubated in guinea pig complement reconstituted with DMEM (1:5, v/v) for an additional 30 min at 37°C. Partially lysed trophectodermal cells were dispersed by gentle pipetting with a flame-pulled Pasteur pipette. ICMs were then rinsed three times with DMEM medium supplemented with 20% FBS. Isolated ICMs were plated onto Nunc 4-well dishes containing a feeder layer of mitomycin-C treated mouse embryonic fibroblasts (MEFs), cultured in KO-DMEM medium containing 10% FBS/10% KO-SR (Invitrogen), supplemented with 4ng/ml bFGF (Abcys; http://www.abcysonline.com), human recombinant LIF (hrLIF; 1000 IU/ml), 1% nonessential amino acids (Invitrogen), 2mM L-glutamine (Invitrogen), 0.1mM β-mercaptoethanol. ICMs that attached to the feeder layer and initiated outgrowth were manually dissociated into small cell clumps with a microscalpel and replated onto new MEFs. When blastocysts exhibited undistinguishable trophectoderm and ICM, immunosurgery was not performed and the whole blastocysts were cultured on MEFs after digestion of zona pellucida with pronase.
Colonies with ESC-like morphology were selected for further propagation, characterization and freezing. During the early stage of ESC derivation, the medium was supplemented with hrLIF (1000 IU/ml), and half of the medium was changed every other day. For expansion and maintenance, ESC were cultured in KO-DMEM containing 20% KO-SR, and 4ng/ml bFGF. Mechanical passaging of the undifferentiated colonies was performed manually every 5–7 days by cutting the colonies in big clumps using a flame-pulled Pasteur pipette. ESC colonies were replated onto dishes with fresh feeder layers. Care was taken so that the differentiated areas were eliminated during passaging. Cultures were maintained at 37 °C, 5% CO2.
Lentiviral infection and cell sorting
We used two Simian Immunodeficiency Virus (SIV)-based vectors, GAE-CAG-eGFP/WPRE (a gift from F.L. Cosset), that harbors the sequence encoding the enhanced Green Fluorescent Protein (eGFP), and GAE-CAG-tau-GFP/WPRE that harbors the sequence encoding Tau-GFP 25, both driven by the CMV enhanced chicken beta-actin (CAG) promoter. GAE-CAG-TauGFP/WPRE was generated by replacing the BamH1/EcoRV restriction fragment containing the eGFP cassette in GAE-CAG-eGFP/WPRE by a EcoRV blunt restriction fragment of the pTP6 vector (a gift from Tom Pratt) containing the tau-GFP cassette. We previously described the lentiviral production 26. Briefly, 293T cells were transfected with a mixture of DNAs containing 10 μg of the pGRev plasmid encoding the vesicular stomatitis virus glycoprotein (VSV-G) envelope, 10 μg of pSIV3+ plasmid encoding the gag, pol, tat and rev proteins, and 13 μg of the R4SA-CAG-EGFP-W plasmid or the R4SA-CAG-TauGFP-W plasmid, using the calcium phosphate precipitation technique. The following day, cells were re-fed 7 ml of DMEM and further cultured for 24 hours. The supernatant was then collected, cleared by centrifugation (3,000RPM, 15 min) and passed through a 0.8 μm filter. Prior to infection, LYON-ES1 cells were treated with collagenase IV (1mg/ml) for 5 min at 37°C. Clumps of undifferentiated cells were manually selected and transferred to fresh medium (200 μl) containing SIV-eGFP or SIV-tau-GFP in the presence of 6μg/ml polybrene (Sigma). Cells were incubated for 3 hrs at 37°C, before being replated on fresh feeder cells. To select for GFP positive LYON-ES cells, GFP and tau-GFP expressing LYON-ES cells were treated with collagenase IV (1mg/ml) (Invitrogen) for 20 min, trypsined, and the single cell preparation was resuspended in PBS before being processed in a FACSVantageTMSE (Becton Dickinson). The viability of LYON-ES1 cells after FACS sorting was >98% as assessed by trypan blue exclusion. The percentage of GFP positive cells was analysed using a FACS Canto2. Data acquisition was performed with Diva software (Becton Dickinson).
Cloning
LYON-ES cells were dissociated to single cells for 7 min with trypsin (0,025%)/EDTA (0,1g/l) (Invitrogen), washed by centrifugation, and individual cells were selected by direct observation under a stereomicroscope and transferred by micropipettes to individual wells of 96-well plates containing MEF and medium supplemented with 20% KOSR and 10ng/ml bFGF. As physiologic oxygen has been reported to enhance clone recovery in hESC lines 27,28, cultures were maintained at 5% O2, at 5% CO2, at 37°C. Emerging clones were first passaged into 24-well plates, subsequently amplified into 35mm well plates, and frozen in liquid nitrogen. All clones were found to be GFP positive using fluorescent microscopy.
In vitro differentiation of LYON-ES cells
For the formation of embryoid bodies (EBs), LYON-ES1 colonies were treated with collagenase IV (1mg/ml) for 30 mins, and clumps of cells were cultured in suspension in KO-DMEM medium containing 20% KO-SR (Invitrogen), and supplemented with 1% non essential amino acids (Invitrogen), 2mM L-glutamine (Invitrogen), 0.1mM β-mercaptoethanol, without bFGF. Medium was changed every other day. For spontaneous differentiation, LYON-ES cells were cultured to subconfluency for 15 days with daily medium change. For neural differentiation, neuroepithelial-like cells spontaneously emerging in culture were selected manually, and cultured in Euromed-N medium (Euroclone; http://www.euroclone.net), supplemented with 20 ng/ml bFGF (Abcys) and 20 ng/ml EGF (Abcys). For neuronal differentiation, LYON-ES derived neural cells were trypsinized (0,025%)/EDTA (0,1g/l) (Invitrogen), and re-plated into 4-well plates (Nunc) coated with matrigel (Bdbiosciences; http://www.bdbiosciences.com), in EuromedN medium supplemented with bFGF (10ng/ml), modified N2, and B27 (Invitrogen). A half volume of medium was replaced every 2 days. After 7 days, the medium was changed to Euromed-N mixed with neurobasal medium (Invitrogen) (1:1) supplemented with 0,5x N2 and B27, bFGF (5ng/ml), BDNF (20ng/ml) (Sigma), and ascorbic acid (200μM). After a further 7 days in these conditions, medium was switched to neurobasal medium supplemented with B27 and BDNF (10ng/ml) without N2 or bFGF. For glial differentiation, LYON-ES derived neural cells were cultured in Euromed-N medium supplemented with 10%FBS (Hyclone; Perbio).
