Abstract
Background
A 776C→G variant (dbSNP ID: rs1801198) in the transcobalamin gene (TCN2; MIM# 275350) decreases the cellular and plasma concentration of transcobalamin and thereby influences the cellular availability of vitamin B12.
Objective
To evaluate the worldwide prevalence of this variant and its association with homocysteine plasma level.
Methods
The study was performed in 1433 apparently healthy subjects, including Afro‐Americans and Afro‐Africans and in 251 Afro‐Africans participants with severe malaria.
Results
The frequencies of the 776G allele were the highest in China (0.607; 95% CI 0.554 to 0.659), low in West Africa (Bénin and Togo, 0.178; 0.154 to 0.206), and intermediate in France (0.445; 0.408 to 0.481), Italy (0.352; 0.299 to 0.409), Morocco (0.370; 0.300 to 0.447) and Mexico (0.374; 0.392 to 0.419). The 776G genotype was more frequent in Afro‐Americans from New York (16.7; 8.4 to 30.7) and in Afro‐African patients with severe malaria (6.0%; 95% CI 3.7 to 9.6) than in healthy Afro‐African volunteers (p = 0.0004 and p = 0.033, respectively), while no difference was observed for MTHFR 677TT and 677T alleles. A disequilibrium of TCN2 genotype distribution was recorded in patients with severe malaria, with a twofold higher GG genotype than expected (p = 0.010). An association between the TCN2 polymorphism and homocysteine was observed only in Mexico and France, the two countries with the highest rate of low plasma concentration of vitamin B12 (<100 pmol/l).
Conclusion
Given the dramatic heterogeneity of the 776G allele frequency worldwide, this polymorphism may be prone to a selective pressure or confers an evolutionary advantage in confronting environmental factors, one of which is malaria.
Accumulating evidence suggests that the metabolisms of homocysteine and vitamin B12 play a role in developmental disorders.1 Homocysteine is a crosspoint substrate of the so‐called one‐carbon metabolism. This metabolism is crucial for DNA synthesis and repair, and for a wide range of transmethylation reactions, including those of DNA and histones that are implicated in the regulation of gene expression.2 The concentration of homocysteine in humans is influenced by the dietary intake of folate and vitamin B12, and by the polymorphisms in genes that encode enzymes or proteins involved in the specific transport of folate and vitamin B12.3 One of these enzymes, methylenetetrahydrofolate reductase (MTHFR), catalyses the synthesis of 5‐methyltetrahydrofolate, the methyl donor for vitamin B12‐dependent re‐methylation of homocysteine to methionine. A 677C→T variant (dbSNP ID rs1801133) of MTHFR (MIM# 607093) decreases by fourfold the catalytic rate of the enzyme and, consequently, increases t‐Hcys, especially in low folate status.4,5 This variant is also a genetic determinant of folate imbalance between DNA synthesis and/or methylation.6 Its worldwide distribution is very heterogeneous, the highest and the lowest T allele frequency being reported in Mexico and sub‐Saharan Africa, respectively.7,8,9 It has been hypothesised that dietary folate is one of the factors that has influenced the prevalence of the 677T allele worldwide.10,11
Another common genetic polymorphism that influences homocysteine and vitamin B12 metabolisms is a 776C→G variant in the transcobalamin gene (TCN2, MIM# 275350) that results in the substitution of proline for arginine in codon 259 (dbSNP ID: rs1801198).12,13 The TCN2 776G allele decreases the transcription and the cellular and plasma concentration of transcobalamin, the carrier protein which delivers vitamin B12 to cells.13 The influence of the TCN2 776G allele on the cellular availability of vitamin B12 has been documented by its association with plasma apotranscobalamin, holotranscobalamin, homocysteine and methylmalonic acid, in Caucasians.13,14,15 This polymorphism seems to be associated with an increased risk of spontaneous abortion.16 The possibility of a gene–gene interaction between the MTHFR and TCN2 polymorphisms has also been suggested in this context.17 More recently, TCN2 polymorphism was found to be associated with non‐syndromic cleft lip with or without cleft palate.18 Taken together, these data suggest an association between the TCN2 776G allele and the pathological and developmental events of the very early steps of life. Whether this association is influenced by environmental factors is not known, and, if it were the case, it would be reasonable to predict its heterogeneous distribution in populations that are exposed to a contrasted environment. To address this issue, we therefore studied the worldwide distribution of the TCN2 776G allele in populations from Europe, Mediterranean area, West Africa, China and Mexico, and compared Afro‐Africans from West Africa with Afro‐Americans. We also evaluated the influence of this variant on plasma homocysteine concentration in those populations that were accessible to plasma collection.
