Abstract
Rats treated with (±)-3,4-methylenedioxymethamphetamine (MDMA) or (+)-methamphetamine (MA) neonatally exhibit long-lasting learning impairments (i.e., after treatment on postnatal days (P)11–15 or P11–20). Although both drugs are substituted amphetamines, they each produce a unique profile of cognitive deficits (i.e., spatial vs. path integration learning and severity of deficits) which may be the result of differential early neurochemical changes. We previously showed that MA and MDMA increase corticosterone (CORT) and MDMA reduces levels of serotonin (5-HT) 24 h after treatment on P11, however, learning deficits are seen after 5 or 10 days of drug treatment, not just 1 day. Accordingly, in the present experiment, rats were treated with MA or MDMA starting on P11 for 5 or 10 days (P11–15 or P11–20) and tissues collected on P16, P21, or P30. Five-day MA administration dramatically increased CORT on P16, whereas MDMA did not. Both drugs decreased hippocampal 5-HT on P16 and P21, although MDMA produced larger reductions. Ten-day treatment with either drug increased dopamine utilization in the neostriatum on P21, whereas 5-day treatment had no effect. No CORT or brain 5-HT or dopamine changes were found with either drug on P30. Although the monoamine changes are transient, they may alter developing neural circuits sufficiently to permanently disrupt later learning and memory abilities.
Keywords: 3,4-methylenedioxymethamphetamine; corticosterone; development; dopamine; methamphetamine; serotonin
Substituted amphetamines, including 3,4-methylenedioxymethamphetamine (MDMA) and methamphetamine (MA), are being abused by women of childbearing age (EMCDDA 2006; Johnston et al. 2007). MDMA is known for its popularity at raves, or all night dance parties, although its use is increasingly being documented in suburbs, rural areas, and college campuses (Wish et al. 2006). The abuse of MA is a worldwide problem because of its ease of production, low cost, stimulatory effects, and addictive properties (Rawson et al. 2002). Both MDMA and MA are thought to increase high-risk sexual behavior that may lead to pregnancy and potentially fetal exposure (Buffum 1988; Degenhardt 2005; McElrath 2005). Pregnant women have been reported to abuse MDMA or MA into the third trimester and up to the point of delivery (Ho et al. 2001; Smith et al. 2003; Chomchai et al. 2004). It is suspected that these exposures to MDMA or MA during fetal development have lasting effects that alter neural development and subsequent cognitive function, but this remains to be proven.
To investigate long-term consequences of MA or MDMA, we have used the neonatal rat as a model of late second through third trimester human brain development, specifically hippocampal and cortical development (Bayer et al. 1993; Herlenius and Lagercrantz 2004; Clancy et al. 2007). Although MA and MDMA are both amphetamine analogues, there are differences between the two in the behavioral outcomes of animals exposed to either drug neonatally. For example, we find that both MDMA or MA, administered from postnatal day (P) 11–20, produce spatial learning deficits in the Morris water maze (MWM), however, neonates exposed to MDMA exhibited more severe deficits (Vorhees et al. 2000a, 2004; Broening et al. 2001; Williams et al. 2002, 2003b,c; Schaefer et al. 2006; Skelton et al. 2006) than those exposed to MA. We also find that MDMA, but not MA, produces deficits in the Cincinnati water maze, a task solved using a combination of spatial and path integration learning when tested under fluorescent or low-level visible red light conditions (Broening et al. 2001; Williams et al. 2003b,c; Skelton et al. 2006). Furthermore, we find that the majority of the spatial learning effects after MA are attributable to the first 5 days (P11–15) of MA treatment (Williams et al. 2003a); shorter treatment periods have not yet been examined with MDMA. In addition to long-term cognitive changes, MDMA and MA exposure produces hypoactivity following neonatal exposure (Vorhees et al. 1994, 2007). Neonatal mice have also been shown to be affected in spatial learning and novel location recognition following neonatal MA administration (Acevedo et al. 2007). These differences in behavior following MA or MDMA treatment are likely the result of differential effects on neural development.
Brain development is influenced by changes in hormonal and monoamine levels including corticosterone (CORT) and serotonin (5-HT). Disruptions of the processes these signaling molecules control during development can cause short-and long-term changes in brain physiology and cognitive function. Although it is recognized that some level of CORT is essential for neuronal development, excess CORT has been shown to produce decreases in hippocampal cell proliferation (Woolley et al. 1990; Gould et al. 1991a; Liu et al. 2003). The hippocampus is important for several types of learning (i.e., spatial learning) and undergoes neurogenesis and neuronal pruning during the first few weeks of life in the rat (Gould et al. 1991a; Bayer et al. 1993). In addition, 5-HT is one of the first monoamines to appear in the developing brain and is thought to be required for neurogenesis, the development of serotonergic target fields, and organization of some areas of the brain (Lauder and Krebs 1978; Lauder 1990; Whitaker-Azmitia et al. 1996). p-Chlorophenylalanine-induced inhibition of 5-HT synthesis, after essentially the same days of exposure (P10–20) that we find to be sensitive for MDMA and MA (i.e., P11–20), altered synaptogenesis and produced deficits in learning (Mazer et al. 1997). Taken together, these data suggest that factors influencing CORT and/or 5-HT may disrupt cognitive development.
We previously showed that 1 day of MDMA treatment produces significant increases in CORT and decreases in 5-HT and MA treatment produces only increases in CORT levels 24 h later with MA producing the largest increase in CORT and MDMA producing the largest decrease in 5-HT (Schaefer et al. 2006). In addition, we showed that P11 exposure to MDMA produces CORT increases and 5-HT decreases within 1 h after exposure and that 5-HT decreases are still apparent 78 h following 1 day of treatment (Williams et al. 2005). In other experiments, we showed that MA produces increases in CORT 30 min following single or multiple days of exposure (Williams et al. 2000, 2006). These experiments represent the effects of these drugs at the beginning of the exposure intervals that lead to later learning and memory impairments, but the effects of multiple day treatment (P11–15 or P11–20) remain to be elucidated. Therefore, the purpose of this experiment was to investigate the effects of MDMA and MA on CORT and monoamine levels 24 h after the first treatment on the last day of exposure (P16 or P20) and on P30 following either 5 days (P11–15) or 10 days (P11–20) of exposure.
Materials and methods
Animals and housing
Nulliparous female Sprague–Dawley CD, International Genetic Strain, rats (Charles River Laboratories, Raleigh, NC, USA) were mated with males of the same strain and supplier. Rats were housed in a 22 ± 1°C environment at 50 ± 10% humidity with a 14/10 h light/dark cycle (lights on at 600 h). Prior to mating, a period of at least 1 week ensued to allow the animals to acclimate to the vivarium. Each female spent 2 weeks housed with one male in hanging wire cages before being housed singly in a polycarbonate cage (46 cm × 24 cm × 20 cm) containing wood chip bedding and ad libitum food and water. Pups from the resulting litters were used as subjects. The Cincinnati Children’s Research Foundation’s Institutional Animal Care and Use Committee approved all protocols and the vivarium was fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC).
The day of birth was considered P0, and on P1 litters were culled to eight pups with six males and two females. On P7, pups were weighed and individually identified by ear-punch. Only males were used for this study for several reasons. First, we have previously shown only subtle if any drug × sex interactions on learning and memory following neonatal MA or MDMA administration in rats (Broening et al. 1994, 2001; Vorhees et al. 2000a,b; Morford et al. 2002; Williams et al. 2003a,b,c). Second, our model produces no significant differences between males and females in adrenal-pituitary hormone levels during neonatal MA exposure (Williams et al. 2000) with only slight differences following exposure from P11–20 (Skelton et al. 2007). In regard to MDMA, no sex differences in CORT or monoamine levels have been observed (Williams et al. 2005). It should be noted that others have suggested that sex dependent changes exist following MA exposure in neonatal rats and mice. For example, some have found sex differences in dopamine (DA) levels in rats (Gomes-da-Silva et al. 2004) and behavioral differences in mice following neonatal MA (Acevedo et al. 2007). Nonetheless, taken together with the fact we wanted to maintain similar litter sizes between the current study and previous experiments, and the minor and sparsely observed sex differences during or after the drug dosing, only males were used.