Immunofluorescence and Alkaline Phosphatase staining
Cells were fixed with 2% PFA in phosphate-buffered saline (PBS) at 4°C for 1 hour, and permeabilized in Tris Buffer Saline (TBS)+0.1%TritonX-100 (3 times 10 min). Non-specific binding was blocked with 10% normal goat serum (NGS) (Jackson immunoresearch Laboratories, West Grove, PA, http://www.jacksonimuno.com) for 20 min at room temperature (RT). Cells were incubated overnight at 4°C, with primary antibodies (see Supplementary Table 2) diluted in Dako diluent (Dako; http://www.dako.fr). After three rinses in TBS, cells were exposed either to affinity-purified goat anti-mouse, anti-rat or anti-rabbit immunoglobulin G or M (IgG, IgM) conjugated either to indocarbocyanine or to cyanin (Cy3 and Cy2, respectively; Jackson ImmunoResearch Laboratories) for 1 hour at RT followed by nuclear staining with 1 ng/ml Hoechst 33258 for 3 min. After three rinses in TBS, coverslips were mounted on slides. Coverslips were examined using an oil objective microscope under UV light to detect fluorescencence in isothiocyanate (FITC) (filter 450–490 nm), indocarbocyanine 3 (filter 550–570 nm), and Hoechst 33258 (filter 355–425 nm). Alkaline phosphatase activity was revealed using the Alkaline Phospatase substrate kit (Ref. 86R; Sigma), according to manufacturer’s instructions.
Karyotype analysis
LYON-ES1 cells were treated with colcemid (0.08μg/ml) (Sigma) for 2 hours. Cells were then trypsinized, resuspended in 0.075M KCl, and incubated for 10 min at RT. The cells were then fixed with fresh Carnoy’s fixative (methanol:glacial acetic acid; 3:1), and dropped onto ice-cold slides. Chromosome spread were Giemsa banded. Images were captured on a Leica microscope using the mosaic imaging system (Explora Nova; http://www.exploranova.com). At least 20 metaphase spreads were counted for each passage and 14 banded karyotypes were evaluated for chromosomal rearrangements.
Telomerase activity
Telomerase activity was determined using the TRAPEZE Telomerase Detection Kit (Chemicon; http://www.chemicon.com) according to the manufacturer’s instructions. Briefly, cell extracts were obtained from one 35mm culture dish. Protein concentrations were normalized using the Coomassie blue–stained protein assay reagent BSA standards (Pierce, Inc., Rockford, IL, http://www.piercenet.com). Heat-inactivated controls were obtained by incubating the samples at 85°C for 10 min. Aliquotes (1μg) of the cell extracts were used for polymerase chain reaction (PCR). The PCR products were electrophoresed on a 12.5% nondenaturing polyacrylamide gel, and telomerase activity was detected by ethidium bromide staining.
Semi quantitative RT-PCR
Total RNA was prepared with a QIAGEN RNeasy kit (QIAGEN, Valencia, CA, http://www.qiagen.com). Standard reverse transcription reactions were performed with 1μg of total RNA primed with random primers using SuperScriptII first strand synthesis system (Invitrogen). PCR was carried out using the following parameters: denaturation at 94°C for 45 seconds, annealing at the suitable annealing temperature (See Table 1) for 1 min, and polymerization at 72°C for 2 min. The sequence, annealing temperature, and cycle number of each pair of primers are listed in supplemental Table 1. An extension step of 7 min at 72°C was added at the end of the cycles. Each PCR was performed under linear conditions. Reactions without reverse transcriptase were performed to control for contaminations with genomic DNA, using β-actin primers. PCR products were analysed on a 1,5% agarose gel and visualized with ethidium bromide.
Teratoma formation
Colonies of LYON-ES cells were selected manually and inoculated beneath the testicular capsule of 7-week-old severe combined immunodeficient (SCID) males (CB17/SCID; Charles River Laboratories, http://www.criver.com). 5 to 10 weeks later, mice were euthanized and lesions surgically removed. Teratomas were fixed in 4% PFA overnight at 4°C, sunk in 10% sucrose for 24 hours, in 20% sucrose for 24 hours, and embedded in OCT embedding medium (CellPath; http://www.cellpath.co.uk). Cryosections (20μm) were washed three times for 10 min in TBS, and processed for immunofluorescent staining (cf above). Primary antibodies are detailed in Supplementary Table 2. Oil red O staining was used to label adipose-like cells, and alizarine red staining to mark cartilage.
RESULTS
Establishment and characterization of the LYON-ES1 cell line
Thirty-four rhesus monkey blastocysts produced by ICSI were used. Seven embryos exhibited a small or undistinguishable ICM, thus immunosurgery was not performed to reduce the risk of cell loss. All of them formed outgrowths. Twenty-seven ICMs were isolated by immunosurgery, of which twenty-two formed outgrowths and generated 5 lines that were expanded for more than 12 passages and frozen. One cell line, named LYON-ES1, established without performing immunosurgery, could be easily maintained and showed rapid expansion in culture. Within the first days of derivation, cells showing an ESC like morphology emerged (Fig. 1A). These putative ESC were manually selected and subcultured on a fresh feeder layer (Passage 1 at day 6). After one day in vitro, colonies of small, tigthly packed cells proliferated from the transferred clumps (Fig. 1B). These colonies were split after 2 days, and passaged every 4 to 7 days. LYON-ES1 cell line was initially derived in a medium containing 10% FBS and 10% serum replacement (KO-SR), and supplemented with bFGF, and rhLIF. Under those conditions, ES cells showed low amplification rates associated with a sustained level of spontaneous differentiation, despite the manual removal of differentiated cells. After 4 –5 passages the cells were adapted to 20% KOSR and FGF2 (4ng/ml) containing medium. Those conditions resulted in a decrease in the incidence of spontaneous differentiation and an increase in proliferation. Under those conditions, cell morphology was homogeneous, with colonies mainly composed of ES-like cells (Fig. 1C). The morphology of LYON-ES1 cells is identical to that reported for other human and non- human primate ECS lines: they formed packed and tight colonies (Fig. 1C), show a high nucleus/cytoplasmic ratio and clearly distinguishable nucleoli (Fig. 1D). Amplification and routine passaging of the cells were performed using a manual dissociation technique so as to avoid the emergence of chromosomal abnormalities induced by enzymatic treatment 29.
Figure 1. Derivation of the LYON-ES1 cell line.
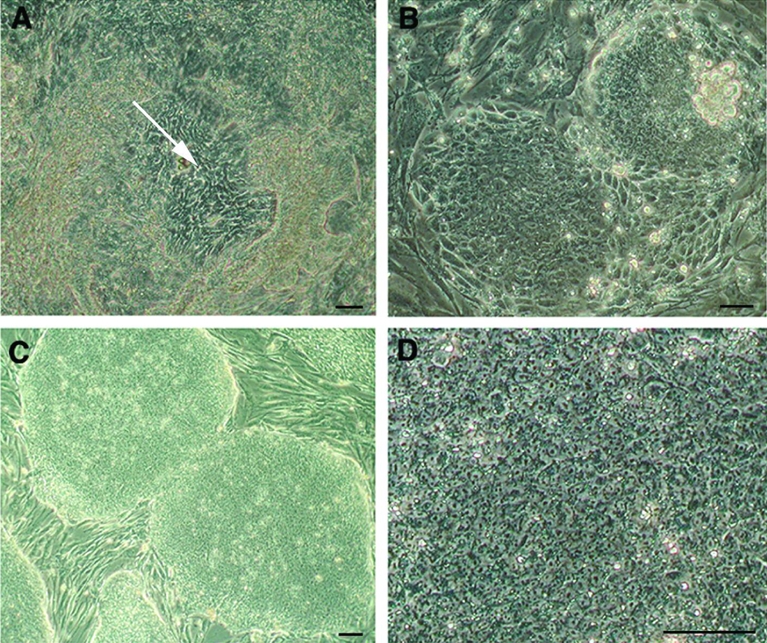
(A) Outgrowth of inner cell mass (white arrow) 10 days after initial plating of the embryo. (B) Resulting colonies 1 day after the first dissociation. Note the high nucleus/cytoplasm ratio and prominent nucleoli. Established LYON-ES1 cell line at passage 7 (C) and passage 28 (D). Scale bars=100μm (A–D).