Methods
The study was carried out on a population of 1433 apparently healthy participants, with an age range of 20–60 years. Ethical approval for study of DNA variants in the untraceable DNA samples was obtained through the Research Ethics Board of Troina (Sicily, Italy). Similarly, local approval was obtained for DNA samples from New York and China. All the other subjects were medically examined to rule out cancer, cardiovascular, renal, hepatic and genetic disease, and recurrent vitamin supplementation prior to inclusion in the study. Institutional review board approval was obtained from the ethical committees of the Medical Center of Troina (IRCCS Associazione Oasi Maria SS, Troina, Sicily, Italy), University Hospital Center of Nancy (Nancy, France), University of Bénin (Lomé, Togo), University of Cotonou (Cotonou, Bénin), Instituto Nacional de Ciencias Medicas y Nutricion (Mexico DF, US of Mexico) and University Hospital of Casablanca (Morocco). Informed consent was obtained from the participants. The volunteers from Sicily were from a rural area, in the north‐east of Sicily. Volunteers from Togo were recruited from the coast (Lomé) and the Savanna. They have already been described in a recent publication.8 The volunteers from Bénin were recruited from the coastal urban area of Cotonou. The volunteers from Mexico were blood donors at the Instituto Nacional de Ciencias Medicas y Nutricion and originated from the periphery of Mexico City, and those from China and New York were blood donors from the region of Wuhan and New York City, respectively. A group of 251 patients with severe malaria were also recruited from the University Hospital Center of Cotonou (Bénin). All blood specimens were tested in a single laboratory (Nancy, France). Fasting venous blood was collected in EDTA‐containing tubes and, after immediate centrifugation, aliquots were stored at −30°C until analysis. Plasma homocysteine concentrations (protein‐bound and free homocysteine) were determined by the fluorescence polarisation immunoassay IMx‐homocysteine method developed by Abbott (Abbott Laboratories Diagnostics Division, Abbott Park, Illinois, USA). Buffy coat was prepared from the previously collected blood and genomic DNA was isolated using the Qiagen kit (Qiagen‐France, Courtaboeuf cedex, France). Plasma vitamin B12 and folate concentrations were determined by the immunoassay kit V B12 and Folates on an ACS 180 automated chemiluminescent system (Chiron Diagnostics Corporation, East Walpole, Massachusetts, USA), with threshold values established, respectively, at 100 pmol/l and 7 nmol/l, respectively.11 Genotyping of the TCN2 776C→G polymorphism was performed by the amplification‐refractory mutation system, as we described recently.12 PCR‐based restriction fragment length polymorphism method was used to determine the genotypes of MTHFR.8 Allele and genotype frequencies were reported as percentages (95% CI), and a Fisher's test was used to assess differences between the groups. Continuous variables were reported as mean (SD). Comparisons of continuous variables were performed by Mann–Whitney U test. p Values <0.05 were considered significant. Data were prospectively collected and analysed using the SAS software v9.1 for Windows.
Results
Table 1 presents the distribution of the genotypes of TCN2 776C→G polymorphism among the seven populations. The highest frequency was reported in China, this genotype being even more frequent than the 776CC genotype in this population. A low frequency of GG genotype was observed in the populations from West Africa (table 2).