Dosing procedures
(+)-Methamphetamine HCl and (±)-3,4-methylenedioxymetham-phetamine HCl (National Institute on Drug Abuse), were dissolved in isotonic saline (SAL) at a dose of 10 mg/kg (each expressed as the freebase; purity >95%) in a volume of 3 mL/kg. On each day of drug administration one animal from each litter received one injection every 2 h for a total of four injections of MA, MDMA, or SAL from P11–15 or P11–20. An animal’s weight was recorded prior to each drug treatment, however, only the first (‘am’) and last (‘pm’) weight taken on each day were used for body weight analysis. Injection sites were varied to prevent irritation to the dermis.
As noted, we investigated two dosing periods (P11–15 and P11–20). These were chosen based on prior behavioral experiments that demonstrated long-term effects using these exposure periods (Vorhees et al. 2000a, 2004; Broening et al. 2001; Williams et al. 2002, 2003a,b,c; Skelton et al. 2006). Two different time points were analyzed for each dosing regimen: 24 h after the first dose on the last day of drug treatment (P16 following P11–15 dosing or P21 following P11–20 dosing) or on P30. Therefore, the treatment groups were as follows: (i) treated from P11–15 and killed 24 h later on P16 (n = 13 litters); (ii) treated from P11–15 and killed on P30 (n = 6 litters); (iii) treated from P11–20 and killed 24 h later on P21 (n = 16 litters); and (iv) treated from P11–20 and killed on P30 (n = 8 litters). Within each litter, three animals (one each treated with MA, MDMA, or SAL) were dosed and killed at the earlier age (P16 or P21) and the other three animals in the litter (one each treated with MA, MDMA, or SAL) were killed on P30. In this design, treatment group was balanced for litter membership.
Blood and tissue collection
Each animal was decapitated at the appropriate time point and blood was collected in tubes containing 2% EDTA (0.05 mL) and then centrifuged (1399 RCF) for 25 min at 4°C. Plasma was collected and stored at −80°C until assayed for CORT.
The brain was simultaneously removed and placed in a chilled brain block (Zivic-Miller, Pittsburgh, PA, USA) to aid in dissection of the neostriatum and hippocampus as defined previously (Williams et al. 2007). Briefly, for the neostriatum, a coronal cut was made at the optic chiasm and then another cut made 2 mm rostral to the first. The neostriatum (caudate-putamen) was dissected from this 2 mm slab. Hippocampi were removed from the remaining tissue. Dissections were done on a chilled dissection plate and tissues were immediately frozen on dry ice. Tissue weights were determined by placing the tissues in pre-weighed tubes and then re-weighing the tubes. All tissues were stored at −80°C until monoamines were quantified by high-pressure liquid chromatography with electrochemical detection.
Corticosterone assessment in plasma
Plasma was diluted 3: 1 for CORT in a kit specific assay buffer prior to the determination of hormone. CORT was assayed in duplicate using a commercially available EIA (Immunodiagnostic Systems Inc., Fountain Hills, AZ, USA) and measured on a SpectraMax Plus microtiter plate reader (Molecular Devices, Sunnyvale, CA, USA). The CORT EIA has little cross-reactivity with other hormones or precursors (<1.4%) with the minor exceptions of 11-dehydrocorticosterone and 11-deoxycorticosterone (<6.7%).
Monoamine determinations
The tissue concentrations of DA, 3,4-dihydroxyphenylacetic acid (DOPAC), 5-HT, and 5-hydroxyindolacetic acid (5-HIAA) in the neostriatum and 5-HT and 5-HIAA in the hippocampus were quantified using high-pressure liquid chromatography with electrochemical detection (Schaefer et al. 2006). Briefly, tissues were homogenized in 50 volumes of 0.2 N perchloric acid and centrifuged for 6 min at 10 000 g. Aliquots of 20 μL were injected onto a C18-column (MD-150, 3 × 150 mm; ESA, Chelmsford, MA, USA) connected to either a LC-4B amperometric detector (Bioanalytical Systems, West Lafayette, IN, USA) or a Coulochem detector (25A, Chemsford) and an integrator recorded the peak heights for each injection. The potential for the LC-4B was 0.6 V and the potentials of E1 and E2 on the analytical cell (model 5014B) of the Coulochem were −150 and 160 mV, respectively. The mobile phase consisted of 35 mmol/L citric acid, 54 mmol/L sodium acetate, 50 mg/L of disodium ethylenediamine tetraacetate, 70 mg/L of octanesulfonic acid sodium salt, 6% (v/v) methanol, 6% (v/v) acetonitrile, pH 4.0, and pumped at a flow rate of 0.4 mL/min. Quantification of the analytes was calculated on the basis of known standards. Retention times for DOPAC, DA, 5-HIAA, and 5-HT were approximately 6, 8, 11, and 17 min, respectively.
Statistics
Data were analyzed with repeated measures ANOVA utilizing the general linear modeling procedure (Proc GLM, SAS Institute, Cary, NC, USA). Body weights during dosing were subjected to a separate ANOVA for the P11–15 and for the P11–20 treatment regimens. Separate ANOVAS were performed on neurochemical and CORT data for each of the two killing days within the two exposure periods (5-day exposure, P16; 5-day exposure, P30; 10-day exposure, P21; 10-day exposure, P30). Litter membership was treated as a matching factor, therefore, drug treatment was handled in the ANOVA as a repeated measures factor as per (Kirk 1995) and (Winer 1978). The experimental unit was the litter at each time-point (Holson and Pearce 1992). Significant main effects were analyzed further to localize differences using the step-down F-test procedure (Kirk 1995). Simple effect F-tests were used to analyze significant interactions followed by step-down F-tests for individual group comparisons. Only drug treatment main effects and interaction F-ratios are shown for clarity of data presentation. Significance was set at p ≤ 0.05.
Results
Body weight
Animals treated from P11–15 showed significant main effects of Treatment (F2,38 = 19.97, p < 0.0001), Day (p < 0.0001), and Dosing time, i.e., am or pm [see Materials and methods for explanation (p < 0.0001)], and the highest order significant interaction was Treatment × Day × Dosing time (F8,152 = 2.96, p < 0.004). All animals showed increased weight gain from P11–15, however, analysis of the interaction showed that MDMA-treated animals weighed less than SAL-treated animals beginning on P12 and through the remainder of drug treatment. MA-treated animals weighed the least compared to SAL- and MDMA-treated animals beginning on P13 and throughout the rest of the treatment period (Fig. 1a).
Fig. 1.