LYON-ES1 cell line has been cultured for more than 60 passages, while maintaining an undifferentiated state.
Immunohistochemistry and RT-PCR were used to analyse the expression of pluripotency markers. LYON-ES1 cells express the transcription factors Oct-4, Nanog, the cell surface markers SSEA4, TRA-1-60, TRA-1-81 and CD90 (Fig. 2A) and show strong alkaline phosphatase activity (Fig. 2A and data not shown). Normal diploid 42XX karyotype and high telomerase activity are maintained after prolonged culture (supplementary Fig. 1A, B). After passaging every 5 days, LYON-ES1 cells show sustained proliferation rates in long term cultures. Bromodeoxyuridine (BrdU) cumulative labeling indicates a total cell-cycle duration of 9 hrs when cultured in 20%KOSR and FGF2 (4ng/ml). Cell cycle duration increased dramatically (15,5 hrs) when cells were cultured in the medium routinely used for culturing monkey ES cells, ie containing 20%FBS (supplementary Fig. 1C) 2, 26, 30, suggesting that FBS containing medium is not optimal for monkey ES cell expansion. The distribution of self-renewing LYON-ES cells in cell-cycle phases, as analysed by flow cytometry, indicates that the fractions of cells in G1, S and G2/M phase are 23%, 58% and 19% respectively (Fig. 2B). Differentiation of LYON-ES1 cells is accompanied by a dramatic increase in the fraction of cells in G1 (Fig. 2B). LYON-ES1 cells have cell cycle characteristics that are typical of primate ES cells 26, 31.
Figure 2. Characterization of the LYON-ES1 cell line.
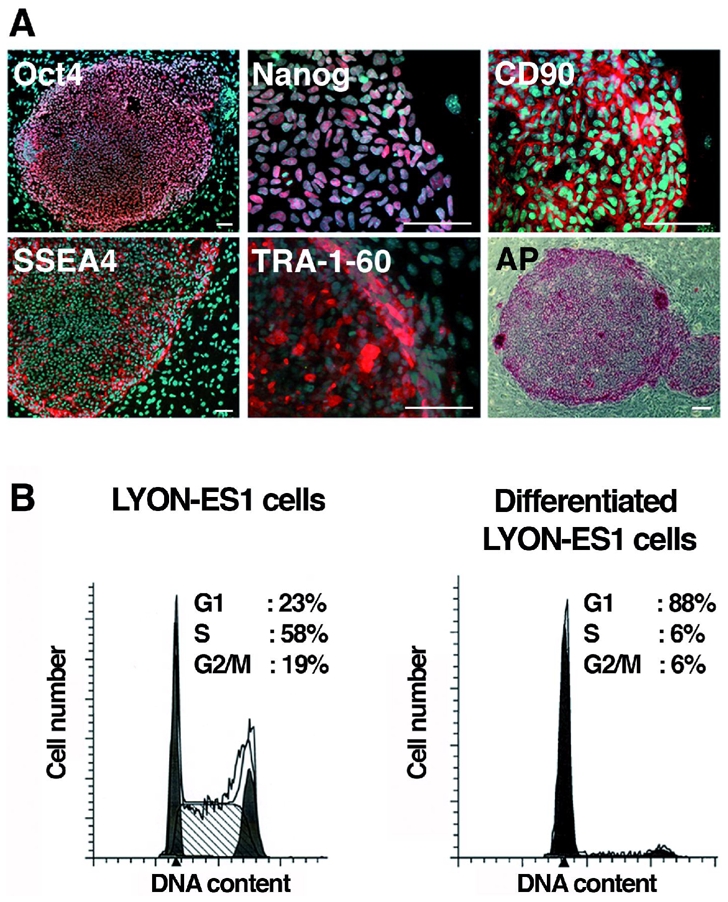
(A) Immunofluorescent staining for Oct4, Nanog, CD90, SSEA-4, TRA-1-60, and staining for alkaline Phosphatase (AP). (B) Histograms showing cell-cycle distribution of LYON-ES1 cells and LYON-ES1 differentiated derivatives as measured by flow cytometry. Scale bars=100μm (A).
LYON-ES1 cells are pluripotent in vitro and in vivo
The capacity of LYON-ES1 cells to form differentiated cell types has been assessed in vitro either by culture in subconfluent conditions, or by formation of embryoid bodies (EBs) (Fig. 3A). The expression of specific markers of primate ESC, ectoderm, endoderm and mesoderm was analysed using semiquantitative RT-PCR (Fig. 3B). Expression of oct4, Nanog and Rex1 gradually decreases during differentiation. All tissue-specific markers are expressed in EBs, and showed increased expression levels with time (Fig. 3B). After more than 40 passages, LYON-ES1 cells retained the potential to differentiate into derivatives of the three germ layers in vitro (data not shown).
Figure 3. Pluripotency of LYON-ES1 cells in vitro and in vivo.
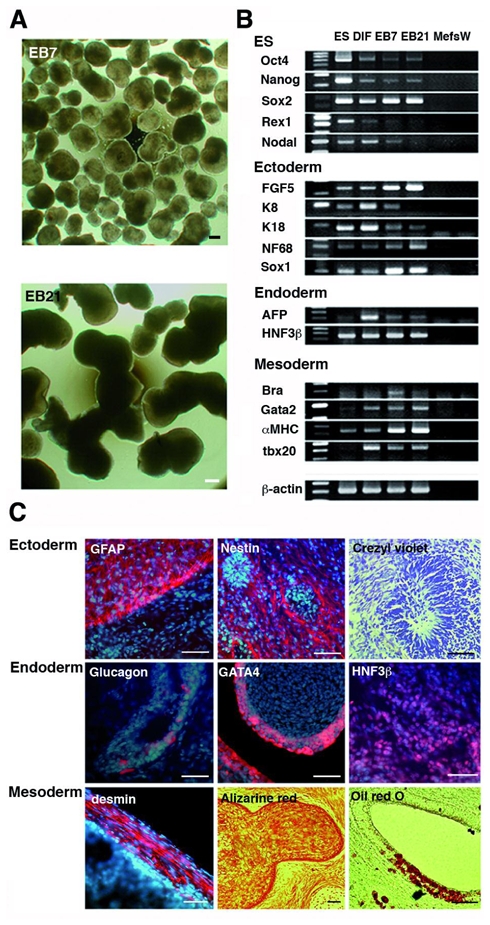
(A) Day-7 and Day-21 embryoid bodies (EB7 and EB21) derived from LYON-ES1cells. (B) RT-PCR analysis of ES cell- and lineage- markers expression in LYON-ES1 cells, spontaneously differentiated cells (DIF), EB7 and EB21. (C) Teratomas sections six weeks after injection of LYON-ES1 cells in the testes of SCID mice, showing derivatives of the ectoderm, endoderm and mesoderm. Scale bars= 500μm (A, C). Abbreviations: Mefs, mouse embryonic fibroblasts; W, water control; Bra, Brachyury; K, keratin.
