Abstract
The hypothalamic paraventricular nucleus (PVN) and angiotensin II (AngII) play critical roles in cardiovascular and neurohumoral regulation ascribed in part to vasopressin (VP) release. The AngII actions in the PVN are mediated largely through AngII type 1 (AT1) receptors. However, there is indirect evidence that the functionally elusive central AngII type 2 (AT2) receptors are also mediators of AngII signaling in the PVN. We used electron microscopic dual immunolabeling of antisera recognizing the AT2 receptor and VP to test the hypothesis that PVN neurons expressing VP are among the cellular sites where this receptor has a subcellular distribution conducive to local activation. Immunoreactivity for the AT2 receptor was detected in somatodendritic profiles, of which ~60% of the somata and ~28% of the dendrites also contained VP. In comparison with somata and dendrites, axons, axon terminals, and glia less frequently contained the AT2 receptor. Somatic labeling for the AT2 receptor was often seen in the cytoplasm near the Golgi lamellae and other endomembrane structures implicated in receptor trafficking. AT2 receptor immunoreactivity in dendrites was commonly localized to cytoplasmic endomembranes, but was occasionally observed on extra- or peri-synaptic portions of the plasma membrane apposed by astrocytic processes or by unlabeled axon terminals. The labeled dendritic plasmalemmal segments containing AT2 receptors received asymmetric excitatory-type or more rarely symmetric inhibitory-type contacts from unlabeled axon terminals containing dense core vesicles, many of which are known to store neuropeptides. These results provide the first ultrastructural evidence that AT2 receptors in PVN neurons expressing vasopressin and other neuromodulators are strategically positioned for surface activation by AngII and/or intracellular trafficking.
Keywords: fluid homeostasis, cardiovascular regulation, neurosecretory neuron
The hypothalamic paraventricular nucleus (PVN) is a complex integrative center important for neurohumoral regulation and the maintenance of cardiovascular and body fluid homeostasis (Benarroch, 2005; Dampney et al., 2005). The vasopressin (VP)-containing neurons in the PVN are among the principal mediators of these critical functions (de Wardener, 2001). The VP-containing neurons are mainly located in the magnocellular subdivision, but are also present in the parvocellular subdivision of the PVN in most species. The majority of VP-containing magnocellular neurons project to the posterior pituitary gland, where VP is stored until its secretion into the venous flow. In addition, however, some VP-containing neurons in the magnocellular and parvocellular subdivisions project to the brainstem and thoracic spinal cord, where the released VP can enhance sympathetic nerve activity (Armstrong et al., 1980; Swanson and Kuypers, 1980; Sawchenko and Swanson, 1981; Cechetto and Saper, 1988; Portillo et al., 1998; Shafton et al., 1998; Pyner and Coote, 1999; Hallbeck et al., 2001). Although lacking clearly segregated magnocellular and parvocellular subdivisions (Schonemann et al., 1995; Van Pett et al., 2000), the mouse PVN contains a subpopulation of vasopressin-containing neurons (Hyodo et al., 1992) as well as cells that express angiotensin II (AngII), angiotensin-converting enzyme, and AngII subtype-selective receptors (Johren et al., 1997).
Systemic AngII administration increases plasma VP levels through central mechanisms involving the PVN (Keil et al., 1975; Veltmar et al., 1992). AngII-induced activation of circumventricular organs outside the blood-brain barrier, such as the subfornical organ (SFO), provides monosynaptic input to the PVN (Miselis, 1981; Tanaka et al., 1986; Ferguson and Kasting, 1988; Wright et al., 1993). Projection neurons from the SFO to the PVN contain glutamate and/or AngII (Lind et al., 1984; Li and Ferguson, 1993b; Ferguson and Washburn, 1998; Benarroch, 2005). SFO stimulation enhances both the excitability of putative hypothalamic VP-secreting neurons (Miselis et al., 1979; Ferguson et al., 1984a, b) and plasma VP levels (Ferguson and Kasting, 1986; Smith et al., 1997). Transection of SFO efferent pathways prevents systemic AngII-induced VP release (Knepel et al., 1982). Taken together, these findings provide strong evidence for the involvement of AngII and/or glutamate containing inputs from the SFO to the PVN in neurohumoral control.
The predominant brain actions of AngII are linked to signaling pathways initiated through the AngII-type 1 (AT1) receptor subtype. The effects of AngII on VP release from PVN neurons are well documented (see above), however, there is uncertainty as to whether AT1 and/or AngII-type 2 (AT2) receptors are mediators of these effects. The ambiguity arises in part because multiple studies in rat have shown that the AT1 receptor is principally expressed in PVN neurons containing corticotropin-releasing hormone, not VP (Aguilera et al., 1995; Lenkei et al., 1995; Armando et al., 2007). AT2 receptor expression levels are generally low except in fetal tissue, which suggests a primary role for this receptor subtype in cellular growth and developmental processes (Gallinat et al., 2000; Steckelings et al., 2005). However, peripheral and/or brain AT2 receptors may also be involved in the regulation of cardiovascular activity and fluid balance. In the mid-1990s, several groups reported that adult mice lacking the AT2 receptor exhibit an increased blood pressure response to systemic AngII (Hein et al., 1995; Ichiki et al., 1995). A more recent study by Li et al. (2003) indicated that the pressor response to intracerebroventricular administration of AngII in wild-type mice was enhanced by pre-injection of the AT2 receptor antagonist PD-123319. Additionally, AT2 receptors in adult rodent tissue have been shown to increase under pathological conditions, such as vascular injury, myocardial infarction, heart failure, and hypertension (Nio et al., 1995; Ohkubo et al., 1997; Barker et al., 2006; Yayama et al., 2006; Hernandez Schulman et al., 2007).
