Abstract
Few studies have assessed whether the patterns of neuropsychological impairment in patients with different frontotemporal lobar degeneration (FTLD) subtypes remain distinct over the duration of their illness or devolve into a common, undifferentiated neuropsychological state. A longitudinal neuropsychological analysis was obtained over 100 months assessing executive control, language/naming, and visuoconstruction in 441 patients diagnosed with Alzheimer’s disease (AD) and four FTLD subtypes, i.e., a social comportment/dysexecutive (SOC/EXEC) disorder; progressive non-fluent aphasia (PNFA); semantic dementia (SemD); and corticobasal degeneration (CBD). Initial group differences on each measure were maintained over the duration of illness, including several double dissociations. For example, AD patients exhibited a decline in ‘animal’ fluency; PNFA patients had difficulty on tests of executive control, SemD maintained their impairment on tests of naming, and CBD had presented with performance on visuoconstructional tests. None of the group by neuropsychological task interactions evaluating longitudinal decline was significant, suggesting that performance does not converge onto a common subtype over time. These data indicate that distinct patterns of neuropsychological impairment are maintained longitudinally, reflecting the unique anatomic distribution of relative disease burden in AD and FTLD.
Keywords: frontotemporal dementia, Alzheimer’s disease, longitudinal assessment
Frontotemporal lobar degeneration (FTLD) is a progressive neurodegenerative illness presenting with imaging and autopsy evidence of frontal and temporal alterations (McKhann et al., 2001; Snowden, Neary, & Mann, 1996). Major FTLD subtypes that have been identified include a social/dysexecutive (SOC/EXEC) syndrome, progressive nonfluent aphasia (PNFA), semantic dementia (SemD), and corticobasal degeneration (CBD). In this study, we examined the longitudinal course of the neuropsychological profiles of these patient groups to determine whether their initial neuropsychological deficits are maintained over time, or whether these distinctions are lost as the disease progresses such that they merge into a single, end-stage clinical dementia profile. This issue has clinical as well as theoretical implications.
Past neuropsychological studies have tended to focus on the initial, cross-sectional differences that may distinguish FTLD from Alzheimer’s disease (AD; Kramer et al., 2003; Libon, Massimo, et al., 2007; Libon, Xie, et al., 2007). For example, Libon, Massimo, et al. (2007) studied a large sample of AD and FTLD patients and subjected their performance on a comprehensive neuropsychological protocol to a principal component analysis. Distinct neuropsychological deficits typified each group. Between- and within-groups analyses revealed that patients with AD obtained their lowest scores on tests of episodic memory, whereas SemD patients were particularly disadvantaged on tests of semantic memory. On tests of processing speed and mental flexibility, time to completion was faster for SOC/EXEC patients, but these patients made many more errors on these tests. Patients with CBD and PNFA were impaired on tests of working memory. CBD patients also obtained low scores on visuospatial and visuoconstructional tests.
In a recent report, Grossman et al. (2007) found distinct neuropsychological as well as distinct neuroimaging profiles associated with pathologically defined groups of AD and FTLD patents. For example, tau-positive FTLD syndromes such as CBD presented with greater visuospatial difficulty and an extrapyramidal disorder coupled with MRI atrophy and greater pathological burden of disease involving the frontal and parietal regions. Clinically, these patients can present with symptoms of CBD or PNFA. By contrast, patients with a tau-negative FTLD syndrome such as FTLD with TDP-43/ubiquitin-positive inclusions presented with behavioral–social comportment difficulties along with significant impairment on language and verbally mediated executive measures. Their MRI cortical atrophy and pathological burden were greatest in frontal and temporal regions. These patients often present clinically with SemD or SOC/EXEC subtypes. Finally, AD patients presented with significant impairment on tests of episodic memory, with MRI studies demonstrating widespread cortical involvement including medial temporal lobe structures. Their neuropathology likewise was widely distributed and included the medial temporal lobe.
A problem associated with studies such as those described above is the lack of longitudinal follow-up on these patients. Indeed, little quantitative research has addressed the critical issue of longitudinal alterations in neuropsychological functioning in FTLD. Kertesz and colleagues (Kertesz, 2003; Kertesz, Davidson, & Munoz, 1999) have suggested that the clinical characteristics of FTLD patients tend to converge and devolve into a single subtype over time. These investigators employ the term Pick complex to emphasize that a common subtype reflects a single disease process despite heterogeneous histopathological abnormalities. Kertesz and colleagues have conducted additional longitudinal studies with a variety of FTLD subgroups to support their claim. For example, in a study examining patients who were initially diagnosed with CBD, Kertesz, Martinez-Lage, Davidson, and Munoz (2000) noted that over time the cognitive deficits associated with primary progressive aphasia (PPA) tend to merge with a dysexecutive disorder. Subsequent autopsy studies of these patients confirmed the presence of CBD in some cases, and other cases presented with pathological evidence of Pick’s disease or other pathological alterations. In another study, Marczinski, Davidson, and Kertesz (2004) assessed the course of behavioral–social comportment symptoms over a 3-year period in SOC/EXEC and PPA patients. The SOC/EXEC group presented with greater impairment on the Frontal Behavioral Index (FBI; Kertesz, Davidson, & Fox, 1997) at their initial assessment, suggesting greater behavioral–social comportment dysfunction than the PPA group. Although the slope for FBI scores remained fairly stable over time in the SOC/EXEC group, after 3 years the FBI score of the PPA group approached the same level of impairment as seen in the SOC/EXEC group. A third study (Kertesz, McMonagle, Blair, Davidson, & Munoz, 2005) charted the longitudinal, clinical–pathological course of FTLD patients over a 3-year period. Patients who initially presented with one FTLD–neurobehavioral syndrome often went on to develop features of other FTLD–neurobehavioral syndromes. For example, patients who initially presented with a SOC/EXEC syndrome might then manifest features of PNFA or clinical characteristics consistent with CBD at follow-up. As in previous work, autopsy revealed considerable overlap and heterogeneity regarding the underlying neuropathological substrate associated with these patients. Finally, Blair, Marczinski, Davis-Faroque, and Kertesz (2007) noted the emergence of a common pattern of impaired language on a survey instrument of language functioning in FTLD. In a statistical sense, these studies suggest that the clinical course of FTLD is best described in terms of an interaction between FTLD subtype and longitudinal course, with different rates of decline across cognitive domains in different patient groups, so that ultimately all patients demonstrate the same level of end-stage functioning regardless of their initial clinical subtype or neuropsychological test performance.