Table 1 Worldwide distribution of TCN2 776C→G genotypes.
| Geographical area | Ethnicity | Number of subjects | Genotype counts, n, % (95% CI) | ||
|---|---|---|---|---|---|
| 776CC | 776CG | 776GG | |||
| Togo | Afro‐Africans | 195 | 134, 68.7 (62.0 to 74.9) | 52, 26.7 (20.8 to 33.1) | 9, 4.6 (2.2 to 8.2) |
| Bénin | Afro‐Africans | 214 | 142, 66.3 (59.9 to 72.4) | 68, 31.8 (25.8 to 38.2) | 4, 1.9 (0.6 to 4.3) |
| West Africa (Togo and Bénin) | Afro‐Africans | 409 | 276, 67.5 (62.8 to 71.8) | 120, 29.3 (25.1 to 33.9) | 13, 3.2 (1.9 to 5.4) |
| New York | Afro‐Americans | 42 | 21, 50.0 (35.4 to 64.5) | 14, 30.3 (21.0 to 48.5) | 7, 16.7 (8.4 to 30.7) |
| North East United States* | Afro‐Americans | 51 | 19, 37.2 (25.9 to 50.7) | 27, 52.9 (39.5 to 19.9) | 5, 9.8 (3.6 to 19.9) |
| New York and North‐East United States | Afro‐Americans | 93 | 40, 43.0 (33.4 to 53.2) | 41, 44.1 (34.4 to 54.2) | 12, 12.9 (7.6 to 21.2) |
| Morocco | Arabs‐Caucasians | 81 | 35, 43.2 (33.0 to 54.1) | 32, 39.5 (29.6 to 50.4) | 14, 17.3 (10.6 to 29.0) |
| France | Caucasians | 353 | 109, 30.9 (26.3 to 35.9) | 174, 49.3 (44.1 to 54.5) | 70, 19.8 (16.0 to 24.3) |
| Italy (Sicily) | Caucasians | 142 | 58, 40.8 (33.1 to 49.1) | 68, 47.9 (39.8 to 56.1) | 16, 11.3 (7.1 to 17.5) |
| Mexico | Hispanics | 239 | 102, 42.7 (36.6 to 49.0) | 95, 39.7 (33.8 to 46.1) | 42, 17.6 (13.3 to 22.9) |
| Central China | Hans | 167 | 34, 20.4 (15.0 to 27.1) | 63, 37.7 (30.7 to 45.3) | 70, 41.9 (34.7 to 49.5) |
*Data from Bowen et al.19
Table 2 TCN2 776G and MTHFR 677T allele frequencies worldwide.
| Geographical area | Ethnicity | Number of alleles | Allele frequency (95% CI) | |
|---|---|---|---|---|
| TCN2 776G | MTHFR 677T | |||
| Togo | Afro‐Africans | 390 | 0.179 (0.144 to 0.219)) | 7.4 (5.1 to 10.3) |
| Bénin | Afro‐Africans | 428 | 0.177 (0.143 to 0.216) | 10.7 (5.1 to 10.3) |
| West Africa | Afro‐Africans | 818 | 0.178 (0.154 to 0.206) | 0.093 (0.075 to 0.115) |
| New York | Afro‐Americans | 84 | 0.333 (0.242 to 0.440) | 0.107 (0.057 to 0.191) |
| North‐East United States* | Afro‐Americans | 102 | 0.363 (0.274 to 0.458) | |
| New York and North‐East United States | Afro‐Americans | 186 | 0.349 (0.284 to 0.349) | |
| Morocco | Arabs‐Caucasians | 162 | 0.370 (0.300 to 0.447) | 0.289 (0.218 to 0.373) |
| France | Caucasians | 706 | 0.445 (0.408 to 0.481) | 0.354 (0.318 to 0.392) |
| Italy (Sicily) | Caucasians | 284 | 0.352 (0.299 to 0.409) | 0.438 (0.382 to 0.496) |
| Mexico | Hispanics | 478 | 0.374 (0.392 to 0.41.9) | 0.581 (0.538 to 0.622) |
| Central China | Hans | 334 | 0.607 (0.554 to 0.659) | 0.392 (0.344 to 0.442) |
*Data from Bowen et al.19
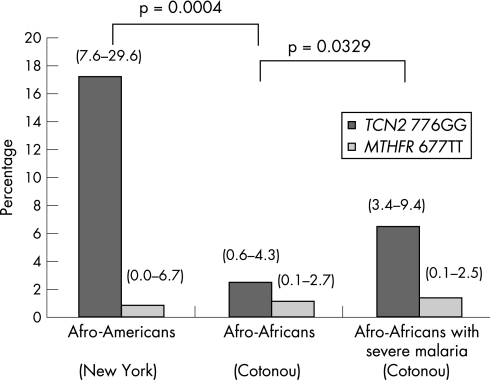
In addition, no difference was observed between the participants from Togo and those from Bénin, nor between those from the coast of Togo and those from the Savannah. In contrast, a twofold higher frequency of TCN2 776GG genotype was reported in the patients from Bénin with severe malaria (6.0% (95% CI 3.7 to 9.6); fig 1). Surprisingly, the frequency of 776GG genotype in Afro‐Americans was not significantly different from that reported in Caucasian populations (p = 0.839), but it was dramatically higher than that observed in the Afro‐African participants (table 1; p = 0.0004). It was in the same order of magnitude as that reported in another group of Afro‐Americans from north‐eastern United States.19 By comparison, the frequency of MTHFR 677TT genotype was similar in both African populations from West Africa and New York,8 indicating that the difference in 776GG genotype was not due to crossbreeding.