A functional role for AT2 receptors in the PVN is suggested by in vivo and in vitro experiments showing that AT2 receptor blockade decreases the effects of locally administered AngII on neuronal excitability (Ambuhl et al., 1992; Li and Ferguson, 1993a; Ferguson and Washburn, 1998). Furthermore, direct injection of AngII into the PVN increases pressor and drinking responses (Bains et al., 1992; Jensen et al., 1992; Ferguson and Washburn, 1998), and several pharmacological studies have demonstrated that AngII-induced changes in blood pressure, drinking, and/or VP release are partially mediated by the AT2 receptor (Hogarty et al., 1992; Rowland and Fregly, 1993; Toney and Porter, 1993; Hohle et al., 1995; do-Prado et al., 1996; Lee et al., 1996; Camara and Osborn, 2001; Li et al., 2003). Considering these findings, AT2 receptors may have subcellular locations in the PVN that would enable active participation in AngII-mediated functions, specifically the regulation of VP release. To test this hypothesis, we examined the electron microscopic dual immunolabeling of antisera for recognizing the AT2 receptor and VP in the PVN of mouse brain. This species was chosen because of their utility in gene deletion studies of AT2 receptor function (Gross et al., 2004). Electron microscopic immunolabeling is one of the few methods having the resolution necessary for detection of even low levels of antigen, such as the AT2 receptor, in neuronal and glial compartments (Wang and Pickel, 2002). This method also enables the assessment of AT2 receptors relative to the many PVN blood vessels. Additionally, we used confocal immunofluorescence to show the selectivity of the AT2 receptor antiserum in AT2, not AT1, transfected cells. Our results show that AT2 receptors have somatodendritic distributions consistent with their activation by axonal and/or glial derived AngII affecting the excitability of PVN neurons, some of which contain VP.
EXPERIMENTAL PROCEDURES
Antisera
A commercially obtained polyclonal antiserum to VP was generated in guinea pig (Peninsula Laboratories Inc., San Carlos, CA; T-5048; Lot # 061305). This antiserum was previously tested using a radioimmunoassay method. The antiserum showed complete recognition of VP, a slight cross-reaction (<0.1%) with other VP derivatives, and no recognition of oxytocin (Peninsula Laboratories).
Affinity purified polyclonal antiserum was raised in rabbits against a synthetic peptide from amino acids 349-363 (CRKSSSLREMETFVS) of the rat AT2 receptor carboxy-terminal domain (Abcam Inc., Cambridge, MA; ab19134; Lot # 292932). This commercially-available AT2 receptor antibody showed specificity for AT2 receptors in human cervical carcinoma HeLa cells transfected with the AT2 receptor (determined in present study).
Transfected cells
HeLa cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2 in Dulbecco’s modified Eagle’s medium (Cellgro) supplemented with 10% fetal bovine serum, 100 units/ml penicillin G, and 100 μg/ml streptomycin B (Atlanta Biologicals, Lawrenceville, GA). Cells grown on glass coverslips in 12 well plates were transfected with 100 ng pEGFP (Clontech, Palo Alto, CA) along with 300 ng empty vector plasmid (pCMVSPORT6, Invitrogen, Carlsbad, CA) and expression vectors for mouse AT1A (IMAGE clone ID: 4989471) or mouse AT2 receptor (IMAGE clone ID: 3497420) using Lipofectamine (Invitrogen) according to manufacturers suggestions. The medium was removed 48 h later and the cells were washed with cold 0.1 M phosphate-buffered saline (PBS; pH 7.4). The cells were fixed with 4% paraformaldehyde in 0.1 M PBS for 20 min on ice. After washing with 0.1 M PBS, the cells were incubated for 20 min at room temperature in 0.1% Triton X-100 in PBS. The cells were washed with PBS alone followed by a wash with PBS containing 0.05% Tween20. The cells were then blocked with 3% bovine serum albumin (BSA) in PBS for 30 min, washed with PBS containing 0.05% Tween20, and incubated in a 1:500 dilution of the AT2 receptor antibody overnight at 4°C. Following the primary antibody incubation, the cells were washed with 0.05% Tween20 in PBS and incubated in the secondary antibody (1:200 dilution; Cy5, Jackson ImmunoResearch Inc., West Grove, PA) for 1 h in the dark at room temperature. The coverslips containing the immunolabeled cells were washed with 0.05% Tween20 in PBS and mounted on glass slides. Cells were visualized by laser scanning confocal microscopy (LSCM) using a Leica SP5 system (Leica Microsystems®, Bannockburn, IL) equipped with a 63X oil immersion objective. Transfected cells were identified by being EGFP positive after 488 nm illumination. Angiotensin receptor immunoreactivity was detected after excitation at 633 nm.
Animals and tissue preparation
All experimental procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committees (IACUC) at Weill Medical College of Cornell University. Adult male C57BL/6J mice (25–30g; Jackson Laboratory, Bar Harbor, ME) were deeply anesthetized by i.p. injection of sodium pentobarbital and perfused through the left ventricle of the heart sequentially with 1) 5–10 ml of heparin-saline, 2) 30 ml of 3.75% acrolein in 2% paraformaldehyde, and 3) 100 ml of 2% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Immediately following perfusion, the brains were removed and post-fixed with 2% paraformaldehyde in 0.1 M PB for 30 min at room temperature. Coronal sections (40 μm) were cut through the region of the paraventricular nucleus of the hypothalamus starting at 0.55 mm caudal to bregma (Paxinos and Franklin 2001) using a vibratome (Leica Microsystems®).
Immunolabeling tissue sections
A pre-embedding dual-labeling protocol was used for the detection of AT2 and VP. In additional tissue sections, the immuno-markers were reversed to ensure specificity of intracellular labeling with both immunoperoxidase and immunogold. The tissue sections were placed for 30 min in 1% sodium borohydride in 0.1 M PB to neutralize reactive aldehydes. The free-floating sections were then rinsed with 0.1 M PB, transferred to 0.1 M Tris-buffered saline (TS; pH 7.6), and incubated for 30 min in 0.5% BSA in 0.1 M TS to minimize nonspecific binding of the antisera. The sections were placed for 48h at 4 °C in a solution of rabbit anti-AT2 receptor (1:2000 dilution immunoperoxidase; 1:1000 dilution immunogold) and guinea pig anti-VP (1:2000 dilution immunoperoxidase; 1:2000 dilution immunogold) antisera in 0.1M TS with 0.1% BSA.
For immunoperoxidase labeling, the sections previously incubated with the primary antisera were placed for 30 min in goat anti-rabbit or donkey anti-guinea pig biotinylated immunoglobulin (IgG; 1:400; Incstar, Stillwater, MN). This was followed by a 30 min incubation in avidin-biotin peroxidase complex (ABC; Vector Laboratories, Burlinghame, CA). The bound peroxidase was visualized by reaction of the sections for 5–6 min in 3,3′-diaminobenzidine (DAB; Sigma-Aldrich, St. Louis, MO) and hydrogen peroxide. All incubations were separated by rinses in 0.1 M TS.
