Abstract
Objective
To assess the cross-sectional association between meniscal status and joint effusion on magnetic resonance imaging (MRI) in knees without radiographic osteoarthritis.
Design
Knees without osteoarthritis (Kellgren/Lawrence grade 0) from the Framingham and MOST studies were examined by MRI. Meniscal status was assessed with a score of 0–4 in the anterior horn/body/posterior horn of the medial/lateral meniscus and effusion was assessed using a score of 0 to 3. The odds ratios (OR) of joint effusion in those with meniscal damage were estimated using a logistic regression model. A subanalysis was performed for knees without MRI detected cartilage damage.
Results
Of 1368 knees, 296 (21.6%) showed meniscal pathology in at least one subregion. Effusion was present in 133 (44.9%) of knees with meniscal damage vs. 328 (30.6%) in those without meniscal damage. The adjusted OR of effusion in a knee with meniscal damage was 1.8, 95% confidence intervals (CI) [1.4, 2.4]. The OR of effusion for the group with meniscal pathology in two compartments was 5.4, 95% CI [2.1, 14.3]. For knees without any cartilage lesions but with meniscal damage in any compartment the OR was 2.3, 95% CI [1.1, 4.5].
Conclusions
Knees without osteoarthritis but with meniscal pathology exhibit joint effusion to a significantly higher degree than knees without meniscal damage. The association persists for knees without cartilage damage. The prevalence of effusion is further increased when present in two compartments. Concomitant occurrence of synovial activation and meniscal damage contributes to understanding the pathophysiology of early degenerative joint disease.
Keywords: Meniscal tear, Magnetic resonance imaging, Effusion, Osteoarthritis, Synovium, Knee
INTRODUCTION
The meniscus may tear due to knee trauma or spontaneously due to aging and degenerative processes1–3. Mechanical impairment of the meniscus will alter the weight-bearing capacities of a joint and may damage the articular chondral surface of the same compartment as well as the subchondral bone4,5. Loss of meniscal function is recognized as a potent risk factor for both the development and progression of knee osteoarthritis (OA)6,7. Meniscal tears are commonly observed in conjunction with radiographic OA in the elderly population8–10, but meniscal damage is also prevalent in younger age groups and even in asymptomatic knees without signs of radiographic OA11.
In addition to being a potent risk factor for knee OA, meniscal damage might also be a trigger of synovial activation. Synovial activation in OA is thought to be triggered by release of detritus from joint structures including cartilage12. To phagocytose and eliminate this detritus macrophages in the synovial lining proliferate, producing a tissue whose lining develops inflammatory features and thickens. In moderate to advanced OA, ligamentous injury, loose bodies or hyaline cartilage deterioration cause synovial activation. In knees without any evidence of OA, damage to knee structures might trigger synovial activation and this activation might, in turn, provide indirect evidence of ongoing structural damage in the joint. Such damage could be present, for example, in the meniscus (Figure 1). Animal models have also shown that synovial cell response plays an important role in meniscal tear repair13,14.
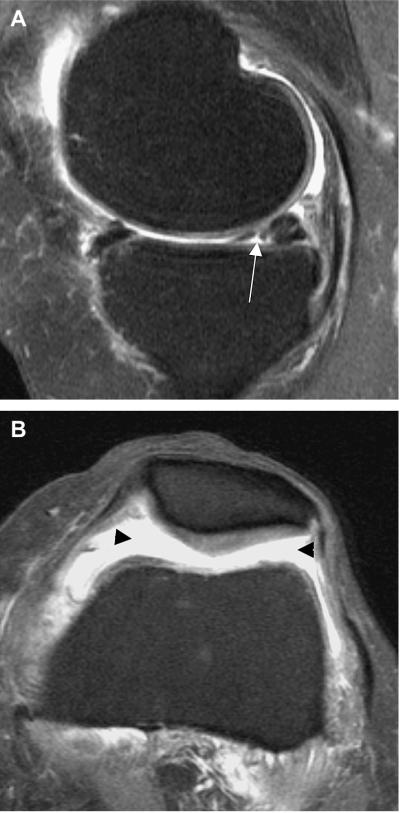
Figure 1.
