Abstract
The presence of DDT and derivatives in the food web of freshwater ecosystems of Rangsit agricultural area, Pathum Thani Province, Thailand were investigated from June 2004 to May 2007. By using gas chromatography (GC) with micro electron capture detector (μ ECD), DDT and derivatives in water, sediment, and fifteen indicator species i.e., 2 producers; Eichhornia crassipes and plankton (phyto- and zoo- plankton), an herbivore; Trichogaster microlepis (3) 3 omnivores; Trichogaster trichopterus, Oreochromis niloticus, and Puntius gonionotus, 6 carnivores; Channa striatus, Oxyeleotris marmoratus, Macrognathus siamensis, Parambassis siamensis, Anabas testudineus, and Pristolepis fasciatus, and 3 detritivores; Macrobrachium lanchesteri, Pomacea sp., and Filopaludina mertensi were measured. Results show low concentration levels (part per billion) of DDT & derivatives in each food web compartment i.e. water, sediment, aquatic plant, plankton, fish, and invertebrates. Magnification patterns, i.e. bioconcentration, bioaccumulation, and biomagnification, based on habitat and foraging behavior of selected freshwater species indicates that DDT & derivatives can accumulate and be magnified through the food chain from the lowest up to the highest trophic level. Therefore, the presence of residues and the evidence of magnification patterns can be observed as ecological indicators for evaluating ecological health risk.
Keywords: Organochlorine pesticides, Bioconcentration, Bioaccumulation, Biomagnification, Aquatic food web
1. Introduction
In Thailand, organochlorine pesticides (OCPs) had been heavily used for agricultural and public health purposes starting in the 1950s and reached maximum use in the 1970s through the 1990s (Thirakhupt et al., 2006). DDT displays biomagnification, an increase in contaminant concentration from one trophic level to the next due to accumulation from ingesting contaminated food (Keithmaleesatti et al., 2007). Although DDT and derivatives had been banned for agricultural and public health purposes in Thailand during 1983–2001, the concentration of DDT and derivatives have been detected in soil and water from many main rivers and agricultural areas (Anat and Paul, 2000; Thirakhupt et al., 2006). These residues are still slowly being released into aquatic and terrestrial food chains and can reach significant concentrations in animals at higher trophic levels (Robinson et al., 1967; Keithmaleesatti, 2007). Direct documentation of ecosystem changes as related to management measures, can be cost and time intensive. Therefore, a more pragmatic approach to communicate information about ecosystems and to monitor the impact of human activities on an ecosystem is through the use of ecological indicators. (Osinski et al., 2003)
This study aimed to investigate the residues and magnification factors i.e. bioconcentration factor (BCF), bioaccumulation factor (BAF), and biomagnification factor (BMF) of DDT and derivatives in the selected predators and preys in the food web of aquatic ecosystems at Khlong 7 (canal), Rangsit Agricultural Area. Fifteen common organisms were selected as the indicator species of the food web such as 2 producers; Eichhornia crassipes and plankton (phyto- and zoo- plankton), an herbivore; Trichogaster microlepis, 3 omnivores; Trichogaster trichopterus, Oreochromis niloticus, and Puntius gonionotus, 6 carnivores; Channa striatus, Oxyeleotris marmoratus, Macrognathus siamensis, Parambassis siamensis, Anabas testudineus, and Pristolepis fasciatus, and 3 detritivores; Macrobrachium lanchesteri, Pomacea sp., and Filopaludina mertensi. The food web relationship was investigated by foraging behavior observation in the laboratory aquarium, stomach analysis, and literature reviews (Nelson, 1976; Rainboth, 1996; Monkolprasit et al, 1997; Vidthayanon, 2002; Vidthayanon, 2004). The BCF, BAF, and BMF of DDT and derivatives were calculated throughout the food chain from the lowest trophic level to highest trophic level.