A different perspective has been offered by Grossman et al. (in press). These investigators carried out a longitudinal analysis of neuropsychological functioning on a cohort of patients with autopsy-proven disease who were divided into tau-positive, tau-negative, and frontal variant-Alzheimer’s disease subgroups. Significant longitudinal decline was seen in all groups for all neuropsychological measures. Moreover, several neuropsychological measures differentiated between patient subgroups throughout the duration of the illness. For example, a significant double dissociation involving persistent relative difficulty on visuoconstructional tests in the tau-positive group contrasted with relatively impaired performance maintained over time on tests of visual confrontation naming in tau-negative patients. Other neuropsychological measures distinguished each of the FTLD patient subgroups from patients with autopsy-proven AD. In contrast to the perspective suggested by Kertesz and colleagues, the findings of Grossman et al. suggest that pathologically defined neurodegenerative patient groups do not necessarily overlap and devolve into a single subtype. These authors argue that differences between disease states, including relative differences in the neuroanatomic distribution of disease at onset, are maintained throughout the course of these conditions.
Additional evidence that patient groups maintain their distinct neuropsychological characteristics comes from a longitudinal study correlating progressive cortical atrophy on MRI with progressive neuropsychological difficulty on language measures (Avants, Grossman, & Gee, 2005). In this study, investigators found declining performance on measures of visual confrontation naming and semantic category fluency, as well as progressive cortical atrophy in frontal and temporal brain regions over 1 year. These investigators also showed that neuropsychological decline correlated with progressive atrophy. Moreover, the correlations of longitudinal cognitive measures with progressive cortical atrophy overlapped only partially, suggesting that the unique neuroanatomic basis for cognitive decline on distinct tasks is maintained over time and does not necessarily converge onto a single subtype with a common neuroanatomic substrate.
In the current research, we undertook a longitudinal analysis of performance on tests of executive control, language/naming, and visuoconstruction obtained from a large group of AD and FTLD patients. Patients were followed up to 100 months. We focused on the longitudinal course of group neuropsychological performance. We expected that relatively distinct subtype characteristics of these patients seen at their initial neuropsychological assessment would persist over the longitudinal course of their illness, as suggested by Grossman et al. (in press). Therefore, our primary prediction was that statistical analyses should be dominated by main effects for group differences and longitudinal decline. We expected to see few interaction effects that would reflect longitudinal convergence onto a common subtype.
Method
Patients
All patients were recruited and evaluated at the Departments of Neurology of the University of Pennsylvania and Thomas Jefferson University. Subsequently, at least two trained reviewers of a consensus committee confirmed the presence of specific diagnostic criteria and also assigned patients to an FTLD subgroup on the basis of an independent review of the semistructured history, a detailed neurologic exam, and a standardized mental status examination (Philadelphia Brief Assessment of Cognition [PBAC]; Libon, Xie, et al., 2007). Data were drawn from a patient corpus consisting of 441 patients. Fourteen participants were excluded because of a diagnosis other than AD or FTLD (Lewy body dementia = 4, vascular dementia = 2, ALS = 1, mild cognitive impairment = 4) or an atypical presentation of AD (visual variant = 3).
The FTLD subgroups were classified on the basis of published criteria that have been modified to improve reliability (Grossman & Ash, 2004). When there was disagreement between reviewers, the case was discussed by the entire committee to arrive at a consensus diagnosis. On a different occasion, trained technicians administered a detailed neuropsychological protocol comprising different tests. This formal neuropsychological evaluation, described in detail below, was not used in the diagnosis of these patients, and the diagnosing neurologists were blind to patients’ performance on the neuropsychological evaluation. These patients and their legal representatives participated in an informed consent procedure approved by the Institutional Review Board at the University of Pennsylvania.
Among the participants in this study, 212 patients were given a clinical diagnosis of AD based on National Institute of Neurologic and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., 1984). In brief, this included a progressive syndrome involving prominent episodic memory difficulty, associated with circumlocutory speech, a visual constructional impairment, and limited executive control impairment.
Another 229 patients were clinically diagnosed with FTLD according to published criteria (Lund and Manchester Groups, 1994; McKhann et al., 2001). Our sample included 87 patients with a SOC/EXEC profile. These patients presented with significant social and behavioral difficulties and alterations of executive functioning. Our sample also included 38 PNFA patients. These patients had effortful speech that may be associated with dysarthria, speech errors, grammatically simplified speech, or impaired grammatical comprehension, but relatively good single word comprehension. Forty-one patients were diagnosed with SemD. The language disorder of these patients was characterized by fluent and circumlocutory spontaneous speech that was often empty in content with a prominent naming deficit and was associated with difficulty understanding single words and impaired object knowledge. Initial research with patients with SemD suggested that episodic memory is relatively unaffected in this patient group (Hodges, Patternson, Oxbury, & Funnell, 1992; Neary et al., 1998). However, subsequent research has shown that SemD patient can present with episodic memory difficulty (Kramer et al., 2003). Also, episodic memory depends on the modality used to assess impairment in dementia patients (Libon, Xie, et al., 2007). Because of these and other considerations, episodic memory tests were not part of the diagnostic algorithm for the diagnosis of SemD patients. Finally, 63 patients were diagnosed with CBD on the basis of criteria derived from clinical–pathological studies reported in the literature and our own autopsy series (Murray et al., 2007; Grimes, Lang, & Bergeron, 1999). These patients had apraxia, visuospatial impairments, gait difficulty, and a lateralized extrapyramidal disorder (e.g., unilateral limb rigidity, myoclonus, dystonia, alien hand phenomena).
Within the entire sample, 352 patients remained in the study cohort after Year 1, 293 patients beyond Year 2, 241 patients beyond Year 3, 182 patients beyond Year 4, 121 patients beyond Year 5, 58 patients beyond Year 6, and 30 patients beyond Year 7. We were able to evaluate 241 patients at least twice. The average follow-up time between two visits was 5.68 months (SD = 3.94). The average number of visits was 3.51 (SD = 1.96) among those who had follow-up visits. The average length of total time interval observed was 20.54 months (SD = 16.07) among those who had follow-up visits. There was no significant group difference in terms of total time interval observed.
