Abstract
Background:
Fibroblast growth factor 23 (FGF-23) is a phosphorus-regulating hormone. In chronic kidney disease (CKD), circulating FGF-23 levels are markedly elevated and independently associated with mortality. Left ventricular hypertrophy (LVH) and coronary artery calcification are potent risk factors for mortality in CKD, and fibroblast growth factors have been implicated in the pathogenesis of both myocardial hypertrophy and atherosclerosis. We conducted a cross-sectional study to test the hypothesis that elevated FGF-23 concentrations are associated with LVH and coronary artery calcification in patients with CKD.
Methods and Results:
162 subjects with CKD underwent echocardiograms and computed tomography scans to assess left ventricular mass index (LVMI, g/m2.71) and coronary artery calcification; echocardiograms were also obtained in 58 subjects without CKD. In multivariable-adjusted regression analyses in the overall sample, increased log FGF-23 concentrations were independently associated with increased LVMI (5% increase per 1 SD increase in log FGF-23, P = 0.01), and risk of LVH (OR per 1 SD increase in log FGF-23 2.1, 95%CI 1.03, 4.2). These associations strengthened in analyses restricted to the CKD subjects (11% increase in LVMI per 1 SD increase in log FGF-23, P = 0.01; OR of LVH per 1 SD increase in log FGF-23 2.3, 95%CI 1.2, 4.2). While the highest tertile of FGF-23 was associated with a 2.4-fold increased risk of coronary artery calcification ≥ vs. < 100 units compared to the lowest tertile (95%CI 1.1, 5.5), the association was no longer significant after multivariable adjustment.
Conclusions:
FGF-23 is independently associated with LVMI and LVH in patients with CKD. Whether increased FGF-23 is a marker or a potential mechanism of myocardial hypertrophy in CKD requires further study.
Keywords: Fibroblast growth factor 23, left ventricular hypertrophy, coronary artery calcification, chronic kidney disease
Introduction
Chronic kidney disease (CKD) is a growing public health epidemic that is associated with markedly increased risk of cardiovascular disease and mortality.1, 2 While CKD populations manifest a high prevalence of traditional risk factors for atherosclerosis, such as hypertension and diabetes, these classic risk factors do not fully account for the burden of cardiovascular disease in patients with CKD.3-5 Left ventricular hypertrophy (LVH) and diffuse arterial calcification are common manifestations of cardiovascular disease that are powerful independent risk factors for mortality in patients with CKD.6-9 Approximately 40% of patients with pre-dialysis CKD and up to 80% of patients initiating hemodialysis manifest LVH.4, 8 Likewise, diffuse arterial calcification begins before patients reach dialysis and significant disease is present in over 60% of new dialysis patients.6, 10 Understanding early mechanisms of LVH and arterial calcification are essential for designing novel therapeutic strategies to attenuate cardiovascular disease in CKD.
Elevated serum phosphate concentrations, even within the normal range, are associated with LVH and increased cardiovascular mortality in both CKD and non-CKD populations.11-14 In addition, high phosphate concentrations promote non-atherosclerotic arterial calcification by stimulating metaplasia of vascular smooth muscle cells into an osteogenic phenotype.15 These results suggest that disordered phosphorus metabolism is a novel risk factor for cardiovascular disease. However, overt hyperphosphatemia is uncommon outside dialysis, and the small absolute increases in serum phosphate concentrations that were associated with poor clinical outcomes in large epidemiological studies13, 14 limit their utility as a tool to detect which individual patients with CKD are at greatest cardiovascular risk. Fibroblast growth factor 23 (FGF-23) is a recently-discovered hormone that helps maintain normal serum phosphate concentrations in patients with kidney disease by stimulating urinary phosphate excretion and decreasing dietary phosphorus absorption through the inhibition of 1,25-dihydroxyvitamin D (1,25(OH)2D) synthesis.16 Importantly, circulating concentrations of FGF-23 increase early in the course of kidney disease, long before the development of hyperphosphatemia, and thus a high FGF-23 concentration is among the earliest markers of disordered phosphorus metabolism in CKD.17
We recently reported that elevated FGF-23 concentrations at the initiation of dialysis are independently associated with increased risk of future mortality.18 The results were not confounded by other risk factors, and demonstrated a strong “dose-response”-type relationship such that ascending quartiles of FGF-23 were associated with a linear increase in risk of mortality.18 Furthermore, increased FGF-23 concentrations were much stronger predictors of mortality than elevated serum phosphate concentrations, and the strongest associations between FGF-23 and mortality were observed in the normal range of serum phosphate. These results suggest that increased FGF-23 may represent an early, more sensitive biomarker of disordered phosphorus metabolism than concomitant serum phosphate measurements. Moreover, elevated concentrations of FGF-23 have been shown to non-selectively activate fibroblast growth factor receptors implicated in the development of cardiac hypertrophy and atherosclerosis,19-23 suggesting a biological basis for an association between increased FGF-23 and mortality in CKD. However, no studies have examined the relationship between FGF-23 and LVH or coronary artery calcification in patients with pre-dialysis CKD. We conducted a cross-sectional study to test the hypothesis that elevated FGF-23 concentrations are independently associated with left ventricular mass index (LVMI), LVH and coronary artery calcification in asymptomatic patients with CKD.
