Abstract
The objective of this study was to evaluate the reproductive risk associated with exposure of adult male Fisher-344 (F-344) rats to inhaled benzo(a)pyrene (BaP), a ubiquitous environmental toxicant present in cigarette smoke, automobile exhaust fumes and industrial emissions. Rats were assigned randomly to a treatment or control group. Treatment consisted of exposure of rats via nose-only inhalation to 75μg BaP/m3, 4 hours daily for 60 days, while control animals were unexposed (UNC). Blood samples were collected immediately on day 60 of exposures (time 0) and subsequently at 24, 48, and 72 hours, to assess the effect of exposures to BaP on plasma testosterone and luteinizing hormone (LH) concentrations. Mean testis weight, total weight of tubules and total tubular length per paired testes were reduced 33% (P< 0.025), 27% (P < 0.01) and 39%, respectively in exposed rats (P < 0.01) compared with UNC rats. The number of homogenization-resistant spermatids was significantly reduced in BaP-exposed versus UNC rats. Plasma testosterone and intra-testicular testosterone (ITT) concentrations were significantly decreased by BaP compared with those of UNC rats. The decreases in circulating plasma testosterone were accompanied by concomitant increases in plasma LH concentrations in BaP-exposed versus control rats (P < 0.05). These data suggest that 60 days exposure to inhaled BaP contribute to reduced testicular endocrine and spermatogenic functions in exposed rats.
Keywords: Benzo(a)pyrene, inhalation, testis, luteinizing hormone, testosterone, sperm production, rats
Introduction
Several reports [reviewed in 1 & 2] show that human health globally, is influenced by exposures to toxic chemicals. Many diseases including infertility are on the rise in recent years and mirror the increase in toxic chemical production, use, and release into the environment [3]. Delicately coordinated neuro-endocrine regulated physiological events that lead to the production of mature spermatozoa must occur to ensure the fertilization of mature ova, the development of normal embryos and eventually, the delivery of viable offspring. The perturbation of any one segment of this delicate sequence of events leads to infertility or abnormal fetal development if spermatozoa carrying genetic defect(s) fertilize normal mature ova. In the United States, approximately 15% of couples experience some difficulties when trying to conceive and in roughly 50% of these couples, male factor is partially responsible for failure to conceive [4]. For 25% of men evaluated, no identifiable cause of their abnormal semen analyses can be found, hence the diagnosis of idiopathic male factor infertility [4], probably due to exposure(s) to environmental/occupational xenobiotics that act as reproductive endocrine disruptors. Reproductive endocrine disruptors can alter the hypothalamic/pituitary and testicular hormones that regulate spermatogenesis. As a consequence of exposures to endocrine disruptive compounds, hypogonadism and/or infertility can ensue. One such xenobiotic that is prevalent in the environment and depresses circulating plasma testosterone and sperm motility is benzo(a)pyrene (BaP; [5]).
Benzo(a)pyrene is a semi-volatile, lipophilic, high molecular weight compound that belongs to the polycyclic aromatic hydrocarbon (PAH) family. Benzo(a)pyrene and other PAHs are products of combustion and can accumulate in crops via absorption from contaminated soils [6]. Exclusive sources for BaP contamination of the environment and consequently human exposures include industrial and automobile emissions, hazardous waste sites, cigarette smoke, biomass burning, municipal incinerators, volcanic eruptions, home heating, and consumption of charcoal broiled and smoked foods [6, 7]. Food ingestion and inhalation are the major routes of entry into the human body for a large section of the general population exposed to PAHs (reviewed in [8]).
The biological effects of BaP are mediated by the aryl hydrocarbon receptor (AhR)-mediated cytochrome P-450 (CYP) genes [9]. Biotransformation of BaP by microsomal epoxide hydrolase and CYP are necessary for this prototypical PAH to elicit toxicity [10]. The initial step in the BaP metabolic pathway is the formation of epoxides, catalyzed by CYP-dependent monooxygenases [11]. Further metabolism involves hydration by microsomal epoxide hydrolase to the dihydrodiols, isomerization to phenols or conjugation with glutathione by glutathione transferase. The phenols and dihydrodiols are further metabolized by conjugation with glucuronic acid or sulphuric acid by glucuronyltransferases and sulfotransferases, respectively to facilitate excretions. During the process of metabolism, reactive metabolites such as BaP-7,8-dihydrodiol 9,10-epoxide (BPDE) are formed that may damage cellular macromolecules including DNA, thus, resulting in the alteration of cell function and cancer. Biotransformation of BaP also leads to production of BaP quinones that may arise from autooxidation of metabolites such as 6-hydroxy BaP. These compounds undergo one electron redox cycling with their semiquinone radicals resulting in the formation of reactive oxygen species (ROS; [12]). The formation of ROS has been associated with altered cell signaling, cell damage and apoptosis [13–15]. Interactions of these reactive oxygen species with cellular nucleophiles such as DNA in the compartments of the testis have the potential to alter transcriptional events required for the production of mature spermatozoa.
The hormonal control of spermatogenesis is based on the action of the pituitary gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH), on the testis. LH stimulates the Leydig cells in the testes to produce testosterone (T). Intratesticular T (ITT) mediates its effects within the testes through the androgen receptor that is found on Leydig cells, Sertoli cells, and peritubular cells [16]. Follicle stimulating hormone activity is also important for quantitatively normal spermatogenesis [17, 18]. Withdrawal of LH and FSH stimulation of the testis reduces sperm production. Suppression of LH levels results in a decrease in ITT [19, 20], which in turn suppresses sperm production in men (World Health Organization; WHO, [21]) and rats [22]. Intratesticular testosterone is believed to stimulate spermatogenesis directly in rats [22] and men [17] although not to quantitatively normal levels in men. We have shown that a 10 day exposure of male rats to inhaled BaP (75μg/kg) causes a significant reduction and increase in plasma testosterone and LH concentrations, respectively [5], suggesting a lack of direct effect of BaP on LH secretion. This reduction in circulating testosterone could be due to: 1) BaP-induced reduction in Leydig cell testosterone synthesis and release or 2) to a higher metabolism of testosterone by the liver due to a higher induction of liver phase II metabolic enzymes by BaP or 3) both. It is likely that the reduction in circulating testosterone by BaP is contributed to by reduced intratesticular testosterone based on the fact that sperm motility was reduced significantly in BaP-exposed rats compared to controls [5]. Motility is acquired in the epididymides under the regulation of high testosterone concentrations contributed to this organ by testicular fluid that aids in the transport of spermatozoa from the testis into the epididymides.