The initial clinical diagnosis of a neurodegenerative disease was consistent with the results of serum studies, clinical structural imaging studies such as MRI or CT, studies of cerebrospinal fluid (when available), and clinical functional neuroimaging studies such as SPECT or PET (these studies were not available to the consensus committee). Exclusion criteria included the presence of other neurologic conditions such as stroke or hydrocephalus, primary psychiatric disorders such as depression or psychosis, or a systemic illness that can interfere with cognitive functioning. Some of these patients were taking a fixed dosage of an acetyl-cholinesterase inhibitor (e.g., donepezil, rivastigmine, galantamine) or memantine. Some of these patients also may have been medicated with a low dosage of a nonsedating antidepressant (e.g., serotonin-specific reuptake inhibitors such as sertraline) or an atypical neuroleptic agent (e.g., quetiapine), as indicated clinically, but none of the patients demonstrated any evidence of sedation suggesting overmedication. Table 1 summarizes the demographic features of these patients.
Table 1.
Demographic Characteristics
| Characteristic | AD (n = 212) | SOC/EXEC (n = 87) | PNFA (n = 38) | SemD (n = 41) | CBS (n = 63) |
|---|---|---|---|---|---|
| Mean (SD) age (years) | 71.99 (8.58) | 62.60 (10.70) | 68.68 (8.08) | 68.02 (8.81) | 65.48 (8.45) |
| Mean (SD) education (years) | 13.68 (3.45) | 15.09 (2.98) | 13.42 (2.67) | 14.96 (2.73) | 14.94 (3.22) |
| Mean (SD) MMSE score | 19.60 (6.88) | 22.73 (6.74) | 22.55 (7.03) | 23.79 (5.05) | 20.17 (6.63) |
| Mean (SD) duration of illness at initial evaluation (months) | 36.82 (25.98) | 39.28 (36.18) | 31.58 (18.46) | 44.28 (26.70) | 39.00 (24.98) |
Note. AD = Alzheimer’s disease; SOC/EXEC = social comportment/dysexecutive; PNFA = primary nonfluent aphasia; SemD = semantic dementia; CBD = corticobasal degeneration; MMSE = Mini-Mental State Examination.
Neuropsychological Protocol
The neuropsychological protocol consisted of six tests that have previously been subjected to a principal component analysis (Libon, Xie, et al., 2007), which suggested that these tests relate to cognitive constructs including executive control (Digit Span, letter fluency; Lamar et al., 2007), language abilities (lexical retrieval, response naming [Boston Naming Test], “animal” fluency; Libon, Massimo, et al., 2007), and visuoconstruction (figure copy test; Freeman et al., 2000), constructs well known to be affected in dementia. The version of the Boston Naming Test and the figure copy test used in this research came from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological protocol (Morris et al., 1989).
Wechsler Adult Intelligence Scale—Revised Digit Span Subtest (Wechsler, 1987)
Forward and backward span was assessed using standardized instructions. The longest forward and backward spans were the two dependent variables derived from this test.
Letter Fluency (Spreen & Strauss, 1990)
Patients were given 60 s to generate words, excluding proper nouns and numbers, beginning with specified letters (FAS). Letter fluency tests provide a measure of executive control. Imaging studies have shown that letter–phoneme fluency tests activate the left dorsolateral prefrontal region in younger (Phelps, Hyder, Blamire, & Shulman, 1997) and older adults (Gourovitch et al., 2000). The dependent variable was the number of responses summed across the three letters.
Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 1983)
Visual confrontation naming was assessed with a 15-item version of the BNT from the CERAD neuropsychological protocol (Morris et al., 1989). The stimuli were equally divided among high-frequency, mid-frequency, and low-frequency items. Patients were given as much time as they needed to respond. The dependent variable was the total number of correct responses.
“Animal” Fluency (Spreen & Strauss, 1990)
The “animal” fluency task was administered by asking patients to produce as many names of animals as possible in 60 s. Recent studies suggest that category fluency tests provide a measure of semantic knowledge and have shown that performance on category fluency tests tends to activate the left temporal lobe (Mummery, Patterson, Hodges, & Wise, 1996). The dependent variable was the total number of responses, excluding perseverations and extracategory intrusion responses.
Geometric Figure Copy (Morris et al., 1989)
To assess visuoconstructional ability, we asked patients to copy four geometric designs graded on their perceptual–spatial complexity. Performance was evaluated on an 11-point scale.
Statistics
Data were analyzed using methods reported by Grossman et al. (2007, in press). Raw scores from neuropsychological data were converted to z scores on the basis of the performance of 25 age-and education-matched healthy seniors for each individual patient group. Analyses of variance indicated that the age and education of these healthy controls did not differ from the patient groups. A between-groups difference was obtained on the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1974). A threshold of z = −2.32 (equivalent to a p value of .01) was used to identify abnormal performance in patient groups relative to controls when first seen.
A mixed-effect model was used to examine the longitudinal patterns of the cognitive variables over time (Laird & Ware, 1982). This statistical procedure accounts for the within-subject correlations in this longitudinal analysis that are due to the repeated measurements of cognitive variables over time in the same patients and accounts for missing data. Specifically, we were particularly interested in examining whether disease diagnosis was related to the longitudinal measures of the neuropsychological variables. Follow-up time was treated as a random effect, and a random intercept was also included in the mixed-effect model. Disease diagnosis and demographic variables such as age and education were treated as fixed effects.
We did not covary for the MMSE for several reasons. First, in a recent study, Jefferson et al. (2002) examined MMSE performance among patients diagnosed with AD, subcortical vascular dementia (VaD), and dementia patients with Parkinson’s disease (PD). Total MMSE scores were equivalent. However, an analysis of the errors revealed substantial differences. As compared with other dementia groups, AD patients were differentially impaired on the MMSE orientation test items, and their lower scores on these MMSE test items were specifically related to episodic memory impairment. Moreover, the orientation questions are disproportionately represented on the MMSE. By contrast, VaD and PD patients were differentially impaired on the MMSE items requiring a motor response, and poor performance on these MMSE test items were related to differential impairment on executive control tests. Similarly, MMSE scores obtained from SemD and CBD patients could also be differentially affected by their language and visuo-spatial problems, respectively, and these cognitive domains are not equally represented on a brief battery such as the MMSE. A second reason for not covarying for the total MMSE score is that there is potential partial overlap between the cognitive skills probed by the MMSE and the neuropsychological tests described above in a multifactorial manner that may or may not be linear. Therefore, the longitudinal changes on the neuropsychological tests described below may not necessarily be adequately controlled by a linear correction such as analysis of covariance. Insofar as neuropsychological test performance reflects both a domain-specific component and a component associated with overall cognitive decline, it could be potentially confounding to covary for the MMSE. Third, a variety of prior studies have reported that performance on the MMSE can be quite variable over time and therefore does not necessarily provide an adequate measure of longitudinal change (Clark, et al., 1999; Galasko, Gould, Abramson, & Salmon, 2000; Gould, Abramson, Galasko, & Salmon, 2001; Han, Cole, Bellavance, McCusker, & Primeau, 2000). Finally, the maximum difference between groups on the MMSE was relatively modest.