Example of meniscal tear and concomitant joint effusion.
A. Sagittal T2w fat suppressed image. Arrow depicts non-displaced grade-2 meniscal tear in the posterior horn of the medial meniscus.
B. Axial T2w fat suppressed image. Grade 2 non-traumatic joint effusion (black arrowheads). Note intact retropatellar cartilage.
Synovial activation of a joint is reflected as synovitis and joint effusion on MRI. Direct measures of synovitis are probably ideally obtained from contrast-enhanced MRI assessment of synovial thickness15,16. However, joint effusion may be assessed and quantified on non-contrast enhanced MRI17–19. It is not known to what extent meniscal damage in subjects without OA is associated with joint effusion.
This study was performed to analyze the association of meniscal damage and joint effusion in knees without radiographic signs of osteoarthritis using non-enhanced MRI in a combined patient sample drawn from the Framingham Osteoarthritis Study and the Multicenter Osteoarthritis (MOST) Study. We combined data from two studies in order to have a large number of knees without OA that would permit a robust evaluation of the question.
METHODS
Subjects
The study cohort consisted of a combined sample from two large osteoarthritis studies – the Framingham Osteoarthritis Study and the MOST Study.
Framingham Subgroup
The Framingham sample was composed of two separate groups, 1) members of the Framingham Heart Study Offspring Cohort and 2) a newly recruited cohort from the town of Framingham, Massachusetts (Community Cohort). Participants in this combined group, designated the Framingham OA Study Cohort, were examined between 2002 and 2005.
The Framingham Heart Study Offspring Cohort participants included surviving descendants (and spouses of descendants) of the Original Framingham Heart Study cohort subjects20. As part of a study of the inheritance of OA, selected participants were originally examined in 1992–94. Members of this group were identified as potential participants of the current study. All were contacted by telephone and invited to participate in the study. A validated survey instrument21 supplemented by questions about medication use that would reflect treated rheumatoid arthritis was used to screen potential subjects. Those who screened positively for rheumatoid arthritis were excluded.
The newly recruited participants to the Framingham OA Study Cohort (Community Cohort) were drawn from a random sample of the Framingham, Massachusetts community. Flyers were hung in public areas to increase awareness of the study, which focused on health including bone health, foot health and arthritis. Participants were recruited using random digit dialing and census tract data to ensure inclusion of a representative sample of the community. To be included, subjects had to be at least 50 years old and ambulatory (use of canes and walkers was allowed). Participants with bilateral total knee replacements (TKR) or a positive screen for rheumatoid arthritis as above21 were excluded. In neither group was participant selection based on the presence or absence of knee OA or knee pain.
In order assess if a subject had experienced previous knee injury the following question was asked: “Have you ever injured your knee badly enough to limit your ability to walk for at least 3days?”
Framingham MRI
Based on an agreement with the parent Framingham Heart Study to limit the respondent burden of the Framingham Offspring subgroup, only those who answered affirmatively to the question, “In the past 30 days, have you had any pain, aching or stiffness in either of your knees?” underwent MRI of the knee(s) In these subjects, both knee MRIs were read when acquired. For the community sample, all subjects had bilateral knee MRIs whether or not they had knee symptoms but, because of funding limitations, only the right knee was read. Those participants with MRI contraindications were not scanned, and those with one TKR had only their native knee scanned.
All studies were performed with a 1.5T MRI system (Siemens, Mountain View, CA) using a phased array knee coil. A positioning device was used to ensure uniform placement of the knees. Imaging sequences included: sagittal (TR 3610 ms, TE 40 ms, 3.5 mm slice thickness, 0 mm interslice gap, 32 slices, 256 × 256 matrix, 139 mm2 FOV, echo train length 7), axial (TR 3610 ms, TE 40 ms, 3.0 mm slice thickness, 0 mm interslice gap, 20 slices, 256 × 256 matrix, 139 mm2 FOV, echo train length 6) and coronal (TR 3610 ms, TE 40 ms, 3.5 mm slice thickness, 0 mm interslice gap, 30 slices, 256 × 256 matrix, 139 mm2 FOV, echo train length 7) T2-weighted turbo spin echo sequences with fat suppression, and a sagittal T1-weighted spin echo sequence without fat suppression (TR 475 ms, TE 24 ms, 3.5 mm slice thickness, 0 mm interslice gap, 32 slices, 256 × 256 matrix, 139 mm2 FOV).