The objective of this study was to determine the effect of a 60 day exposure to inhaled BaP on intratesticular function.
Materials and Methods
Animals and Exposure
Adult male Fisher-344 rats, approximately 12–13 weeks of age and weighing approximately 340–360g, were purchased from Harlan Sprague Dawley (Indianapolis, IN). Animals were housed in pairs, in polyethylene cages and allowed to acclimatize to the animal care facilities for one week prior to initiation of studies. Rats were maintained in an environmentally controlled room with a 14 hour light: 10 hour dark cycle (lights on at 6.0 AM.), 22°C and humidity range of 50–60% and allowed ad libitum access to commercial rat chow (5001 Lab meal, Ralston Purina Co., MO, USA) and water. Before initiation of animal exposures to BaP via inhalation, all rats were acclimated to 52 port Cannon nose-only exposure chambers, 4 hrs a day for 3 days. Subsequently, rats were randomly assigned to a treatment and a control group (N = 10 per group). Treatment consisted of exposure of rats to 75 μg BaP/m3 (98% pure, Sigma Chemical Co., St. Louis, MO) via nose-only inhalation, 4 hrs daily for 60 days, using a state-of-the-art dual-component aerosol generator developed in our laboratory [23]. Carbon black (CB) was used as a carrier for BaP because it adsorbs and strongly binds to PAHs [24] and was not known to be mutagenic or carcinogenic in mammalian systems [25]. Rats in the control group served as unexposed controls (UNC). We did not control for the carrier of BaP (CB) because of the lack of effect of 10- and 60-days exposure to CB on the endocrine and reproductive characteristics of rats [5, 25, 26]. Even though rats in the UNC group were unexposed, they were subjected to conditions of restraint similar to those imposed on rats exposed to BaP via inhalation. Details on the design, fabrication, installation, and characterization of the exposure system are reported in Hood et al. [23]. The methods for aerosol generation, preparation of carbon black cakes and impactor substrates as well as the characterization and quantitation of BaP aerosol and substrate extraction, analysis and quality assurance/control are detailed in our previous study [5].
Rats in the BaP and UNC group were weighed prior to initiation of exposures, followed subsequently by weekly weight monitoring throughout the duration of the study to determine whether BaP affected thriftiness among treated animals.
Post-exposure Processing Of Tissue Samples
Blood samples were collected via sinus orbital puncture using heparinized pulled Pasteur pipettes, between 17:00 and 18:00 h immediately following the last day of exposure (on day 60; 0 hr) and at 24, 48 and 72 hrs post initial sampling. Subsequently, plasma was harvested post centrifugation at 2000-× g at 4°C for 10 minutes from each sample and stored at −20°C until assayed for testosterone and luteinizing hormone (LH). Following the last blood sampling, animals were sacrificed by CO2 asphyxiation, testes immediately harvested and weighed. The right testes from UNC and BaP-exposed rats (N = 6/group) were used for the determination of daily sperm production and intra-testicular testosterone (ITT) concentrations while the left testes were prepared for histological evaluation according to the modified method of Weibel [27] by Lunstra et al [28]. Briefly, fixed testis pieces from UNC and exposed rats were washed in PBS (1 × 1 h), dehydrated through graded ethanol (50, 70, 80, 90, 100; 2 × 1 hr each), cleared in xylene (2 × 1 h; Sigma, St. Louis, MO), infiltrated with paraffin wax (60°C; 4 × 1 hr), and embedded in paraffin wax. Serial sections (5 μm) were cut from the middle of each testis preparation. Sections were dried overnight onto glass slides at 37°C and stored at room temperature until processed for histology. For staining and morphometric evaluation, sections were deparaffinized in Microclear (2 × 5min; Micron Environmental Industries, Fairfax, VA) and rehydrated through graded ethanol (2 × 100%, 2 × 95%, 1 × 70%). Sections were rinsed thoroughly in water, stained with haematoxylin, dehydrated, cleared in Microclear and mounted using DPX mounting media (Sigma-Aldrich, Milwaukee, WI). Stained sections were stored at room temperature (26 ± 2°C) until morphometric analysis was conducted.
Morphometry & Histopathology
Stained sections were evaluated at 50x magnification using a Zeiss Axioplan-2 Photomicroscope equipped with planaphotochromat objectives for DIC microscopy, coupled to a computerized morphometric planimetry system (Bioquant Nova 2000 Advanced Image Analysis, R&M Biometrics, Nashville, TN) to obtain tubule diameters and area percentages (volume percentages) occupied by seminiferous tubules and interstitium. Two full cross-sections from near the middle of each testis were used in these evaluations. Four areas randomly selected to represent each quadrant on each section were evaluated. Briefly, images from an Optronics DEI-750 triple-CCD color camera attached to the microscope were displayed on a high resolution (1600 lines/inch) color video monitor and the tubular components of the parenchyma were measured by tracing outlines of whole seminiferous tubules (about 60 tubules per rat) using a computer mouse connected to a high-resolution digital pad. Thus, approximately 10 × 106 μm2 was evaluated per testis (approximately 1.2 × 106 μm2 evaluated per quadrant). For all morphometric calculations, specific gravity of the testicular tissue was assumed to be 1.0, and area percentages were assumed equal to volume percentages [28]. No correction factor for shrinkage was applied, since the samples used for comparison were fixed and processed into paraffin blocks using identical conditions. Morphometric assessments included the evaluation of the percentage tubular volume, percentage interstitial volume, average tubule diameter, and percentage tubules displaying elongated spermatids (i.e., full spermatogenesis), as described previously [27, 29]. The volume percentage of seminiferous tubules was multiplied by testicular volume (i.e., testis weight) per paired testes to obtain total tubular volume, and then divided by average area per round tubule profile, using the formula for volume of a cylinder, to obtain total length of seminiferous tubules per paired testes [30].