Methods
Study Population
The study population consisted of 162 patients with pre-dialysis CKD and 58 patients with preserved kidney function. Consecutive patients with CKD were recruited from outpatient nephrology clinics at the Massachusetts General Hospital (MGH), Boston, MA; the University of Maryland, Baltimore, MD; and the Baltimore Veteran's Administration Medical Center. Patients were eligible for the study if they were 30 years of age or older and had a sustained reduction (≥ 3 months) in estimated glomerular filtration rate (eGFR) of ≤ 60 ml/min/1.732, based on the simplified Modification of Diet in Renal Disease formula.24 Exclusion criteria included stage 5 kidney disease (eGFR < 15 ml/min/1.732), renal replacement therapy (dialysis or kidney transplant), prior history of coronary artery bypass grafting, or a history of myocardial infarction within 90 days of enrollment. In addition, in order to focus the study on patients with early cardiac disease, patients with symptoms consistent with New York Heart Association > class 1 heart failure or Canadian Cardiovascular Society > class 1 angina were excluded. We also included 58 subjects with preserved kidney function (eGFR > 60 ml/min/1.73m2) in order to examine the relationship between FGF-23 and myocardial disease in non-CKD patients. These subjects were recruited from inpatient services at MGH, were at least 18 years old and clinically stable, did not have acute myocardial infarction, known cardiomyopathy or ejection fraction < 40%, or known mitral or aortic valve disease and were scheduled for an echocardiogram for diagnostic purposes per the primary admitting team. All studies were approved by the Institutional Review Boards of the Massachusetts General Hospital, the University of Maryland School of Medicine, and the Baltimore Veteran's Administration Medical Center, and all patients provided written informed consent.
Clinical Data and Laboratory Results of Interest
Data on demographic characteristics, past medical history, current medications and blood samples were collected in all subjects at the time of enrollment. Blood samples were immediately centrifuged, separated into aliquots, and stored at −80°C for future batched assays. Serum creatinine, calcium, phosphate and albumin were measured using standard commercial assays. Intact parathyroid hormone (PTH) concentrations were measured using the Roche Elecsys PTH assay (Roche, Indianapolis, IN). FGF-23 concentrations were measured in duplicate in the Core Laboratory of the MGH General Clinical Research Center using a two-site ELISA that detects two epitopes in the carboxyl-terminal portion of FGF-23 (Immutopics, San Clemente, CA). Serum B-type natriuretic peptide (BNP) and cardiac c-reactive protein (CRP) concentrations were measured in CKD patients using the Access 2 Immunoassay System (Beckman Coulter, Inc., Fullerton, CA) and Siemens Dimension RxL Max (Newark, DE), respectively. Serum was available in 69 CKD subjects for measurement of 1,25(OH)2D concentrations using extraction/liquid chromatography-tandem mass spectrometry (Mayo Medical Laboratories, Rochester, MN). Besides FGF-23 and 1,25(OH)2D, all other blood tests in CKD subjects were processed in a central laboratory at the University of Maryland following a single thaw.
Echocardiography
All subjects underwent two-dimensional transthoracic echocardiograms. In the CKD subjects, the studies were interpreted by a single reviewer at the University of Maryland who was blinded to subjects' clinical and laboratory data; for the subjects with preserved kidney function, studies were interpreted as part of routine clinical care. Left ventricular ejection fraction was determined using biplane modified Simpson's measurements. For the primary analysis, LVMI was calculated using the modified American Society of Echocardiography equation25 indexed to height2.71. This formula may be preferable to BSA-indexed formulas in CKD patients because it mitigates the potential effects of extracellular volume overload on weight-based calculations of LVMI.26 Relative wall thickness (RWT) was calculated using the following formula: (posterior wall thickness + septal wall thickness)/ end-diastolic cavity dimension.27 LVH was defined as LVMI > 49.2 g/m2.71 for men and > 46.7 g/m2.71 for women.27 LVH was further characterized as eccentric hypertrophy if RWT ≤ 0.43 or concentric hypertrophy if RWT > 0.43.28 Normal left ventricular geometry was defined as RWT ≤ 0.43 and normal LVMI. To test whether the results were robust to the method of calculating LVMI, BSA-indexed values were also examined; since the BSA-indexed and height-indexed results were qualitatively similar, only the latter are presented.
Coronary Artery Calcification
All patients with CKD underwent cardiac computed tomography (CT) scans to assess coronary artery calcification. CT examinations were performed on a 64 slice scanner (Sensations 64, Siemens Medical Solutions, Forchheim, Germany at MGH; Brilliance 64, Philips Healthcare, Cleveland, OH at the University of Maryland) using standardized protocols. Calcifications were quantified with dedicated scoring software (Brilliance Workspace, Philips Healthcare) by one blinded observer at the University of Maryland, using the method of Agatston et al.29
Statistical Analyses
Baseline characteristics were assessed using standard descriptive statistics. Estimated GFR was examined both on a continuous scale, and categorically according to level of kidney function: preserved kidney function (eGFR > 60 ml/min/1.73 m2), stage 3a CKD (eGFR 45 – 60), stage 3b (eGFR 30 – 44), or stage 4 (eGFR 15 – 29).30 Coronary artery calcification scores were examined on a continuous scale (log-transformed to approximate a normal distribution), and dichotomized by presence (score ≥ 100 units) or absence (< 100) of moderate to severe calcification.31, 32
Linear regression was used to examine the association between LVMI and baseline demographic, clinical, and laboratory variables. We utilized multivariable models to examine the relationship between LVMI and FGF-23 concentrations, adjusting for age, gender, race, eGFR, phosphate concentrations, body mass index (BMI), diabetes, hypertension and covariates that were significantly (P < 0.05) associated with LVMI in univariate analyses. We used analysis of covariance to test for different relationships between FGF-23 and LVMI among the CKD and non-CKD populations, and conducted pre-specified analyses restricted to patients with CKD. We adjusted for exposure to phosphorus binders or activated vitamin D analogs because they may influence FGF-23 concentrations,16 and for serum CRP and BNP concentrations as surrogate markers of inflammation, sodium intake, volume overload, and sympathetic activity, which are non-traditional risk factors for increased LVMI in CKD.33-36 Decreased vitamin D receptor activation by 1,25(OH)2D has been associated with increased left ventricular mass,37 and elevated FGF-23 inhibits the synthesis of 1,25(OH)2D in CKD.17 Therefore, we also examined the impact of 1,25(OH)2D on the relationship between FGF-23 and LVMI in CKD, modeling 1,25(OH)2D as a categorical variable with a separate category for missing values. We tested regression assumptions by examining transformations of LVMI and excluded non-linear relationships between FGF-23, LVMI, and coronary artery calcification by testing models that included polynomial terms.