For immunogold labeling, the tissue sections were rinsed in 0.01 M PBS (pH 7.4) and blocked for 10 min in 0.1% gelatin and 0.8% BSA to reduce non-specific binding of gold particles. The sections were then placed for 2 h in the secondary antisera solution containing donkey anti-rabbit or goat anti-guinea pig IgG (1:50 dilution) conjugated with 1 nm colloidal gold (Electron Microscopy Science, Hatfield, PA). The tissue sections were rinsed extensively with 0.01 M PBS and then incubated for 10 min in 2% glutaraldehyde in PBS. After washing the sections with 0.2 M citrate buffer, a Silver IntenSEM kit (Amersham, Arlington Heights, IL) was used for 6 min to enhance bound gold particles.
To prepare preadsorbed solutions for immunolabeling, the primary AT2 receptor antiserum (1:2000 dilution) was incubated with the AT2 receptor antigenic peptide (100 μg/ml; Biosynthesis Inc., Lewisville, TX) overnight at 4° C. Following the incubation, the antibody and peptide solution was centrifuged at 14,000 RPM for 30 min. The tissue was processed for immunoperoxidase labeling, as described above, using the adsorbed AT2 receptor antiserum. Following the DAB reaction, the tissue was washed and mounted on slides for visualization of the peroxidase reaction product using a Nikon Eclipse 80i microscope (Nikon Instruments, Melville, NY). Images were captured using a Micropublisher 5.0RTV camera (Q Imaging, Burnaby, BC, Canada) and IP Lab Scientific Image Processing software (version 3.9.3 r3, Scanalytics Inc., Rockville, MD).
Tissue sections were prepared for electron microscopy by post-fixing in 2% osmium tetroxide in 0.1M PB for 1 hr, dehydrating with a graded series of ethanols and propylene oxide, and incubating overnight in a 50:50 mixture of propylene oxide and Epon (Embed-812 kit, Electron Microscopy Science). The next day the tissue was placed in 100% Epon for 2 h and then flat-embedded between two sheets of Aclar plastic. The flat-embedded tissue was hardened in an oven for 48 h at 60 °C.
Ultrastructural analysis
The tissue area of interest within the hypothalamus was identified and selected using a light microscope. Excised embedded tissue was glued to a plastic block and trimmed. Ultrathin sections (70 nm) were cut with a diamond knife (Diatome, Fort Washington, PA) using a Leica EM UC6 ultratome (Leica Microsystems®). The ultrathin sections were collected and dried on 400-mesh copper grids (Electron Microscopy Sciences), and then counterstained with uranyl acetate and lead citrate. The tissue was examined using a Philips CM10 transmission electron microscope (FEI, Hillsboro, OR).
Electron micrographs (magnification 19,000X) were taken of labeled profiles in ultrathin sections containing the PVN from three mice. The ultrastructural analysis was done exclusively on portions of the tissue in contact with the overlying Epon where penetration of the antisera and immunoreagents is optimal. A total area of 3387 μm2 was examined in three vibratome sections processed for immunogold labeling of the AT2 receptor and immunoperoxidase labeling of VP. In addition, 3408 μm2 from three sections was processed with reversal of the markers. Micrographs used for illustration were captured using AMT Advantage HR/HR-B CCD Camera System (Advanced Microscopy Techniques, Danvers, MA). The images were adjusted for brightness and sharpness using Adobe Photoshop (version 7.0.1, Adobe Systems Inc., Mountain View, CA) and then imported into Powerpoint (Microsoft Office, 2003).
The tissue was quantitatively examined to determine the frequency with which the immunoreactive products were localized in somata, dendrites, axons, axon terminals, or glial processes. All neuronal, glial, and vascular structures were classified according to the descriptions of Peters et al. (1991). Asymmetric synapses (excitatory-type) were identified by thick postsynaptic densities, whereas symmetric (inhibitory-type) synapses had thin pre- and post-synaptic specializations. Structures were classified as apposed if their plasma membranes were in contact, but lacked a recognizable synaptic density.
Immunoperoxidase-labeled profiles had an electron dense precipitate greater than that seen in other morphologically similar profiles in the surrounding neuropil. Profiles containing one or more gold particles were considered to be immunogold-labeled. We used this quantitative approach because previous studies in mouse have shown that AT2 receptor expression levels are low in the PVN (Johren et al., 1997). Profiles with only one gold particle were identified as labeled only if the particle was contacting the plasmalemma or an intracellular membrane. Similar labeling patterns were observed in the present study for the AT2 receptor using immunoperoxidase. The validity of this approach also depends on 1) minimal labeling of tissue not known to express AT2 receptors, such as myelin, and 2) little if any spurious distribution of gold-silver deposits at the tissue/epon interface.
RESULTS
All transfected HeLa cells were positively identified by the presence of green fluorescent protein (GFP; Fig. 1A–C). The cells transfected with the AT2 receptor were immunolabeled with the AT2 receptor antiserum (Fig. 1A). Intense fluorescent product was seen in the cytoplasm surrounding the nuclear envelope. Immunoreactivity was not detected in cells transfected with the AT1 receptor (Fig. 1B) or the empty vector plasmid (Fig. 1C).
Fig. 1.
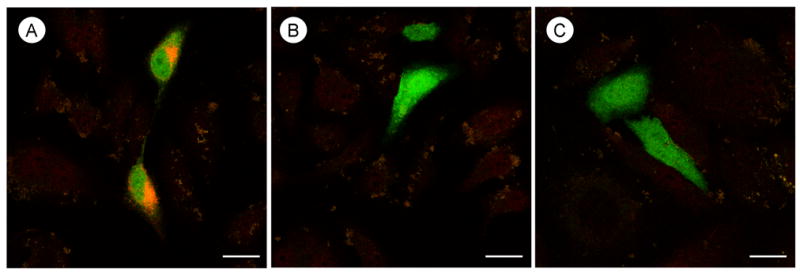
Confocal microscopic immunolabeling for the AT2 receptor antiserum in HeLa cells differentially transfected with AngII receptor subtypes. A, Immunofluorescence labeling (orange) is seen in the cytoplasm near the nucleus in cells transfected with the AT2 receptor. B and C, There is no observable immunolabeling in cells transfected with either the AT1A receptor in panel B or an empty vector plasmid in panel C. Green fluorescent protein (green) is present in all transfected cells. Scale bar=10 μm.