Approval for the study was obtained from the Boston University Medical Center Institutional Review Board (IRB).
MOST Subgroup
The MOST study is an NIH-funded prospective epidemiological study of 3,026 persons aged 50 to 79 years, with a goal of identifying risk factors for incident and progressive knee OA in a sample with either OA or at high risk of developing it. Those considered at high risk included persons who were overweight or obese, those with knee pain, aching or stiffness on most of the last 30 days, a history of knee injury that made it difficult to walk for at least one week, or previous knee surgery.
Subjects were recruited from two U.S. communities, Birmingham, Alabama and Iowa City, Iowa through mass mailing of letters and study brochures, supplemented by media and community outreach campaigns. The study protocol was approved by IRB's at the University of Iowa, University of Alabama, Birmingham, University of California, San Francisco and Boston University Medical Campus.
Subjects were excluded from MOST if they screened positive for rheumatoid arthritis21, had ankylosing spondylitis, psoriatic arthritis, Reiter's syndrome, had kidney problems requiring hemo- or peritoneal dialysis, a history of cancer (except for non-melanoma skin cancer), had or planned to have bilateral knee replacement surgery, were unable to walk without the help of another person or walker, or were planning to move out of the area in the next three years. History of past knee injury was assessed in the same manner than Framingham study.
MOST MRI
MRIs in MOST were obtained in both knees with a 1.0T dedicated MR system (OrthOne™, ONI Medical Systems, Inc., Wilmington, MA) with a circumferential extremity coil using fat suppressed fast spin echo proton density (PD) weighted sequences in two planes, sagittal (TR 4800 ms, TE 35 ms, 3 mm slice thickness, 0 mm interslice gap, 32 slices, 288 × 192 matrix, 140 mm2 FOV, echo train length 8) and axial (TR 4680 ms, TE 13 ms, 3 mm slice thickness, 0 mm interslice gap, 20 slices, 288 × 192 matrix, 140 mm2 FOV, echo train length 8) and a STIR sequence in the coronal plane (TR 6650 ms, TE 15 ms, TI 100 ms, 3 mm slice thickness, 0 mm interslice gap, 28 slices, 256 × 192 matrix, 140 mm2 FOV, echo train length 8). Only one knee per patient was randomly selected to be read when both knees had been scanned and had available MRI readings.
Radiographic assessment in both studies
In both studies, all subjects underwent weight-bearing posteroanterior (PA) fixed flexion knee radiographs using the protocol by Peterfy et al. and a plexiglass positioning frame (SynaFlexer™)22.
A musculoskeletal radiologist and a rheumatologist experienced in reading study films, both blinded to case/control status and clinical data, graded all PA films according to the Kellgren and Lawrence (K/L) scale23. If readers disagreed on the presence of radiographic OA, the film readings were adjudicated by a panel of 3 readers.
Out of the above defined study samples, we excluded all subjects who exhibited any sign of tibiofemoral radiographic osteoarthritis (K/L grades 1 through 4) and retained only knees with K/L Grade 0.
MRI assessment for both studies
In both studies two musculoskeletal radiologists (for the Framingham study FWR and AM; for the MOST study FWR and AG), blinded to clinical and x-ray data, read the MRIs.
Meniscal integrity was graded according to the Whole-Organ MRI Score (WORMS) method17. The anterior horn, body segment, and the posterior horn of the medial and lateral menisci were scored separately from 0–4: 0 = intact; 1 = minor radial or parrot-beak tear; 2 = non-displaced tear; 3 = displaced tear; 4 = complete maceration or destruction (Inter-observer reliability for meniscal scoring in the Framingham study: weighted-kappa 0.70, 95% confidence intervals [0.64,0.76]; in the MOST study: weighted-kappa 0.79, 95% confidence intervals [0.59,0.97]). According to the WORMS system meniscal damage or pathology was defined as a tear, maceration, and (or) destruction (=any grades ≥1 on scale above). The readers regarded intrameniscal signal as a meniscal tear only when it communicated with the meniscal inferior or superior margin on at least two slices. Intrameniscal signal alterations that did not fulfill the aforementioned criteria of a tear were scored as no tear (Grade 0).