Determination of Daily Sperm Production (DSP)
The number of homogenization-resistant testicular spermatids was determined according to the method of Goyal et al. [31]. Briefly, testes from BaP-exposed and UNC rats were decapsulated, weighed and each testicular parenchyma was homogenized in 50 ml of PBS using a motorized homogenizer (Brinkmann Instruments, Westbury, NY). The homogenate was filtered through a metal sieve, and an aliquot of the filtrate was mixed with PBS and 0.6% trypan blue in a ratio of 2:1:2, respectively. An 8.5 μl aliquot of the latter mixture was then used to determine the average number of homogenization-resistant spermatids in each sample in duplicate, by hemocytometric counting. Daily sperm production was calculated by dividing the total number of spermatids per gram of testicular parenchyma (testis weight minus weight of the capsule) by 6.1 days, the duration of step 19 spermatids in the seminiferous epithelial cycle [32].
Plasma and Intratesticular Testosterone and Plasma LH Determination
Testosterone was extracted from testis homogenates with ether followed by evaporation of ether and reconstitution of extracted testosterone in 0.1% gel PBS. Subsequently, testis homogenate extracts and plasma samples from UNC and BaP-exposed rats were analyzed for total testosterone by radioimmunoassay while analysis for LH was conducted in plasma samples using the same assay method previously validated in our laboratory [5, 26]. The sensitivity of testosterone assay was 2 pg/tube and the intra-assay coefficient of variation was 8%. The inter-assay coefficient of variation for this assay is not available because testosterone in all samples was measured in a single assay. The sensitivity of LH assay was 0.08 ng/tube and the intra- and inter-assay coefficients of variation were 6.5 and 10.9%, respectively.
Statistical Analyses
Data on weekly body weights, total plasma testosterone and LH concentrations were analyzed by ANOVA with repeated measures and the differences among means were tested with orthogonal contrasts. Those on testis weight, ITT concentrations and daily sperm production were compared by unpaired t-test. Data on morphometric assessments of testicular histologies were analyzed by GLM procedure of SAS Version 6.12 (SAS/STAT User’s Guide, [33]).
Results
Weekly body weights of UNC and BaP-exposed rats were similar during the nine weeks of this study (complete data not shown), suggesting that the exposure concentration of BaP used in this study, the route of administration and duration of exposures per day for 60 days did not affect body weights in rats. Mean weekly weights (Mean ± SE) of rats increased from 356.1 ± 8 to 430.3 ± 7 and from 351.4 ± 11 to 433.7 ± 8 gm for UNC and BaP-exposed rats, respectively.
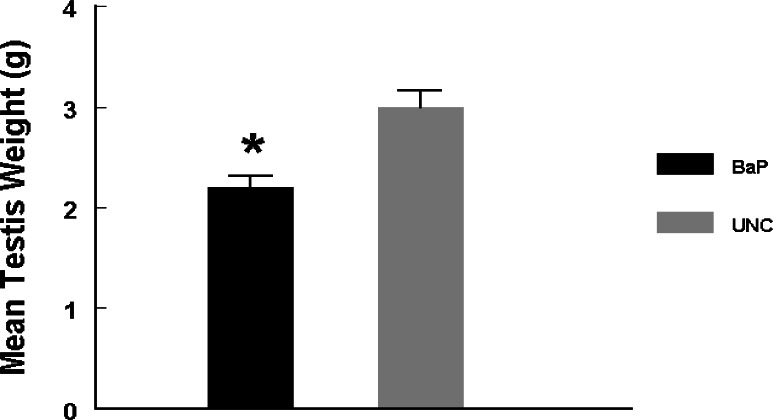
Testes recovered from rats exposed to BaP weighed less than those from their UNC counterparts (Fig. 1; P < 0.025).
Figure 1:
Mean testis weight of BaP-exposed versus UNC rats.
Table 1 depicts the morphometric characteristics of rat testis exposed to BaP via inhalation versus UNC. Testes from BaP-exposed rats weighed 34% less (P < 0.025) than those recovered from their UNC counterparts. Tubule diameter (μM) and percentage of tubules exhibiting elongated spermatids were similar between UNC and BaP-exposed rats. However, exposure to BaP caused a reduction in total tubular volume (P < 0.002), total weight of tubules (P < 0.002) and total tubular length (P < 0.01) per paired testes compared with those of their control counterparts. Similarly, both total volume and total weight of interstitium per paired testes were reduced by approximately 12% due to exposure of rats to BaP compared with those of testes recovered from control rats (P < 0.05). Collectively, these morphometric data suggest that exposure of rats to inhaled BaP contributed significantly to the reduction in testis weight (Fig. 1).
Table 1:
Testicular morphometric data of BaP (75ug/kg)-exposed rats after 60 days
| Parameter | Control | BaP-exposed |
|---|---|---|
| Tubule diameter (μm) | 250 ± 8.5 | 230 ± 8.2 |
| Tubules with elongated spermatids (%) | 99 ± 1.0 | 99 ± 1.0 |
| Tubular volume (X 109 μm3) | 2.0 ± 0.7 | 1.6 ± 0.004*** |
| Total weight of tubules (gm) | 2.20 ± 0.7 | 1.6 ± 0.004*** |
| Total tubular length (μm) | 55 ± 1.0 | 33 ± 1.0** |
| Total volume of interstitium per paired testis (μm3) | 0.43 ± 0.01 | 0.38 ± 0.01* |
| Total weight of interstitium per paired testis (gm) | 0.45 ± 0.01 | 0.37 ± 0.01* |
p < 0.05;
p < 0.01;
p < 0.002
Some of the above mentioned pathological changes are depicted in the photomicrographs of BaP and UNC testis histologies (Fig. 2).