Multivariable logistic regression analyses were performed to examine whether increased FGF-23 concentrations were associated with coronary artery calcification scores ≥ vs. < 100 and LVH, adjusted for the same covariates as above. In addition, we examined the likelihood of having concentric or eccentric hypertrophy vs. normal left ventricular geometry as a function of log FGF-23 concentrations. For all analyses, FGF-23 was examined both on a continuous scale, using natural log-transformed FGF-23 values to achieve a normal distribution, and in tertiles defined according to its distribution in the overall study sample. Two-tailed P-values < 0.05 were considered statistically significant. All statistical analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC, USA).
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Patient Characteristics
Tables 1 and 2 depict demographic and laboratory data, coronary artery calcification scores, and echocardiogram results of the 220 subjects according to level of kidney function. Mean LVMI in the overall population was 40 ± 12 g/m2.71. Coronary artery calcification scores ranged from 0 to 3132, with 48% of the patients having scores ≥ 100 units. Whereas the prevalence of coronary artery calcification scores ≥ vs. < 100 significantly increased with worsening eGFR, there were no significant differences in the indices of cardiac structure and function across the spectrum of CKD. When compared to patients with preserved kidney function, however, patients with CKD had significantly higher mean LVMI and lower mean ejection fraction. Mean LVMI and the prevalence of LVH among CKD subjects in this study were lower than in previous studies,36, 38 confirming that the majority of patients recruited for this study were free of significant cardiac disease, consistent with the study design.
Table 1.
Description of subjects by level of kidney function. Results are expressed as frequencies, mean ± standard deviation or median (interquartile range) as appropriate.
| Variables | eGFR >60 N=58 |
eGFR 45-60 N=34 |
eGFR 30-44 N=69 |
eGFR < 30 N=59 |
P (trend) |
|---|---|---|---|---|---|
| Age | 57 ± 17 | 60 ± 10 | 64 ± 11 | 65 ± 12 | <0.001 |
| Female gender (%) | 43 | 21 | 32 | 37 | 0.10 |
| Black (%) | 7 | 59 | 43 | 21 | 0.10 |
| eGFR (ml/min/1.73 m2) | 93 ± 23 | 53 ± 5 | 37 ± 5 | 23 ± 6 | <0.001 |
| Blood pressure (mmHg) | |||||
| Systolic | 120 ± 17 | 135 ± 19 | 134 ± 20 | 135 ± 22 | <0.001 |
| Diastolic | 68 ± 13 | 76 ± 10 | 76 ± 11 | 75 ± 13 | 0.002 |
| Body mass index (kg/m2) | 28 ± 7 | 32 ± 6 | 31 ± 7 | 29 ± 7 | 0.87 |
| Co-morbidities (%) | |||||
| Diabetes | 19 | 62 | 52 | 39 | 0.04 |
| Hypertension | 57 | 85 | 93 | 95 | <0.001 |
| Coronary artery disease | 14 | 6 | 19 | 17 | 0.32 |
| Congestive heart failure | 14 | 6 | 9 | 7 | 0.25 |
| Tobacco use* | 55 | 66 | 64 | 68 | 0.12 |
| Medications (%) | |||||
| Anti-hypertensive | 59 | 80 | 75 | 71 | 0.01 |
| Phosphorus binders | 0 | 0 | 0 | 15 | <0.001 |
| Activated Vitamin D | 0 | 3 | 9 | 25 | 0.001 |
| Laboratory values | |||||
| Creatinine (mg/dl) | 0.9 ± 0.2 | 1.5 ± 0.2 | 1.9 ± 0.3 | 3.0 ± 1.5 | <0.001 |
| Calcium (mg/dl) | 9.2 ± 0.5 | 9.1 ± 1.2 | 9.4 ± 0.5 | 9.4 ± 0.6 | 0.01 |
| Albumin (mg/dl) | 3.4 ± 0.5 | 3.8 ± 1.1 | 3.9 ± 0.5 | 3.8 ± 0.6 | 0.005 |
| PTH (pg/ml) | 40 (29, 58) | 79 (50, 135) | 64 (41, 95) | 73 (49, 104) | <0.001 |
includes current and former smokers
eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone
Table 2.
Coronary artery calcification scores and echocardiographic characteristics by level of kidney function. Results are expressed as frequencies, mean ± standard deviation or median (interquartile range) as appropriate.