2. Materials and Methods
2.1 Study area and sampling
Rangsit agricultural area is located at the central part of Thailand in Pathum Thani Province. This agricultural area has a man-made irrigation-network-system consisting of 14 sub-canals (Khlong). These sub-canals are divided by Rangsit-Prayulasakdi canal into an upper and lower part. The study area is situated at Khlong 7, a 20-km sub-canal, on the upper part of the irrigation-network-system. Field samplings and field procedure were conducted following U.S. EPA (2000) recommendation from June 2004 to May 2007. Triplicate samples of water, sediment, plankton, aquatic plants, invertebrates and fish were collected monthly from the upper stream (U), middle stream (M), and lower stream (L) of Khlong 7 (figure 1).
Figure 1.
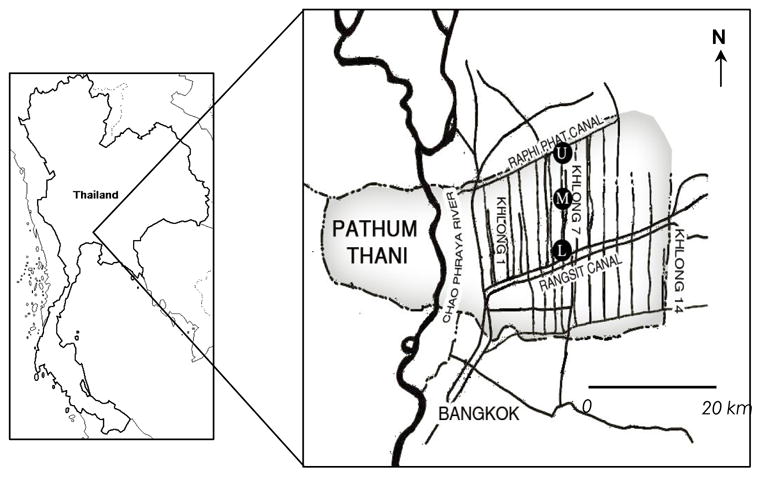
Map of Rangsit agricultural area, Pathum Thani Province, Thailand. The sampling stations are at Khlong 7; where (U) = upper stream, (M) = middle stream, and (L) = lower stream
2.2 Sample extraction and clean up
2.2.1 Extraction of OCPRs in water
Using liquid-liquid extraction (LLE) as described in APHA (1975), the total amount of each surface water sample, 800 mL, was filtered with Whatman® filter paper (i.d. 70 mm) then poured into a 2-L separatory funnel. For the first LLE, the mixture of 100 mL n- hexane and dichloromethane (1:1 v/v) was added and shaken vigorously for 2 min before 2-phase separation. The water-phase was drained from the separatory funnel into a 1,000 mL beaker. The organic phase was carefully poured into a glass funnel containing 20 g of anhydrous sodium sulfate through a 200-mL concentrator tube. Following the second and third LLE, the water-phase was poured back into the separatory funnel to re-extract with 50 mL of the same solvent mixture. The extract was concentrated to the volume of 2 mL under a gentle stream of nitrogen using Turbo Vap® evaporator, and then analyzed with Gas Chromatography with micro Electron Capture Detector (GC-μECD) (2.4).
2.2.2 Extraction of OCPRs in sediment
Each sediment sample was well-mixed and dried in circulating air at room temperature without sunlight exposure for 3–4 days. Dried sample was ground and sieved (500 μm) to remove stones and shells (Pridmore et al., 1992). Using accelerated solvent extractor (ASE, Dionex Canada Ltd. Oakville, ON, Canada); 5 g of the sediment sample was mixed with 5 g anhydrous sodium sulfate (1:1 w/w) and placed into a 34-mL ASE-vessel, then extracted with 1:1 v/v 95% n-hexane: dichloromethane. The sample was preheated for 5 min and extracted at 100°C with a pressure of 1,500 psi for 10 min. Finally, the sample was purged with nitrogen for 60 sec.