Figure 2:
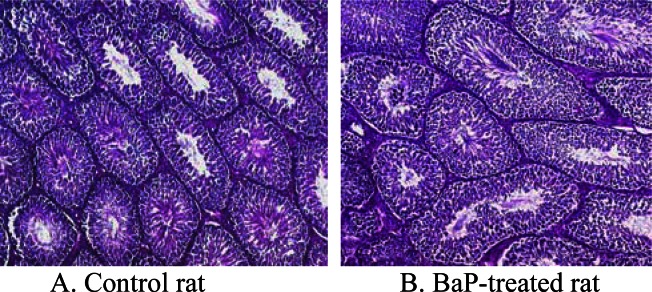
Photomicrographs of testes histologies from A) unexposed control F-344 rat; B) F-344 rat exposed to 75 μg BaP /m3 for 60 days. Magnification bar = 250 μm. Though the seminiferous tubules appear qualitatively similar, the size of tubular lumens and length decreased in BaP-exposed rats, compared with controls. This indicates a reduction in spermatogenic activity and loss of fluid in the seminiferous tubules due to decreased testosterone production, thus the observed decreased testis size.
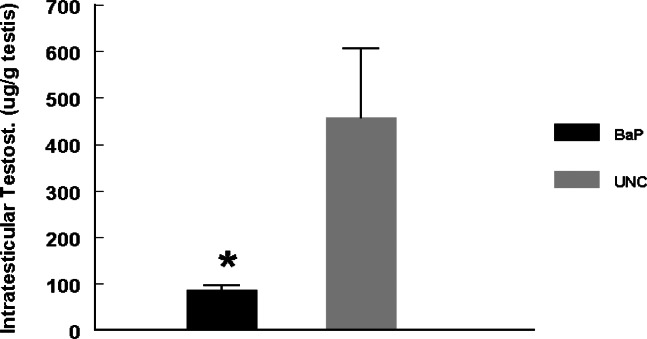
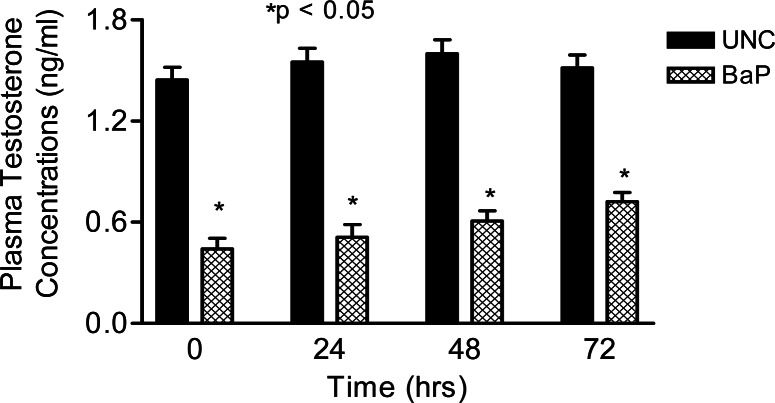
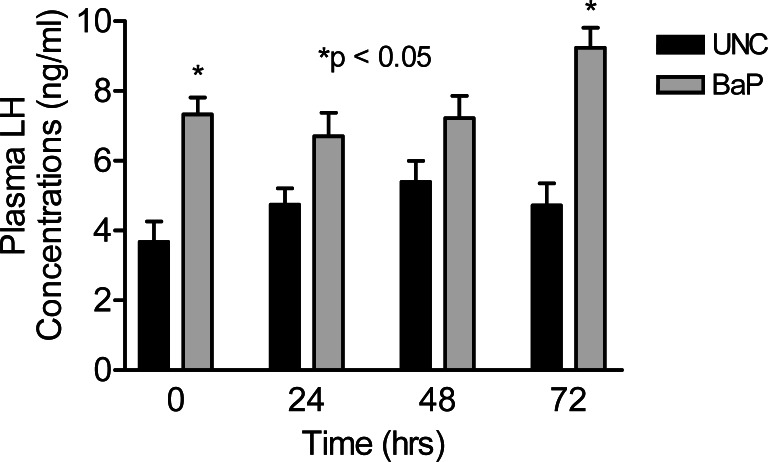
Daily sperm production was reduced (P <0.025) in rats exposed to inhaled BaP for 60 days compared with rats in the UNC group (Fig. 3). Intratesticular testosterone concentrations per gram of testicular tissue harvested on day 60 of BaP exposures were also reduced (P < 0.025; Fig. 4) as well as plasma testosterone concentrations (P < 0.05; Fig. 5) on the last day of exposures (day 60) and at 24, 48 and 72hr later compared with controls. However, LH concentrations in plasma samples of BaP-exposed rats were elevated throughout the above mentioned four time periods studied compared with controls (P < 0.05; Fig. 6).
Figure 3:
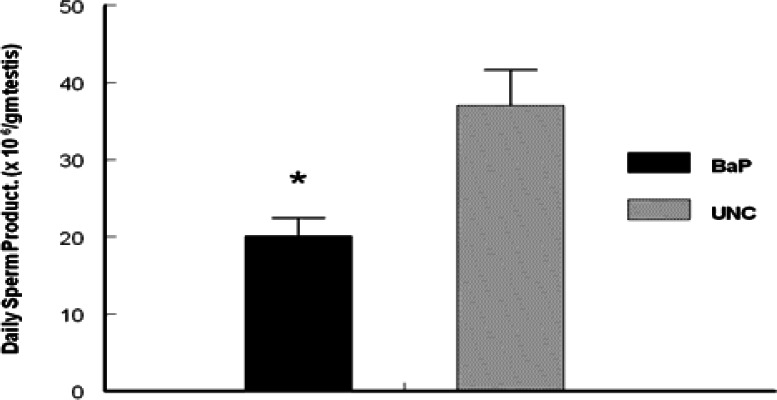
Effect of inhaled BaP on daily sperm production per gram of testis in F-344 male rats exposed to 75 μg BaP/m3 for 60 days; n = 10 per treatment or control group. Results are expressed as mean ± SE (UNC = unexposed control; BaP = BaP-inhaled rats. Asterisks indicate a significant difference from controls (P < 0.05).