The coefficient for the fixed effect estimating monthly change for each neuropsychological measure was used to illustrate longitudinal decline. For between-groups longitudinal analysis using the mixed-effect model, we computed F statistics for the overall effect of covariates, and t statistics are reported for any two-disease group comparisons that are significant. Longitudinal decline may be linear or curvilinear, and we examined both longitudinal effects in the statistical models reported below. Analyses were performed using SAS software with the proc mixed program (v9.1, SAS Institute Inc., Cary, NC). All statistical tests were two-tailed. In these contrasts, statistical significance was set at the p < .05 level unless otherwise indicated.
Results
Demographic Data
One-way analysis of variance (ANOVA) revealed between-groups differences for age, F(4, 436) = 19.01, p < .001, education, F(4, 436) = 15.81, p < .001, and performance on the MMSE, F(4, 410) = 5.93, p < .001, at initial evaluation. At the initial neuropsychological evaluation, there was no between-groups difference for duration of illness. For age, AD patients were older than SOC/EXEC (p < .001) and CBD (p < .001) patients, PNFA patients were older than CBD (p < .005) patients, and SemD patients were older than SOC/EXEC (p < .013) patients. AD patients had fewer years of education compared with SOC/EXEC (p < .005) and CBD (p < .048) patients. The AD group had a lower score on the MMSE compared with SOC/EXEC (p < .004) and SemD (p < .003) patients when first seen. To further assess the possible effects of education or age on neuropsychological functioning, we subjected all six neuropsychological measures to three-way interactions (Duration of Illness × Education or Age × Group). A significant three-way interaction involving education, group, and illness duration was obtained only for the BNT, F(1, 915) = 4.60, p < 001.
Neuropsychological Analyses
All cross-sectional neuropsychological test performance (i.e., performance at initial presentation) was analyzed using one-way ANOVA followed by Tukey post hoc tests (see Table 2). Statistics describing significant main effects for group and illness duration are listed in Table 3. Below, the initial and longitudinal test performances are described separately for each neuropsychological domain. Follow-up analyses for longitudinal course are listed in Table 4.
Table 2.
Neuropsychological Test Performance—Initial Presentation
| AD |
SOC/EXEC |
PNFA |
SemD |
CBD |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test | Raw score | z score | Raw score | z score | Raw score | z score | Raw score | z score | Raw score | z score |
| Digits Forward | 5.59 (1.32) | −0.64 (0.88) | 5.78 (1.46) | −0.52 (0.97) | 4.96 (1.99) | −1.06 (1.32) | 5.30 (1.63) | −0.84 (1.08) | 5.61 (1.53) | −0.63 (1.02) |
| Digits Backward | 3.42 (1.60) | −0.99 (1.22) | 3.76 (1.62) | −0.73 (1.23) | 3.04 (1.22) | −1.28 (0.93) | 4.00 (1.67) | −0.54 (1.27) | 2.76 (1.53) | −1.49 (1.16) |
| Letter fluency | 22.28 (13.14) | −1.43 (1.01) | 21.08 (14.16) | −1.53 (1.09) | 12.95 (10.38) | −2.15 (0.79) | 24.09 (13.41) | −1.29 (1.03) | 19.22 (13.80) | −1.67 (1.06) |
| BNT | 10.51 (3.49) | −2.72 (2.60) | 12.15 (3.49) | −1.49 (2.54) | 11.74 (3.31) | −1.80 (2.46) | 8.85 (3.98) | −3.96 (2.97) | 11.24 (3.57) | −2.17 (2.66) |
| “Animal” fluency | 8.39 (5.06) | −2.40 (0.96) | 10.62 (5.65) | −1.97 (1.07) | 8.93 (5.53) | −2.29 (1.04) | 8.47 (4.89) | −2.38 (.92) | 9.00 (5.01) | −2.28 (0.95) |
| Figure Copy Test | 8.24 (2.90) | −1.95 (2.68) | 9.50 (1.85) | −0.79 (1.70) | 9.03 (1.63) | −1.22 (1.50) | 9.88 (2.11) | −0.44 (1.95) | 5.44 (3.34) | −4.55 (3.09) |
Note. AD = Alzheimer’s disease; SOC/EXEC = social comportment/dysexecutive; PNFA = primary nonfluent aphasia; SemD = semantic dementia; CBD = corticobasal degeneration; BNT = Boston Naming Test.
Table 3.
Longitudinal Assessment: Effects of Group and Duration of Illness
| Test | Main effect group | Main effect illness duration (linear or quadratic) | Group × Illness Duration (linear or quadratic) |
|---|---|---|---|
| Digits Forward | F(4, 286) = 3.40, p < .010 | Quadratic: F(1, 204) = 8.08, p < .005 | ns |
| Digits Backwards | F(4, 264) = 2.37, p < .053 | Quadratic: F(1, 191) = 13.69, p < .001 | ns |
| Letter fluency (FAS) | F(4, 110) = 2.38, p < .056 | Linear: F(1, 105) = 31.80, p < .001 | ns |
| BNT | F(4, 333) = 6.15, p < .001 | Quadratic: F(1, 221) = 21.74, p < .001 | ns |
| “Animal” fluency | F(4, 346) = 2.44, p < .046 | Linear: F(1, 225) = 97.40, p < .001 | ns |
| Figure Copy Test | F(4, 328) = 16.46, p < .001 | Quadratic: F(1, 215) = 4.65, p < .032 | ns |
Note. BNT = Boston Naming Test.
Table 4.