Joint effusion was graded from 0 to 3 in terms of the estimated maximal distention of the synovial cavity according to the WORMS scoring system: 0=normal; 1=<33% of maximum potential distention; 2=33%–66% of maximum potential distention; 3=>66% of maximum potential distention. Joint effusion was defined as any joint effusion score ≥1. Image examples of the different effusion scores are presented in Figure 1. (Inter-observer reliability for effusion scoring in the Framingham study: weighted-kappa 0.70, 95% confidence intervals [0.62,0.79]; in the MOST study: weighted-kappa 0.73, 95% confidence intervals [0.53,0.92]).
Statistical Analysis
We first computed the overall prevalence of meniscal damage in the study sample. We then evaluated the association between meniscal damage and presence of joint effusion using logistic regression adjusting for gender, age, body mass index (BMI) and study site, with knees without meniscal damage as the reference group. Before combining the subsamples we examined the data to be sure that results were consistent in both sites. A subanalysis was performed concerning joint effusion present in knees with meniscal pathology in only one or in both, the medial and lateral tibiofemoral compartments. An additional analysis was performed for knees with no cartilage damage (WORMS score ≤1) in the tibiofemoral and patellofemoral compartments.
RESULTS
Patient Demographics
The combined study sample included 1368 knees (918 from the Framingham study and 451 from the MOST study) with no signs of radiographic OA (K/L grade 0). Of these, 387 (28.3%) were also free of MRI-defined cartilage damage. On average the subjects in both studies were elderly and overweight, and there were more women than men. Significant differences between the two study samples were found for age and BMI with the MOST participants being slightly younger, more overweight and having experienced more knee injuries in the past. When comparing the subgroups without cartilage damage, no significant demographic differences were observed between the Framingham and MOST participants (Table 1). Regarding maximal meniscal damage severity, also no significant demographic differences were observed (Table 2). P values ranged from 0.10 for age to 0.87 for gender.
Table 1.
Demographic overview of the subjects in the Framingham and MOST study
| All subjects without radiographic osteoarthritis (K/L grade = 0) |
|||
|---|---|---|---|
| Both studies combined (N=1368) | Framingham Study (N=918) | MOST Study (N=450) | |
| Age | 61.9 ±8.2 | 62.6 ±8.4 | 60.6 ±7.5* |
| % women | 57.5 | 56.8 | 59.1 |
| BMI | 28.5 ±5.2 | 28.2 ±5.4 | 29.0 ±4.5* |
| % previous knee injury | 12.1 | 9.3 | 17.6* |
| Subjects with no radiographic osteoarthritis and no cartilage damage on MRI |
|||
|---|---|---|---|
| Both studies (N=387) | Framingham Study (N=274) | MOST Study (N=113) | |
| Age | 60.7 ±7.9 | 60.7 ±8.0 | 60.6 ±7.6 |
| % women | 58.5 | 57.6 | 60.8 |
| BMI | 27.9 ±4.9 | 27.8 ±5.2 | 28.1 ±4.0 |
| % previous knee injury | 9.5 | 6.8 | 16.0* |
statistically significant defined as p< 0.05
BMI body mass index K/L Kellgren/Lawrence
Table 2.
Demographic overview concerning the distribution of meniscal damage severity
| Maximal meniscal damage in knees with meniscal damage (N=296) |
|||
|---|---|---|---|
| 1 N=164 (55.4%) | 2 N=107 (36.2%) | 3–4 N=25 (8.4%) | |
| Age | 63.5 ± 8.8 | 65.8 ± 8.8 | 63.8 ± 8.5 |
| BMI | 28.0 ± 5.2 | 28.1 ± 4.7 | 29.8 ± 5.7 |
| Women, % | 41.5 | 41.1 | 36.0 |
BMI body mass index
Meniscal Status
296 (21.6%) of the knees showed evidence of meniscal damage. Of these, 276 (93.2%) showed damage in only one compartment, and 20 (6.8%) showed damage in both the medial and lateral compartments. Of the 60 knees with meniscal damage and without cartilage lesions only one (1.7%) knee had signs of meniscal damage in both compartments.