| Variables | eGFR > 60 N=58 |
eGFR 45-60 N=34 |
eGFR 30-44 N=69 |
eGFR < 30 N=59 |
P (trend) |
|---|---|---|---|---|---|
| CAC Score | -- | 10 (0, 269) | 81 (13, 300) | 136 (10, 362) | 0.02* |
| % ≥ 100 | -- | 35 | 46 | 56 | 0.05* |
| LVEF (%) | 67 ± 7 | 59 ± 4 | 58 ± 5 | 57 ± 6 | <0.001 |
| LVMI (g/m2.71) | 35 ± 11 | 41 ± 11 | 40 ± 12 | 41 ± 13 | 0.02 |
| LVH (%) | 9 | 26 | 16 | 22 | 0.13 |
test for trend only includes patients with CKD
eGFR, estimated glomerular filtration rate; CAC, coronary artery calcification; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; LVH, left ventricular hypertrophy
FGF-23 and Phosphate Concentrations
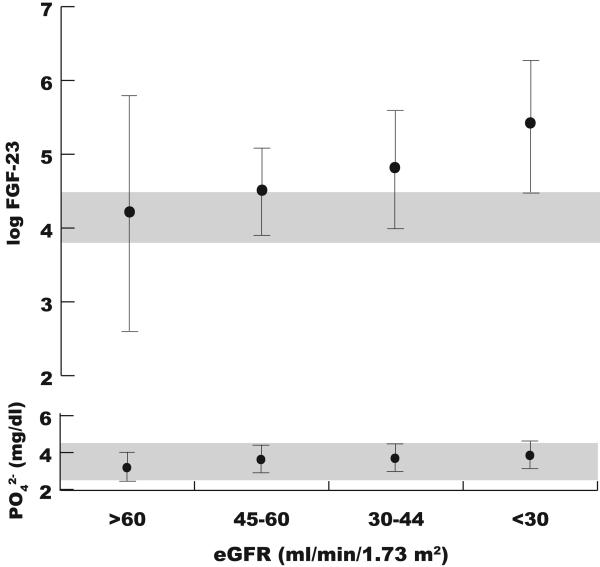
The median FGF-23 concentration in the overall population was 109 RU/ml (interquartile range, 65, 204); mean log FGF-23 was 4.74 ± 1.12. Mean phosphate and log FGF-23 concentrations significantly increased with decreasing levels of eGFR (Figure 1, P for trend <0.001 for both), however, the absolute difference in mean serum phosphate concentrations between the lowest and highest eGFR groups was only 0.6 mg/dl (relative difference of 14%). Furthermore, the mean serum phosphate concentration was within the normal range in all groups, including the group with the most severe CKD (eGFR <30 ml/min/1.73m2). In contrast, the analogous percent increase in mean log FGF-23 concentrations was substantially larger (68%), and levels were already above the normal range in patients with mild to moderate CKD (eGFR 30-60 ml/min/1.73m2), as has been reported previously.17
Figure 1.
Mean concentrations of log FGF-23 and phosphate according to level of kidney function. Bars represent standard deviations, and shaded areas represent normal ranges for each analyte.
FGF-23 and Left Ventricular Structure
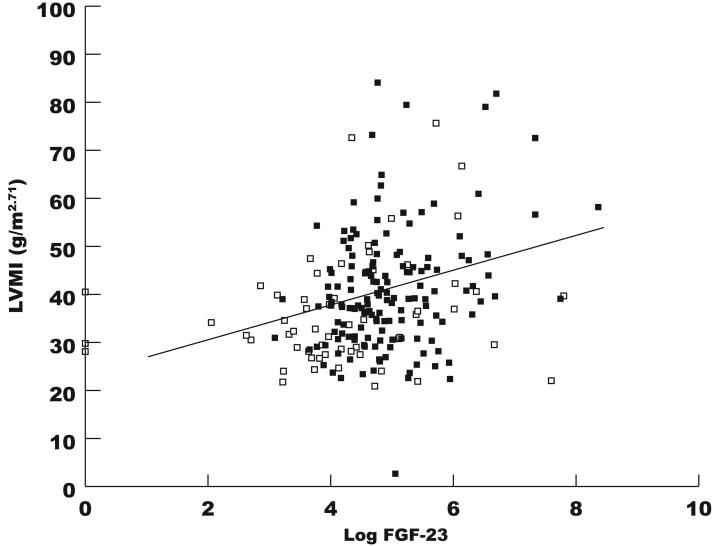
In univariable analyses, increased log FGF-23 concentrations were significantly associated with increased LVMI (9% increase per 1 SD increase in log FGF-23, P<0.001, Figure 2); the relationship was not modified by presence of CKD. Other variables that were associated with increased LVMI in univariable analyses included BMI (2% increase per 1 kg/m2 increase in BMI, P<0.001), eGFR (2% increase per 10 ml/min/1.73m2 decrease in eGFR, P<0.001), diabetes (13% increase compared to none, P=0.002), and hypertension (20% increase compared to none, P<0.001). When adjusted for age, gender, race, diabetes, BMI, hypertension, eGFR, and serum phosphate concentrations, there was minimal change in the point estimate for log FGF-23, which remained significantly associated with increased LVMI (5% increase per 1 SD increase in log FGF-23, P=0.01). In the multivariable model, BMI was the only other factor that remained significantly associated with LVMI (P<0.001). When examined in tertiles, mean LVMI increased with increasing tertiles of FGF-23 (Table 3), and the step-wise increase in LVMI with increasing tertiles of FGF-23 remained significant in multivariable-adjusted analysis (tertile 1 reference; tertile 2, 5% increase in LVMI; tertile 3, 11% increase in LVMI, P for linear trend = 0.04). In contrast to FGF-23, serum phosphate concentrations were not associated with LVMI or LVH in univariable or multivariable analyses. In analyses restricted to subjects with CKD, log FGF-23 remained independently associated with LVMI (Table 4), and the results were unchanged when further adjusted for vitamin D and phosphorus binder use, and serum concentrations of BNP, CRP and 1,25(OH)2D, even though the latter was inversely associated with log FGF-23 (P=0.008). In addition, accounting for recruitment site did not alter the results (data not shown).
Figure 2.
Correlation between log FGF-23 and left ventricular mass index (r=0.27, P<0.001). Open squares refer to non-CKD subjects and closed squares to those with CKD.
Table 3.
Coronary artery calcification scores and echocardiographic characteristics by tertile of FGF-23. Results are expressed as frequencies, mean ± standard deviation or median (interquartile range) as appropriate.
| FGF-23 Tertile 1 (< 75 RU/ml) |
FGF-23 Tertile 2 (75-150 RU/ml) |
FGF-23 Tertile 3 (>150 RU/ml) |
P (trend) |
|
|---|---|---|---|---|
| N | 69 | 71 | 72 | |
| CAC Score | 32 (0, 270) | 121 (0, 302) | 117 (14, 384) | 0.2* |
| % ≥ 100 | 33 | 53 | 55 | 0.04* |
| LVMI (g/m2.71) | 35 ± 8 | 40 ± 12 | 42 ± 15 | <0.001 |
| LVH (%) | 7 | 21 | 25 | 0.006 |
test for trend only includes patients with CKD
CAC, coronary artery calcification; LVMI, left ventricular mass index; LVH, left ventricular hypertrophy
Table 4.