Pluripotency of the LYON-ES1 cells has been also assessed in vivo through teratoma formation. Injection of LYON-ES1 cells in the testis of SCID mice consistently results in teratomas formation. Immunohistochemistry shows that tumors included derivatives of the ectoderm, that are positive for GFAP and Nestin, and contained rosettes of neuroepithelium (Fig. 3C, upper panels). Derivatives of the endoderm stained for glucagon, GATA4 or HNF3β (Fig. 3C, middle panels) are observed. The teratomas also contained mesodermal tissues such as muscle-like structures stained for desmin, cartilage-like tissue revealed by alizarine red staining, and adipose-like cells, revealed by oil-red staining (Fig. 3C, lower panels). The range of differentiation observed within the teratomas of high passage LYON-ES cells (passage 58) is comparable to that observed with low passage LYON-ES (passage 7) (data not shown). Proliferating cells are often observed as shown by Ki67 expression. The expression of the ES-cell markers Oct4 and TRA-1-60 is not detected (data not shown), as is also shown with human ES cells 32, suggesting that proliferating cells correspond to precursors of immature tissue or even differentiated cells, as has been recently shown in human ES cell derived tumors 33.
These data show that the LYON-ES1 cell line shows all the characteristics of a genuine monkey ES cell line and retain its undifferentiated and pluripotent properties after long term culture.
Derivation of a LYON-ES1 cell line stably expressing tau-GFP over long term cultures and throughout differentiation
Stable expression of GFP fused to the microtubule-associated protein tau (tau-GFP) was obtained via lentiviral infection of LYON-ES1 cells with a SIV-based lentiviral vector expressing tau-GFP under the control of the CAG promoter (see materials and methods). As lentiviral infection enabled to transduce a small percentage of monkey ES cells 26, cell sorting was implemented to obtain a pure population of tau-GFP expressing LYON-ES cells. Tau-GFP enables cell morphology of living LYON-ES cells to be visualized in exquisite detail. The fluorescence is distributed evenly throughout the cytoplasm, while being excluded from the nucleus (Fig. 4A). Tau-GFP fluorescence is stable for more than 45 passages (Fig. 4D), and remains detectable several weeks after fixation (data not shown). Tau-GFP LYON-ES cells showed similar characteristics to the parental wild type LYON-ES1 cells, and to genetically modified LYON-ES cells stably expressing eGFP (Supplementary Fig. 2A, B): they express the pluripotent stem cell markers Oct4, Nanog, Rex1, sox2, and alkaline phosphatase as well as the cell surface markers SSEA4, and TRA-1-60 (Supplementary Fig. 3A and data not shown). The karyotype and the level of telomerase activity of tau-GFP LYON-ES cells are similar to the parental wild type LYON-ES cells (Supplementary Fig. 3B, C).
Figure 4. Stable tau-GFP expression in LYON-ES cells and their in vitro differentiated derivatives.
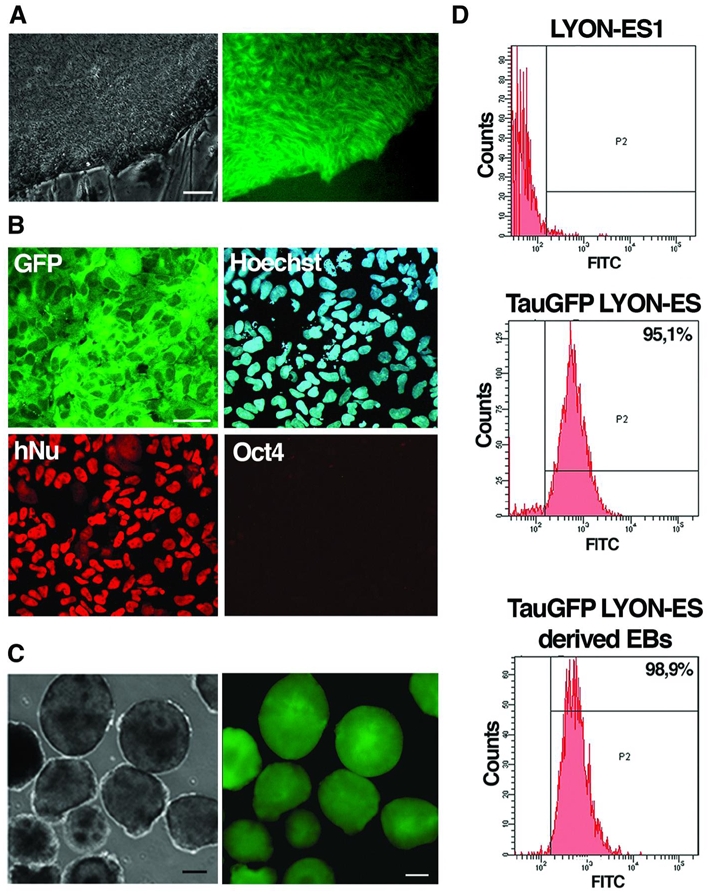
(A) Phase contrast and corresponding live fluorescence images of tau-GFP LYON-ES cells. (B) Immunofluorescent staining for GFP, hNu and Oct4 in spontaneously differentiated tau-GFP LYON-ES cells at passage 45. (C) Phase contrast and corresponding live fluorescence images of tau-GFP day-5 EBs. (D) Proportion of tau-GFP positive cells as measured by flow cytometry in living LYON-ES1 cells, tau-GFP LYON-ES cells (Passage 47) and tau-GFP LYON-ES derived EBs (Passage 45).
Tau-GFP LYON-ES cells are pluripotent and clonogenic
The pluripotency of the transgenic LYON-ES cells was assessed via culture in suspension and in subconfluent conditions. Fluorescent microscopy observations showed that tau-GFP expression is retained during differentiation in vitro (Fig. 4C), even after numerous passages (Fig. 4B, D). RT-PCR and immunohistochemical analysis shows that tau-GFP LYON-ES cells give rise to cells expressing ectoderm (Sox1, nestin, GFAP, βIIItubulin), endoderm (HNF3β, AFP), and mesoderm (αMHC, tbx20, desmin) markers (data not shown).