Concerning maximal meniscal damage in any subregion, 164 (55.4%) knees exhibited grade 1 tears. 107 (36.2%) showed grade 2 tears, and 25 (8.4%) had grade 3 or 4 tears with only one knee exhibiting a grade 4 meniscal lesion. No significant differences were observed between subjects with grade 1, grade 2 and grade 3–4 meniscal lesions concerning age, BMI and gender (Table 2).
Joint effusion in K/L 0 knees and its association with meniscal damage
Joint effusion (any grade) was present in 461 (33.7%) knees in the combined study sample (K/L grade 0 knees). 328 (30.6%) knees without meniscal damage and 133 (44.9%) knees with meniscal damage exhibited joint effusion. 119 (43.1%) knees with unicompartmental meniscal damage and 14 (70.0%) knees with pathology in two compartments showed joint effusion. The large majority of joint effusions were small (Table 3).
Table 3.
Prevalence of joint effusion and relation to meniscal damage
| Meniscal damage | Joint effusion |
||
|---|---|---|---|
| 0 (%) | 1 (%) | 2–3 (%) | |
| Absence (N= 1072) | 744 (69.4) | 295 (27.5) | 33 (3.1) |
| Presence (N= 296) | 163 (55.1) | 109 (36.8) | 24 (8.1) |
| Present in one compartment (N=276) | 157 (56.9) | 96 (34.8) | 23 (8.3) |
| Present in both compartments (N=20 ) | 6 (30.0) | 13 (65.0) | 1 (5.0) |
The adjusted odds ratio (95% confidence interval) of any joint effusion in knees with meniscal damage was 1.8 (1.4, 2.4) using knees without meniscal damage as the reference. The odds ratio of joint effusion for the subgroup with damage in only one compartment was 1.7 (1.3, 2.2) and 5.4 (2.1, 14.3) for the group with pathology in two compartments (Table 4).
Table 4.
Cross-sectional association of joint effusion (all grades ≥ 1) with meniscal damage (any grades ≥1 in any subregion) on MRI for all knees without radiographic osteoarthritis (K/L grade = 0)
| Meniscal damage | Joint effusion |
Adjusted OR* | |
|---|---|---|---|
| 0 (%) | 1–3 (%) | ||
| Absence (N=1073) | 744 (69.4) | 328 (30.6) | 1.0 (reference) |
| Presence (N=296) | 163 (55.1) | 133 (44.9) | 1.8 (1.4, 2.4) |
| Present in one compartment (N=276) | 157 (56.9) | 119 (43.1) | 1.7 (1.3, 2.2) |
| Present in both compartments (N=20) | 6 (30.0) | 14 (70.0) | 5.4 (2.1, 14.3) |
adjusting for age, sex, BMI, and study site
Joint effusion in K/L grade 0 knees without cartilage defects and its association with meniscal damage
Joint effusion (any grade) was present in 60 (15.7%) knees in the subgroup without MRI-detected cartilage damage. 45 (14.0%) knees without meniscal damage and 15 (25.0%) knees with meniscal damage exhibited joint effusion.
The adjusted odds ratio (95% confidence interval) of any joint effusion in a knee with meniscal damage was 2.3 (1.1, 4.5), with knees without meniscal damage as the reference group (Table 5).
Table 5.
Cross-sectional association of joint effusion (all grades ≥ 1) with meniscal damage (any grades ≥1 in any subregion) on MRI for knees without cartilage damage only (n=381)
| Meniscal damage | Joint effusion |
Adjusted OR* | |
|---|---|---|---|
| 0 (%) | 1–3 (%) | ||
| Absence (N=321) | 276 (86.0) | 45 (14.0) | 1.0 (reference) |
| Presence (N=60) | 45 (75.0) | 15 (25.0) | 2.3 (1.1, 4.7) |
adjusting for age, sex, BMI, and study site
DISCUSSION
In a large sample of middle-aged and elderly adults with no signs of radiographic OA from two large osteoarthritis studies, we examined the association of meniscal damage and joint effusion. A significant association was observed that was also confirmed for the subgroup without cartilage damage.