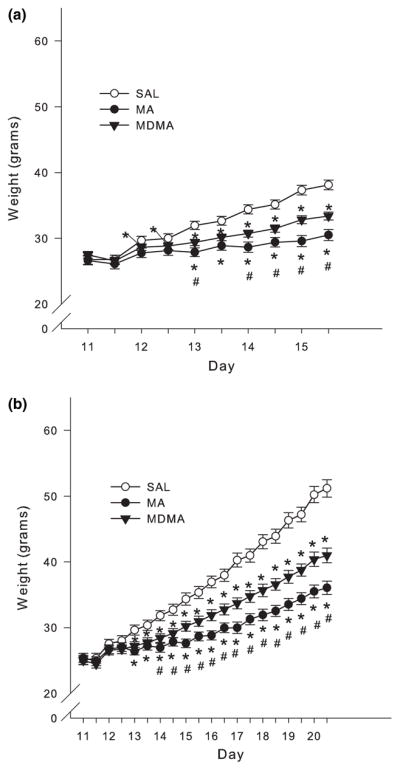
Body weights taken during the first and last dose (mean ± SEM). (a) P11–15 and (b) P11–20 administration of MA or MDMA (10 mg/kg) four times each day with an inter dose interval of 2 h caused a decrease in body weight gain compared to SAL controls beginning on approximately the third day of dosing. MA produced the least amount of body weight gain compared to MDMA and SAL which was evident beginning on approximately P14 in both groups. *p < 0.05 from SAL control; #p < 0.05 from MDMA and MA are different.
For animals treated from P11–20 there were significant effects of Treatment (F2,40 = 33.18, p < 0.0001), Day (p < 0.0001), Dosing time (p < 0.0001), and the interaction of Treatment × Day × Dosing time (F18,360 = 2.02, p < 0.009). Examination of the interaction revealed that MDMA and MA treatment prevented animals from gaining weight similarly to SAL-treated animals beginning on P13 and these differences continued through the remainder of the treatment period. MA treatment produced the greatest decrease in weight gain since it was different from MDMA treatment beginning on P14 and lasted through the remainder of dosing (Fig. 1b). This pattern in body weights continued through P28 (data not shown).
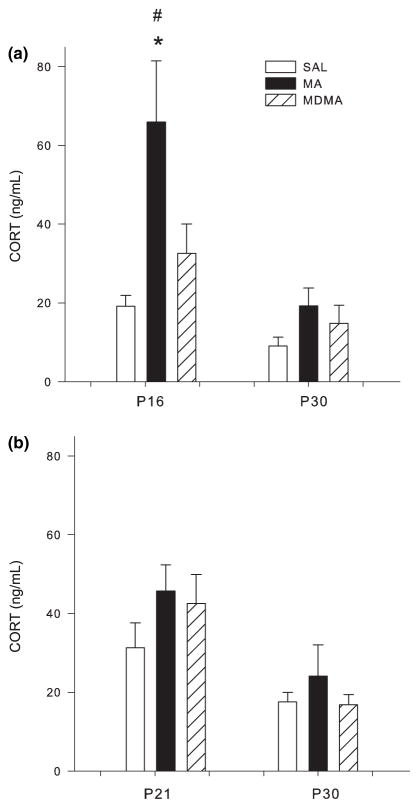
Corticosterone
Following drug treatment from P11–15, CORT in plasma on P16 was significantly affected by treatment (F2,24 = 6.77 p < 0.005). Step-down analysis demonstrated significant CORT increases in the animals treated with MA compared to those treated with either SAL or MDMA (Fig. 2a). No effect of MDMA was observed at this time point. For animals examined on P30, no differences in CORT were observed in either drug group (Fig. 2a).
Fig. 2.
Corticosterone (CORT) concentrations in plasma (mean ± SEM). (a) CORT levels following P11–15 dosing of MDMA or MA (10 mg/kg) 24 h after the first of four doses on P15 (killed on P16) and CORT levels on P30 following P11–15 dosing. (b) CORT levels on P21 following P11–20 dosing and CORT levels on P30 following P11–20 dosing. On P16 CORT levels were significantly increased following MA treatment compared to both SAL and MDMA treatments. There were no changes observed in CORT levels on P21 or on P30 following either drug or dosing regimen. *p < 0.05 from SAL control; #p < 0.05 from MDMA and MA are different.
Following P11–20 treatment, no differences in CORT were observed on either P21 or on P30 (Fig. 2b).
Monoamines
Neostriatum
5-HT levels in the neostriatum following P11–15 dosing were significantly affected by treatment on P16 (F2,24 = 8.01 p < 0.003). Post hoc analysis demonstrated a significant decrease in neostriatal 5-HT only in MDMA-treated animals compared to SAL-treated animals. No differences were detected among the treatments on P30 (Fig. 3a).
Fig. 3.
Serotoninergic markers in neostriatum following P11–15 drug administration on P16 (n = 13/group) and P30 (n = 6/group; mean ± SEM). (a) P16 concentrations of 5-HT and P30 5-HT. (b) P16 5-HIAA and P30 5-HIAA. (c) P16 5-HIAA/5-HT ratio and P30 5-HIAA/5-HT ratio in the neostriatum of animals exposed to 5 days of MDMA or MA. Decreases in 5-HT were observed in the MDMA-treated animals on P16. 5-HIAA levels were decreased following MDMA and MA on P16. There were no differences in the 5-HIAA/5-HT ratio on P16 or any of the neostriatal serotonergic markers on P30. *p < 0.05 from SAL control; #p < 0.05 from MDMA.
Neostriatal 5-HIAA was also affected by treatment on P16 (F2,24 = 46.8 p < 0.0001). Post hoc analysis showed significantly decreased 5-HIAA levels in MA- and MDMA-treated animals compared to SAL-treated animals. Animals exposed to MDMA had the largest decreases in 5-HIAA. No differences were observed on P30 among treatment groups (Fig. 3b).
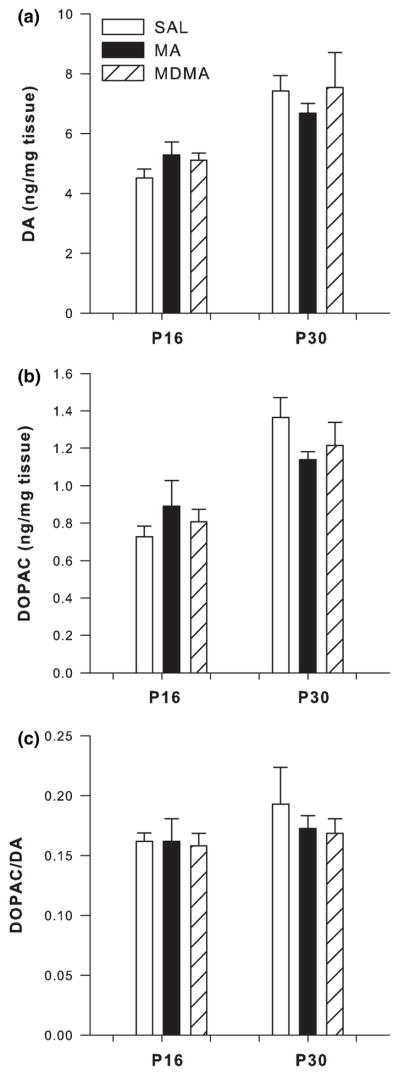
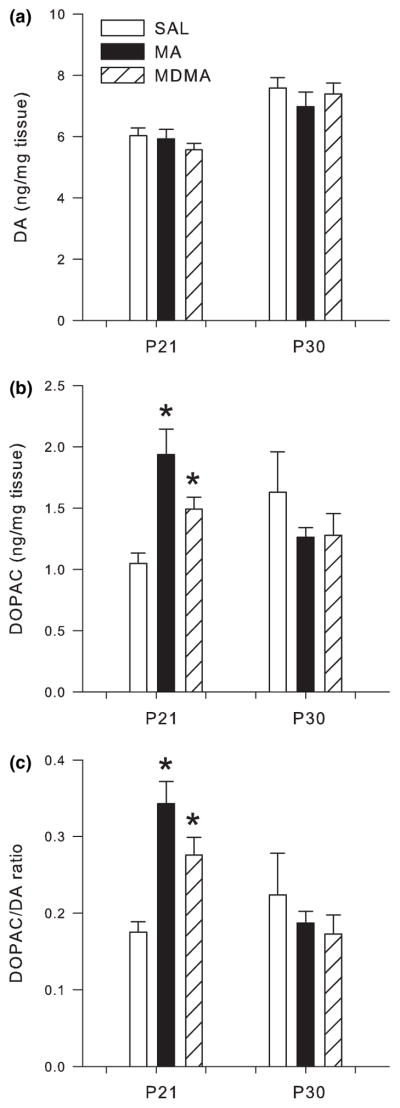
In the neostriatum no changes were observed on either P16 or on P30 in the ratio of 5-HIAA/5-HT or DA, DOPAC, or the ratio of DOPAC/DA (Figs 3c, 4a–c, respectively).