Figure 1 Comparison (by Fisher's test) of the frequency of TCN2 776GG and MTHFR 677TT in healthy volunteers from New York, Cotonou (Bénin) and in patients with severe malaria from Cotonou. 95% confidence intervals are indicated in brackets.
The same contrasted groups were evidenced when considering the allele frequencies of TCN2 polymorphism, instead of TCN2 776GG genotype. The 776G allele was the most predominant allele in the Chinese population (p<0.001), the less frequent allele in West Europe, Mediterranean areas and Mexico, and its frequency was dramatically lower in Afro‐African participants than in participants of any other ethnicity (table 2; p<0.001). The 776G allele frequency was twofold lower in Afro‐Africans, compared with Afro‐Americans from New York (table 2; p = 0.001) and with the sum of Afro‐Americans from New York and from the study of Bowen et al19 (table 2; p<0.001). The allele frequency in France was not different from that previously reported in Sweden, The Netherlands, Ireland, Greece and the Caucasian populations from North America.13,19,20,21,22,23,24 In contrast, it was significantly higher than that reported in Sicily (p = 0.0084) and in Mexico (p = 0.0161). The distribution of MTHFR 677T allele frequency among the seven populations was similar to previous reports of the literature.7,8 In particular, it was in the same order of magnitude in the Afro‐Africans from West Africa and in the Afro‐Americans from New York. In addition, similar results were reported in the Afro‐African population from Atlanta.9 We investigated the hypothesis of a gene–gene influence of MTHFR on the distribution of TCN2 polymorphism and vice versa. We failed to find any difference in 776G allele frequency between the subjects carrying the MTFHR 677T allele and those carrying the 677C allele in any of the seven populations. There was no evidence of Hardy–Weinberg disequilibrium of the genotype distribution in West Africa, north‐eastern United States, Morocco, France and Italy (χ2: 0.0002, p = 0.989; 2.619, p = 0.106; 0.049, p = 0.825; 1.869, p = 0.169; 0.001, p = 0.920 and 0.350, p = 0.554, respectively). In contrast, the frequency of observed TCN2 776CG genotypes was slightly higher than expected in the population samples from China and Mexico, and as much as twofold higher than expected in the African patients with severe malaria (table 3 and fig 1).
Table 3 Hardy–Weinberg disequilibrium in healthy volunteers from Mexico, China and in patients with severe malaria from Bénin.