To remove sulfur contamination as previously described in Pan et al. (2004), the elute was cleaned with 30-cm chromatographic column by packing 6 g of florisil layer between 2 g of activated copper powder and 10 g of anhydrous sodium sulfate layer. Three fractions of eluents were used specifically: 50 mL of 6% and 15% of diethyl ether in petroleum ether, respectively. The elution rate was 5 mL/min by gravity. The eluates were collected in concentrator tubes and the volume was reduced to 2 mL under a gentle stream of nitrogen for quantification with GC-μECD (2.4).
2.2.3 Extraction of OCPRs in plankton
The extraction method was modified from DeLorenzo et al. (2002). The plankton mass was separated from an aliquot (30 mL) by centrifugation (2500 rpm. for 30 min). The supernatant was decanted. The plankton pellet was then washed with deionized water and recentrifuged twice as before. Afterward, the plankton pellet was weighed using a 4-digit balance, dissolved in 2 mL methanol and vortexed. An equal amount of hexane was then added and the contents were mixed. After phase separation, a 1-mL aliquot of hexane layer was transferred for clean up. A florisil Solid Phase Extraction (SPE) cartridge was applied for clean up using three fraction eluents: 10 mL of 6% and 15% of diethyl ether in petroleum ether, respectively (Caleste Matos Lino and Irene Noronha da Silveira, 1997; Alvin and Lau, 2004). The elution rate was 1 mL/min by gravity. The eluates were collected in a concentrator tube and volume was reduced to 2 mL under a gentle stream of nitrogen for quantification with GC-μECD (2.4).
2.2.4 Extraction of OCPRs in aquatic plants
Using Accelerated Solvent Extraction (ASE), a mixture of 1:1 v/v 95% n-hexane:dichloromethane was used as an extracting solvent. Five grams of blended aquatic plant was mixed with 20 g anhydrous sodium sulfate contained in the ASE-vessel. ASE conditions were the same as for prior sediment extraction. Following the pigment removal, the same clean up technique during the plankton extraction was used and then the sample will be analyzed by GC-μECD (2.4)
2.2.5 Extraction of OCPRs in invertebrates
Using the standard operating procedure (SOP) for determination of chlorinated pesticides, PCB Arochlor(s) and PCB congeners in fish and biological tissue (AOAC, 2002); the whole body of each invertebrate tissue was homogenized. A 5 g sample was mixed with 10 g anhydrous sodium sulfate in the ASE-vessel and then extracted with n-hexane:dichloromethane (1:1 v/v) using ASE (Aaron et al., 2003; Thongkongoum, 2005). Following the removal of fat and pigment, the same clean up technique during the plankton extraction was used and then the sample will be analyzed by GC-μECD (2.4).
2.2.6 Extraction of OCPRs in fish
Five grams of homogenized fish was mixed with anhydrous sodium sulfate to remove water. Mixed fillet was placed into the ASE-vessel. The mixture of hexane:acetone (3:1 v/v) was used as the extracting solvent with the same operating condition as described previously in the sediment extraction (AOAC, 2002; Zhuang et al., 2004; Rohitrattana, 2005). The same clean up technique that was used during the plankton extraction was used and then the sample will be analyzed by GC-μECD (2.4).
2.3 Gas chromatography analysis
An Agilent 6890N GC equipped with micro Electron Capture Detector (μECD) was used for the quantification. Compound separation was completed using DB-35MS fused silica capillary column (30 m length, 0.25 mm i.d., 0.25 μm film thickness) coated with 35% diphenyl polysiloxane (J&W Scientific). Sample quantification was performed using multiple external standards. 1.0 μL of sample was injected into the GC on splitless mode with 0.75 min vent delay. The injector and detector temperature were maintained at 260 °C and 300 °C, respectively. The oven temperature was initially maintained at 100 °C for 2 min, and then programmed to increase at 12 °C/min to 280 °C and held for 10 min. Total run time was calculated to be 27.00 min. For optimum performance, the ultra-high-pure (UHP, 99.999%) helium was used as carrier gas with a flow rate at 2 mL/min linear velocity, and nitrogen (UHP) was set at 60 mL/min as make-up gas.