Figure 4:
Effect of inhaled BaP on ITT concentrations in F-344 male rats exposed to 75 μg BaP/m3 for 60 days; n = 10 per treatment or control group. Results are expressed as mean ± SE (UNC = unexposed control; BaP = BaP-inhaled rats. Asterisks indicate a significant difference from controls (P < 0.05).
Figure 5:
Effect of inhaled BaP on plasma testosterone concentrations in F-344 male rats exposed to 75 μg BaP/m3 on the last day of exposures (day 60) and at 24, 48 and 72hr later; n = 10 per treatment or control group. Results are expressed as mean ± SE (UNC = unexposed control; BaP = BaP-inhaled rats. Asterisks indicate a significant difference from controls (P < 0.05).
Figure 6:
Effect of inhaled BaP on plasma LH concentrations in F-344 male rats exposed to 75 μg BaP /m3 on the last day of exposures (day 60) and at 24, 48 and 72hr later; n = 10 per treatment or control group. Results are expressed as mean ± SE (UNC = unexposed control; BaP = BaP-inhaled rats. Asterisks indicate a significant difference from controls (P < 0.05).
Discussion
The exposure concentration of BaP (75μg/m3) used in this study is one that adversely affected reproductive phenomena in our previous studies in both adult male and female rats [5, 26] and is within the range (10μg–2 mg/m3) present in a variety of sources in the environment. Some of these sources include: aluminum smelter and coke industries [7]; ambient air of highly polluted industrial cities [34]; cooking oil and wood combustion fumes [35]; home heating with coal-gas [7]. Furthermore, the exposure concentration of BaP to which rats in this study were exposed, is close to the legally enforceable limit of 100μg/m3 established for PAHs by the Occupational Safety and Health Administration [36].
Rats exposed to BaP in this study had similar weekly weight gains compared with UNC rats, suggesting that the exposure of animals to 75μg/m3 BaP, daily exposure durations and method of restraint, did not impede growth through undue stress compared with controls.
Mean testis weight among BaP-exposed rats was significantly reduced compared with their UNC counterparts. Similar observation was made by Blazak et al. [37] for adult rats and by Singh and Tate [38] in adult hamsters, indicating toxicity by this PAH on the testis [39]. Testicular morphometric data obtained in this study indicate that BaP exposure reduced components of both the steroidogenic and spermatogenic compartments of the testis. However, more of the BaP-influenced reduction was brought to bear on the spermatogenic compartment compared with controls (12% reduction in both total volume and total weight of interstitium per paired testis [steroidogenic compartment] versus 20% reduction in both tubular volume and tubular weight and 40% reduction in tubular length [spermatogenic compartment]). This is not surprising in as much as the testis bulk (85%; [40]) is involved in sperm production; consequently, when seminiferous tubular cells such as Sertoli cells undergo apoptosis due to exposure to BaP [41], paracrine mediators involved in the regulation of spermatogenesis [42] are reduced, leading to a reduction in spermatogenetic support and testicular mass.
The morphometric data on the effects of BaP on spermatogenic and steroidogenic compartments of the testis were predictive of the functions of these testicular compartments. We observed a significant reduction in DSP per gram of testicular tissue in BaP exposed rats compared with their control counterparts, probably due to BaP-induced dysfunction of exposed Sertoli cells. Raychoudhury and Kubinski [41] reported that in vitro exposure of isolated rat Sertoli cells to BaP resulted in cellular changes characteristic of apoptosis. Although Sertoli cells were not enumerated in this study, the dramatic reduction (39%) of total tubular length in BaP-treated rats implies that a significant reduction in Sertoli cell numbers also occurred during BaP exposure. Sertoli cells initiate, support, maintain and regulate spermatogenesis in mammals by (1) providing structural and functional barrier that regulate the movement of extratubular blood-borne components into the seminiferous tubular environment; (2) synthesizing and secreting various nutrients utilized by developing germ cells; and (3) synthesizing and secreting paracrine mediators proposed to be involved in the regulation of spermatogenesis [42]. Thus, the reduced DSP per gram testis observed in this study may have been due to reduced spermatogenesis resulting from BaP-induced DNA damage and apoptosis in Sertoli cells [41, 43].
High ITT concentrations are required for the regulation of spermatogenesis [44], the reduction of which results in fewer mature sperm produced by the testis [45]. Our data demonstrate that reduced DSP was accompanied by approximately 20% reduction in ITT concentrations per gram of testicular tissue. Our data also indicate that the effect of BaP on testicular testosterone synthesis was a direct effect on the Leydig cells. This conclusion is based on increased plasma LH concentrations observed in this study due to the abrogation of the negative feedback on this pituitary gonadotropin by decreased plasma concentrations of testosterone in BaP exposed versus controls during the four time periods studied. The reduction in intratesticular synthesis of testosterone may have resulted from the exposure of Leydig cells to sequestered BaP in high-density lipoproteins that are essential for steroid hormone biosynthesis [46]. Mandal et al. [47] have demonstrated that DMBA inhibits the ability of Leydig cells to synthesize and release testosterone.