The injection of tau-GFP LYON-ES cells in the testis of SCID mice results in teratoma which contain solid tissues and fluid-filled cystic masses, comprising derivatives of the three germ layers (Fig. 5A, C, D and data not shown), as in the case of the wildtype LYON-ES cell line. Strong tau-GFP fluorescence is readily visible 10 weeks after the injection, before any immunohistochemical amplification (Fig. 5A). Immunostaining with an anti human nuclear antigen (hNA) antibody, which specifically labels cells of primate origin, shows that all the cells of monkey origin expressed tau-GFP (Fig. 5B). These data indicate that tau-GFP expression is maintained during differentiation in vivo.
Figure 5. Analysis of teratomas sections ten weeks after injection of tau-GFP LYON-ES cells in the testes of SCID mice.
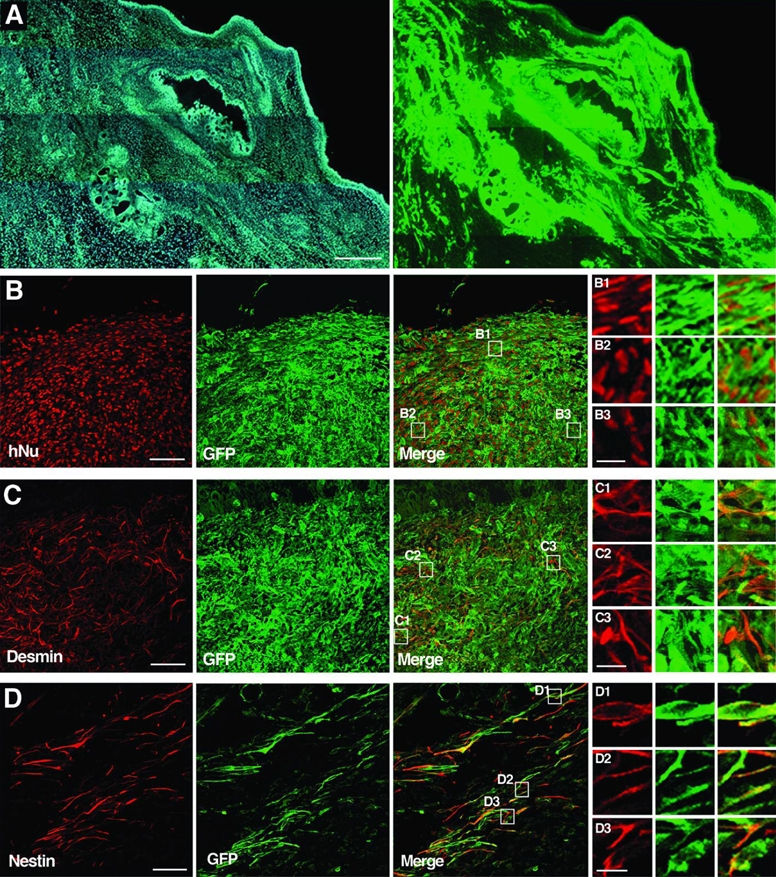
(A) Low magnification of tau-GFP labeled teratoma stained with Hoechst (left) and GFP (right). Confocal images showing co-expression of tau-GFP and hNU (B), coexpression of tau-GFP and desmin in derivatives of the mesoderm (C), co-expression of tau-GFP and nestin in derivatives of the ectoderm (D). B1–3, C1–3, D1–3 are high magnifications of the fields shown in the merge images B, C and D respectively. Scale bars = 500μm (A); 50μm (B–D); 10μm (B1–3; C1–3; D1–3).
So as to get a homogeneous ES cell line, we isolated clones of tau-GFP expressing cells. Tau-GFP expressing cells were manually selected and cultured individually in 96-wells. 5 clones were isolated from the tau-GFP LYON-ES parental cell line. The average cloning efficiency was 0,65%, similar to that obtained with the eGFP expressing LYON-ES cells (data not shown), and with human ES cells 34, 35. All clones express tau-GFP in living cells, as is the case for eGFP expressing clones (supplementary Fig. 4A and unpublished results). The fluorescence was retained after extended period of culture (more than 12 passages). Whereas differences in tau-GFP expression levels are noted between different clones, transgene expression within individual clones is uniform. When tau-GFP expressing clones were induced to differentiate in non-adhesive or in subconfluent culture conditions, all the resulting cells expressed tau-GFP (supplementary Fig. 4B, C).
Tau-GFP LYON-ES clones maintain the characteristics of the parental (tau-GFP) LYON-ES cell line. The cell morphology is typical of LYON-ES cells and they exhibit a high level of alkaline phosphatase activity, as well as continue to express the markers of pluripotency Oct4, Nanog, and Sox2, and the cell surface markers (SSEA4, TRA-1-60, TRA-1-81 and CD90) (supplementary Fig. 4A, D).
Pluripotency of the tauGFP-clones was assessed in vivo via teratoma formation. We injected three tau-GFP expressing clones, and three GFP-expressing clones as controls, in the testis of SCID mice. Teratomas with pronounced differentiation into multiple somatic tissues were found in the injected testes analysed 10 weeks after grafting. They retain tau-GFP expression, as revealed by fluorescent microscopy (data not shown). Similar results were obtained with the eGFP LYON-ES clones (unpublished results). These data indicate that tau-GFP LYON-ES cells are pluripotent in vitro and in vivo, and are clonogenic, and that transgene expression does not alter the properties of LYON-ES cells.
Tau-GFP expression is retained throughout neural differentiation in vitro, and in vivo after transplantation in the rat brain
One of the major advantage of the tau–GFP label is that it makes it possible to follow the fate of neural cell precursors and their derivatives in exquisite details in vitro and in vivo 25. Tau-GFP expression also provides a detailed labeling of dendritic and axonal morphologies, revealing important details concerning the connections formed by neurons derived from tau-GFP expressing ESC after grafting in the brain.
To determine whether tau-GFP expression is retained throughout neural differentiation in vitro, we cultured tau-GFP LYON-ES cells in subconfluent conditions. This results in extensive spontaneous differentiation notably into neuroepithelial rosettes labeled with tau-GFP (Fig. 6A). These rosettes were selected manually, and re-plated in basal medium (Euromed-N plus N2 supplement) in the presence of FGF2 plus EGF. The resulting neural precursors were further propagated for analysis. They express tau-GFP, as revealed by fluorescence microscopy in living cells (Fig. 6B), and express the neural precursor markers sox2, nestin, emx2, sox1, FGF5, NF68, and the radial glia markers BLBP and Glast (data not shown). After induction of neuronal differentiation (see materials and methods), cells with tau-GFP expressing processes appear in culture, and express the neuronal markers βIIItubulin, microtubule-associated protein-2 (MAP2), and neurofilament 165 (NF165) (Fig. 6C, and data not shown). Following exposure to serum, neural precursors retained the tau-GFP fluorescence and differentiated into GFAP-positive astrocytes and into Galactocerebroside (GalC)-positive oligodendrocytes (Fig. 6D). These results show that tau-GFP LYON-ES neural precursors have the capacity to differentiate in vitro into all three fundamental neural lineages (neurons, astrocytes and oligodendrocytes), while retaining tau-GFP expression.