2.4 Quality control
DDT and derivatives peaks and retention times were confirmed with DB-1701 fused silica capillary column (30 m length, 0.25 mm i.d., 0.25 μm film thickness) coated with 14% cyanopropylphenyl and 86% diphenyl polysiloxane (J&W Scientific). A calibration curve using the external mixed standard of DDT and derivatives was performed for each compound to be quantified at concentrations of 5, 10, 20, 50, and 100 ng/mL. Calibration standards were run every 10 samples and all measurements were performed in the ranges of linearity found for each compound. The limit of detections (LODs) and the limit of quantifications (LOQs) were calculated from DDT and derivatives mixed standard to give a response of 3 and 10 times the signal/noise ratio and in the order of 0.002–0.04 ng/mL and 0.01–0.10 ng/mL, respectively. The validation data showed quantitative recoveries at the 10 and 50 ng/mL fortified raw matrices i.e. water, sediment, fish, Lanchester’s freshwater prawn, freshwater snail, and vegetables were in the range of 77–116, 86–91, 82–109, 88–103, 78–106, and 71–103%, respectively. The precision of the matrices were in the range of 4.22–7.64, 1.87–3.69, 4.99–7.59, 3.74–7.52, 1.42–8.38, and 5.06–8.60% RSD, respectively. The method detection limits (MDLs) were determined by multiplying the appropriate (i.e., n-1 degree of freedom) one-sided 95 percent Student’s t-statistic (t0.95) by the standard deviation (SD) obtained from seven replicate analyses of spiked matrices containing 50 ng/mL of DDT and derivatives. MDLs of this study were in the range of 0.002–0.003, 0.66–0.74, 1.06–1.40, 1.46–3.32, 0.64–3.89, and 1.93–2.34 ng/g wet wt. for DDT and derivatives in water, sediment, fish, Lanchester’s freshwater prawn, freshwater snail, and vegetables, respectively. We considered the methods to be reliable to quantify the concentration of DDT and derivatives in those aquatic organisms following the AOAC Peer Verified Methods Program (1993).
3. Results and Discussion
Low concentrations of DDT and derivatives were found in water (0.02 ng/mL) and higher concentrations of DDT and derivatives were found in sediment (12.05 ng/g). This is because they are less soluble, but they are well-trapped in sediment particles (Thirakhupt et al., 2006). Mean concentrations of DDT and derivatives in fish, vertebrate organisms, ranged from 4.16 to 57.66 ng/g wet wt., which increased through the higher trophic levels of the food chain. The highest amount of DDT and derivatives residues were found in carnivore fish species, C. striatus which is the top predator of food web. In contrast, the lowest residue levels of DDT and derivatives were found in the omnivores, P. gonionotus. Furthermore, macroinvertebrate species, bottom feeders, can be exposed to contaminants either from sediment or water through their diet; this resulted in higher concentrations of DDT and derivatives found in Pomacea sp., M. lanchesteri, and F.mertensi.
Aquatic macroinvertebrates have long been used as indicators of environmental conditions. They are species-rich, respond to a broad range of environmental conditions, and are relatively immobile and live in close contact with both bottom sediments and the water column, thereby having the potential for exposure to stressors via both sediment and aqueous pathways (Brazner et al., 2007). In this study, the primary consumers such as Pomacea sp., M. lanchesteri, and F. mertensi showed a BAF of 4.0, 4.4, and 6.6, respectively for DDT and derivatives.