Fig. 4.
Dopaminergic markers in neostriatum following P11–15 drug administration on P16 (n = 13/group) and P30 (n = 6/group; mean ± SEM). (a) P16 concentrations of DA and P30 DA. (b) P16 DOPAC and P30 DOPAC. (c) P16 DOPAC/DA ratio and P30 DOPAC/DA ratio in the neostriatum on P16 following administration of MDMA or MA. No changes in any dopaminergic markers were observed following 5 days of drug treatment at the 24 h time-point or on P30.
Following 10 days of drug administration from P11–20, 5-HT in the neostriatum was significantly affected by Treatment on P21 (F2,30 = 11.27 p < 0.0002) but not on P30 (Fig. 5a). Post hoc analysis revealed that both MA and MDMA treatment significantly decreased neostriatal 5-HT on P21.
Fig. 5.
Serotonergic markers in the neostriatum following P11–20 drug administration on P21 (n = 16/group) and P30 (n = 8/group; mean ± SEM). (a) P21 concentrations of 5-HT and P30 5-HT. (b) P21 5-HIAA and P30 5-HIAA. (c) P21 5-HIAA/5-HT ratio and P30 5-HIAA/5-HT ratio in the neostriatum of animals dosed for 10 days with MDMA or MA. On P21, 5-HT, and 5-HIAA levels were decreased following both MDMA and MA treatments. No other differences were noted in the neostriatum of P11–20 treated animals. *p < 0.05 relative to saline controls.
Neostriatal 5-HIAA was also affected by Treatment on P21 following P11–20 dosing (F2,30 = 27.87 p < 0.0001). Post hoc analysis showed significantly decreased 5-HIAA levels in MA- and MDMA-treated animals compared to SAL-treated animals on P21. No differences were demonstrated among treatment groups on P30 (Fig. 5b). Similarly, no changes in the 5-HIAA/5-HT ratio were observed on P21 or P30 (Fig. 5c).
In the neostriatum, DA levels were unaffected on P21 or P30 (Fig. 6a). However, DOPAC levels were affected by treatment (F2,30 = 14.70 p < 0.0001) on P21. Post hoc analysis revealed neostriatal DOPAC was significantly increased on P21 in both the MA- and MDMA-treated groups compared to SAL-treated animals. No treatment group differences were observed on P30 (Fig. 6b).
Fig. 6.
Dopaminergic markers in neostriatum following P11–20 drug administration on P21 (n = 16/group) and P30 (n = 8/group; mean ± SEM). (a) P21 concentrations of DA and P30 DA. (b) P21 DOPAC and P30 DOPAC. (c) P21 DOPAC/DA ratio and P30 DOPAC/DA ratio in the neostriatum on P21 following administration of MDMA or MA. No changes in DA were observed following 10 days of drug administration. DOPAC and the DOPAC/DA ratio were increased following MDMA and MA compared to SAL treatment on P21. No differences in dopaminergic markers were noted on P30. *p < 0.05 relative to saline controls.
The DOPAC/DA ratio in the neostriatum showed a significant treatment effect (F2,28 = 14.75, p < 0.0001) on P21, but not on P30. As with DOPAC, both MA and MDMA treatments produced a significant increase in the DOPAC/DA ratio compared to SAL treatment (Fig. 6c).
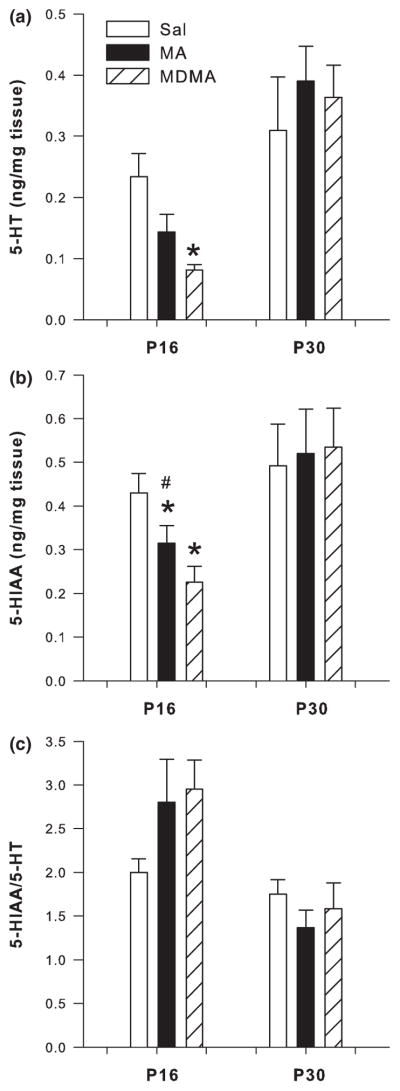
Hippocampus
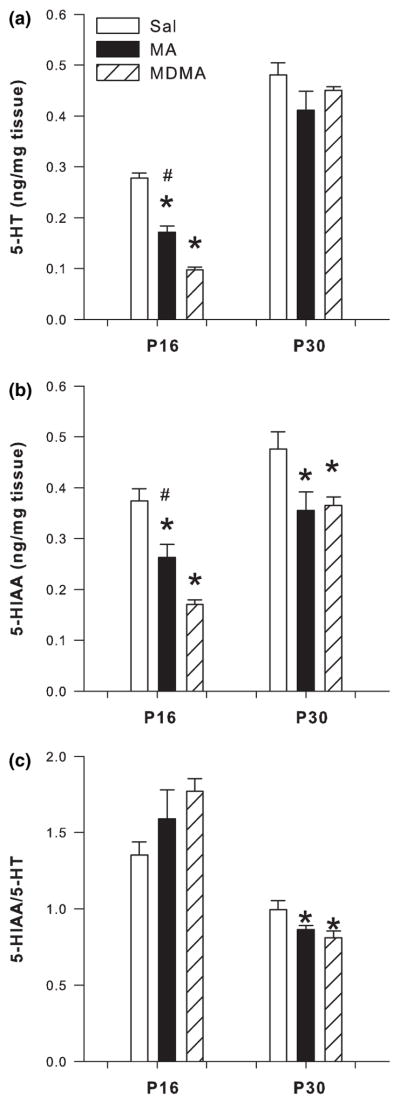
Analysis of the hippocampus following P11–15 dosing revealed that both 5-HT (F2,24 = 73.09 p < 0.0001) and 5-HIAA (F2,24 = 30.47 p < 0.0001) were affected on P16 by treatment. Post hoc analysis revealed significant decreases in 5-HT and 5-HIAA in the hippocampus of MA- and MDMA-treated animals when compared to SAL-treated animals (Fig. 7a and b, respectively). 5-HT and 5-HIAA levels in the MA and MDMA groups were also different from each other with MDMA producing the larger decrease. No changes in 5-HT in the hippocampus were noted on P30 (Fig. 7a), however, for 5-HIAA there was a significant treatment effect. Both MA and MDMA treatment produced a decrease in 5-HIAA compared to SAL controls (Fig 7b).