Furthermore, it could be surmised that the metabolism of BaP by Leydig cells or BaP metabolites produced elsewhere in the body and transported to the testis may have adversely affected Leydig cell function. In this context, it is worth mentioning that the tissues in the male reproductive organs and the Leydig cells in particular extensively metabolize PAHs including BaP [48–51]. Consequently, the metabolites of BaP may have significantly reduced the ability of the Leydig cell to synthesize and release testosterone in this study, due to ROS-induced aging [47, 52]. Peltola et al. [53] have shown that ROS can damage critical components of the steroidogenic pathway in Leydig cells, including steroidogenic acute regulatory (StAR) protein [54]. The reduction in the synthesis and release of testosterone could also be exacerbated by a reduction in the population of Leydig cells via apoptosis as exemplified in data generated on Leydig cells from rats exposed to cigarette smoke [55]. A reduction in ITT normally results in reduced spermatogenesis [56] and could explain the reduced DSP per gram of testicular tissue among BaP-exposed rats compared with controls in this study. Additionally, decreased circulating testosterone levels similar to that observed in the present study upon prolonged exposure to BaP may have implications on successful mating of females by exposed males [57]. It is also very likely that the reduction in the circulating testosterone concentrations by BaP is primarily contributed to by reduced ITT synthesis and release and secondarily by the heightened induction of the liver cytochrome P450s that are necessary for detoxification of BaP [10].
Our data raise the awareness that exposure to BaP contributes to the declining fertility in men due to declining sperm production and altered endocrine regulated events that lead to sperm maturation, gamete interaction, embryo development and the delivery of viable young. The adverse effect of this endocrine disruptor on reproduction will not abate in as much as exposures of men to this xenobiotic continue. It is important therefore, for healthcare givers to advise patients during infertility counseling sessions, to curtail exposures to BaP in order to reduce the risk to infertility, should the patients fall within ‘at risk’ category for BaP exposure. This ‘at risk’ category includes smokers; workers in coke oven, coal tar, distillery, iron foundry, aluminum, bitumen, creosote and carbon electrode manufacturing industries; thermo-electric power plants; roofers; ship builders; painters; fire fighters; auto and aircraft mechanics; incineration plant workers; restaurant cooks, and individuals that live near hazardous waste sites.
Acknowledgments
This work was supported in part by PHS grants No. U50ATU3989-48-06 (Meharry), 1U54HD0431501-09, RO1 HD020419-19S1 (AA), G12RRO3032 (AA & AR), S11ES014156-01 and 1RO3CA130112-01 (AR).
References
- 1.Moline JM, Golden AL, Bar-Chama N, Smith E, Rauch ME, Chapin RE, Perreault SD, Schrader SM, Suk WA, Landrigan PJ. Exposure to hazardous substances and male reproductive health: a research framework. Environ Health Perspect. 2000;108:803–813. doi: 10.1289/ehp.00108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon GM, Schettler T. Environment and health: 6. Endocrine disruption and potential human health implications. CMAJ. 2000;163:1471–1476. [PMC free article] [PubMed] [Google Scholar]
- 3.Arcand-hoy LD, Nimrod AC, Benson WH. Endocrine-modulating substances in the environment:estrogenic effects of pharmaceutical products. Int. J. Toxicol. 1998;17:139–158. [Google Scholar]
- 4.Jarow JP, Zirkin B. The androgen microenvironment of the human testis and hormonal control of spermatogenesis. Ann NY Sci. 2005;1061:208–220. doi: 10.1196/annals.1336.023. [DOI] [PubMed] [Google Scholar]
- 5.Inyang F, Ramesh A, Kopsombut P, Niaz MS, Hood DB, Nyanda AM, et al. Disruption of testicular steroidogenesis and epididymal function by inhaled benzo(a)pyrene. Reprod Toxicol. 2003;17:527–537. doi: 10.1016/s0890-6238(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 6.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for Polycyclic Aromatic Hydrocarbons (PAHs) US Department of Health & Human Services; Atlanta, Georgia: 1995. p. 458. [PubMed] [Google Scholar]
- 7.WHO . Selected non-heterocyclic polycyclic aromatic hydrocarbons. Environmental Health Criteria 202. World Health Organization; Geneva: 1998. p. 883. [Google Scholar]
- 8.Ramesh A, Walker SA, Hood DB, Guillen MD, Schneider H, Weyand EH. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int. J. Toxicol. 2004;23:301–333. doi: 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- 9.Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem. Pharmacol. 2000;59:65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- 10.Shimada T, Guengerich FP. Inhibition of cytochrome P450 1A1-, 1A2-, and 1B1-mediated activation of procarcinogens to genotoxic metabolites by polycyclic aromatic hydrocarbons. Chem. Res. Toxicol. 2006;19:288–294. doi: 10.1021/tx050291v. [DOI] [PubMed] [Google Scholar]
- 11.Nebert DW, Gonzalez FJ. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- 12.Flowers L, Ohnishi S, Penning TM. DNA strand scission by polycyclic aromatic hydrocarbon o-quinones: role of reactive oxygen species, Cu (II)/Cu (I) redox cycling, and o-semiquinone anion radicals. Biochemistry. 1997;36:8640–8648. doi: 10.1021/bi970367p. [DOI] [PubMed] [Google Scholar]