Aquatic vegetation is also an excellent indicator of the health of aquatic ecosystems, in both inlands and wetlands (Albert and Minc, 2004; Brazner et al., 2007). The BCF for DDT and derivatives were measured in E. crassipes, macrophyton as a producer, which was 462.5. This BCF was compared with values found in other plant species such as Chaetomorpha linum, which was 10,460 (Pérez-Ruzafa et al, 2001) which is much greater than this study observed. This may be due to differing plant uptake of lipophilic contaminants (Siriwong et al., 2007).
This study found DDT and derivatives in plankton (phyto- and zoo- plankton) and reported a BCF of 182.5, as compared to Siriwong et al. (2008). This indicates that the presence of organochlorine pesticide residues in plankton in this agricultural area may have been a result of historical usage and some illegal usage at the present time. For plankton-eating invertebrate such as M. lanchesteri, the BMF (M. lanchesteri/plankton) for DDT and derivatives was 14.5. The BMF of plankton-eating fish for DDT and derivatives was 1.1 for P. gonionotus, 6.5 for P. siamensis, 3.5 for T. trichopterus, and 6.5 for T. microlepis.
Fish have long been included as key indicators in the assessment of biotic integrity in streams (Lyons, Wang and Simonson, 1996) and their ecological significance in Great Lakes coastal wetlands (Jude and Pappas, 1992) has recently generated interest (Wilcox et al., 2002; Uzarski et al., 2005). In regards to fish BMF, the uptake of contaminants generally takes place from water moving across the respiratory surface and from food ingestion (Borgå et al, 2001). DDT and derivatives presented an increase in BMF (BMF > 1.0) through the food web including both interspecific relationship and intraspecific relationship trophic levels due to rapid and high efficient energy transfer coupled with lipid content in predators (Norstrom et al., 1988). Remarkably in broad perspective, the more prey species along the food chain that were taken up, the lower the BMF value between fish and their prey. The BMF (C. striatus/P. fasciatus) and BMF (P. fasciatus/P. gonionotus) for DDT and derivatives were 4.0 and 3.5, respectively, while the BMF (C. striatus/P. gonionotus) for DDT and derivatives was 13.9. On the other hand, the contaminants may be eliminated through metabolism and excretion resulting in biomagnification reduction (Borgå et al., 2001) as seen with BMF (P. gonionotus/E. crassipes) for DDT and derivatives which were less than 1.0. This phenomenon is called trophic depletion or trophic dilution (Newman, 1998).
In conclusion, the environmental data from this study provides a better understanding of the fate of DDT and derivatives in tropical aquatic ecosystems. Although these DDT and derivatives were banned, their residues are still circulated and magnified in multiple food chains over different trophic levels. BAF, BCF, and BMF can be observed as ecological indicators for evaluating ecological health risk, especially for DDT and derivatives, which can cycle in the environment and the organism.
Acknowledgments
This research was supported by the National Center of Excellence for Environmental and Hazardous Waste Management (NCE-EHWM), Chulalongkorn University and Thai Fogarty Project D43 TW007849-01 Fogarty International Center - National Institutes of Health – NIEHS, USA. Particularly, we also appreciate Saran Kiethmaleesatti for field assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaron TF, Paul FH, Jean-Marc G, Jason D, Ross JN, Keith AH, Michael K, Derek CGM. Influence of habitat, trophic ecology and lipids on, and spatial trends of, organochlorine contaminants in Arctic marine invertebrates. Marine Ecology Progress Series. 2003;262:201–214. [Google Scholar]