Fig. 7.
Serotonergic markers in the hippocampus following P11–15 drug administration on P16 (n = 13/group) and P30 (n = 6/group; mean ± SEM). (a) P16 concentrations of 5-HT and P30 5-HT. (b) P16 5-HIAA and P30 5-HIAA. (c) P16 5-HIAA/5-HT ratio and P30 5-HIAA/5-HT ratio in the hippocampus of animals dosed for 5 days with MDMA or MA. Decreases in 5-HT were observed following MDMA and MA treatment at the 24 h time-point on P16. MDMA produced the greatest decrease in hippocampal 5-HT which was significantly different from SAL and MA. 5-HIAA levels were also decreased on P16 and on P30 in both the MDMA- and MA- treated groups with MDMA producing the greatest 5-HIAA decreases. The 5-HIAA/5-HT ratio was not affected on P16, however, on P30 the ratio was lower in the MDMA and MA groups when compared to SAL. *p < 0.05 from SAL control; #p < 0.05 from MDMA and MA are different.
The 5-HIAA/5-HT ratio in the hippocampus was unaffected by MDMA or MA treatment on P16. On P30 the 5-HIAA/5-HT ratio showed a significant treatment effect (F2,10 = 12.42 p < 0.002). Post hoc analysis revealed decreases in the 5-HIAA/5-HT ratio in MA- and MDMA-treated animals compared to SAL-treated animals (Fig. 7c).
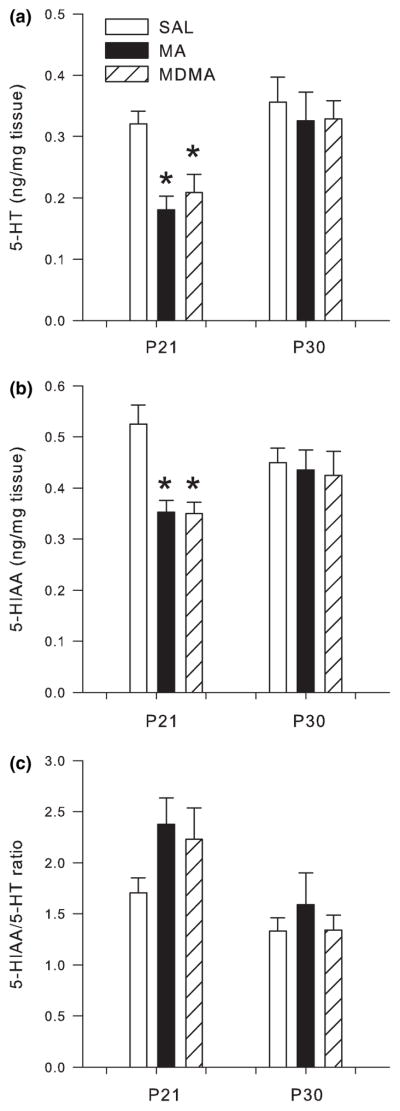
Following P11–20 drug treatment, analysis of the hippocampus revealed that both 5-HT (F2,28 = 64.52 p < 0.0001) and 5-HIAA (F2,28 = 14.64 p < 0.0001) were affected on P21 (Fig. 8a and b, respectively), and only 5-HIAA/5-HT ratio was altered on P30 (Fig. 8b), but not on P21 (Fig. 8a). Post hoc analysis for P21 revealed significant decreases in 5-HT and 5-HIAA in the hippocampus of MA- and MDMA-treated animals when compared to SAL-treated animals. MDMA treatment produced the largest decrease in 5-HT since levels were significantly lower than MA (Fig. 8a). On P30, 5-HIAA levels in animals treated with MA were decreased compared to SAL-treated animals (Fig. 8b); MDMA had no significant effect at this time point.
Fig. 8.
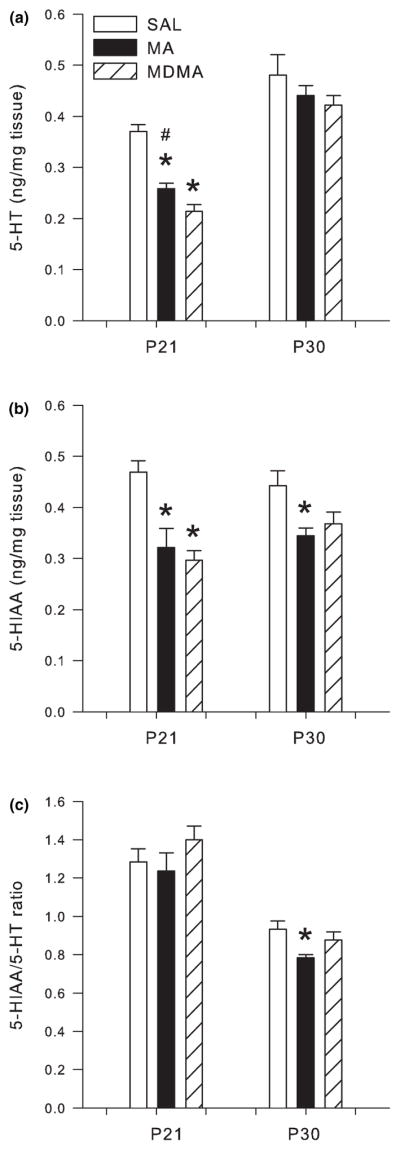
Serotonergic markers in the hippocampus following P11–20 drug administration on P21 (n = 16/group) and P30 (n = 8/group; mean ± SEM). (a) P21 concentrations of 5-HT and P30 5-HT. (b) P21 5-HIAA and P30 5-HIAA. (c) P21 5-HIAA/5-HT ratio and P30 5-HIAA/5-HT ratio in the hippocampus of animals dosed for 10 days with MDMA or MA. Decreases in 5-HT and 5-HIAA were observed following MDMA and MA treatment at the 24 h time-point on P21. MDMA produced the greatest decrease in hippocampal 5-HT which was significantly different from SAL and MA. On P30, 5-HIAA and the 5-HIAA/5-HT ratio were significantly decreased compared to SAL and MDMA treatments. *p < 0.05 from SAL control; #p < 0.05 from MDMA and MA are different.
The 5-HIAA/5-HT ratio in the hippocampus was not affected on P21. However, the 5-HIAA/5-HT ratio in the hippocampus on P30 showed a significant treatment effect (F2,14 = 3.91 p < 0.05). Post hoc analysis revealed decreases in 5-HIAA/5-HT ratio in MA-treated animals compared to SAL-treated animals (Fig. 8c), while MDMA-treated animals showed no effect.
Time-course of CORT and hippocampal 5-HT changes
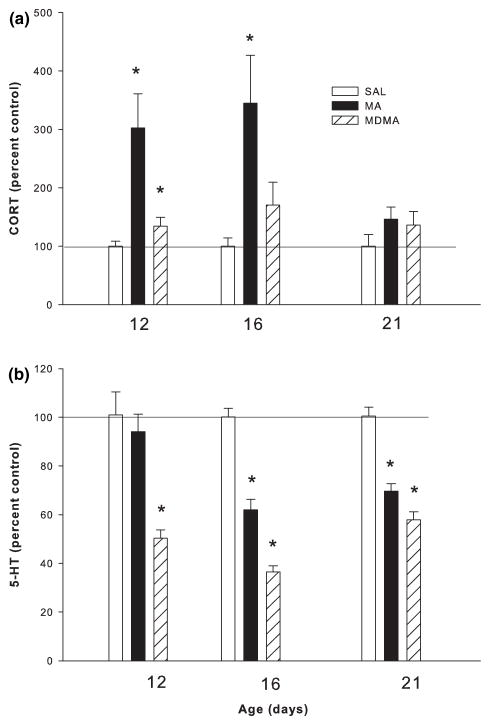
We also examined the CORT and 5-HT changes induced by drug treatment as a percent of control at three different time periods. The time periods selected were all 18 h from the last drug administration which was 24 h from the first administration on the last day of dosing. The days selected were P12, 16, and 21. The P12 data were from a previous study (Schaefer et al. 2006). An ANOVA performed on the data, expressed as percent control, showed that MA produced a significant increase in CORT on P12 and P16, but not on P21. MDMA produced an initial increase in CORT on P12, but significant increases were not detected on P16 or P21. In the hippocampus, MA produced moderate decreases in 5-HT on P16 and P21 and MDMA administration lead to a 5-HT decrease at all three ages (Fig. 9).