Figure 6. Tau-GFP LYON-ES derived neural precursors retain tau-GFP expression and are multipotent.
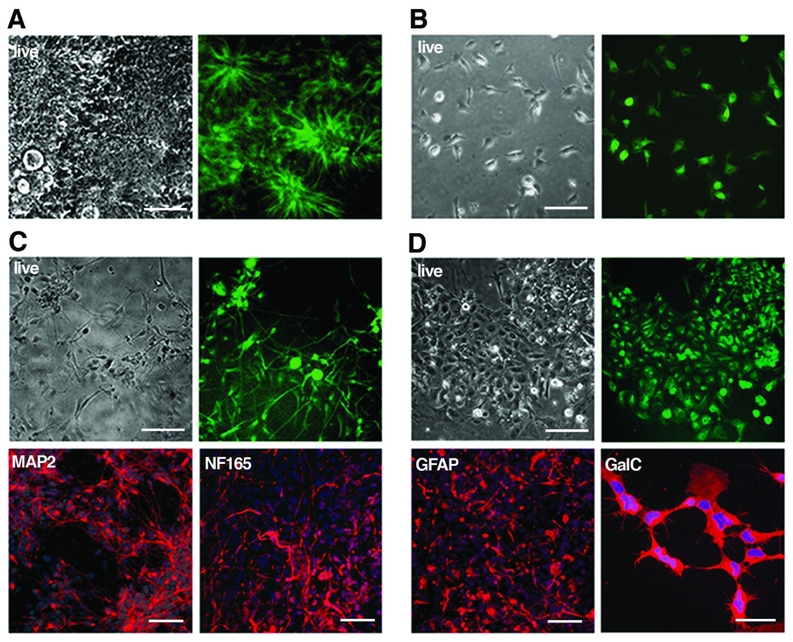
Phase contrast and corresponding live fluorescence images of (A) rosettes and (B) neural precursors at passage 6, derived from tau-GFP LYON-ES cells. (C) Differentiation of tau-GFP neural precursors into MAP2 and NF165 positive neurons. (D) Serum induced differentiation of tau-GFP neural precursors into GFAP or GalC expressing cells. Scale bars= 100μm (A, B); 50μm (C–D).
Finally, we investigated the behaviour of tau-GFP LYON-ES derived precursors in vivo, upon transplantation into the adult rat brain. Tau-GFP LYON-ES cells were induced to differentiate into neural precursors, that were subsequently injected in the dorsal cerebral cortex of adult rats (see supplementary materials and methods). Eighteen or twenty-eight days after transplantation, the brains were fixed and sections were analysed histologically. We observed excellent graft acceptance and sustained survival with cyclosporine A immunosupression. Tau-GFP was readily detected by GFP immunostaining in animals sacrificed 18 days and 28 days after transplantation (Fig. 7A–G). Grafts consist of large numbers of tau-GFP positive cells at the injection site, with some cells migrating laterally, away from the core of the injection site (Fig. 7A–C). Some neurons grow extended axonal projections over several millimetres, crossing the interhemispheric border as seen on Figure 7C. Immunostaining with the anti-hNA antibody showed that all the monkey cells maintained tau-GFP expression after integration in the brain (Fig 7E). Only few tau-GFP cells expressed the proliferative marker Ki67 (Fig. 7F), indicating that most neural precursors withdraw from the cell cycle in vivo. Double immunostaining for GFP and GFAP or βIII tubulin show that tau-GFP neural precursors differentiated along the neuronal and the astroglial pathways after transplantedin the rat brain. (Fig. 7G, H).
Figure 7. Integration of tau-GFP LYON-ES-derived neural precursors 28 days after transplantation in the adult rat brain.
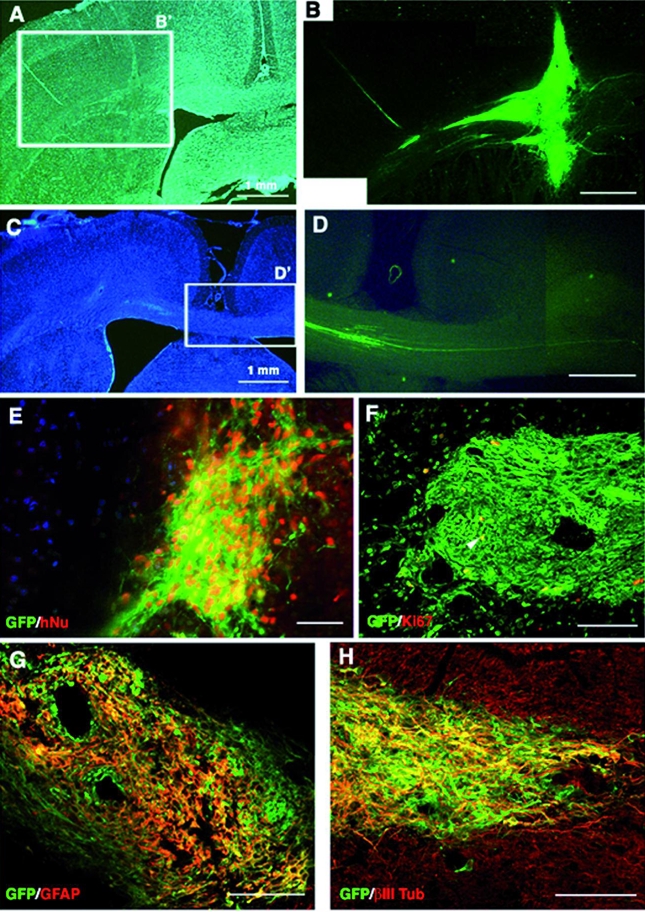
(A, C) Low power microphotographs of hoechst stained brain sections (B) High magnification of field B′ showing the site of the grafted cells. (D) Higher magnification of the field D′, showing tau-GFP expressing axons crossing the interhemispheric border (C); (E) Colocalization of tau-GFP and human nuclear antigen (hNu). (F) Few tau-GFP cells express the proliferative marker Ki67 (yellow, arrow). Coexpression (yellow) of tau-GFP and neuronal marker MAP2 (G), and astroglial marker GFAP (H). Scale bars= 500μm (A–D); 50 μm (E–H).
These data indicate that neural precursors derived from tau-GFP LYON-ES cells can survive, differentiate, and retain tau-GFP expression in the adult brain environment several weeks after transplantation.