Percent increase in mean LVMI and odds ratio of LVH per 1 SD increase in log FGF-23 adjusted for medication use and serum concentrations of CRP, BNP, and 1,25(OH)2D in 162 CKD subjects.
| % increase in mean LVMI (95% CI) per 1 SD increase in log FGF-23 |
OR (95% CI) of LVH per 1 SD increase in log FGF-23 |
|
|---|---|---|
| Unadjusted model | 12% (4%, 18%, P < 0.001) | 2.0 (1.2, 3.4, P = 0.006) |
| Multivariable-adjusted model* | 11% (3%, 18%, P = 0.01) | 2.3 (1.2, 4.2, P = 0.01) |
| + active vitamin D use | 11% (4%, 18%, P = 0.005) | 2.2 (1.2, 4.3, P = 0.01) |
| + phosphorus binder use | 11% (4%, 18%, P = 0.003) | 2.2 (1.2, 4.2, P = 0.01) |
| + log CRP | 11% (3%, 18%, P = 0.005) | 2.3 (1.2, 4.2, P = 0.01) |
| + log BNP | 11% (5%, 16%, P = 0.006) | 2.3 (1.1, 4.6, P = 0.01) |
| + 1,25(OH)2D† | 10% (4%, 18%, P = 0.003) | 2.0 (1.4, 3.0, P = 0.008) |
adjusted for age, gender, race, BMI, eGFR, diabetes, hypertension, and serum phosphate.
1,25(OH)2D was measured in 69 subjects; therefore, 1,25(OH)2D was analyzed as a categorical variable with a separate category for missing values.
FGF-23, fibroblast growth factor 23; LVMI, left ventricular mass index (g/m2.71); LVH, left ventricular hypertrophy; SD, standard deviation; CRP, c-reactive protein; BNP, B-type natriuretic peptide; 1,25(OH)2D, 1,25-dihydroxyvitamin D; CKD, chronic kidney disease.
Increasing log FGF-23 concentrations were also significantly associated with the presence of left ventricular hypertrophy (OR per 1 SD increase in log FGF-23 2.0, 95%CI 1.4, 3.0), a result that was virtually unchanged in the fully adjusted analysis (OR per 1 SD increase in log FGF-23, 1.8, 95%CI 1.2, 2.9). The prevalence of LVH significantly increased with ascending tertiles of FGF-23 in univariate analysis (Table 3), but this relationship was attenuated after multivariable adjustment (tertile 1 reference; tertile 2, OR 2.2, 95% CI 0.7, 6.8; tertile 3, OR 2.7, 95% CI 0.8, 8.6). In the analyses restricted to subjects with CKD, log FGF-23 was significantly associated with increased risk of LVH, independent of traditional risk factors, vitamin D and phosphorus binder use, and serum concentrations of CRP, BNP, and 1,25(OH)2D (Table 4).
In order to further characterize the relationship between increased FGF-23 and LVH, we examined the likelihood of eccentric or concentric hypertrophy vs. normal left ventricular geometry as a function of FGF-23 in the CKD patients. Whereas log FGF-23 was not significantly associated with risk of eccentric LVH, log FGF-23 was significantly associated with increased risk of concentric LVH in univariable (OR 2.4, 95%CI 1.1, 5.1) and multivariable-adjusted analyses (OR 3.4, 95%CI 1.2, 9.9).
FGF-23 and Coronary Artery Calcification
In univariable analyses, increasing age (OR 1.1 per 1 year increase in age, 95%CI 1.04, 1.2), and a history of coronary artery disease (OR 7.6, 95%CI 2.5, 23) and smoking (OR 2.9, 95%CI 1.6, 5.9) were associated with a significantly increased risk of coronary artery calcification score ≥ vs. < 100; serum phosphate concentrations were not associated with coronary artery calcification. On a continuous scale, increased log FGF-23 concentrations were not significantly associated with log-transformed coronary artery calcification scores (P=0.38) or scores ≥ vs. < 100 (OR per 1 SD increase in log FGF-23 1.2, 95%CI 0.8, 1.7); however, the highest tertile of FGF-23 was associated with 2.4-fold (95%CI 1.1, 5.5) greater risk of coronary artery calcification ≥ vs. < 100 when compared to the lowest tertile. In fully adjusted models, the point estimate for the highest versus the lowest FGF-23 tertile was only minimally attenuated, but the association was no longer statistically significant (OR 2.1, 95%CI 0.7, 6.5).
Discussion
In this cross-sectional study of patients with CKD not yet requiring dialysis, increased FGF-23 concentrations were associated with increased LVMI and increased prevalence of LVH independent of established risk factors, such as older age, declining eGFR, diabetes and hypertension. Importantly, the associations were also independent of serum phosphate concentrations, which were not associated with LVMI or LVH. Although previous studies found hyperphosphatemia to be associated with increased LVMI and LVH, these results suggest that FGF-23 may be superior to serum phosphate measurements as a marker of the early pathophysiological mechanisms that link disordered phosphorus metabolism with cardiovascular disease in CKD, especially when serum phosphate levels are in the normal range. Whether chronically elevated FGF-23 concentrations may also directly promote myocardial hypertrophy in patients with CKD is an intriguing possibility that requires further study.