- Albert DA, Minc LD. Plants as regional indicators of Great Lakes coastal wetland health. Aquatic Ecosystem Health & Management. 2004;7(2):233–247. [Google Scholar]
- Alvin CK, Lau S. Solid Phase Extraction Cleanup for the Determination of Organochlorine Pesticides in Vegetable. Malaysian Journal of Chemistry. 2004;6(1):39–47. [Google Scholar]
- Anat T, Paul FH. Pesticide use and occurrence in Thailand. Environmental Monitoring and Assessment. 2000;60:103–144. [Google Scholar]
- AOAC Peer Verified methods Program. Manual on policies and procedures. Arlington, VA: 1993. [Google Scholar]
- AOAC. Massachusetts Department of Environmental Protection. Division of Environmental Analysis; 2002. Standard operating procedure for AOAC Method 983.21 Determination of chlorinated pesticides, PCB Arochlor(s), and PCB congeners in fish and biological tissue. [Google Scholar]
- APHA. Standard methods for the examination of water and waste water and wastewater AWWA/WPCE. 14. Washington, DC: 1975. [Google Scholar]
- Borgå K, Gabrielsen GW, Skaare JU. Biomagnification of organochlorines along a Barents Sea food chain. Environmental Pollution. 2001;113(2):187–198. doi: 10.1016/s0269-7491(00)00171-8. [DOI] [PubMed] [Google Scholar]
- Brazner JC, Danz NP, Niemi GJ, Regal RR, Trebitz AS, Howe RW, Hanowski JM, Johnson LB, Ciborowski JJH, Johnston CA, Reavie ED, Brady VJ, Sgro GV. Evaluation of geographic, geomorphic and human influences on Great Lakes wetland indicators: A multi-assemblage approach. Ecological Indicators. 2007;7 (3):610–635. [Google Scholar]
- Lino Caleste Matos, da Silveira Irene Noronha. Extraction and clean-up methods for the determination of organochlorine pesticide residues in medical plants. Journal of chromatography A. 1997;769:275–283. [Google Scholar]
- DeLorenzo ME, Taylor LA, Lund SA, Pennington PL, Strozier ED, Fulton MH. Toxicity and Bioconcentration Potential of the Agricultural Pesticide Endosulfan in Phytoplankton and Zooplankton. Bulletin of Environmental Contamination and Toxicology. 2002;42:173–181. doi: 10.1007/s00244-001-0008-3. [DOI] [PubMed] [Google Scholar]
- Jude DJ, Pappas J. Fish utilization of Great Lakes coastal wetlands. J Great Lakes Res. 1992;18:651–672. [Google Scholar]
- Keithmaleesatti S, Thirakhupt K, Pradatsudarasar A, Varanusupakul P, Kitana N, Robson M. Concentration of organochlorine in egg yolk and reproductive success of Egretta garzetta (Linnaeus, 1758) at Wat Tan-en non-hunting area, Phra Nakhorn Si Ayuthaya Province, Thailand. Ecotoxicology and Environmental Safety. 2007;68:79–83. doi: 10.1016/j.ecoenv.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Lyons J, Wang L, Simonson TD. Development and validation of an index of biotic integrity for coldwater streams in wisconsin. North American Journal of Fisheries Management. 1996;16:241–256. [Google Scholar]
- Monkolprasit S, Sontirat S, Vimollohakarn S, Songsirikul T. Checklist of Fished in Thailand. Office of Environmental Policy and Planning; Thailand: 1997. [Google Scholar]
- Nelson JS. Fishes of the World. Willey-Interscience publication; USA: 1976. [Google Scholar]
- Newman MC. Fundamentals of ecotoxicology. CRC Press LLC; USA: 1998. [Google Scholar]
- Norstrom RJ, Simon M, Muir DCG, Schweinsburg RE. Organochlorine contaminants in Arctic marine food-chains identification, geographical distribution, and temporal trends in polar bears. Environmental Science and Technology. 1988;22:1063–1071. doi: 10.1021/es00174a011. [DOI] [PubMed] [Google Scholar]