Discussion
In this report, we describe the derivation of a new Rhesus ES cell line stably expressing tau-GFP. First, we generated a new line of ESC in the rhesus monkey (LYON-ES1) and demonstrated that they express markers and have cell cycle characteristics typical of primate ESC 2–4, 26. LYON-ES1 cells are pluripotent, giving rise to derivatives of the three germ layers after culture in subconfluent conditions or in suspension in vitro and in vivo through teratoma formation. LYON-ES1 cells have been maintained in culture for more than 60 passages retaining all their undifferentiated characteristics and a normal karyotype, and show high telomerase activity, consistent with their extended lifespan property. Taken together, these observations indicate that the LYON-ES1 cell line meets all the standard criteria for a pluripotent monkey ES cell line.
Using lentiviral vectors, we then generated LYON-ES cells that ubiquitously and stably express tau-GFP. Monkey ES cells have been labelled with GFP using lipofection or electroporation 36, 37. Transduction of monkey ES cells with a lentiviral vector encoding GFP has only been reported in cynomolgus monkey 38. However, GFP expression was not ubiquituous, and the undifferentiated properties of the infected cells were not described. Here, we show that despite lentiviral infection and cell sorting, tau-GFP expressing cells retained the undifferentiated characteristics of the parental wild type LYON-ES cell line and the ability to differentiate into the derivatives of the three germ layers in vitro and in vivo, while retaining transgene expression. Thus, genetic modification of LYON-ES cells is compatible with maintenance of their undifferentiated and pluripotent properties.
We demonstrated that tau-GFP labeling can be readily detected in vitro in living LYON-ES cells and their differentiated derivatives using fluorescent imaging, and is stable after long term culture. In vivo, the fate of the labeled cells can be mapped during their differentiation in teratomas, and during their integration in the adult rat brain, and most importantly remained identifiable several weeks after injection without using immunohistochemical detection. Tau is a microtubule-binding protein principally expressed in neurons 39, and it is conceivable that ectopic expression of tau-GFP might compromise cell function by interfering with microtubule assembly and could potentially predispose grafted animals to neural pathologies. Indeed, disruption of normal tau function is associated with neurodegenerative disorders, like Alzheimer’s disease 40. However, ESC and transgenic lines carrying the tau-GFP transgene have been produced in the mouse, with no report of altered behavior 24, 25. Consistent with these observations in the mouse, our data show that LYON-ES cells stably expressing tau-GFP have the ability to differentiate in vitro into multipotent neural precursors, survive and colonize the brain after transplantation in vivo. This suggests that tau-GFP labeling did not have any obvious consequences on the potentialities of the labeled cells. As the gene is introduced as a random integration event, it is possible that this insertion event modifies an unknown genomic locus, and could induce an alteration in cell behaviour or characteristics. In our study, we have shown that tau-GFP LYON-ES cells proliferated like their wild type counterparts, and retained a normal karyotype after transduction. More importantly, all tau-GFP generated clones stably expressed tau-GFP and retained tau-GFP expression after in vitro and in vivo differentiation. These clones presented similar characteristics to those of the parental tau-GFP ES cells, suggesting that the site of integration of the transgene did not produce a silencing of tau-GFP expression, and did not have any deleterious effects on the phenotype or the potentialities of the cells. The efficiency of cloning was comparable to the results obtained with eGFP expressing LYON-ES cells, and to previous results with non transduced human ES cells 34. This suggests that transduction of tau-GFP transgene does not alter the potential of monkey ES cells to be cloned. It cannot be ruled out that more subtle cell-type-specific phenotypes could be identified if more detailed analysis were to be undertaken. Thus, experiments using tau-GFP LYON-ES clones should be designed for studying possible effects of the transgene after the induction of differentiation in vitro and in vivo.
Conclusion
The tau-GFP LYON-ES cell line is the first tau-GFP expressing ES cell line derived in primates, including human. While further experiments using tau-GFP LYON-ES cells should be designed for establishing their functional integration in vivo, we anticipate that the tau-GFP LYON-ES cells will prove a powerful tool for a wide variety of applications including developmental studies aimed at tracing the fate and confirming the pluripotency of monkey ESC in chimerae, as well as the development of neural transplantation technologies in monkey.
Supplementary Material
Acknowledgments
We are indebted to Shoukrat Mitalipov, Yves Menezo, Michel Berland, Anne-Catherine Fluckiger for their invaluable help and advice with hormonal stimulations, sperm harvesting and ESC culture. We thank Marielle Afanassieff and Pierre-Yves Bourillot for the production of the eGFP and TauGFP expressing lentiviruses, Camille Lamy for help with the transplantation experiments and PY Bourillot for helpful discussions. This work was supported by INSERM/AFM Cellules Souches Thérapeutiques grant n°4CS016F and by Région Rhône-Alpes (Emergence, n°0101681601 « Thématique prioritaire cellules souches », n°0301455301).
Footnotes
Supporting Information. Detailed materials and methods are presented as Supporting Materials and Methods, which is published as supporting information on the Stem Cells web site.
References
- 1.Svendsen CN, Smith AG. New prospects for human stem-cell therapy in the nervous system. Trends Neurosci. 1999;22:357–364. doi: 10.1016/s0166-2236(99)01428-9. [DOI] [PubMed] [Google Scholar]
- 2.Thomson JA, Kalishman J, Golos TG, et al. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci U S A. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson JA, Kalishman J, Golos TG, et al. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol Reprod. 1996;55:254–259. doi: 10.1095/biolreprod55.2.254. [DOI] [PubMed] [Google Scholar]
- 4.Suemori H, Tada T, Torii R, et al. Establishment of embryonic stem cell lines from cynomolgus monkey blastocysts produced by IVF or ICSI. Dev Dyn. 2001;222:273–279. doi: 10.1002/dvdy.1191. [DOI] [PubMed] [Google Scholar]
- 5.Vrana KE, Hipp JD, Goss AM, et al. Nonhuman primate parthenogenetic stem cells. Proc Natl Acad Sci U S A. 2003;100 (Suppl 1):11911–11916. doi: 10.1073/pnas.2034195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pau KY, Wolf DP. Derivation and characterization of monkey embryonic stem cells. Reprod Biol Endocrinol. 2004;2:41. doi: 10.1186/1477-7827-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitalipov SM, Wolf DP. Nuclear transfer in nonhuman primates. Methods Mol Biol. 2006;348:151–168. doi: 10.1007/978-1-59745-154-3_10. [DOI] [PubMed] [Google Scholar]