FGF-23 is a hormone secreted by osteoblasts and osteocytes that acts primarily in renal proximal tubules to increase urinary phosphorus excretion through down-regulation of sodium-phosphate co-transporters, and to decrease 1,25(OH)2D concentrations through inhibition of renal 25-hydroxyvitamin D-1-α-hydroxylase.16 Reductions in FGF-23 levels have the opposite effects.16 The main physiological stimulus of FGF-23 secretion is increased dietary phosphorus intake.39 In patients with CKD, FGF-23 concentrations are constitutively elevated and increase progressively as kidney function worsens, likely as an appropriate compensation to help maintain normal serum phosphate concentrations in the face of declining nephron mass.17 By the time patients reach end stage renal disease, FGF-23 concentrations are often 100- to 1000-fold above the normal range, whereas serum phosphate concentrations are only modestly increased or even normal.18 Indeed, as demonstrated in Figure 1, serum phosphate concentrations remained within the normal range across the spectrum of eGFR, whereas FGF-23 concentrations were already elevated in early stages of CKD.
Although increased FGF-23 concentrations in advancing CKD appear to be an appropriate compensation to prevent hyperphosphatemia, in the long-term, increased FGF-23 may have adverse end-organ effects. In a prospective study of patients with pre-dialysis CKD, elevated FGF-23 concentrations were independently associated with more rapid progression to renal failure, suggesting a direct link between increased FGF-23 and CKD progression.40 Importantly, the results were independent of serum phosphate concentrations, which were normal in the vast majority of patients. In a more recent prospective cohort study, we observed that increased FGF-23 concentrations at the initiation of hemodialysis were independently associated with increased one-year all-cause mortality in a dose-dependent fashion.18 Not only were the results independent of baseline serum phosphate concentrations, but the magnitude of the risk of death associated with the highest concentrations of FGF-23 (odds ratio 5.7 for highest versus lowest quartile) dwarfed the corresponding risk associated with the highest concentrations of phosphate (odds ratio 1.2 for highest versus lowest quartile). The results of the current study provide further evidence to suggest that markedly elevated FGF-23 concentrations may be toxic in CKD. Furthermore, since LVH is a potent risk factor for cardiovascular mortality in CKD,9 these results suggest one potential mechanism to explain the link between increased FGF-23 and mortality on dialysis.
Fibroblast growth factor receptors, particularly FGFR1, are expressed in adult myocardial cells,41, 42 and their activation by locally-secreted growth factors can stimulate myocardial hypertrophy and interstitial fibrosis. For example, FGF-2 promotes adult cardiac myocyte hypertrophy in vitro,20 and excess exposure to FGF-2 in animal models results in enhanced myocardial hypertrophy and interstitial fibrosis after infarction.22, 23 Furthermore, FGF2−/− mice developed significantly less ventricular hypertrophy than wild-type mice following transverse aortic coarctation, suggesting an important role for fibroblast growth factors in mediating pressure-overload cardiac hypertrophy.43 Thus, it is intriguing to speculate whether chronic exposure to elevated FGF-23 concentrations in CKD may similarly accelerate myocardial hypertrophy and fibrosis, which characterize uremic cardiomyopathy. For example, at the markedly elevated circulating concentrations seen in patients with CKD, FGF-23 may non-selectively bind to FGF receptors that are normally activated by locally-active growth factors such as FGF-2.19 Indeed, it is intriguing that increased FGF-23 was significantly associated with increased risk of concentric but not eccentric hypertrophy, since concentric hypertrophy was observed in animal models of excess FGF-2 exposure described above. Given that FGF-23 concentrations can be lowered in patients with kidney disease using routine clinical interventions that restrict dietary phosphorus absorption,44 whether “treatment” of elevated FGF-23 concentrations with dietary phosphorus restriction or oral phosphorus binders could be a therapeutic target to attenuate the progression of myocardial disease in early kidney disease is an exciting possibility. Of note, although 1,25(OH)2D was inversely associated with FGF-23 in a subset of CKD subjects, adjusting for 1,25(OH)2D did not attenuate the magnitude or strength of the associations between FGF-23 and LVMI or LVH. While this suggests that the relationship between FGF-23 and left ventricular disease may be independent of 1,25(OH)2D, further studies are needed to examine these relationships in more detail.
Vascular calcification is a highly prevalent risk factor for mortality among patients with CKD and is characterized by metaplasia of vascular smooth muscle cells into osteogenic cell-types that mineralize surrounding arterial tissue.15 The univariate association between elevated FGF-23 concentrations and severe coronary artery calcification in this study may reflect a potential link between FGF-23 and diffuse arterial calcification, as has been reported in dialysis patients.45 However, given that the association was no longer significant after multivariable adjustment and the lack of an association when calcification was examined on a continuous scale, we must interpret these findings with caution, especially since a previous study failed to show an association between FGF-23 and coronary artery calcification.46
We acknowledge several limitations in this study. First, the exclusion of CKD subjects with symptomatic heart disease may have limited our ability to detect more robust associations between FGF-23 and LVH, and phosphate and LVH. Nevertheless, this was also an important strength in that we were able to detect an independent association between FGF-23 and increased LVMI despite this potential limitation. Second, the CKD subjects were recruited from outpatient clinics while the non-CKD subjects were inpatients. This likely introduced more variability in the measurements of the non-CKD group, who likely had higher LVMI than if they had been recruited from the outpatient setting. Indeed, non-CKD subjects in this study had wider variation in their FGF-23 concentrations and a higher prevalence of congestive heart failure than the subjects with CKD. However, we would expect these limitations to bias the results to the null, and yet we were still able to detect significant associations. Finally, given that these results are cross-sectional, we cannot draw definitive inferences on their direction or causality. Nevertheless, this is the first report to link FGF-23 with LVMI and LVH in CKD, observations that may contribute to the robust association between FGF-23 and mortality.18 Given the growing worldwide burden of CKD and its strong association with cardiovascular mortality, prospective human studies and laboratory experiments are needed to further explore the effects of FGF-23 on the cardiovascular system in CKD.