| Geographical area | Genotype | Observed | Expected | χ2 | p Value (two tailed) |
|---|---|---|---|---|---|
| Mexico | CC | 102 | 93.5 | 0.769 | |
| CG | 95 | 112.0 | 2.572 | ||
| GG | 42 | 33.5 | 2.151 | ||
| Total | 5.492 | 0.019 | |||
| China | CC | 34 | 25.7 | 2.696 | |
| CG | 63 | 79.6 | 3.473 | ||
| GG | 70 | 61.7 | 1.119 | ||
| Total | 7.288 | 0.007 | |||
| Bénin—severe malaria | CC | 172 | 165.8 | 0.232 | |
| CG | 64 | 76.4 | 2.018 | ||
| GG | 15 | 8.8 | 4.367 | ||
| Total | 6.617 | 0.010 | |||
| Bénin —healthy volunteers | |||||
| CC | 142 | 144.7 | 0.052 | ||
| CG | 68 | 62.5 | 0.482 | ||
| GG | 4 | 6.7 | 1.114 | ||
| Total | 214 | 1.648 | 0.199 |
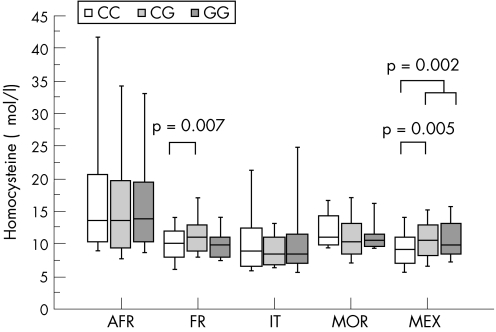
The homocysteine plasma level was the highest in Africa, intermediate in Morocco and France, and the lowest in Italy and Mexico, with respective values of 18.2 (13.9), 11.4 (3.2), 10.7 (3.3), 9.9 (5.9) and 10.3 (3.7) µmol/l. The serum folate level was the highest in Mexico, intermediate in Italy, France and Morocco, and the lowest in Africa, with respective values of 19.5 (9.4), 13.4 (6.3), 12.8 (5.0), 11.3 (5.1) and 10.8 (6.8) nmol/l. By comparison, the vitamin B12 level was the highest in Africa and Morocco, intermediate in Mexico and Italy, and the lowest in France, with respective values of 662.4 (506.4), 590.7 (428.5), 422.7 (658.8), 347.5 (158.7) and 296.8 (191.4) pmol/l. We could evaluate the phenotypic influence of TCN2 on homocysteine plasma concentration in the study groups, except for the subjects recruited from China and New York. An association between TCN2 polymorphism and homocysteine was observed in the subjects from Mexico and France, but not in those from Italy, Morocco and Africa (fig 2).
Figure 2 Plasma homocysteine level (box plots represent median, inter‐quartiles and range) according to the genotypes of TCN2 776C→G in West Africa (AFR), Italy (IT), France (FR), Morocco (MOR) and Mexico (MEX).
Discussion
Our study demonstrated a heterogeneity in the allele distribution of TCN2 776C→G polymorphism worldwide, with the highest 776G allele frequency reported in China. By comparison, the allele frequency was, respectively, 3.4‐fold and 1.5‐fold lower, in African and Caucasian populations. The 776G allele was even more frequent than the 776C allele in China. We did not observe any difference in 776G allele frequency among Caucasian populations from West Europe and the Mediterranean area. These frequencies were also in the same order of magnitude as those reported in Caucasian populations from North Europe and North America. In contrast, a dramatic difference of 776G allele frequency was observed between populations that have the same ethnicity. The Afro‐African populations from New York and the north‐east of the United States originated from the coastal regions of West Africa, respectively, as the populations from Bénin and Togo. However, they had a twofold higher frequency of TCN2 776G allele. It is noteworthy that such a difference was not observed with the MTHFR 677T allele, showing the absence of crossbreeding in the group of Afro‐Americans. The TCN2 776G allele frequency was not related to its phenotypic influence on homocysteine, as the lowest frequency was observed in the African countries and in Sicily—for example, in areas where this polymorphism had no effect on homocysteine plasma concentration.