Light microscopic examination revealed a diffuse distribution of AT2 receptor immunoperoxidase labeling in the mouse PVN. Peroxidase reaction product was primarily localized to thin processes, however, cell somata also showed AT2 receptor labeling (Fig. 2A). The AT2 receptor immunoreactivity was sparsely localized within the cytoplasmic domains of somata and proximal dendritic shafts. Prior adsorption of the primary antiserum with the antigenic peptide sequence resulted in a reduction of the intense punctate immunolabeling for the AT2 receptor (Fig. 2B).
Fig. 2.
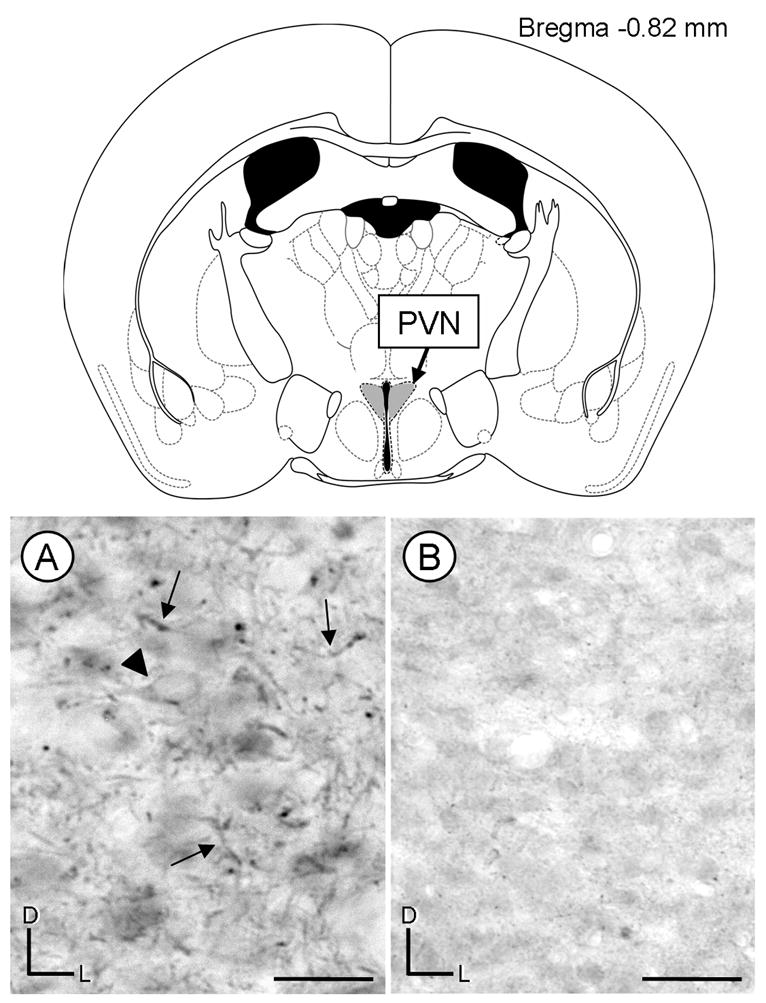
Light micrographs of coronal sections showing immunoperoxidase labeling for AT2 receptors in the paraventricular nucleus (PVN). A coronal diagram from the mouse brain atlas (Paxinos and Franklin, 2001) indicates the level and region (shaded in gray) where tissue was analyzed. A, The peroxidase reaction product is seen mainly in processes in the PVN (arrows). The arrow head is pointing to a soma with diffuse AT2 receptor peroxidase immunoreactivity. B, A reduction of immunoreactivity is observed in a coronal section that was processed using an AT2 receptor antiserum preadsorbed with the antigenic peptide. D, dorsal; L, lateral; scale bar=10 μm.
Electron microscopy of the PVN confirmed the presence of AT2 receptor immunoreactivity primarily in neuronal somata and dendrites, but also revealed the expression of this receptor in axonal and glial profiles. Because the proportion of labeling among the different profiles was quantitatively similar using immunogold or immunoperoxidase for detection of the AT2 receptor, the datasets for the two detection methods were combined. A total of 463 AT2 receptor-labeled profiles were counted in the sampled neuropil. Background immunolabeling with the AT2 receptor antiserum was negligible. Only 1 of 94 (approximately 1%) myelinated axons in the neuropil (3387 μm2) had a single AT2 gold particle over the myelin, where AT2 receptors would not be expected to be expressed. The subcellular (somatodendritic, axonal, and glial) distribution of AT2 receptors and their cellular relationship to VP in the PVN are described in detail below.
AT2 receptor distribution in VP and non-VP containing somatodendritic profiles
Immunoreactivity for the AT2 receptor was predominantly associated with cytoplasmic organelles and less frequently with the plasma membrane in somata and dendrites regardless of whether they contained VP (Figs. 3–5). In somata, intracellular immuno-peroxidase or -gold labeling for the AT2 receptor was commonly found near long, tubular structures and small, round vesicles resembling the Golgi apparatus (Fig. 3A and B, respectively). Immunoreaction product for the AT2 receptor was also present on, or near, the outer membrane of other structures, including mitochondria (Fig. 3A) as well as small and large endomembranes (Fig. 3C). The AT2-labeled somata were contacted by unlabeled axon terminals, 50% (32/64) of which contained dense core vesicles (Fig. 3C). Diffuse immunoperoxidase labeling for the AT2 receptor was associated with the plasma membrane in 15% (4/26) of somata (Fig. 3A and 4). The somatic surface labeling for the AT2 receptor was apposed by unlabeled glial processes (Figs. 3A and 4). Sixty percent (38/64) of AT2 receptor-labeled somata contained VP immunoreactivity in a single plane of section (Fig. 4).