Funding Sources
Funding for the main study in CKD subjects was provided by Siemens. For the present study, all of the hypotheses and analyses were generated and tested by the authors independent of industry support. In addition, this study was supported by the American Kidney Fund Clinical Scientist in Nephrology Fellowship (to Dr. Gutiérrez), and by grants from the American Society of Nephrology-Alaska Kidney Foundation (to Dr. Wolf), the American Heart Association (to Dr. Wang), and the National Institutes of Health (M01RR01066, Mallinckrodt General Clinical Research Center Program at MGH; K30RR02229207; K23DK081673 to Dr. Gutiérrez; R01HL086875 to Dr. Wang; K23RR017376 and R01DK076116 to Dr. Wolf)
Footnotes
Commentary
Chronic kidney disease (CKD) is a growing public health epidemic that is an established risk factor for cardiovascular morbidity and mortality. An emerging body of evidence suggests that, in addition to traditional risk factors, a number of “uremic” factors contribute to the markedly high prevalence of cardiovascular disease in this population. Left ventricular hypertrophy is a common manifestation of cardiovascular disease in CKD that is associated with markedly increased risk of mortality. Fibroblast growth factor 23 (FGF-23) is a novel phosphate-regulating hormone that is markedly elevated in patients with CKD and is independently associated with mortality among patients initiating hemodialysis. In the current report, we present cross-sectional data from 162 subjects with pre-dialysis CKD and 58 subjects with preserved kidney function demonstrating that increased FGF-23 concentrations are associated with increased left ventricular mass index and left ventricular hypertrophy independent of known risk factors in CKD, such as age, diabetes, and hypertension. Given that circulating FGF-23 concentrations are elevated early in the course of kidney failure, and fibroblast growth factors have been implicated in the development of myocardial hypertrophy, this study suggests a novel biological mechanism that may contribute to the accelerated development of myocardial disease in CKD. In addition, given that FGF-23 can be lowered using routine clinical interventions that restrict dietary phosphorus absorption, these results may suggest novel therapeutic strategies for ameliorating the development of left ventricular hypertrophy in millions of patients with CKD.
Disclosures
Dr. Wolf reports having a pending patent application on FGF-23 measurements as a diagnostic aid, and having received grant support from Shire, honoraria from Abbott and Genzyme, and consulting fees from Ineos. Dr. Januzzi reports having received grant support from Roche Diagnostics, Siemens, Critical Diagnostics, and Inverness Medical; speaker fees from Roche Diagnostics, Siemens, and Ortho Clinical Diagnostics; and consulting income from Roche Diagnostics, Siemens, Critical Diagnostics, and BG Medicine. Dr. Christenson reports having received research support from Siemens, Roche, and Inverness Diagnostics, and consulting income from Siemens and Inverness Diagnostics. Dr. Wang reports having received a stipend for serving on the scientific advisory board of Diasorin. Dr. DeFilippi reports having received grant support from Siemens and Roche Diagnostics; speaker fees from Siemens and Roche Diagnostics; and consulting income from Siemens, BG Medicine, and Critical Diagnostics.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Hostetter TH. Chronic kidney disease predicts cardiovascular disease. N Engl J Med. 2004;351:1344–1346. doi: 10.1056/NEJMe048211. [DOI] [PubMed] [Google Scholar]
- 3.Mall G, Rambausek M, Neumeister A, Kollmar S, Vetterlein F, Ritz E. Myocardial interstitial fibrosis in experimental uremia--implications for cardiac compliance. Kidney Int. 1988;33:804–811. doi: 10.1038/ki.1988.71. [DOI] [PubMed] [Google Scholar]
- 4.Middleton RJ, Parfrey PS, Foley RN. Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol. 2001;12:1079–1084. doi: 10.1681/ASN.V1251079. [DOI] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 6.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 7.Kramer H, Toto R, Peshock R, Cooper R, Victor R. Association between chronic kidney disease and coronary artery calcification: the Dallas Heart Study. J Am Soc Nephrol. 2005;16:507–513. doi: 10.1681/ASN.2004070610. [DOI] [PubMed] [Google Scholar]
- 8.Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis. 1996;27:347–354. doi: 10.1016/s0272-6386(96)90357-1. [DOI] [PubMed] [Google Scholar]
- 9.Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36:286–290. doi: 10.1038/ki.1989.192. [DOI] [PubMed] [Google Scholar]
- 10.Garland JS, Holden RM, Groome PA, Lam M, Nolan RL, Morton AR, Pickett W. Prevalence and Associations of Coronary Artery Calcification in Patients With Stages 3 to 5 CKD Without Cardiovascular Disease. Am J Kidney Dis. 2008 doi: 10.1053/j.ajkd.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Ayus JC, Mizani MR, Achinger SG, Thadhani R, Go AS, Lee S. Effects of short daily versus conventional hemodialysis on left ventricular hypertrophy and inflammatory markers: a prospective, controlled study. J Am Soc Nephrol. 2005;16:2778–2788. doi: 10.1681/ASN.2005040392. [DOI] [PubMed] [Google Scholar]
- 12.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 13.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 14.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 15.Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19:1092–1105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez O, Mannstadt M, Isakova T, Rauh-Hain J, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jueppner H, Wolf M. Fibroblast Growth Factor 23 and Mortality among Hemodialysis Patients. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, Garringer HJ, Linhardt RJ, Ornitz DM, Mohammadi M, White KE. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146:4647–4656. doi: 10.1210/en.2005-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corda S, Mebazaa A, Gandolfini MP, Fitting C, Marotte F, Peynet J, Charlemagne D, Cavaillon JM, Payen D, Rappaport L, Samuel JL. Trophic effect of human pericardial fluid on adult cardiac myocytes. Differential role of fibroblast growth factor-2 and factors related to ventricular hypertrophy. Circ Res. 1997;81:679–687. doi: 10.1161/01.res.81.5.679. [DOI] [PubMed] [Google Scholar]