Longitudinal Assessment: Between-Groups Follow-Up Analysis
| Test | |||
| Digits Forward | PNFA < AD, t(286) = 2.67, p <.008 CBD < SemD, t(286) = 2.39, p <.018 |
PNFA < CBD, t(286) = 3.21, p <.001 | PNFA < SOC/EXEC, t(286) = 2.37, p <.020 |
| Digits Backwards | SemD < CBD, t(264) = 2.83, p < .005 PNFA < SemD, t(264) = 2.28, p < .023 |
SemD < AD, t(264) = 1.77, p < .078 | CBD < AD, t(264) = 1.87, p <.063 |
| Letter fluency (FAS) | PNFA < AD, t(110) = 2.58, p < .011 | PNFA < CBD, t(110) = 2.53, p < .013 | PNFA < SemD, t(110) = 2.48, p < .014 |
| BNT | SemD < AD, t(333) = 2.90, p < .004 SemD < SOC/EXEC, t(333) = 3.81, p < .001 |
SemD < CBD, t(333) = 3.71, p < .001 | SemD < PNFA, t(333) = 4.28, p < .001 |
| “Animal” fluency | AD < CBD, t(346) = 2.37, p <.018 | AD < SOC/EXEC, t(346) = 2.47, p <.014 | |
| Figure Copy Test | CBD < AD, t(328) = 6.50, p < .001 CBD < SOC/EXEC, t(328) = 5.76, p < .001 |
CBD < SemD, t(328) = 6.96, p < .001 AD < SemD, t(328) = 2.86, p < .005 |
CBD < PNFA, t(328) = 5.87, p < .001 SOC/EXEC < SemD, t(328) = 1.99, p < .047 |
Note. AD = Alzheimer’s disease; SOC/EXEC = social comportment/dysexecutive; PNFA = primary nonfluent aphasia; SemD = semantic dementia; CBD = corticobasal degeneration; BNT = Boston Naming Test.
Executive Control
Initial neuropsychological test performance
Significant differences on the Digit Backward Test, F(4, 337) = 4.90, p < .001, and the letter fluency test, F(4, 219) = 2.73, p < .030, were observed. Follow-up tests on the Digit Backward Test found that CBD patients were more impaired compared with AD (p < .045), SOC/EXEC (p < .004), and SemD (p < .002) patients. On the letter fluency test, PNFA patients were more impaired compared with AD (p < .041) and SemD (p < .023) patients.
Longitudinal neuropsychological test performance
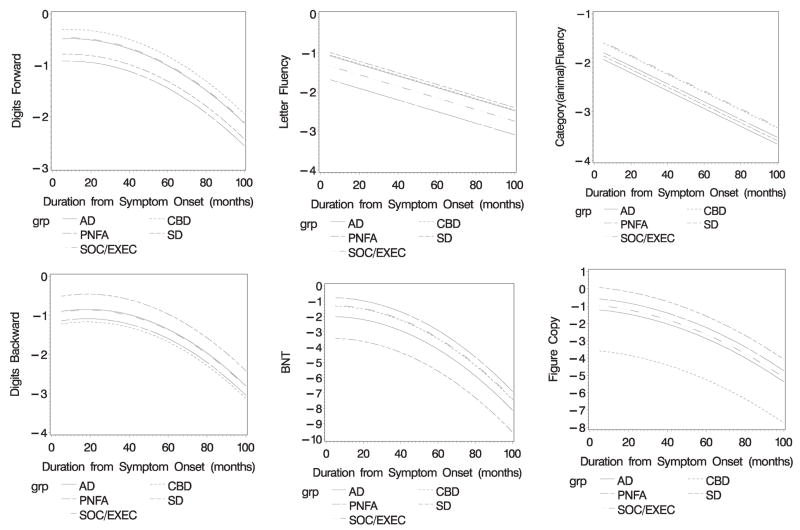
Analyses for the Digit Forward Test produced a significant quadratic effect for longitudinal course (p < .010; see Figure 1). PNFA patients demonstrated greater difficulty throughout the course of their disease compared with AD (p < .008), SOC/EXEC (p < .020), and CBD (p < .001) patients. Similarly, CBD patients exhibited greater difficulty throughout the course of their disease compared with SemD patients (p < .018).
Figure 1.
Longitudinal decline on neuropsychological tests. The slope indicates the rate of monthly decline (x-axis) as reflected by z-score performance on each task (y-axis).
A quadratic effect for longitudinal course was also noted for the Digit Backward Test (p < .001), with PNFA patients again demonstrating greater difficulty throughout the course of their disease compared with SemD patients (p < .023). Also, SemD patients exhibited more striking impairment throughout the course of their illness compared with CBD patients (p < .005).
On the letter fluency test, a quadratic effect for longitudinal course was obtained (p < .001). PNFA patients exhibited greater difficulty throughout the course of their illness compared with AD (p < .001), SemD (p < .014), and CBD (p < .013) patients. In sum, consistent with the initial neuropsychological assessment, PNFA and CBD patients generally showed difficulty throughout the course of their illness on executive tests compared with other patient groups.
Language Tests
Initial neuropsychological test performance
On tests related to language, between-groups differences were observed on the BNT, F(4, 399) = 6.74, p < .001, and the “animal” fluency test, F(4, 397) = 2.48, p < .043. Follow-up tests revealed that SemD patients obtained a lower score on the BNT compared with SOC/EXEC (p < .001), PNFA (p < .006), and CBD (p < .008) patients. On the “animal” fluency test, AD patients generated fewer responses compared with SOC/EXEC patients (p < .016).
Longitudinal neuropsychological test performance
A quadratic main effect for longitudinal course was noted on the BNT (p < .001). SemD patients demonstrated greater difficulty throughout the course of their illness compared with all other patient groups (p < .004, all analyses).
A quadratic main effect for longitudinal course was found for the “animal” fluency test (p < .001), with AD patients demonstrating greater difficulty throughout the course of their illness compared with SOC/EXEC (p < .014) and CBD (p < .018) patients. These longitudinal analyses are very similar to the initial, cross-sectional between-groups analyses, showing that worse performance on the BNT in SemD patients and worse performance on the “animal” fluency in AD patients are maintained throughout the course of the illness.
Visuoconstructional Tests
Initial neuropsychological test performance
CBD patients obtained a lower score on the figure copy test compared with all other groups, F = (4, 328) = 15.19, p < .001.
Longitudinal neuropsychological test performance
A quadratic main effect for longitudinal course was obtained for the figure copy test (p < .032). CBD patients uniformly demonstrated greater difficulty throughout the course of their illness compared with all other groups (p < .001). For this test, AD patients also demonstrated worse performance throughout the course of their disease compared with SemD patients (p < .005). SOC/EXEC patients exhibited worse performance throughout the course of their disease compared with SemD patients (p < .047). These test results tend to mirror the initial, cross-sectional between-groups analyses.
Double Dissociations on Neuropsychological Tests
An inspection of Table 4 suggests that longitudinal decline is associated with several double dissociations on neuropsychological tests. These double dissociations are consistent with the pattern of initial neuropsychological test performance.
SemD versus PNFA
When assessed over the longitudinal course of their disease, the SemD and PNFA groups dissociated on tests of naming and executive control such that the SemD group was significantly more impaired on the BNT compared with the letter fluency test. The PNFA group displayed the opposite pattern.
SemD versus CBD
Differences between the CBD and SemD patients were noted such that the SemD group was particularly impaired on the BNT compared with the figure copy test. The opposite profile was noted for the CBD group.