Fig. 9.
Time-course of CORT and hippocampal 5-HT changes 24 h following 1, 5, and 10 days of exposure. Data are presented as percent of SAL control and the P11 data are from a previous experiment (Schaefer et al. 2006). (a) CORT and (b) 5-HT levels following MA and MDMA exposure. Following MA, CORT levels are elevated from P12 through P16 and 5-HT levels are decreased by at least P16, remain depleted through P21. After MDMA exposure CORT levels are elevated only on P12 while 5-HT levels are decreased on P12, 16 and 21. *p < 0.05 relative to saline controls.
Discussion
We have shown differential CORT and 5-HT alterations 24 h following 5 or 10 days of MA or MDMA treatment during a period of development when cognitive deficits are induced by these drugs at these doses. We showed that MA treatment increased levels of CORT in plasma by approximately threefold compared to SAL and twofold compared to MDMA following 5 days of exposure when examined 24 h later (cf. Figs 2 and 9). However, no differences in CORT among treatments were observed on P21 following 10 days of exposure. In the hippocampus, we identified significant 5-HT decreases of approximately 50% after 5 and 10 days of MDMA exposure, while MA administration produced a decrease following 5 days of exposure (~40%) which was attenuated on P21 (~30%; Figs 7–9). 5-HT decreases in the neostriatum were similar to changes in the hippocampus for MDMA-exposed animals; however, we only observed significant neostriatal 5-HT decreases following 10 days of MA injections (Figs 3 and 5), not after 5 days of MA treatment. These findings suggest that MA and MDMA affect CORT and 5-HT differentially and that these differences may contribute to the unique pattern of long-term behavioral changes following administration of these drugs.
The increases in CORT following neonatal MA administration are likely to affect cell proliferation and we have shown that neonatal MA affects dendritic morphology in the hippocampus (Williams et al. 2004); an area important for spatial learning and memory as assessed using the MWM (Morris et al. 1982). The greatest number of proliferating dentate granular cells, the dominant neuronal type in the hippocampus, occurs during the neonatal period at a time when environmental stress-induced CORT increases are normally blunted (Schlessinger et al. 1975; Sapolsky and Meaney 1986; Liu et al. 2003). Humans are also thought to experience a period of blunted cortisol (the major glucocorticoid in humans) induction following stress during prenatal development; in humans rapid cell proliferation in the dentate gyrus occurs from second through third trimester (Bayer et al. 1993; Rice and Barone 2000; Brosnan 2001; Clancy et al. 2007). During the period when CORT should be blunted, large increases in CORT or exposure to stress can prevent the proliferation of dentate granular cells in such a way that the number of proliferating cells is inversely proportional to the concentration of CORT and this alteration in development may influence long-term cognitive function (Sapolsky and Meaney 1986; Aisa et al. 2007). We previously showed the most sensitive period for MA induced spatial learning deficits occurs following P11–15 exposure (Williams et al. 2003a) which coincides with the observed elevated CORT levels at the 24 h time point in the current study. These data along with previously reported results suggest that MA-treated animals experience CORT increases beginning just after the first dose on P11 and repeatedly through at least P16 at the 24 h time point following 5 days of dosing (Fig. 9) (Williams et al. 2000, 2006; Schaefer et al. 2006). Although following MDMA treatment we do not find any significant CORT changes following 5 or 10 days of exposure at the 24 h time-point we have previously shown CORT increases on P11 1 h after a single dose (~2.5 × higher), 1 h following three doses (~5 × higher), and 24 h following four doses on P11 (<2 × higher, Fig. 9) compared to SAL controls (Williams et al. 2005; Schaefer et al. 2006). Although we suspect that increased CORT following these drugs is affecting later spatial learning performance, no direct evidence currently exists showing that neonatal CORT treatment impairs spatial learning when given at the specific ages we use or at the levels of CORT increase that MA induces. One study exposed pups to CORT in the dam’s drinking water (P13–17) and found CORT increases smaller than those observed in the current study and did not show detrimental effects in spatial learning; in fact, these smaller CORT increases even appeared to be beneficial to learning (McCormick et al. 2001). Others have demonstrated long-term learning and memory deficits in novel object recognition and spatial memory following neonatal maternal separation, a stressor that also causes CORT release (Aisa et al. 2007), however, this form of stress has multiple effects and may be different than CORT release caused by MA treatment. Nevertheless, from these data, it appears that the magnitude of CORT increase is important in determining whether or not detrimental changes in spatial learning are obtained.
In addition to glucocorticoids, the developing brain requires critical concentrations of monoamines for proper development including long-term cognitive ability. For example, temporal lobe morphological changes are induced by depletion of 5-HT, and spatial learning deficits have been observed following P10–20 administration of p-chlorophenylalanine, a tryptophan hydroxylase inhibitor (Mazer et al. 1997; Yan et al. 1997). The decreases in 5-HT following both MA and MDMA may hinder proliferation, migration, and synaptogenesis and this could contribute to the impaired cognitive ability we see given that 5-HT levels are altered throughout a known critical period (i.e., hippocampal neurogenesis) (Bayer et al. 1993; Herlenius and Lagercrantz 2004). During the 24 h time points investigated in this study, MDMA had larger and more protracted effects on the 5-HT system in the hippocampus than MA since there is an earlier-onset of 5-HT decreases observed following MDMA (Figs 3a, 7a, 8a and 9). The 5-HT depletions resulting from MA or MDMA treatment may influence cognitive development since there is a rough correlation between the magnitude of the 5-HT reductions and the magnitude of the later spatial learning impairment (i.e., MDMA > MA). It will be important to test this hypothesis, perhaps by interfering with 5-HT reductions by pre-treatment with another drug to determine the role of 5-HT in mediating later spatial learning effects. Others have also reported a decrease in 5-HT on P21 following P11–20 MDMA treatment (Koprich et al. 2003) and the present study shows similar reductions on P21 and shows that even greater depletions are seen on P16. Taken together with the CORT data, the learning and memory deficits in MDMA-treated animals may be more dependent on 5-HT depletions, whereas the large CORT increases in MA-treated animals may account for the learning and memory deficits in those animals. Disentangling these two effects on learning will require additional studies.