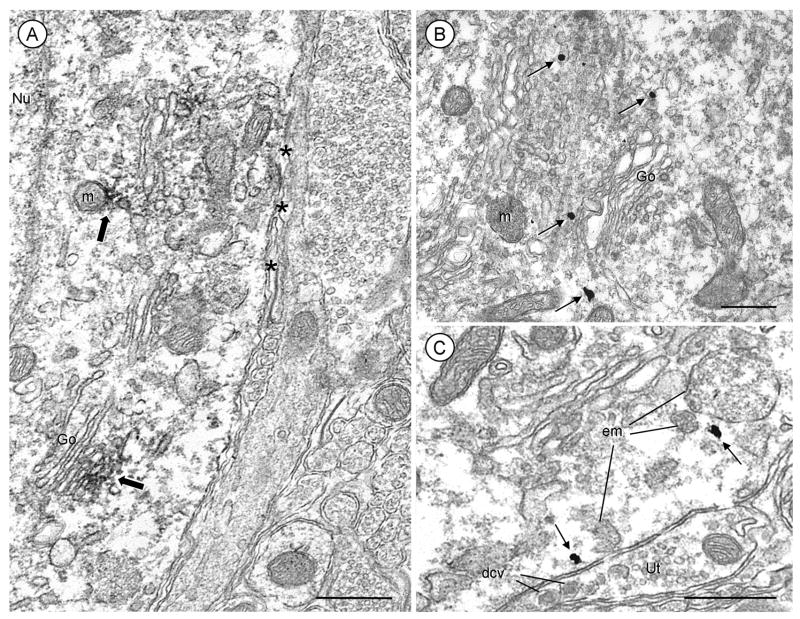
Fig. 3.
Electron micrographs showing cytoplasmic and plasmalemmal localization of AT2 receptors in somata of the mouse PVN. Sections were processed for AT2 receptor and VP dual-labeling, but the somata shown here are without detectable vasopressin immunoreactivity. A, Immunoperoxidase labeling for the AT2 receptor (block arrows) is seen within the cytoplasm along or near membranes of the Golgi apparatus (Go) and mitochondria (m). Diffuse labeling for the AT2 receptor is seen throughout the cytoplasm and associated with portions of the plasma membrane. B, Reversal of the immunolabels demonstrates that AT2 receptor immunogold (arrows) is present near saccules of Go. C, One AT2 receptor immunogold particle is seen in the cytoplasm next to a large endomembrane (em). Another AT2 receptor immunogold particle is located beneath portions of the plasma membrane that is apposed to an unlabeled terminal (Ut) enriched in large dense core vesicles (dcv). Nu= nucleus; asterisks=glial processes; scale bars=0.5 μm.
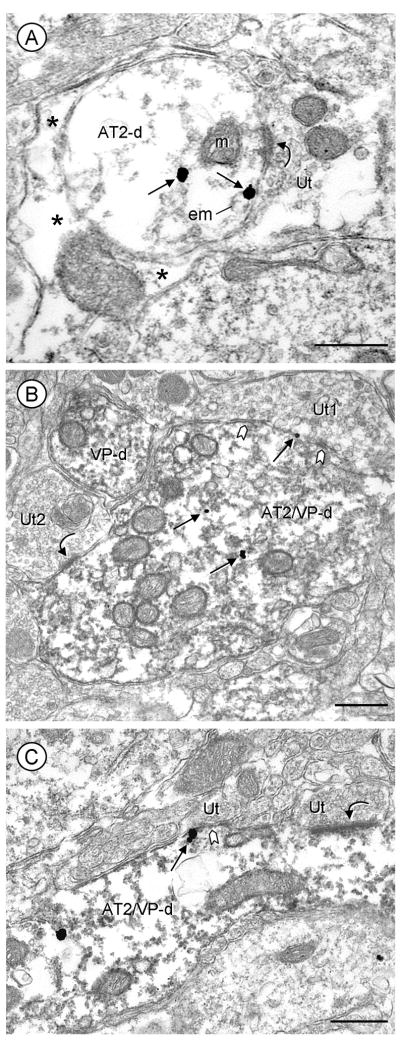
Fig. 5.
Distribution of AT2 receptor immunogold in dendrites without (A) or with (B, C) peroxidase labeling for VP in mouse PVN. A, An AT2-labeled dendrite (AT2-d) receives an asymmetric synapse (curved arrow) from an unlabeled terminal (Ut). One immunogold particle is located in the cytoplasm, while another particle is located on the plasma membrane near the synaptic junction. B, AT2-gold and VP immunoperoxidase product are seen in a large dually labeled dendrite (AT2/VP-d). AT2 receptor immunogold particles are sparsely located in the central portion of the cytoplasm and on the plasma membrane of a dendrite receiving a symmetric synapse (chevron) from an unlabeled terminal (Ut1). This terminal contains dense core vesicles, whereas a second unlabeled terminal (Ut2) forms an asymmetric synapse (curved arrow) and contains small clear vesicles. C, A cluster of AT2 receptor gold particles is located on the plasma membrane of a longitudinally sectioned dendrite (AT2/VP-d) near a symmetric synapse (chevron). This dual labeled dendrite also receives an asymmetric synapse (curved arrow) from an unlabeled terminal. Asterisks=glial processes; scale bars=0.5 μm.
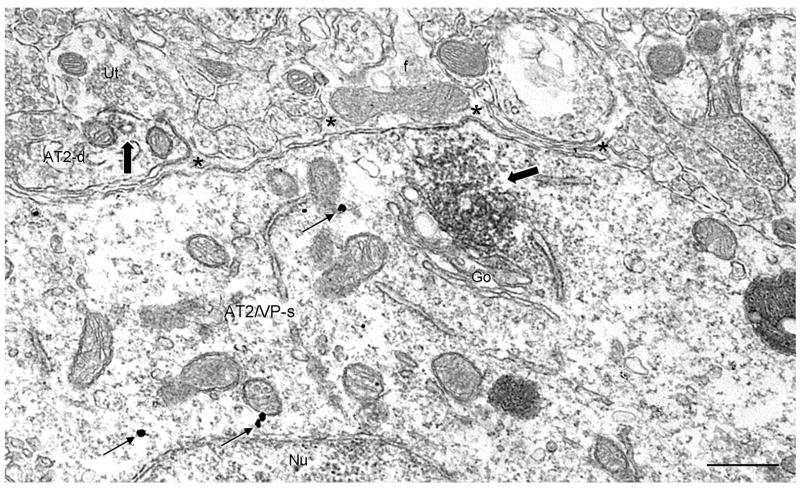
Fig. 4.
Distribution of the AT2 receptor in PVN neuronal and glial profiles in a section processed for dual labeling. Immunoperoxidase AT2 receptor labeling (block arrow) in a soma (AT2/VP-s) that also contains vasopressin immunogold particles (arrows) is heavily associated with the Golgi apparatus (Go). Diffuse labeling for the AT2 receptor is seen along a small portion of the soma plasma membrane apposed by an unlabeled glial process (*) that contains a large mitochondrion and microfilaments (f) typical of astrocytes. In the neuropil, diffuse AT2 receptor immunoreactivity is localized to a dendrite (AT2-d) that is partially surrounded by glia. Scale bars=0.5 μm.