PNFA versus CBD
A double dissociation was found between CBD and PNFA patients on the Figure Copy and letter fluency tests. Here, the PNFA patients were significantly more impaired on the letter fluency test compared with the figure copy test. The CBS group displayed the opposite pattern.
Discussion
FTLD is a major cause of dementia in persons younger than 65 years. Some of the initial neuropsychological studies attempting to show specific subtype profiles for AD versus FTLD and between FTLD subtypes were hampered, in part, because of small sample sizes, variability with respect to the selection of neuropsychological tests, and how patients were diagnosed (Perri et al., 2005; Starkstein et al., 1994; Thomas-Anterion, Jacquin, & Laurent, 2000).
However, over the past several years considerable knowledge regarding the neurobiology of FTLD has accumulated. This information has been incorporated into the newer schemes for the diagnosis of FTLD (McKhann et al., 2001). More recent research has shown that specific neuropsychological and behavioral deficits can distinguish AD from FTLD and between FTLD subtypes (Grossman et al., 2007; Kramer et al., 2003; Libon, Massimo, et al., 2007; Libon, Xie, et al., 2007). This has been confirmed in clinical–pathological studies distinguishing FTLD from AD (Rascovsky et al., 2002; Rascovsky, Salmon, Hansen, Thal, & Galasko, 2007) and studies comparing pathologically confirmed subgroups of FTLD patients (Forman et al., 2006; Grossman et al., in press; Murray et al., 2007). Clinically, these data may be useful in the future for identifying participants for novel pharmacological treatment paradigms for FTLD.
In the current study, we addressed an issue that has received comparatively little attention, that is, the longitudinal course or outcome of patients with FTLD. Kertesz and colleagues (Kertesz, 2003; Kertesz et al., 1999) have introduced into the literature a hypothetical construct labeled Pick complex as a means of understanding the brain–behavior relationships that underlie FTLD. The origin of the term is based on research suggesting that the neuropsychological and behavioral characteristics of many FTLD patients overlap or devolve into a single, undifferentiated subtype over time. Although we do not question the internal validity of these findings, certain aspects of the methodology described in these reports may limit their generalizability. First, the data are based on survey protocols that do not necessarily reflect fine-grained distinctions within verbally mediated tasks (Blair et al., 2007) or patterns of social disorder (Marczinski et al., 2004). Although this is a reasonable initial approach to the study of longitudinal decline, these measures tend to average scores across potentially dissociable areas, and thus can blur distinctions between subgroups. Second, the statistical methods used in studies such as these allowed longitudinal observations for only 36 months (Kertesz et al., 2005). Finally, the diagnostic algorithms do not always differentiate the SemD from the PNFA subtypes, and AD patients were not generally included in these reports (Blair et al., 2007; Kertesz et al., 2005). In the current research, the statistical treatment of the data allowed us to report follow-up on patients up to 100 months; a well-researched neuropsychological protocol was administered that has demonstrated dissociations between neurodegenerative patients on measures of executive control, language, visuoconstruction, and semantic memory (Libon, Massimo, et al., 2007; Grossman et al., 2007); subgroup dissociations have been validated by evaluations in autopsy-confirmed cases (Grossman et al., in press); and several clinically distinct FTLD subtypes were studied and compared with AD patients.
The present longitudinal analysis of neuropsychological test performance was designed to test the hypothesis that the pattern of relative neuropsychological impairment seen at the initial assessment of FTLD persists over the duration of illness. If this is true, statistical analyses should be dominated by main effects for group as well as longitudinal course, suggesting that FTLD patients do not converge onto a single, undifferentiated state. The alternative pattern, interaction effects, would be consistent with a single end-state.
Overall, we found support for our hypothesis. At their initial evaluation, distinct patterns of neuropsychological impairment were noted in FTLD and AD. Impaired performance on tests of category fluency (“animals”) was seen in patents with AD. Pronounced impairment on visuoconstructional and working memory tests was observed in patients with CBD. Low scores on tests of visual confrontation naming were noted among SemD patients. Finally, PNFA patients obtained low scores on tests of executive control.
With respect to longitudinal neuropsychological test performance, the statistical analyses described above yielded main effects for group and longitudinal course. No interactions between group and longitudinal course were found. For many domains of cognitive function, the patterns of impairment observed at the initial neuropsychological assessment were maintained over the course of the illness. The longitudinal course for AD patients continued to be dominated by their low scores on the “animal” fluency test, CBD patients continued to display disproportionate impairment on visuoconstructional tests, SemD patients performed relatively poorly on tests of visual confrontation naming, and PNFA patients displayed disproportionate impairment on tests of executive control.
The current research also produced several double dissociations on neuropsychological tests. In the present research, the SemD group obtained a low score on a test of naming but performed better on tests of working memory. The PNFA group produced the opposite profile. Likewise, the low score on the BNT in the SemD group was worse than their score on the figure copy test. CBD patients presented with the opposite pattern on these tests. Finally, a double dissociation was found for the CBD and PNFA patients on visuoconstruction and letter fluency tests. Consistent with the autopsy series described by Grossman et al. (in press), these double dissociations persisted during the entire course of the illness.
From a neuropsychological perspective, these data are particularly valuable for several reasons. First, the longitudinal data presented above are consistent with prior reports that demonstrated patterns of cognitive deficit associated with specific dementia syndromes in cross-sectional studies, including studies with pathologically defined cases (Grossman et al., 2007; Libon, Massimo, et al., 2007; Libon, Xie, et al., 2007; Kramer et al., 2003; Rascovsky et al., 2002, 2007). Second, we have now extended these findings to show that patients maintained a relatively unique pattern of neuropsychological deficits well into the course of their dementia. Relative impairments are likely to be of diagnostic value at any point during the course of a dementing condition. Third, the dissociations reported above suggest the existence of cognitive networks where behavior is organized around specific but broadly encompassing cognitive functions involving large-scale cortical networks. The importance of the current data is that these networks of cognitive dysfunction are shown to persist well into course of a dementia.