- 21.Li G, Oparil S, Kelpke SS, Chen YF, Thompson JA. Fibroblast growth factor receptor-1 signaling induces osteopontin expression and vascular smooth muscle cell-dependent adventitial fibroblast migration in vitro. Circulation. 2002;106:854–859. doi: 10.1161/01.cir.0000024113.26985.cc. [DOI] [PubMed] [Google Scholar]
- 22.Scheinowitz M, Kotlyar A, Zimand S, Ohad D, Leibovitz I, Bloom N, Goldberg I, Nass D, Engelberg S, Savion N, Eldar M. Basic fibroblast growth factor induces myocardial hypertrophy following acute infarction in rats. Exp Physiol. 1998;83:585–593. doi: 10.1113/expphysiol.1998.sp004140. [DOI] [PubMed] [Google Scholar]
- 23.Virag JA, Rolle ML, Reece J, Hardouin S, Feigl EO, Murry CE. Fibroblast growth factor-2 regulates myocardial infarct repair: effects on cell proliferation, scar contraction, and ventricular function. Am J Pathol. 2007;171:1431–1440. doi: 10.2353/ajpath.2007.070003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 25.Schillaci G, Verdecchia P, Borgioni C, Ciucci A, Guerrieri M, Zampi I, Battistelli M, Bartoccini C, Porcellati C. Improved electrocardiographic diagnosis of left ventricular hypertrophy. Am J Cardiol. 1994;74:714–719. doi: 10.1016/0002-9149(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 26.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Cataliotti A, Seminara G, Stancanelli B, Malatino LS. Prognostic impact of the indexation of left ventricular mass in patients undergoing dialysis. J Am Soc Nephrol. 2001;12:2768–2774. doi: 10.1681/ASN.V12122768. [DOI] [PubMed] [Google Scholar]
- 27.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 29.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 30.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 31.Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, Witteman JC. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–577. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 32.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 33.Damgaard M, Goetze JP, Norsk P, Gadsboll N. Altered sodium intake affects plasma concentrations of BNP but not proBNP in healthy individuals and patients with compensated heart failure. Eur Heart J. 2007;28:2726–2731. doi: 10.1093/eurheartj/ehm396. [DOI] [PubMed] [Google Scholar]
- 34.Brunner-La Rocca HP, Kaye DM, Woods RL, Hastings J, Esler MD. Effects of intravenous brain natriuretic peptide on regional sympathetic activity in patients with chronic heart failure as compared with healthy control subjects. J Am Coll Cardiol. 2001;37:1221–1227. doi: 10.1016/s0735-1097(01)01172-x. [DOI] [PubMed] [Google Scholar]
- 35.Kutlay S, Dincer I, Sengul S, Nergizoglu G, Duman N, Erturk S. The long-term behavior and predictors of left ventricular hypertrophy in hemodialysis patients. Am J Kidney Dis. 2006;47:485–492. doi: 10.1053/j.ajkd.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 36.Khan IA, Fink J, Nass C, Chen H, Christenson R, deFilippi CR. N-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide for identifying coronary artery disease and left ventricular hypertrophy in ambulatory chronic kidney disease patients. Am J Cardiol. 2006;97:1530–1534. doi: 10.1016/j.amjcard.2005.11.090. [DOI] [PubMed] [Google Scholar]
- 37.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 38.Verma A, Anavekar NS, Meris A, Thune JJ, Arnold JM, Ghali JK, Velazquez EJ, McMurray JJ, Pfeffer MA, Solomon SD. The relationship between renal function and cardiac structure, function, and prognosis after myocardial infarction: the VALIANT Echo Study. J Am Coll Cardiol. 2007;50:1238–1245. doi: 10.1016/j.jacc.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 40.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, Konig P, Kraatz G, Mann JF, Muller GA, Kohler H, Riegler P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 41.Hughes SE. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J Histochem Cytochem. 1997;45:1005–1019. doi: 10.1177/002215549704500710. [DOI] [PubMed] [Google Scholar]
- 42.Liu L, Pasumarthi KB, Padua RR, Massaeli H, Fandrich RR, Pierce GN, Cattini PA, Kardami E. Adult cardiomyocytes express functional high-affinity receptors for basic fibroblast growth factor. Am J Physiol. 1995 doi: 10.1152/ajpheart.1995.268.5.H1927. [DOI] [PubMed] [Google Scholar]
- 43.Schultz JE, Witt SA, Nieman ML, Reiser PJ, Engle SJ, Zhou M, Pawlowski SA, Lorenz JN, Kimball TR, Doetschman T. Fibroblast growth factor-2 mediates pressure-induced hypertrophic response. J Clin Invest. 1999;104:709–719. doi: 10.1172/JCI7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koiwa F, Kazama JJ, Tokumoto A, Onoda N, Kato H, Okada T, Nii-Kono T, Fukagawa M, Shigematsu T. Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients. Ther Apher Dial. 2005;9:336–339. doi: 10.1111/j.1744-9987.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 45.Jean G, Bresson E, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol Dial Transplant. 2008 doi: 10.1093/ndt/gfn571. [DOI] [PubMed] [Google Scholar]
- 46.Roos M, Lutz J, Salmhofer H, Luppa P, Knauss A, Braun S, Martinof S, Schomig A, Heemann U, Kastrati A, Hausleiter J. Relation between plasma fibroblast growth factor-23, serum fetuin-A levels and coronary artery calcification evaluated by multislice computed tomography in patients with normal kidney function. Clin Endocrinol (Oxf) 2008;68:660–665. doi: 10.1111/j.1365-2265.2007.03074.x. [DOI] [PubMed] [Google Scholar]