Our findings suggest that synovial activation reflected as joint effusion on MRI may be triggered by meniscal pathology, which is supported by the findings of previous work on animal models. These studies described marked synovial stimulation during the healing phase of meniscal tears or enhancement of repair through synovial activation13,14,24. However, we cannot comment on whether tears and effusion are truly subsequent events or if effusion might precede meniscal damage or might even have a damaging effect on meniscal composition, which will ultimately result in a tear. Only a longitudinal study with multiple visits over short periods of time might clinically be able to show the train of events.
MRI diagnosis of meniscal pathology is reliable and well-accepted in clinical practice, and has been reported to have a sensitivity and specificity in the range of 80–95% compared with a reference standard of arthroscopic inspection and probing25,26. Our findings suggest that meniscal tears are common in knees without radiographic OA. About a fifth of the knees (all K/L grade 0) exhibited meniscal damage in our study sample, while in the whole population-based Framingham study sample of middle-aged and elderly persons (irrespective of radiographic OA status and symptoms) about one third of knees had meniscal damage10. In addition, a recent study that examined a small cohort of asymptomatic postmenopausal women reported meniscal tears in nearly half of the knees27.
We found that one third of the knees in our sample showed joint effusion. In another large study examining the probability of radiographic OA in elderly patients with knee pain, the subgroup without radiographic OA (K/L =0 and 1) exhibited palpable joint effusion in 21%28. The amount of moderate to gross effusion in that study was higher than in our cohort, which showed large grade 2 and 3 effusions in only 4%. These discrepant findings are explained by the higher sensitivity of MRI for the detection of joint effusion compared to physical examination. Secondly, the two study samples are only marginally comparable despite having no radiographic OA. Hayes et al. detected joint effusion in about 10% of the knees in their mixed study sample concerning OA status and meniscal tears in 27%29. This group used a different effusion definition which might further explain the discrepant number to our findings. A large study assessing inflammation in painful knee OA using ultrasound found a prevalence of effusion in 44% of the examined knees30. There are no comparable population-based studies available that describe non-traumatic joint effusion in a population without knee OA. In the subgroup without any cartilage damage joint effusion was observed in several knees that exhibited no concomitant meniscal damage. One reason explaining this finding is that intrameniscal pathology that did not fulfill the criteria of damage or other intraarticular lesions might have been present in these knees. It also has been shown that a large number of radiographically normal knees exhibit some intraarticular pathology on MRI31.
There are several limitations to our study that bear mention. Some other underlying conditions that may trigger non-traumatic effusion despite meniscal damage must be considered in the differential diagnosis. The most common reasons for non-traumatic effusion beside osteoarthritis are infection, rheumatic disease, crystal induced arthropathies, other more rare rheumatic conditions and tumor32–34. Patients were screened for rheumatoid arthritis with a validated screening tool and those who screened positive were excluded prior to participation in the study. The MRI analysis did not suggest a knee tumor or signs of a septic joint that might be attributable to effusion. A limitation of the WORMS method of semiquantitative meniscal scoring is that it does not differentiate between a meniscal tear and partial resection or maceration. Also mucoid degeneration, a common finding of unknown clinical relevance is not scored in WORMS.
In summary, we found a prevalence of meniscal damage in about a fifth and effusion in about one third of knees. After adjusting for age, gender, BMI and study cohort we found a significant association of joint effusion for knees with meniscal damage when compared to knees without damage. Also, the presence of joint effusion was even more frequent when meniscal tears were present in both tibiofemoral compartments. As meniscal tears are a potent risk factor for the development of OA35 the presence of an isolated joint effusion might place subjects also at greater risk for subsequent OA. The described association of meniscal damage and concomitant joint effusion should warrant further longitudinal investigation to help understand the role of synovial activation in early knee OA.
Acknowledgments
We wish to acknowledge the support of the staff of the Framingham and MOST osteoarthritis studies, especially at the Coordinating Center in San Francisco. We further would like to thank the participants of the Framingham and MOST studies.