Other longitudinal research studies have also reported main effects for group or illness course for tests of category (“animals”) and letter fluency in patients with AD and several FTLD subtypes (Rogers, Ivanoiu, Patterson, & Hodges, 2006). Ratcliff and colleagues (Ratcliff, Dodge, Birzescu, & Ganguli, 2003) found that performance on tests of executive control and category fluency remained stable over time, with no interaction reported between groups and longitudinal course. Specific neuropathological substrates are also associated with a particular clinical presentation in dementia. In AD, neurofibrillary tangles compromise medial temporal lobe functioning and correlate with impaired episodic memory; in tau-positive FTLD such as CBD, the histopathological burden of tau in frontal and parietal regions correlates with visual–constructional difficulty; and ubiquitin/TDP-43 pathology seen in patients with SemD and SOC/EXEC subtypes is associated with disease in frontal and temporal brain regions (Grossman et al., in press; Murray et al., 2007). The underlying cytoarchitectonic distribution of pathology found in some FTLD subtypes also may help explain the core findings of the current research. Some early onset FTLD subtypes may disproportionately affect superficial layers of the brain (Amunts et al., 2004). This may interfere with local neuronal networks that support specific cognitive measures. Other FTLD subtypes might affect the neurons in both superficial and deep layers of the cortex. Such neuropathology might disturb the pyramidal cell neurons located in the deeper cortical layers (Cairns et al., 2004; Simon et al., 2004). These neurons are important for interregion connectivity and complex cognitive processes supported by these large-scale neural networks. Clearly, additional work is needed to assess clinical–pathological hypotheses such as these in the role of longitudinal decline seen in neurodegenerative diseases.
The current research is not without limitations, and several caveats should be kept in mind when considering our observations. First, although the neuropsychological protocol we used is relatively comprehensive, not all domains of cognitive functioning, such as episodic and semantic memory, were represented. Also, our findings may have been different if a wider array of tests, particularly other tests that assess executive control (Stroop, Card Sorting, Trail Making, etc.), had been used. Another problem is the fact that we had no longitudinal measures of behavior–social comportment. Although we examined longitudinal performance for a longer duration and later in the course of disease compared with most other research, it is possible that we did not examine patients sufficiently late in the disease process to demonstrate converging group profiles. Finally, although our results resemble the observations of the autopsy-proven cohort reported by Grossman et al. (in press), we do not have histopathologic evidence for the diseases causing impairments in the patients participating in this study.
With these caveats in mind, our findings indicate that different profiles of longitudinal decline are present in AD and FTLD. Relatively distinct patterns of neuropsychological impairment appear to be maintained over time, suggesting that AD and FTLD patients do not devolve into a single undifferentiated clinical dementia syndrome.
Acknowledgments
This work was supported in part by Grants AG17586, AG15116, AG44266, and AG10124 from the National Institutes of Health and the Dana Foundation.
Contributor Information
David J. Libon, Department of Neurology, Drexel University College of Medicine
Sharon X. Xie, Department of Biostatistics and Epidemiology, University of Pennsylvania School of Medicine
Xingmei Wang, Department of Biostatistics and Epidemiology, University of Pennsylvania School of Medicine.
Lauren Massimo, Department of Neurology, University of Pennsylvania School of Medicine.
Peachie Moore, Department of Neurology, University of Pennsylvania School of Medicine.
Luisa Vesely, Department of Neurology, University of Pennsylvania School of Medicine.
Alea Khan, Department of Neurology, University of Pennsylvania School of Medicine.
Anjan Chatterjee, Department of Neurology, University of Pennsylvania School of Medicine.
H. Branch Coslett, Department of Neurology, University of Pennsylvania School of Medicine.
Howard I. Hurtig, Department of Neurology, University of Pennsylvania School of Medicine
Murray Grossman, Department of Neurology, University of Pennsylvania School of Medicine.
Tsao-Wei Liang, Department of Neurology, Thomas Jefferson University Hospital.
References
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, et al. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space: The roles of Brodmann areas 44 and 45. NeuroImage. 2004;22:42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Avants B, Grossman M, Gee JC. The correlation of cognitive decline with frontotemporal dementia induced annualized gray matter loss using diffeomorphic morphometry. Journal of the Alzheimer Disease Association. 2005;19(Suppl 1):S25–S28. doi: 10.1097/01.wad.0000183083.14939.82. [DOI] [PubMed] [Google Scholar]
- Blair M, Marczinski CA, Davis-Faroque N, Kertesz A. A longitudinal study of language decline in Alzheimer’s disease and fron-totemporal dementia. Journal of the International Neuropsychological Society. 2007;13:237–245. doi: 10.1017/S1355617707070269. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Grossman M, Arnold SE, Burn DJ, Jaros E, Perry RH, et al. Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology. 2004;63:1376–1384. doi: 10.1212/01.wnl.0000139809.16817.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Sheppard L, Fillenbaum GG, Galasko D, Morris JC, Koss E, et al. Variability in annual Mini-Mental State Examination score in patients with probable Alzheimer’s disease: A clinical perspective of data from the Consortium to Establish a Registry for Alzheimer’s Disease. Archives of Neurology. 1999;56:857–862. doi: 10.1001/archneur.56.7.857. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the mental state of patients for a clinician. Journal of Psychiatric Residency. 1974;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, et al. Frontotemporal dementia: Clinicopathological correlations. Annals of Neurology. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman RQ, Giovannetti T, Lamar M, Cloud BS, Kaplan E, Stern R, Libon DJ. Visuoconstructional deficits in dementia. Neuropsychology. 2000;14:415–426. doi: 10.1037//0894-4105.14.3.415. [DOI] [PubMed] [Google Scholar]
- Galasko DR, Gould RL, Abramson IS, Salmon DP. Measuring cognitive change in a cohort of patients with Alzheimer’s disease. Statistical Methods. 2000;19:1421–1432. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1421::aid-sim434>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Gould R, Abramson I, Galasko D, Salmon D. Rate of cognitive change in Alzheimer’s disease: Methodological approaches using random effects models. Journal of the International Neuropsychological Society. 2001;7:813–824. [PubMed] [Google Scholar]
- Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G, et al. A comparison of RCBF patterns during letter and semantic fluency. Neuropsychology. 2000;14:353–360. doi: 10.1037//0894-4105.14.3.353. [DOI] [PubMed] [Google Scholar]