- 13.Aust SD, Chignell CF, Bray TM, Kalyanaraman B, Mason RP. Free radicals in toxicology. Toxicol. Appl. Pharmacol. 1993;120:168–178. doi: 10.1006/taap.1993.1100. [DOI] [PubMed] [Google Scholar]
- 14.Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem. Res. Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 16.Burchiel SW, Luster MI. Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. Clin. Immunol. 2001;98:2–10. doi: 10.1006/clim.2000.4934. [DOI] [PubMed] [Google Scholar]
- 17.McLachlan RI, O’Donnell L, Stanton PG, Balourdos G, Frydenberg M, de Kretser DM, Robertson DM. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. J Clin Endocrinol Metab. 2002;87:546–556. doi: 10.1210/jcem.87.2.8231. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto AM, Bremner WJ. Stimulation of sperm production by human chorionic gonadotropin after prolonged gonadotropin suppression in normal men. J Androl. 1985;6:137–143. doi: 10.1002/j.1939-4640.1985.tb00829.x. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto AM. Effects of chronic testosterone administration in normal men: safety and efficacy of high dosage testosterone and parallel dose-dependent suppression of luteinizing hormone, follicle-stimulating hormone, and sperm production. J Clin Endocrinol Metab. 1990;70:282–287. doi: 10.1210/jcem-70-1-282. [DOI] [PubMed] [Google Scholar]
- 20.Morse HC, Horike N, Rowley MJ, Heller CG. Testosterone concentrations in testes of normal men: Effects of testosterone propionate administration. J Clin Endocrinol Metab. 1973;37:882–886. doi: 10.1210/jcem-37-6-882. [DOI] [PubMed] [Google Scholar]
- 21.Huhtaniemi I, Nikula H, Rannikko S. Pituitary-testicular function of prostatic cancer patients during treatment with a gonadotropin-releasing hormone agonist analog. I. Circulating hormone levels. J Androl. 1987;8:355–362. doi: 10.1002/j.1939-4640.1987.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 22.WHO Contraceptive efficacy of testosterone-induced azoospermia in normal men. Lancet. 1990;336:995–999. [PubMed] [Google Scholar]
- 23.Hill CM, Anway MD, Zirkin BR, Brown TR. Intratesticular androgen levels, androgen receptor localization, and androgen receptor expression in adult rat Sertoli cells. Biol. Reprod. 2004;71:1348–1358. doi: 10.1095/biolreprod.104.029249. [DOI] [PubMed] [Google Scholar]
- 24.Hood DB, Nayyar T, Ramesh A, Greenwood M, Inyang F. Modulation in the developmental expression profile of Sp1 subsequent to transplacental exposure of fetal rats to desorbed benzo(a)pyrene following maternal inhalation. Inhal Toxicol. 2000;12:511–535. doi: 10.1080/089583700402897. [DOI] [PubMed] [Google Scholar]
- 25.Accardi-Dey A, Gschwend PM. Reinterpreting literature sorption data considering both absorption into organic carbon and adsorption onto black carbon. Environ Sci Technol. 2003;37:99–106. doi: 10.1021/es020569v. [DOI] [PubMed] [Google Scholar]
- 26.Anon Carbon black users guide. Safety, health and environmental information. International Carbon Black Association. 2004 [Google Scholar]
- 27.Archibong AE, Inyang F, Ramesh A, Greenwood M, Nayyar T, Kopsombut P, et al. Alteration of pregnancy related hormones and fetal survival in F-344 rats by inhaled benzo(a)pyrene. Reprod Toxicol. 2002;16:801–808. doi: 10.1016/s0890-6238(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 28.Weibel ER. Stereological techniques for electron microscopic morphometry. In: Hayat MA, editor. Principles and Techniques of Electron Microscopy, Vol 3, Biological Applications. Van Nostrand Reinhold Co; New York: 1973. pp. 237–296. [Google Scholar]
- 29.Lunstra DD, Ford JJ, Christenson RK, Allrich RD. Changes in Leydig cell ultrastructure and function during pubertal development in the boar. Biol Reprod. 1986;34:145–158. doi: 10.1095/biolreprod34.1.145. [DOI] [PubMed] [Google Scholar]
- 30.Jiménez-Severiano H, Mussard ML, Fitzpatrick LA, D’Occhio MJ, Ford JJ, Lunstra DD, et al. Testicular development of Zebu bulls after chronic treatment with a gonadotropin-releasing hormone agonist. J. Anim Sci. 2005;83:2111–2122. doi: 10.2527/2005.8392111x. [DOI] [PubMed] [Google Scholar]
- 31.Okwun OE, Igboeli G, Ford JJ, Lunstra DD, Johnson L. Sertoli cell number and function, spermatogonial number and yield and daily sperm production in three breeds of boars. J. Reprod Fertil. 1996;107:137–149. doi: 10.1530/jrf.0.1070137. [DOI] [PubMed] [Google Scholar]
- 32.Goyal HO, Braden TD, Mansour M, Williams CS, Kamaleldin A, Srivastava KK. Diethylstilbestrol-treated adult rats with altered epididymal sperm numbers and sperm motility parameters, but without alterations in sperm production and sperm morphology. Biol Reprod. 2001;64:927–934. doi: 10.1095/biolreprod64.3.927. [DOI] [PubMed] [Google Scholar]
- 33.Robb GW, Amann RP, Killian GJ. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fertil. 1978;54:103–107. doi: 10.1530/jrf.0.0540103. [DOI] [PubMed] [Google Scholar]
- 34.SAS/STAT . Cary, North Carolina: Statistical Analysis System Institute, Inc. 1990. SAS Institute SAS/STAT Statistical Analysis System User’s Guide (Version 6, 5th Edition) pp. 113–709. [Google Scholar]
- 35.Chorazy M, Szeliga J, Strozyk M, Cimander B. Ambient air pollutants in Upper Silesia: partial chemical composition and biological activity. Environ Health Perspect. 1994;102(Suppl 4):61–66. doi: 10.1289/ehp.94102s461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viau C, Hakizimana G, Bouchard M. Indoor exposure to polycyclic aromatic hydrocarbons and carbon monoxide in traditional houses in Burundi. Int Arch Occup Environ Health. 2000;73:331–338. doi: 10.1007/s004209900112. [DOI] [PubMed] [Google Scholar]