TCN2 polymorphism may have a deleterious influence on very early events of life and on the normal development of the embryo. The prevalence of the mutated TCN2 776G allele is significantly higher in spontaneous abortion of embryos, and the frequency of wild‐type TCN2 776CC embryos is much lower among spontaneously aborted embryos, compared with a reference adult population.1,16 Embryos that had combined MTHFR 677TT and TN2 776CG or 776GG genotypes are at increased risk for spontaneous abortion compared with embryos that had only one of these genotypes.17 In addition, TCN2 776C→G was the single polymorphism associated with the onset of non‐syndromic cleft lip with or without cleft palate, in a recent study.18 Contrasting results have been observed with regard to the association between TCN2 776C→G and the risk of neural tube defect, with no association reported in Ireland and The Netherlands and an increased risk in the cases with the predominant 677CC genotype of MTHFR polymorphism in Sicily.20,24,25
With regard to its worldwide distribution, the phenotypic influence of the TCN2 polymorphism seems not to be primarily associated with its effect on homocysteine metabolism. An effect of the 776G allele on homocysteine concentration was observed only in Mexico and in France, in the two populations that had the highest rate of low concentration of vitamin B12. In fact, we have previously shown in different cell lines that this polymorphism was a determinant of the intracellular concentration of transcobalamin.12,13 This polymorphism had a negative influence on the transcription level, leading to a dramatically lower concentration of the transcript in the 259RR variant than in the 259PP variant.12,13
Environmental factors may confer a selective advantage or exert a selective pressure on the frequency of the TCN2 776G allele, with regard to the dramatic differences observed in Africa, in the Afro‐American populations and in China. One of the factors that may exert a pressure could be the dietary intake of vitamin B12, which is one of the strongest nutritional determinants of homocysteine metabolism. However, this hypothesis is not sustained by the lack of association between the allele frequency and the vitamin B12 status of the study groups. In addition, the lowest frequency of TCN2 776GG was observed in Bénin and Togo, two countries where the polymorphism had no influence on plasma homocysteine level. Another hypothesis could be the influence of this polymorphism on the gravity of pathologies such as diseases from infections in young populations. Among the pathologies, malaria is a good candidate for such an investigation. Plasmodium falciparum requires substrates of the one‐carbon metabolism that are provided by the host.26,27 In addition, cellular vitamin B12 is involved in the synthesis of succinyl‐CoA,28 the first substrate of the haeme pathway, and haemoglobin is a molecule that is crucial for the growth of the parasite within the erythrocyte. The prevalence of malaria is very high in West Africa and null in north‐west America. We reported a twofold increased frequency of the 776GG genotype in patients with severe malaria, compared with the apparently healthy sample of population from the same urban area of Cotonou, in Bénin. The criteria of severity were those defined by the World Health Organization. In addition, a disequilibrium of genotype distribution was recorded in these patients, with a twofold higher rate of GG genotype than expected (table 1). Hence, these data indicated that malaria may exert a selective pressure on the frequency of GG genotype in Afro‐Africans, considering that the mortality for malaria in Bénin is one of the highest in the world, with the rate of complicated malaria estimated at 4.2% of children.29 By comparison, the prevalence of mortality related to malaria is very low in the province of Hubei (central China)30 and is null in Mexico city and in Europe. In addition, it is noteworthy that the lowest frequency of the TCN2 776G allele in Europe was reported in Sicily, a region where the prevalence of malaria was high until the middle of the 20th century. Finally, the dramatic difference in GG genotype frequency between Afro‐African and Afro‐American populations suggests that this variant may confer a selective advantage when the selective pressure is removed or absent. This could explain the Hardy–Weinberg disequilibrium that was observed in healthy volunteers from Mexico, with the frequency of GC and GG genotypes higher than expected (table 3).
In conclusion, we have observed a worldwide heterogeneity in the distribution of the TCN2 776C→G polymorphism, which provides evidence for its association with environmental factors in early events of life. One of the environmental factors that may exert a selective pressure could be malaria, as the TCN2 776GG genotype was associated with the severity of this disease.
Acknowledgements
This work was supported by grants from Conseil Régional de Lorraine, Fondation de France, Institut National de la Santé et de la Recherche Médicale, French Ministère de la Recherche et de la Technologie and the Consejo Nacional de Ciencia y Tecnología (CONACYT ‐ M0237) México.
Abbreviations
MTHFR - methylenetetrahydrofolate reductase
Footnotes
Competing interests: None.