Many unlabeled as well as AT2 receptor-labeled dendrites were seen in the PVN (Table 1). In dendrites, AT2 receptor immunoreactivity was associated with cytoplasmic endomembranes and mitochondria (Figs. 4 and 5). Nearly all AT2-labeled dendrites (289/312) were partially surrounded by glia. Immunogold surface labeling was detected in 12% (18/145) of the dendrites containing AT2 receptors. The immunogold particles were distributed on extra- or peri-synaptic portions of the plasma membrane contacted by unlabeled axon terminals (Fig. 5A-C) or glia. In randomly sampled single sections, 28% (88/312) of the AT2 receptor-labeled dendrites contained VP immunoreactivity (Fig. 5B and C).
Table 1.
PVN distribution of AT2 receptors within neuronal profilesa
| Percentage of profiles (AT2-labeled/unlabeled) | |
|---|---|
| Dendritic profiles | 14.6% (117/799) |
| Axon profiles | 0.5% (28/5752) |
| Axon terminal profiles | 0.5% (4/884) |
Numbers are labeled and unlabeled profiles in 3408 square micrometers of neuropil in coronal sections through the PVN of three mice.
The PVN dendrites containing AT2 receptors received synaptic input from morphologically heterogeneous axon terminals. Of all the axon terminals contacting AT2 receptor-labeled dendrites without VP, 38% (37/98) formed asymmetric excitatory-type synapses, whereas only 12% (12/98) formed symmetric inhibitory-type synapses. Likewise, axon terminals formed more asymmetric (23%, 8/35) than symmetric (9%, 3/35) synapses with AT2 receptor-labeled dendrites that contained VP. Therefore, AT2 receptors in the PVN are mainly localized to dendritic segments that are receptive to excitatory inputs as indicated by the asymmetry of the postsynaptic membranes (Rollenhagen and Lubke, 2006). Other terminals apposing AT2 or AT2 and VP-labeled dendritic profiles were without recognizable synaptic junctions.
Several single (AT2) and dual (AT2 and VP) labeled somatodendritic profiles were in close proximity to blood vessels in the PVN (Fig. 6A and B). These profiles were either in contact with the basal lamina or separated from the basal lamina of microvessels by only thin glial processes.
Fig. 6.
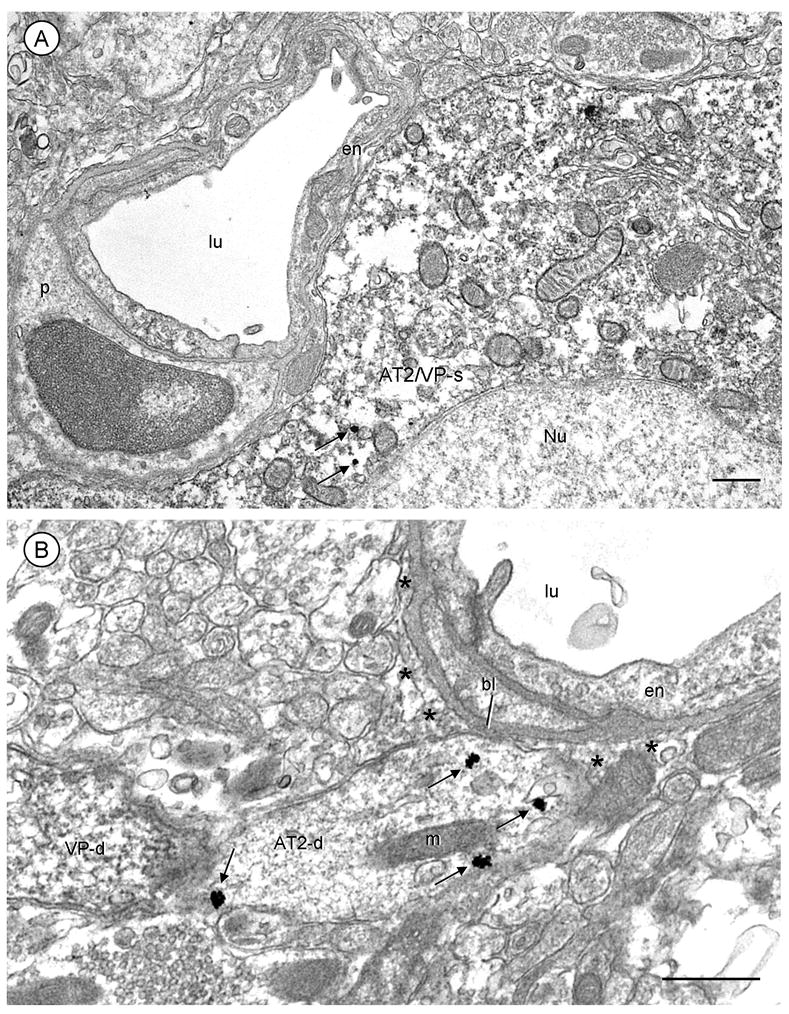
Immunogold localization of AT2 receptors to somatodendritic profiles contacting microvessels in the PVN. A, The soma (AT2/VP-s) contains abundant peroxidase reaction product indicating vasopressin immunoreactivity and a few cytoplasmic AT2 receptor immunogold particles (arrows). This soma contacts a small blood vessel. B, An AT2-labeled dendrite (AT2-d) with cytoplasmic and plasmalemmal gold particles (arrows) is apposed to perivascular astrocytic processes (*) and a small portion of the vascular basal lamina (bl). The endothelial cells (en) are without immunoreactivity in A and B. p, pericyte; lu, blood vessel lumen; scale bars=0.5 μm.
AT2 receptor axonal distribution
Small (<0.2 micron in diameter) axons were prevalent in the PVN, but only a small proportion were AT2 receptor immunoreactive (Table 1). The peroxidase reaction product for the AT2 receptor was seen in the cytoplasm and on the plasma membrane of axonal profiles (Fig. 7B). Similar patterns were observed in axons with immunogold labeling for the AT2 receptor. Of all AT2-labeled profiles, 9% (42/463) were unmyelinated axons. The AT2-labeled axons were without detectable VP immunoreactivity and were apposed to unlabeled axons, as well as small dendrites and glial processes.
Fig. 7.