- Osinski E, Meier U, Büchs W, Weickel J, Matzdorf B. Application of biotic indicators for evaluation of sustainable land use – current procedures and future developments. Agriculture, Ecosystems and Environment. 2003;98:407–421. [Google Scholar]
- Pan B, Liu WX, Shi Z, Cao J, Shen WR, Qing BP, Sun R, Tao S. Sample Purification for Analysis of Organochlorine Pesticides in Sediment and Fish Muscle. Journal of Environmental Science and Health. 2004;39(3):353–365. doi: 10.1081/pfc-120035922. [DOI] [PubMed] [Google Scholar]
- Pérez-Ruzafa A, Navarro S, Barba A, Marcos C, Cámara MA, Salas F, Gutiérrez JM. Presence of Pesticides throughout Trophic Compartments of the Food Web in the Mar Menor Lagoon (SE Spain) Marine Pollution Bulletin. 2000;40 (2):140–151. [Google Scholar]
- Pridmore RD, Thrush SF, Cummings VJ, Hewitt JE. Effect of the Organochlorine Pesticide Technical Chlordane on Intertidal Macrofauna. Marine Pollution Bulletin. 1992;24(2):98–102. [Google Scholar]
- Rainboth WJ. Fishes of the Cambodian Mekong. Food and Agriculture Organization of the United Nations; Rome: 1996. [Google Scholar]
- Robinson J, Richardson A, Crabtree AN, Coulson JC, Potts GR. Organochlorine Residues in Marine Organisms. Nature. 1967;214:1307–1311. doi: 10.1038/2141307a0. [DOI] [PubMed] [Google Scholar]
- Rohitrattana J. Thesis. Graduate school Chulalongkorn University; Thailand: 2005. Accumulation of organochlorine insecticide residues in food chain of fish at Khlong 7, Rangsit agricultural area, Pathum Thani province. [Google Scholar]
- Siriwong W, Thirakhupt K, Sitticharoenchai D, Robson M. Accumulation of organochlorine pesticide residues in aquatic plants. J Sci Res Chulalongkorn Univ. 2007;32:7–14. [Google Scholar]
- Siriwong W, Thirakhupt T, Sitticharoenchai D, Borjan M, Robson M. Organochlorine pesticide residues in plankton, Rangsit agricultural area, Central Thailand. Bull Environ Contam Toxicol. 2008 doi: 10.1007/s00128-008-9532-4. (article in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirakhupt K, Sitthicharoenchai D, Keithmaleesatti S, Siriwong W. Organochlorine Pesticides and Their Usages in Thailand: A Review. Journal of Scientific Research Chulalongkorn University. 2006;31(2):1–15. [Google Scholar]
- Thongkongoum P. Thesis. Graduate school Chulalongkorn University; Thailand: 2005. Accumulation of organochlorine residues in water, sediment, and aquatic invertebrate at Khlong 7, Rangsit agricultural area, Pathum Thani province. [Google Scholar]
- U.S. EPA. Guidance for assessing chemical contaminant data for use in fish advisories Volume 1: Fish Sampling and Analysis. 3. U.S. EPA; Washington, DC: 2000. [Google Scholar]
- Uzarski DG, Burton TM, Cooper MJ, Ingram JW, Timmermans S. Fish habitat use within and across wetland classes in coastal wetlands of the five Great Lakes: development of a fish-based index of biotic integrity. J Great Lakes Res. 2005;31(Suppl 1):171–187. [Google Scholar]
- Vidthayanon C. Peat Swamp Fishes of Thailand. Office of Environmental Policy and Planning; Thailand: 2002. [Google Scholar]
- Vidthayanon C. Manual of freshwater fishes. Bangkok Printing; Thailand: 2004. [Google Scholar]
- Wilcox DA, Meeker JE, Hudson PL, Armitage BJ, Black MG, Uzarski DG. Hydrologic variability and the application of index of biotic integrity metrics to wetlands: a Great Lakes evaluation. Wetlands. 2002;22:588–615. [Google Scholar]
- Zhuang W, McKague B, Reeve D, Carey J. A comparative evaluation of accelerated solvent extraction and polytron extraction for quantification of lipids and extractable organochlorine in fish. Chemosphere. 2004;54:467–480. doi: 10.1016/j.chemosphere.2003.08.024. [DOI] [PubMed] [Google Scholar]