Interestingly, neonatal MA and MDMA treatment affect the dopaminergic system differently than in adult animals. We have observed no changes in DA following 5 or 10 days of drug exposure 24 h later, even though a single day of MA treatment, at the same dose as used in this study, to an adult rat produces a 40–60% DA decrease that lasts for days and even weeks or months (Cass and Manning 1999; Wallace et al. 1999; Cappon et al. 2000; Cass 2000; O’Callaghan and Miller 2002). Although we do not show changes in DA or 5-HT on P30, it is evident that lasting alterations to the 5-HT system remain because 5-HIAA and the 5-HIAA/5-HT ratio are decreased following both drugs on P30. Interestingly, we have previously demonstrated changes in 5-HT and DA in adults following similar neonatal drug exposure including decreases in striatal DA following MA treatment (Crawford et al. 2003) and hippocampal 5-HT and striatal DA following MDMA (Broening et al. 2001; Crawford et al. 2006). This may be attributed to changes in monoamine innervation stemming from disruptions of differentiation, migration, and synapse formation or alterations in the rate of monoamine synthesis and/or degradation. It may be that developmental mechanisms are disrupted in such a way that the full effect of the drugs do not become apparent until later in life. Similar age-dependent changes in [3H]paroxetine binding to 5-HT transporters following P1–4 MDMA administration have been reported in which no differences were observed on P25, however, a decrease in binding was observed on P60 (Meyer et al. 2004). Hence, early changes produced by MA or MDMA treatment may alter the wiring of developing neural circuits such that absolute levels of neurotransmitters at P30 are not as indicative of the damage as are cytoarchitectural changes (Williams et al. 2004). This notion will require further experimental investigation.
In conclusion, MA produces increases in CORT and changes in 5-HT during the neonatal period that are associated with spatial learning deficits in the MWM whereas MDMA produces a smaller initial increase in CORT and larger decreases in 5-HT than MA that result in augmented long-term decreases in 5-HT, larger MWM deficits, and impaired learning in other tasks, such as the Cincinnati water maze. The mechanism by which MA or MDMA prevent proper spatial learning ability is unknown, however, we have shown protracted changes in two neurochemicals that are known to affect the hippocampus (Gould et al. 1991a,b; Gould and Tanapat 1999). It is likely that two different but perhaps overlapping mechanisms lead to the resulting spatial learning disruptions following neonatal MA or MDMA treatment.
Acknowledgments
Portions of these data were presented at the 13th annual meeting of the International Behavioral Neuroscience Society meeting in Key West, Florida, 2004. Supported by National Institutes of Health grants DA014269 (MTW) and DA006733 (CVV), DA007427 (GAG) and training grant ES007051 (TLS and MRS).
Abbreviations
- 5-HIAA
5-hydroxyindolacetic acid
- 5-HT
serotonin
- CORT
corticosterone
- DA
dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- MA
(+)methamphetamine
- MDMA
(±)3,4-methylenedioxymethamphetamine
- MWM
Morris water maze
- P
postnatal day
- SAL
saline
- SHRP
stress hyporesponsive period
References
- Acevedo SF, de Esch IJP, Raber J. Sex- and histamine-dependent long-term cognitive effects of methamphetamine exposure. Neuropsychopharmacology. 2007;32:665–672. doi: 10.1038/sj.npp.1301091. [DOI] [PubMed] [Google Scholar]
- Aisa B, Tordera R, Lasheras B, Del RJ, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Bayer SL, Altman J, Russo RJ, Zhang X. Timetables of Neurogenesis in the Human Brain Based on Experimentally Determined Patterns in the Rat. Neuro Toxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Broening HW, Bacon L, Slikker W., Jr Age modulates the long-term but not the acute effects of the serotonergic neurotoxicant 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1994;271:285–293. [PubMed] [Google Scholar]
- Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV. 3,4-methylenedioxymethamphetamine (ecstasy)-induced learning and memory impairments depend on the age of exposure during early development. J Neurosci. 2001;21:3228–3235. doi: 10.1523/JNEUROSCI.21-09-03228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan PG. The hypothalamic pituitary axis in the fetus and newborn. Semin Perinatol. 2001;25:371–384. doi: 10.1053/sper.2001.29038. [DOI] [PubMed] [Google Scholar]
- Buffum J. Substance abuse and high-risk sexual behavior: drugs and sex–the dark side. J Psychoactive Drugs. 1988;20:165–168. doi: 10.1080/02791072.1988.10524489. [DOI] [PubMed] [Google Scholar]
- Cappon GD, Pu C, Vorhees CV. Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Res. 2000;863:106–111. doi: 10.1016/s0006-8993(00)02107-7. [DOI] [PubMed] [Google Scholar]
- Cass WA. Attenuation and recovery of evoked overflow of striatal serotonin in rats treated with neurotoxic doses of methamphetamine. J Neurochem. 2000;74:1079–1085. doi: 10.1046/j.1471-4159.2000.0741079.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Manning MW. Recovery of presynaptic dopaminergic functioning in rats treated with neurotoxic doses of methamphetamine 1. J Neurosci. 1999;19:7653–7660. doi: 10.1523/JNEUROSCI.19-17-07653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomchai C, Na MN, Watanarungsan P, Yossuck P, Chomchai S. Methamphetamine abuse during pregnancy and its health impact on neonates born at Siriraj Hospital, Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 2004;35:228–231. [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJS, Finlay BL. Web-based method for translating neuro-development from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Williams MT, Newman ER, McDougall SA, Vorhees CV. Methamphetamine exposure during the pre-weanling period causes prolonged changes in dorsal striatal protein kinase A activity, dopamine D2-like binding sites, and dopamine content. Synapse. 2003;48:131–137. doi: 10.1002/syn.10197. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Williams MT, Kohutek JL, Choi FY, Yoshida ST, McDougall SA, Vorhees CV. Neonatal 3,4-methylenedioxymethamphetamine (MDMA) exposure alters neuronal protein kinase A activity, serotonin and dopamine content, and [35S]GTPgammaS binding in adult rats. Brain Res. 2006;1077:178–186. doi: 10.1016/j.brainres.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L. Drug use and risk behaviour among regular ecstasy users: Does sexuality make a difference? Cult Health Sex. 2005;7:599–614. doi: 10.1080/13691050500349875. [DOI] [PubMed] [Google Scholar]
- EMCDDA. Annual Report 2006: The State of the Drugs Problem in the Europe. European Monitoring Centre for Drugs and Drug Addiction; Luxembourg: 2006. [Google Scholar]
- Gomes-da-Silva J, de MR, Fernandez-Ruiz J, Summavielle T, Tavares MA. Effects of neonatal exposure to methamphetamine: catecholamine levels in brain areas of the developing rat. Ann NY Acad Sci. 2004;1025:602–611. doi: 10.1196/annals.1316.075. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell birth. J Comp Neurol. 1991a;313:486–493. doi: 10.1002/cne.903130309. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J Comp Neurol. 1991b;313:479–485. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp Neurol. 2004;190(Suppl 1):S8–21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Ho E, Karimi-Tabesh L, Koren G. Characteristics of pregnant women who use ecstasy (3, 4-methylenedioxymethamphetamine) Neurotoxicol Teratol. 2001;23:561–567. doi: 10.1016/s0892-0362(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug use: Overview of key Findings, 2006 (NIH Publication No. 07-6202) National Institute on Drug Abuse; Bethesda: 2007. [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Science. Brooks/Cole Publishing Co.; Pacific Grove: 1995. [Google Scholar]