Funding Sources This work was supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (Contract No. N01-HC-25195). The Framingham Osteoarthritis Study is supported by NIH grants AG18393 and AR47785.
The MOST Study is supported by NIH grants from the National Institute on Aging to Drs. Lewis (U01-AG-18947), Torner (U01-AG-18832), Nevitt (U01-AG-19069), and Felson (U01-AG-18820). The study sponsors were not involved in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflict of interest statement Ali Guermazi is president of Boston Imaging Core Lab, LLC (BICL), a company providing radiological image assessment services. He is shareholder of Synarc, Inc.
Frank Roemer and David Hunter are shareholders of BICL.
REFERENCES
- 1.Poehling GG, Ruch DS, Chabon SJ. The landscape of meniscal injuries. Clin Sports Med. 1990;9:539–49. [PubMed] [Google Scholar]
- 2.Noble J, Hamblen DL. The pathology of the degenerate meniscus lesion. J Bone Joint Surg Br. 1975;57:180–6. [PubMed] [Google Scholar]
- 3.Noble J. Lesions of the menisci. Autopsy incidence in adults less than fifty-five years old. J Bone Joint Surg Am. 1977;59:480–3. [PubMed] [Google Scholar]
- 4.Kurosawa H, Fukubayashi T, Nakajima H. Load-bearing mode of the knee joint: physical behavior of the knee joint with or without menisci. Clin Orthop Relat Res. 1980:283–90. [PubMed] [Google Scholar]
- 5.Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975:184–92. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Hunter DJ, Zhang YQ, Niu J, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 7.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50:2811–9. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale ME, McLaughlin S, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am. 2003;85-A:4–9. doi: 10.2106/00004623-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Gale DR, Chaisson CE, Totterman SM, Schwartz RK, Gale ME, Felson D. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage. 1999;7:526–32. doi: 10.1053/joca.1999.0256. [DOI] [PubMed] [Google Scholar]
- 10.Englund M, Hunter DJ, Gale D, Clancy M, Guermazi A, Aliabadi P, et al. Meniscal MRI signal abnormalities including tears and their association with knee symptoms and osteoarthritis in the community. Arthritis Rheum. [abstract] 2006 Sep;54(9):S512–13. 2006. [Google Scholar]
- 11.Zanetti M, Pfirrmann CW, Schmid MR, Romero J, Seifert B, Hodler J. Patients with suspected meniscal tears: prevalence of abnormalities seen on MRI of 100 symptomatic and 100 contralateral asymptomatic knees. AJR Am J Roentgenol. 2003;181:635–41. doi: 10.2214/ajr.181.3.1810635. [DOI] [PubMed] [Google Scholar]
- 12.Aigner T, van der Kraan P, van den Berg W. Osteoarthritis and Inflammation - inflammatory changes in osteoarthritis synoviopathy. In: Buckwalter JA, Lotz M, Stoltz JF, editors. Osteoarthritis, Inflammation and Degradation: a Continuum. IOS Press; Amsterdam: 2007. pp. 219–35. [Google Scholar]
- 13.Okuda K, Ochi M, Shu N, Uchio Y. Meniscal rasping for repair of meniscal tear in the avascular zone. Arthroscopy. 1999;15:281–6. doi: 10.1016/s0749-8063(99)70035-6. [DOI] [PubMed] [Google Scholar]
- 14.Jitsuiki J, Ochi M, Ikuta Y. Meniscal repair enhanced by an interpositional free synovial autograft: an experimental study in rabbits. Arthroscopy. 1994;10:659–66. doi: 10.1016/s0749-8063(05)80065-9. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes LA, Grainger AJ, Keenan AM, Thomas C, Emery P, Conaghan PG. The validation of simple scoring methods for evaluating compartment-specific synovitis detected by MRI in knee osteoarthritis. Rheumatology (Oxford) 2005;44:1569–73. doi: 10.1093/rheumatology/kei094. [DOI] [PubMed] [Google Scholar]
- 16.Grainger AJ, Rhodes LA, Keenan AM, Emery P, Conaghan PG. Quantifying peri-meniscal synovitis and its relationship to meniscal pathology in osteoarthritis of the knee. Eur Radiol. 2007;17:119–24. doi: 10.1007/s00330-006-0282-6. [DOI] [PubMed] [Google Scholar]