- Grimes DA, Lang AE, Bergeron CB. Dementia as the most common presentation of cortical–basal ganglionic degeneration. Neurology. 1999;53:1969–1974. doi: 10.1212/wnl.53.9.1969. [DOI] [PubMed] [Google Scholar]
- Grossman M, Ash S. Primary progressive aphasia: A review. Neurocase. 2004;10:3–18. doi: 10.1080/13554790490960440. [DOI] [PubMed] [Google Scholar]
- Grossman M, Libon DJ, Forman MS, Wood EM, Moore P, Farmer J, et al. Distinct neuropsychological profiles in pathologically defined patients with frontotemporal lobe dementia. Archives of Neurology. 2007;64:1601–1609. doi: 10.1001/archneur.64.11.1601. [DOI] [PubMed] [Google Scholar]
- Grossman M, Xie S, Libon DJ, Xingmei Massimo L, Moore P, et al. Longitudinal decline in autopsy-defined frontotemporal dementia. Neurology. doi: 10.1212/01.wnl.0000303816.25065.bc. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Cole M, Bellavance F, McCusker J, Primeau F. Tracking cognitive decline in Alzheimer’s disease using the Mini-Mental State Examination: A Mets analysis. International Psychogeriatrics. 2000;12:231–247. doi: 10.1017/s1041610200006359. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patternson K, Oxbury S, Funnell E. Semantic dementia: Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Cosentino S, Ball SK, Bogdanoff B, Leopold N, Kaplan E, Libon DJ. Errors produced on the Mini-Mental State Examination and neuropsychological test performance among patients with Alzheimer’s disease, ischaemic vascular dementia, and Parkinson’s disease. Journal of Neuropsychiatry and Clinical Neuroscience. 2002;14:311–320. doi: 10.1176/jnp.14.3.311. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kertesz A. Pick complex: An integrative approach to frontotemporal dementia: Primary progressive aphasia, corticobasal degeneration, and progressive supranuclear palsy. Neurologist. 2003;6:311–317. doi: 10.1097/01.nrl.0000094943.84390.cf. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: Diagnostic criteria for frontal lobe dementia. Canadian Journal of Neuroscience. 1997;24:29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, Munoz DG. Clinical and pathological overlap between frontotemporal dementia, primary progressive aphasia, and corticobasal degeneration: The Pick complex. Dementia and Geriatric Cognitive Disorders. 1999;10(Suppl 1):46–49. doi: 10.1159/000051212. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Martinez-Lage P, Davidson W, Munoz DG. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology. 2000;55:1368–1375. doi: 10.1212/wnl.55.9.1368. [DOI] [PubMed] [Google Scholar]
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cognitive and Behavioral Neurology. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lamar M, Price CC, Libon DJ, Penney DL, Kaplan E, Grossman M, Heilman KM. Alterations in working memory as a function of leukoaraiosis in dementia. Neuropsychologia. 2007;45:245–254. doi: 10.1016/j.neuropsychologia.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, Massimo L, Moore P, Coslett HB, Chatterjee A, Aguirre G, Grossman M. The Philadelphia Brief Assessment of Cognition (PBAC): A brief neuropsychological protocol that distinguishes frontotemporal dementia from Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2007;24:441–447. doi: 10.1159/000110577. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Xie S, Moore P, Farmer J, Antani S, McCawley G, Grossman M. Patterns of neuropsychological impairment associated with frontotemporal dementia: A factor analytic study. Neurology. 2007;68:368–375. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Davidson W, Kertesz A. A longitudinal study of behavior in frontotemporal dementia and primary progressive aphasia. Cognitive and Behavioral Neurology. 2004;17:185–190. [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadian EM. Clinical diagnosis of Alzheimer’s disease: Report on the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann G, Trojanowski JQ, Grossman M, Miller BL, Dickson D, Albert M. Clinical and pathological diagnosis of frontotemporal dementia: Report of a work group on frontotemporal dementia and Pick’s disease. Archives of Neurology. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Wise RJ. Generating “tiger” as an animal name or a word beginning with t: Differences in brain activation. Proceedings: Biological Sciences. 1996;263:989–995. doi: 10.1098/rspb.1996.0146. [DOI] [PubMed] [Google Scholar]
- Murray R, Neumann M, Forman MS, Farmer J, Miller BL, Johnson JK, et al. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology. 2007;68:1274–1283. doi: 10.1212/01.wnl.0000259519.78480.c3. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Perri R, Koch G, Carlesimo GA, Serra L, Fadda L, Pasqualetti P, et al. Alzheimer’s disease and frontal variant of frontotemporal dementia: A very brief battery for cognitive and behavioural distinction. Journal of Neurology. 2005;252:1238–1244. doi: 10.1007/s00415-005-0849-1. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Hyder F, Blamire AM, Shulman RG. fMRI of the prefrontal cortex during overt verbal fluency. NeuroReport. 1997;8:561–565. doi: 10.1097/00001756-199701200-00036. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Hansen LA, Thal LJ, Galasko D. Disparate letter and semantic category fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer’s disease. Neuropsychology. 2007;21:20–30. doi: 10.1037/0894-4105.21.1.20. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Ho GJ, Galasko D, Peavy GM, Hansen LA, Thal LJ. Cognitive profiles differ in autopsy-confirmed frontotemporal dementia and AD. Neurology. 2002;58:1801–1808. doi: 10.1212/wnl.58.12.1801. [DOI] [PubMed] [Google Scholar]
- Ratcliff G, Dodge H, Birzescu M, Ganguli M. Tracking cognitive functioning over time: Ten-year longitudinal data from a community-based study. Applied Neuropsychology. 2003;10:76–88. doi: 10.1207/S15324826AN1002_03. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Ivanoiu A, Patterson K, Hodges JR. Semantic memory in Alzheimer’s disease and the frontotemporal dementias: A longitudinal study of 236 patients. Neuropsychology. 2006;20:319–335. doi: 10.1037/0894-4105.20.3.319. [DOI] [PubMed] [Google Scholar]
- Simon O, Kherif F, Flandin G, Poline JB, Riviere D, Mangin JF, Le Bihan D, Dehaene S. Automatized clustering and functional geometry of human parietofrontal networks for language, space, and number. NeuroImage. 2004;23:1192–1202. doi: 10.1016/j.neuroimage.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Neary D, Mann DM. Fronto-temporal lobar degeneration: Fronto-temporal dementia, progressive aphasia, semantic dementia. 1. New York: Churchill Livingstone; 1996. [Google Scholar]
- Spreen O, Struss EA. Compendium of neuropsychological tests. New York: Oxford University Press; 1990. [Google Scholar]
- Starkstein SE, Migliorelli R, Tesón A, Sabe L, Vázquez S, Turjanski M, Robinson RG, Leiguarda R. Specificity of changes in cerebral blood flow in patients with frontal lobe dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:790–796. doi: 10.1136/jnnp.57.7.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Anterion C, Jacquin K, Laurent B. Differential mechanisms of impairment of remote memory in Alzheimer’s disease and frontotemporal lobe dementia. Dementia and Geriatric Cognitive Disorder. 2000;11:100–106. doi: 10.1159/000017221. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Revised. San Antonio, TX: The Psychology Corporation; 1987. [Google Scholar]