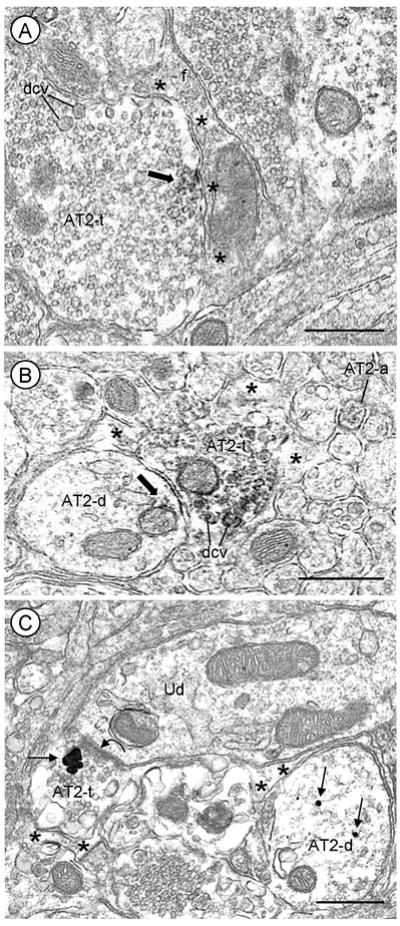
Presynaptic localization of AT2 receptors in axon terminals apposing astrocytic processes in mouse PVN. Immunoperoxidase (A and B) or immunogold (C) labeling for the AT2 receptor is distributed in axon terminals, many of which contain dense core vesicles (dcv) and are contacted by astrocytic processes (*). A, AT2 receptor immunoreactivity (block arrow) is localized to the plasma membrane and associated membranes of small vesicles in an axon terminal (AT2-t) also containing numerous dcv. The portion of the plasma membrane immunoreactive for the AT2 receptor is apposed by a filamentous (f) astrocytic process. B, Peroxidase reaction product is associated with a non-synaptic portion of the plasma membrane and on membranes of a mitochondrion and nearby dcv in a terminal (AT2-t). This labeled terminal is apposed to a dendrite (AT2-d) that contains diffuse AT2 receptor peroxidase labeling along a mitochondrion (m) near the plasma membrane. A small AT2-immunoreactive axon (AT2-a) is seen in the neuropil. C, An AT2 receptor immunogold aggregate (arrow) is seen in a terminal forming an asymmetric synapse (curved arrow) with an unlabeled dendrite (Ud). The AT2 receptor aggregate is located in the cytosol near the presynaptic specialization. Nearby, there is an AT2 receptor-labeled dendrite (AT2-d) that does not co-express vasopressin. Scale bars=0.5 μm.
Axon terminals comprised 5% (23/463) of all AT2-labeled profiles in the PVN even though unlabeled terminals were more prevalent than dendrites in this region (Table 1). These terminals ranged in size from 0.1 μm to 1.3 μm in diameter and contained all small clear or a mixture of small clear and large dense core vesicles. Immuno-peroxidase (Fig. 7A, B) or -gold (Fig. 7C) labeling for the AT2 receptor was present in the cytoplasm overlying membranes of synaptic vesicles and along extrasynaptic plasma membranes. The AT2-receptor labeling on extrasynaptic areas of the membrane was often apposed by unlabeled astrocytic processes (Fig. 7). The axon terminals containing AT2 receptor immunoreactivity made asymmetric contacts with dendrites, some of which expressed AT2 receptors (Fig. 7B and C). None of these terminals co-expressed AT2 receptors and VP, which was rarely observed in any axon terminals within the PVN.
AT2 receptor glial distribution
The AT2 receptor labeling in the PVN was sparsely distributed in astrocytic processes distinguished by their cytological features and perineuronal or vascular associations (Peters et al., 1991). Astrocytic immunoreactivity for the AT2 receptor was present throughout the cytoplasm and also distributed along the plasma membrane (Fig. 8A–C). These AT2-labeled glia were often apposed to unlabeled axons and terminals. Five percent (22/463) of all AT2-labeled profiles were filamentous glial processes. None of the AT2-labeled glia contained VP, which was seen exclusively in PVN neurons.
Fig. 8.
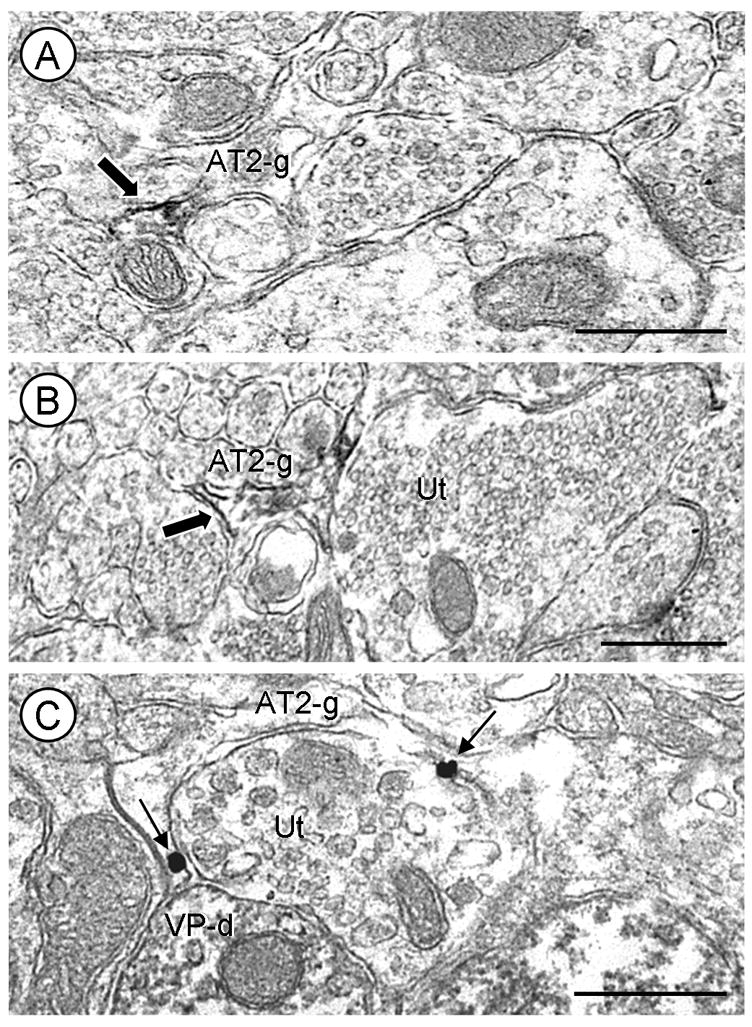
Glial localization of AT2 receptors in the PVN. A and B, Glial profiles (AT2-g) containing AT2 receptor immunoperoxidase labeling (block arrow). Both AT2-labeled glial processes are apposed to unlabeled axons and terminals. C, AT2 immunogold labeling (arrows) is located on the plasma membrane of a glial process partially surrounding an unlabeled axon terminal (Ut). One astrocytic immunogold particle is found near a contact between the Ut, containing large dense core vesicles, and a VP-containing dendrite (VP-d). Scale bars=0.5 μm.