- 17.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The development and reliability of a new scoring system for knee osteoarthritis MRI: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67(2):206–11. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 19.Kornaat PR, Ceulemans RY, Kroon HM, Riyazi N, Kloppenburg M, Carter WO, et al. MRI assessment of knee osteoarthritis: Knee Osteoarthritis Scoring System (KOSS)--inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skeletal Radiol. 2005;34:95–102. doi: 10.1007/s00256-004-0828-0. [DOI] [PubMed] [Google Scholar]
- 20.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–25. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 21.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5:297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 22.Peterfy CG, Lynch JA, Miaux Y, Genant HK. Nonfluoroscopic method for flexed radiography of the knee that allows reproducible joint-space width measurement. Arthritis Rheum. 1998;41:S361. [Google Scholar]
- 23.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobuna Y, Shirakura K, Niijima M. Meniscal repair using a flap of synovium. An experimental study in the dog. Am J Knee Surg. 1995;8:52–5. [PubMed] [Google Scholar]
- 25.De Smet AA, Tuite MJ. Use of the “two-slice-touch” rule for the MRI diagnosis of meniscal tears. AJR Am J Roentgenol. 2006;187:911–4. doi: 10.2214/AJR.05.1354. [DOI] [PubMed] [Google Scholar]
- 26.Crues JV, 3rd, Mink J, Levy TL, Lotysch M, Stoller DW. Meniscal tears of the knee: accuracy of MR imaging. Radiology. 1987;164:445–8. doi: 10.1148/radiology.164.2.3602385. [DOI] [PubMed] [Google Scholar]
- 27.Davies-Tuck ML, Martel-Pelletier J, Wluka AE, Pelletier JP, Ding C, Jones G, et al. Meniscal tear and increased tibial plateau bone area in healthy post-menopausal women. Osteoarthritis Cartilage. 2007 doi: 10.1016/j.joca.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Peat G, Thomas E, Duncan R, Wood L, Wilkie R, Hill J, et al. Estimating the probability of radiographic osteoarthritis in the older patient with knee pain. Arthritis Rheum. 2007;57:794–802. doi: 10.1002/art.22785. [DOI] [PubMed] [Google Scholar]
- 29.Hayes CW, Jamadar DA, Welch GW, Jannausch ML, Lachance LL, Capul DC, et al. Osteoarthritis of the knee: comparison of MR imaging findings with radiographic severity measurements and pain in middle-aged women. Radiology. 2005;237:998–1007. doi: 10.1148/radiol.2373041989. [DOI] [PubMed] [Google Scholar]
- 30.D'Agostino MA, Conaghan P, Le Bars M, Baron G, Grassi W, Martin-Mola E, et al. EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: prevalence of inflammation in osteoarthritis. Ann Rheum Dis. 2005;64:1703–9. doi: 10.1136/ard.2005.037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guermazi A, Hunter DJ, Roemer FW, Niu J, McLennan CE, Felson DT. MRI prevalence of different features of knee osteoarthritis in persons with normal knee X-rays. [abstract] Arthritis Rheum. 2007;56(9):S128–129. [Google Scholar]
- 32.Munn RK, Pierce ST, Sloan D, Weeks JA. Malignant joint effusions secondary to solid tumor metastasis. J Rheumatol. 1995;22:973–5. [PubMed] [Google Scholar]
- 33.Kane D, Balint PV, Sturrock RD. Ultrasonography is superior to clinical examination in the detection and localization of knee joint effusion in rheumatoid arthritis. J Rheumatol. 2003;30:966–71. [PubMed] [Google Scholar]
- 34.Johnson MW. Acute knee effusions: a systematic approach to diagnosis. Am Fam Physician. 2000;61:2391–400. [PubMed] [Google Scholar]
- 35.Englund M, Guermazi A, Roemer FW, Yang M, Lewis CE, Torner J, et al. The Effect of Meniscal Damage on Incident Radiographic Knee Osteoarthritis [abstract] Arthritis Rheum. 2007;56(9):S316. doi: 10.1002/art.23071. [DOI] [PubMed] [Google Scholar]