- Koprich JB, Campbell NG, Lipton JW. Neonatal 3,4-methylenedioxymethamphetamine (ecstasy) alters dopamine and serotonin neurochemistry and increases brain-derived neurotrophic factor in the forebrain and brainstem of the rat. Brain Res Dev Brain Res. 2003;147:177–182. doi: 10.1016/s0165-3806(03)00219-0. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann NY Acad Sci. 1990;600:297–313. doi: 10.1111/j.1749-6632.1990.tb16891.x. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Krebs H. Serotonin as a differentiation signal in early neurogenesis. Dev Neurosci. 1978;1:15–30. doi: 10.1159/000112549. [DOI] [PubMed] [Google Scholar]
- Liu H, Kaur J, Dashtipour K, Kinyamu R, Ribak CE, Friedman LK. Suppression of hippocampal neurogenesis is associated with developmental stage, number of perinatal seizure episodes, and glucocorticosteroid level. Exp Neurol. 2003;184:196–213. doi: 10.1016/s0014-4886(03)00207-3. [DOI] [PubMed] [Google Scholar]
- Mazer C, Muneyyirci J, Taheny K, Raio N, Borella A, Whitaker-Azmitia P. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Res. 1997;760:68–73. doi: 10.1016/s0006-8993(97)00297-7. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Rioux T, Fisher R, Lang K, MacLaury K, Teillon SM. Effects of neonatal corticosterone treatment on maze performance and HPA axis in juvenile rats. Physiol Behav. 2001;74:371–379. doi: 10.1016/s0031-9384(01)00574-1. [DOI] [PubMed] [Google Scholar]
- McElrath K. MDMA and sexual behavior: ecstasy users’ perceptions about sexuality and sexual risk. Subst Use Misuse. 2005;40:1461–1477. doi: 10.1081/JA-200066814. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Grande M, Johnson K, Ali SF. Neurotoxic effects of MDMA (“ecstasy”) administration to neonatal rats. Int J Dev Neurosci. 2004;22:261–271. doi: 10.1016/j.ijdevneu.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Morford LL, Inman-Wood SL, Gudelsky GA, Williams MT, Vorhees CV. Impaired spatial and sequential learning in rats treated neonatally with D-fenfluramine. Eur J Neurosci. 2002;16:491–500. doi: 10.1046/j.1460-9568.2002.02100.x. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB. Neurotoxic Effects of Substituted Amphetamines in Rats and Mice. In: Massaro EJ, editor. Handbood of Neurotoxicology. Vol. 2. Humana Press Inc.; Totowa: 2002. pp. 269–301. [Google Scholar]
- Rawson RA, Anglin MD, Ling W. Will the methamphetamine problem go away? J Addict Dis. 2002;21:5–19. doi: 10.1300/j069v21n01_02. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schaefer TL, Ehrman LA, Gudelsky GA, Vorhees CV, Williams MT. Comparison of monoamine and corticosterone levels 24 h following (+)methamphetamine, (+/−)3,4-methylenedioxymethamphetamine, cocaine, (+)fenfluramine or (+/−)methylphenidate administration in the neonatal rat. J Neurochem. 2006;98:1369–1378. doi: 10.1111/j.1471-4159.2006.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159:149–175. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Vorhees CV. Treatment with MDMA from P11-20 disrupts spatial learning and path integration learning in adolescent rats but only spatial learning in older rats. Psychopharmacology (Berl) 2006;189:307–318. doi: 10.1007/s00213-006-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Schaefer TL, Vorhees CV. Neonatal (+)-methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits. Psychoneuroendocrinology. 2007;32:734–745. doi: 10.1016/j.psyneuen.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Yonekura ML, Wallace T, Berman N, Kuo J, Berkowitz C. Effects of prenatal methamphetamine exposure on fetal growth and drug withdrawal symptoms in infants born at term. J Dev Behav Pediatr. 2003;24:17–23. doi: 10.1097/00004703-200302000-00006. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Ahrens KG, Acuff-Smith KD, Schilling MA, Fisher JE. Methamphetamine exposure during early postnatal development in rats: II. Hypoactivity and altered responses to pharmacological challenge. Psychopharmacology (Berl) 1994;114:402–408. doi: 10.1007/BF02249329. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to D-methamphetamine: selective effects on spatial navigation and memory. J Neurosci. 2000a;20:4732–4739. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Inman-Wood SL, Morford LL, Reed TM, Moran MS, Pu C, Cappon GD. Evaluation of neonatal exposure to cocaine on learning, activity, startle, scent marking, immobility, and plasma cocaine concentrations. Neurotoxicol Teratol. 2000b;22:255–265. doi: 10.1016/s0892-0362(99)00071-9. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Skelton MR, Williams MT. Exposure to 3,4-methylenedioxymethamphetamine (MDMA) on postnatal days 11–20 induces reference but not working memory deficits in the Morris water maze in rats: implications of prior learning. Int J Dev Neurosci. 2004;22:247–259. doi: 10.1016/j.ijdevneu.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Schaefer TL, Williams MT. Developmental effects of +/−3,4-methylenedioxymethamphetamine on spatial versus path integration learning: Effects of dose distribution. Synapse. 2007;61:488–499. doi: 10.1002/syn.20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV. Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J Neurosci. 1999;19:9141–9148. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Inman-Wood SL, Morford LL, McCrea AE, Ruttle AM, Moran MS, Rock SL, Vorhees CV. Preweaning treatment with methamphetamine induces increases in both corticosterone and ACTH in rats. Neurotoxicol Teratol. 2000;22:751–759. doi: 10.1016/s0892-0362(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Vorhees CV, Boon F, Saber AJ, Cain DP. Methamphetamine exposure from postnatal day 11 to 20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Res. 2002;958:312–321. doi: 10.1016/s0006-8993(02)03620-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology (Berl) 2003a;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Rock SL, McCrea AE, Fukumura M, Wallace TL, Broening HW, Moran MS, Vorhees CV. Developmental 3,4-methylenedioxymeth-amphetamine (MDMA) impairs sequential and spatial but not cued learning independent of growth, litter effects or injection stress. Brain Res. 2003b;968:89–101. doi: 10.1016/s0006-8993(02)04278-6. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW, Vorhees CV. Developmental D-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse. 2003c;48:138–148. doi: 10.1002/syn.10159. [DOI] [PubMed] [Google Scholar]
- Williams MT, Brown RW, Vorhees CV. Neonatal methamphetamine administration induces region-specific long-term neuronal morphological changes in the rat hippocampus, nucleus accumbens and parietal cortex. Eur J Neurosci. 2004;19:3165–3170. doi: 10.1111/j.0953-816X.2004.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Schaefer TL, Ehrman LA, Able JA, Gudelsky GA, Sah R, Vorhees CV. 3,4-Methylenedioxymethamphetamine administration on postnatal day 11 in rats increases pituitary-adrenal output and reduces striatal and hippocampal serotonin without altering SERT activity. Brain Res. 2005;1039:97–107. doi: 10.1016/j.brainres.2005.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Schaefer TL, Furay AR, Ehrman LA, Vorhees CV. Ontogeny of the adrenal response to (+)-methamphetamine in neonatal rats: The effect of prior drug exposure. Stress. 2006;9:153–163. doi: 10.1080/10253890600902842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Herring NR, Schaefer TL, Skelton MR, Campbell NG, Lipton JW, McCrea AE, Vorhees CV. Alterations in body temperature, corticosterone, and behavior following the administration of 5-methoxy-diisopropyltryptamine (‘foxy’) to adult rats: a new drug of abuse. Neuropsychopharmacology. 2007;32:1404–1420. doi: 10.1038/sj.npp.1301232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ. Statistics and data analysis: trading bias for reduced mean squared error. Annu Rev Psychol. 1978;29:647–681. doi: 10.1146/annurev.ps.29.020178.003243. [DOI] [PubMed] [Google Scholar]
- Wish ED, Fitzelle DB, O’Grady KE, Hsu MH, Arria AM. Evidence for significant polydrug use among ecstasy-using college students. J Am Coll Health. 2006;55:99–104. doi: 10.3200/JACH.55.2.99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Yan W, Wilson CC, Haring JH. Effects of neonatal serotonin depletion on the development of rat dentate granule cells. Brain Res Dev Brain Res. 1997;98:177–184. doi: 10.1016/s0165-3806(96)00176-9. [DOI] [PubMed] [Google Scholar]