References
- 1.Zetterberg H. Methylenetetrahydrofolate reductase and transcobalamin genetic polymorphisms in human spontaneous abortion: biological and clinical implications. Reprod Biol Endocrinol 200427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler B. Homocysteine: overview of biochemistry, molecular biology, and role in disease processes. Semin Vasc Med 2005577–86. [DOI] [PubMed] [Google Scholar]
- 3.Bollander‐Gouaille C.Focus on homocysteine and the vitamins involved in its metabolism. 2nd edn. Paris: Spinger‐Verlag, 2002
- 4.Frosst P, Blom H J, Milos R, Goyette P, Sheppard C A, Matthews R G, Boers G J H, den Heijer M, Kluijtmans L A J, van den Heuve L P, Rozen R. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 199510111–113. [DOI] [PubMed] [Google Scholar]
- 5.Ueland P M, Hustad S, Schneede J, Refsum H, Vollset S E. Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci 200122195–201. [DOI] [PubMed] [Google Scholar]
- 6.Friso S, Choi S W, Girelli D, Mason J B, Dolnikowski G G, Bagley P J, Olivieri O, Jacques P F, Rosenberg I H, Corrocher R, Selhub J. A common mutation in the 5,10‐methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction in the folate status. Proc Natl Acad Sci USA 2002995606–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pepe G, Camacho Vanegas O, Giusti B, Brunelli T, Marcucci R, Attanasio M, Rickards O, De Stefano G F, Prisco D, Gensini G F, Abbate R. Heterogeneity in word distribution of the thermolabile C677T mutation in 5,10‐methylenetetrahydrofolate reductase. Am J Hum Genet 199863917–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amouzou E K, Chabi N W, Adjalla C E, Rodriguez‐Guéant R M, Feillet F, Villaume C, Sanni A, Guéant J L. High prevalence of hyperhomocysteinemia related to folate deficiency and the 677C→T mutation of the gene encoding methylenetetrahydrofolate reductase in coastal West Africa. Am J Clin Nutr 200479619–624. [DOI] [PubMed] [Google Scholar]
- 9.Wilcken B, Bamforth F, Li Z, Zhu H, Ritvanen A, Renlund M, Stoll C, Alembic Y, Dott B, Czeizel A E, Gelman‐Kohan Z, Scarano G, Bianca S, Ettore G, Tenconi R, Bellato S, Scala I, Mutchinick O M, Lopez M A, de Walle H, Hofstra R, Joutchenko L, Kavteladze L, Bermejo E, Martinez‐Frias M L, Gallagher M, Erickson J D, Vollset S E, Mastroiacovo P, Andria G, Botto L D. Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas worldwide. J Med Genet 200340619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munoz‐Moran E, Dieguez‐Lucena J L, Fernandez‐Arcas N, Peran‐Mesa S, Reyes‐Engel Genetic selection and folic acid intake during pregnancy. Lancet 19983521120–1121. [DOI] [PubMed] [Google Scholar]
- 11.Guéant‐Rodriguez R M, Guéant J L, Debard R, Thirion S, Xiao Hong L, Bronowicki J P, Namour F, Chabi N W, Sanni A, Anello G, Bosco P, Romano C, Amouzou E, Heidy R, Arrieta B, Sánchez B E, Romano A, Herbeth B, Guilland J C, Mutchinick O M. Prevalence of methylenetetrahydrofolate reductase 677T and 1298C alleles and folate status: a comparative study among Mexican, West African and West European populations. Am J Clin Nutr 200683701–707. [DOI] [PubMed] [Google Scholar]
- 12.Namour F, Guy M, Aimone‐Gastin I, de Nonancourt M, Mrabet N, Gueant J L. Isoelectrofocusing phenotype and relative concentration of transcobalamin II isoprotein related to the codon 259 Arg/Pro polymorphism. Biochem Biophys Res Commun 1998251769–774. [DOI] [PubMed] [Google Scholar]
- 13.Namour F, Olivier J, Abdelmouttaleh I, Adjalla C, Debard R, Salvat C, Guéant J L. Transcobalamin codon 259 polymorphism in HT‐29 and Caco‐2 cells and in Caucasians: relation to transcobalamin and homocysteine concentration in blood. Blood 2001971092–1098. [DOI] [PubMed] [Google Scholar]
- 14.Miller J W, Ramos M I, Garrod M G, Flynn M A, Greeen R. Transcobalain Ii 775G>C polymorphism and indices of vitamin B12 status in healthy older adults. Blood 2002100718–720. [DOI] [PubMed] [Google Scholar]