- 8.Navara CS, Mich-Basso JD, Redinger CJ, et al. Pedigreed Primate Embryonic Stem Cells, Express Homogeneous Familial Gene Profiles. Stem Cells. 2007 doi: 10.1634/stemcells.2007-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinoda G, Umeda K, Heike T, et al. alpha4-Integrin(+) endothelium derived from primate embryonic stem cells generates primitive and definitive hematopoietic cells. Blood. 2007;109:2406–2415. doi: 10.1182/blood-2006-06-031039. [DOI] [PubMed] [Google Scholar]
- 10.Saito K, Yoshikawa M, Ouji Y, et al. Promoted differentiation of cynomolgus monkey ES cells into hepatocyte-like cells by co-culture with mouse fetal liver-derived cells. World J Gastroenterol. 2006;12:6818–6827. doi: 10.3748/wjg.v12.i42.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lester LB, Kuo HC, Andrews L, et al. Directed differentiation of rhesus monkey ES cells into pancreatic cell phenotypes. Reprod Biol Endocrinol. 2004;2:42. doi: 10.1186/1477-7827-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosseinkhani M, Hosseinkhani H, Khademhosseini A, et al. Bone morphogenetic protein-4 enhances cardiomyocyte differentiation of cynomolgus monkey ESCs in knockout serum replacement medium. Stem Cells. 2007;25:571–580. doi: 10.1634/stemcells.2006-0225. [DOI] [PubMed] [Google Scholar]
- 13.Thomson JA, Marshall VS, Trojanowski JQ. Neural differentiation of rhesus embryonic stem cells. Apmis. 1998;106:149–156. doi: 10.1111/j.1699-0463.1998.tb01330.x. discussion 156–147. [DOI] [PubMed] [Google Scholar]
- 14.Calhoun JD, Lambert NA, Mitalipova MM, et al. Differentiation of rhesus embryonic stem cells to neural progenitors and neurons. Biochem Biophys Res Commun. 2003;306:191–197. doi: 10.1016/s0006-291x(03)00937-9. [DOI] [PubMed] [Google Scholar]
- 15.Chen SS, Revoltella RP, Papini S, et al. Multilineage differentiation of rhesus monkey embryonic stem cells in three-dimensional culture systems. Stem Cells. 2003;21:281–295. doi: 10.1634/stemcells.21-3-281. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki H, Suemori H, Mizuseki K, et al. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc Natl Acad Sci U S A. 2002;29:29. doi: 10.1073/pnas.032662199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo HC, Pau KY, Yeoman RR, et al. Differentiation of monkey embryonic stem cells into neural lineages. Biol Reprod. 2003;68:1727–1735. doi: 10.1095/biolreprod.102.012195. [DOI] [PubMed] [Google Scholar]
- 18.Tibbitts D, Rao RR, Shin S, et al. Uniform adherent neural progenitor populations from rhesus embryonic stem cells. Stem Cells Dev. 2006;15:200–208. doi: 10.1089/scd.2006.15.200. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda R, Kurokawa MS, Chiba S, et al. Transplantation of neural cells derived from retinoic acid-treated cynomolgus monkey embryonic stem cells successfully improved motor function of hemiplegic mice with experimental brain injury. Neurobiol Dis. 2005;20:38–48. doi: 10.1016/j.nbd.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Takagi Y, Takahashi J, Saiki H, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115:102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Pernaute R, Studer L, Ferrari D, et al. Long-term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (cyno-1) after transplantation. Stem Cells. 2005;23:914–922. doi: 10.1634/stemcells.2004-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrari D, Sanchez-Pernaute R, Lee H, et al. Transplanted dopamine neurons derived from primate ES cells preferentially innervate DARPP-32 striatal progenitors within the graft. Eur J Neurosci. 2006;24:1885–1896. doi: 10.1111/j.1460-9568.2006.05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi J, Takagi Y, Fukuda H, et al. Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain. J Cereb Blood Flow Metab. 2006;26:906–914. doi: 10.1038/sj.jcbfm.9600247. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez I, Feinstein P, Mombaerts P. Variable patterns of axonal projections of sensory neurons in the mouse vomeronasal system. Cell. 1999;97:199–208. doi: 10.1016/s0092-8674(00)80730-8. [DOI] [PubMed] [Google Scholar]
- 25.Pratt T, Sharp L, Nichols J, et al. Embryonic stem cells and transgenic mice ubiquitously expressing a tau- tagged green fluorescent protein. Dev Biol. 2000;228:19–28. doi: 10.1006/dbio.2000.9935. [DOI] [PubMed] [Google Scholar]
- 26.Fluckiger AC, Marcy G, Marchand M, et al. Cell cycle features of primate embryonic stem cells. Stem Cells. 2006;24:547–556. doi: 10.1634/stemcells.2005-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forsyth NR, Musio A, Vezzoni P, et al. Physiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalities. Cloning Stem Cells. 2006;8:16–23. doi: 10.1089/clo.2006.8.16. [DOI] [PubMed] [Google Scholar]
- 28.Hewitt Z, Forsyth NR, Waterfall M, et al. Fluorescence-activated single cell sorting of human embryonic stem cells. Cloning Stem Cells. 2006;8:225–234. doi: 10.1089/clo.2006.8.225. [DOI] [PubMed] [Google Scholar]
- 29.Mitalipova M, Palmarini G. Isolation and characterization of human embryonic stem cells. Methods Mol Biol. 2006;331:55–76. doi: 10.1385/1-59745-046-4:55. [DOI] [PubMed] [Google Scholar]
- 30.Mitalipov S, Kuo HC, Byrne J, et al. Isolation and characterization of novel rhesus monkey embryonic stem cell lines. Stem Cells. 2006;24:2177–2186. doi: 10.1634/stemcells.2006-0125. [DOI] [PubMed] [Google Scholar]
- 31.Becker KA, Ghule PN, Therrien JA, et al. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J Cell Physiol. 2006;209:883–893. doi: 10.1002/jcp.20776. [DOI] [PubMed] [Google Scholar]
- 32.Gertow K, Wolbank S, Rozell B, et al. Organized development from human embryonic stem cells after injection into immunodeficient mice. Stem Cells Dev. 2004;13:421–435. doi: 10.1089/scd.2004.13.421. [DOI] [PubMed] [Google Scholar]
- 33.Blum B, Benvenisty N. Clonal analysis of human embryonic stem cell differentiation into teratomas. Stem Cells. 2007;25:1924–1930. doi: 10.1634/stemcells.2007-0073. [DOI] [PubMed] [Google Scholar]
- 34.Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 35.Sidhu KS, Tuch BE. Derivation of three clones from human embryonic stem cell lines by FACS sorting and their characterization. Stem Cells Dev. 2006;15:61–69. doi: 10.1089/scd.2006.15.61. [DOI] [PubMed] [Google Scholar]
- 36.Takada T, Suzuki Y, Kondo Y, et al. Monkey embryonic stem cell lines expressing green fluorescent protein. Cell Transplant. 2002;11:631–635. doi: 10.3727/000000002783985350. [DOI] [PubMed] [Google Scholar]
- 37.Ueda S, Yoshikawa M, Ouji Y, et al. Cynomolgus monkey embryonic stem cell lines express green fluorescent protein. J Biosci Bioeng. 2006;102:14–20. doi: 10.1263/jbb.102.14. [DOI] [PubMed] [Google Scholar]
- 38.Asano T, Hanazono Y, Ueda Y, et al. Highly efficient gene transfer into primate embryonic stem cells with a simian lentivirus vector. Mol Ther. 2002;6:162–168. doi: 10.1006/mthe.2002.0655. [DOI] [PubMed] [Google Scholar]
- 39.Binder LI, Frankfurter A, Rebhun LI. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebneth A, Godemann R, Stamer K, et al. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. J Cell Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