- 37.Occupational Safety and Health Administration (OSHA) Occupational Safety and Health Standards, Toxic and Hazardous Substances. Code of Federal Regulations. 1998 29 CFR 1910.1000. [Google Scholar]
- 38.Blazak WF, Ernst TL, Stewart BE. Potential indicators of reproductive toxicity: testicular sperm production and epididymal sperm number, transit time, and motility in Fischer 344 rats. Fundam Appl Toxicol. 1985;5:1097–1103. doi: 10.1016/0272-0590(85)90145-9. [DOI] [PubMed] [Google Scholar]
- 39.Singh H, Tate F. Antispermatogenic effects of ethylmethanesulfonate and benzo(a)pyrene in PD4 Lakeview hamsters. J. Toxicol. Environ. Health. 1981;8:929–937. doi: 10.1080/15287398109530127. [DOI] [PubMed] [Google Scholar]
- 40.Lipshultz LI, Corriere JN., Jr Progressive testicular atrophy in the varicocele patient. J Urol. 1977;117:175–176. doi: 10.1016/s0022-5347(17)58387-1. [DOI] [PubMed] [Google Scholar]
- 41.Lipshultz LI, Witt MA. Infertility in the male. In: Hammond MG, Talbert LM, editors. Infertility, a practical guide for the physician. Blackwell Scientific Publications; Boston: 1993. pp. 26–55. [Google Scholar]
- 42.Raychoudhury SS, Kubinski D. Polycyclic aromatic hydrocarbon induced cytotoxicity in cultured rat sertoli cells involves differential apoptotic response. Environ. Hlth. Perspect. 2003;111:33–38. doi: 10.1289/ehp.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ku WW, Chapin RE. Preparation and use of Sertoli cell-enriched cultures from 18-Day-old rat. In: Chapin RE, Heindel JJ, editors. Male Reproductive Toxicology, Methods in Toxicology. Vol. 3. Academic Press; San Diego: 1993. pp. 210–229. [Google Scholar]
- 44.Revel A, Roanani H, Younglai E, Xu J, Han R, Savouret J-F, et al. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects sperm from DNA damage and apoptosis caused by benzo(a)pyrene. Reprod. Toxicol. 2001;15:479–486. doi: 10.1016/s0890-6238(01)00149-6. [DOI] [PubMed] [Google Scholar]
- 45.Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocrine Reviews. 2001;22:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- 46.Page ST, Kalhorn TF, Bremner WJ, Anawalt BD, Matsumoto AM, Amory JK. Intratesticular androgens and spermatogenesis during severe gonadotropin suppression induced by male hormonal contraceptive treatment. J. Androl. 2007;28:734–741. doi: 10.2164/jandrol.107.002790. [DOI] [PubMed] [Google Scholar]
- 47.Polyakov LM, Chasovskikh MI, Panin LE. Binding and transport of benzo(a)pyrene by blood plasma lipoproteins: the possible role of apolipoprotein B in this process. Bioconjugate Chem. 1996;7:396–400. doi: 10.1021/bc960005e. [DOI] [PubMed] [Google Scholar]
- 48.Mandal PK, McDaniel LR, Prough RA, Clark BJ. 7, 12-Dimethlbenz(a)anthracene inhibition of steroid production in MA-10 mouse Leydig tumor cells is not directly linked to induction of CYP1B1. Toxicol Appl Pharmacol. 2001;175:200–208. doi: 10.1006/taap.2001.9241. [DOI] [PubMed] [Google Scholar]
- 49.Ramesh A, Inyang F, Hood DB, Knuckles ME. Aryl hydrocarbon hydroxylase activity in F-344 rats subchronically exposed to benzo(a)pyrene and fluoranthene through diet. J Biochem Mol Toxicol. 2000;14:155–161. doi: 10.1002/(sici)1099-0461(2000)14:3<155::aid-jbt5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 50.Ramesh A, Hood DB, Inyang F, Greenwood M, Archibong AE, Knuckles ME, et al. Comparative metabolism, bioavailability and toxicokinetics of benzo(a)pyrene in rats after acute oral, inhalation, and intravenous administration. Polycyclic Aromatic Compounds. 2002;22:969–980. [Google Scholar]
- 51.Lee IP, Nagayama J. Metabolism of benzo(a)pyrene by the isolated perfused rat testis. Cancer Res. 1980;40:3297–3303. [PubMed] [Google Scholar]
- 52.Williams JA, Martin FC, Muir GH, Hewer A, Grover PL, Phillips DH. Metabolic activation of carcinogens and expression of various cytochromes P450 in human prostate tissue. Carcinogenesis. 2000;21:1683–1689. doi: 10.1093/carcin/21.9.1683. [DOI] [PubMed] [Google Scholar]
- 53.Senft AP, Dalton TP, Nebert DW, Genter MB, Puga A, Hutchinson RJ, et al. Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free Radic Biol Med. 2002;33:1268–1278. doi: 10.1016/s0891-5849(02)01014-6. [DOI] [PubMed] [Google Scholar]
- 54.Peltola V, Huhtaniemi I, Metsa-Ketela T, Ahotupa M. Induction of lipid peroxidation during steroidogenesis in the rat testis. Endocrinology. 1996;137:105–112. doi: 10.1210/endo.137.1.8536600. [DOI] [PubMed] [Google Scholar]
- 55.Diemer T, Allen JA, Hales KH, Hales DB. Reactive oxygen disrupts mitochondriain MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology. 2003;144:2882–2891. doi: 10.1210/en.2002-0090. [DOI] [PubMed] [Google Scholar]
- 56.Yardimci S, Atan A, Delibasi T, Sunguroglu K, Guven MC. Long-term effects of cigarette-smoke exposure on plasma testosterone, luteinizing hormone and follicle-stimulating hormone levels in male rats. Br J Urol. 1997;79:66–69. doi: 10.1046/j.1464-410x.1997.28314.x. [DOI] [PubMed] [Google Scholar]
- 57.Jeyaraj DA, Grossman G, Petrusz P. Altered bioavailability of testosterone in androgen-binding protein-transgenic mice. Steroids. 2005;70:704–714. doi: 10.1016/j.steroids.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 58.Karabelyos CS, Csaba G. Benzpyrene treatment decreases the sexual activity of adult rats, what is reversed in neonatally allylestrenol treated animals. Acta Physiol Hung. 1996;84:131–137. [PubMed] [Google Scholar]