- 15.Zetterberg H, Nexo E, Regland B, Minthon L, Boson R, Palmer M, Rymo L, Blennow K. The transcobalamin (TC) codon 259 genetic polymorphism influences holo‐TC concentration in cerebrospinal fluid from patients with Alzheimer disease. Clin Chem 2003491195–1198. [DOI] [PubMed] [Google Scholar]
- 16.Zetterberg H, Regland B, Palmer M, Rymo L, Zafiropoulos A, Arvanitis D A, Spandidos D A, Blennow K. The transcobalamin codon 259 polymorphism influences the risk of human spontaneous abortion. Hum Reprod 2002173033–3036. [DOI] [PubMed] [Google Scholar]
- 17.Zetterberg H, Zafiropoulos A, Spandidos D A, Rymo L, Blennow K. Gene‐gene interaction between fetal MTHFR 677C>T and transcobalamin 776C>G polymorphisms in human spontaneous abortion. Hum Reprod 2003181948–1950. [DOI] [PubMed] [Google Scholar]
- 18.Martinelli M, Scapoli L, Palmieri A, Pezzetti F, Baciliero U, Padula E, Carinci P, Morselli P G, Carinci F. Study of four genes belonging to the folate pathway: transcobalamin 2 is involved in the onset of non‐syndromic cleft lip with or without cleft palate. Hum Mutat 200627294. [DOI] [PubMed] [Google Scholar]
- 19.Bowen R A, Wong B Y, Cole D E. Population‐based differences in frequency of the transcobalamin II Pro259Arg polymorphism. Clin Biochem 200437128–133. [DOI] [PubMed] [Google Scholar]
- 20.Swanson D A, Pangilinan F, Mills J L, Kirke P N, Conley M, Weiler A, Frey T, Parle‐McDermott A, O'Leary V B, Seltzer R R, Moynihan K A, Molloy A M, Burke H, Scott J M, Brody L C. Evaluation of transcobalamin II polymorphisms as neural tube defect risk factors in an Irish population. Birth Defects Res A Clin Mol Teratol 200573239–244. [DOI] [PubMed] [Google Scholar]
- 21.Lievers K J A, Afman L A, Kluitmans L A J, Boers G H, Verhoef P, den Heijer M, Trijbels F J, Blom H J. Polymorphism in the transcobalamin gene: association with plasma homocysteine in healthy individuals and vascular disease patients. Clin Chem 2002481383–1389. [PubMed] [Google Scholar]
- 22.McCaddon A, Blennow K, Hudson P, Regland B, Hill D. Transcobalamin polymorphism and homocysteine. Blood 2001983497–3499. [DOI] [PubMed] [Google Scholar]
- 23.Namour F, Guéant J L. Transcobalamin polymorphism, homocysteine and ageing. Blood 2001983497–3499. [DOI] [PubMed] [Google Scholar]
- 24.Afmann L A, Lievers K J, van der Put N M, Trijbels F J, Blom H J. Single nucleotide polymorphisms in the transcobalamin gene: relationship with transcobalamin concentrations and risk for neural tube defects. Eur J Hum Genet 200210433–438. [DOI] [PubMed] [Google Scholar]
- 25.Guéant‐Rodriguez R M, Rendeli C, Namour B, Venuti L, Romano A, Anello G, Bosco P, Debard R, Gérard P, Viola M, Salvaggio E, Guéant J L. Transcobalamin and methionine synthase reductase mutated polymorphisms aggravate the risk of neural tube defects. Neurosci Lett 2003344189–192. [DOI] [PubMed] [Google Scholar]
- 26.Nzila A, Ward S A, Marsh K, Sims P, Hyde J E. Comparative folate metabolism in humans and malaria parasites (part I): pointers for malaria treatment from cancer chemotherapy. Trends Parasitol 200521292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nzila A, Ward S A, Marsh K, Sims P, Hyde J E. 2005. Comparative folate metabolism in humans and malaria parasites (part II): activities as yet untargeted or specific to Plasmodium, Trends Parasitol 200521334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenblatt D S, Fenton W. 2001. Inherited disorders of folate and cobalamin transport and metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds, The metabolic and molecular bases of inherited disease. New York: McGraw‐Hill, 3897–933
- 29.Wang S J, Lengeler C, Smith T A, Vounatsou P, Akogbeto M, Tanner M. Rapid Urban Malaria Appraisal (RUMA) IV: epidemiology of urban malaria in Cotonou (Benin). Malaria 2006545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou S S, Tang L H, Sheng H F, Wang Y. Malaria situation in the People's Republic of China. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2004241–3. [PubMed] [Google Scholar]