DISCUSSION
Our results provide the first ultrastructural immunocytochemical evidence for a prominent distribution of the AngII AT2 receptor along cytoplasmic endomembranes and plasmalemma of somatodendritic profiles containing VP in the mouse PVN. Moreover, we observed a similar subcellular distribution of AT2 receptors in PVN neurons without detectable VP immunoreactivity, suggesting the involvement of these receptors in the regulation of functionally diverse PVN neurons. The plasmalemmal AT2 receptors in somatodendritic profiles were contacted by axon terminals, but were more often apposed by unlabeled astrocytic processes, separating them from other neurons and/or microvessels of the PVN. Together, these results suggest that both neurons and glia are potential sources of AngII effective at AT2 receptors in the PVN. They also provide support for the concept that AT2 receptors play a role in determining the postsynaptic excitability of neurons expressing VP and other modulators in the PVN. A major involvement of AT2 receptors in modulating the postsynaptic excitability of PVN neurons is further substantiated by our infrequent detection of these receptors in small axons and axon terminals, none of which contained VP. These results are discussed together with the relevant methodological considerations and implications for understanding the contribution of AT2 receptors to cardiovascular function influenced by hypothalamic regulation of VP secretion.
Methodological considerations
The diffuse light and electron microscopic immunolabeling of AT2 receptors using the rabbit antiserum is consistent with low binding levels, as previously reported for the AT2 receptor, in the mouse PVN (Johren et al., 1997). Immunoreactivity was observed in HeLa cells transfected with the AT2 receptor, but not in cells transfected with the AT1 receptor or an empty plasmid vector. Moreover, mouse hypothalamic sections processed with preadsorbed AT2 receptor antiserum showed that the antigenic peptide selectively removes immunoreactivity. The recognition of the AT2 receptor in the PVN by the commercial antiserum used in the present study is similar to findings from a previous immunocytochemistry study in rat brain using an antiserum raised against AT2 receptors from solubilized N1E-115 cells (Reagan et al., 1993; Reagan et al., 1994). The previous study, however, reported a higher labeling density in the PVN than that observed in the present study. A BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) revealed no other proteins with a similar amino acid sequence to the AT2 receptor sequence used for generation of the AT2 receptor antiserum. However, we cannot exclude possible recognition of unknown homologous proteins or inactive carboxyl terminus fragments of the AT2 receptor.
Neuronal and glial AT2 receptor immunoreactivity in the PVN was indicated by small aggregates of peroxidase reaction product or one or more gold particles. Immunolabeling was localized to intracellular or plasmalemmal membranes, which fits accordingly with trafficking and surface availability of receptor proteins. Reversal of the markers for the antisera resulted in a similar number of immunolabeled neuronal and glial profiles. However, slightly fewer dendritic profiles were labeled with immunogold compared to those labeled with immunoperoxidase. This result can be attributed to the lower sensitivity of the immunogold method (Wang et al., 1996). Thus, analysis of tissue processed by immunogold labeling may underestimate the prevalence of the receptor in neurons and the extent of AT2 and VP co-existence. In addition, the higher detection of AT2 receptor labeling in dendrites compared to axons and axon terminals may be accounted for, in part, by the fact that most dendrites are larger than axons (Peters et al., 1991) and might therefore be sectioned more frequently. This seems unlikely, however, since there were more unlabeled axonal profiles than dendrites in randomly sampled tissue sections (Table 1).
Somatodendritic distribution of AT2 receptors
Immunoreactivity for the AT2 receptor in somata was prominently displayed around tubules and saccules of the Golgi apparatus, whereas the dendritic labeling was most evident along cytoplasmic endomembranes and/or plasmalemmal surfaces. These distributions are similar to those of the recently discovered ATIP1, an AT2 receptor interacting protein implicated in AT2 receptor trafficking to the cell surface (Nouet et al., 2004; Wruck et al., 2005; Di Benedetto et al., 2006). Together, these observations suggest that AT2 receptors are synthesized in somata and transported through the endomembrane system of dendrites in the PVN. In addition, we detected AT2 receptor labeling along mitochondria-associated endomembranes of the PVN. This location is comparable to the sites occupied by the AT1 receptor and the NADPH oxidase subunit, gp91phox in the NTS (Huang et al., 2003; Wang et al., 2004; Glass et al., 2005), suggesting that activation of AT2 receptors in the PVN may functionally oppose the generation of NADPH oxidase-derived reactive oxygen species mediated through AT1 receptors (Schumacker, 2002; Wang et al., 2004; Zimmerman et al., 2005; Wang et al., 2006) in multiple central autonomic networks (Wang et al., 2004; Peterson et al., 2006). This speculation is supported by evidence that AT2 receptor signaling opposes AT1 receptor-mediated functions (Inagami et al., 1999; Hernandez Schulman et al., 2007). Such opposition might involve direct binding between AT2 and AT1 receptors as has been shown in transfected cells and human myometrial biopsies (AbdAlla et al., 2001) or by other undisclosed mechanisms.
The AT2 receptor also was observed along portions of the plasma membranes of a few somata and many dendrites of the PVN. The plasmalemmal AT2 receptor labeling was most evident near appositions with filamentous glial processes. This observation is consistent with studies showing that astrocytes provide the main source of the AngII precursor, angiotensinogen (Stornetta et al., 1988; Milsted et al., 1990). Moreover, AngII-like immunoreactivity has been found in astrocytes in several brain regions, including the nucleus of the solitary tract (NTS) and the cerebellar cortex (Lippoldt et al., 1994; Huang et al., 2003). Schinke et al. (1999) have demonstrated that transgenic rats deficient in brain astrocytic angiotensinogen exhibit a decreased blood pressure compared to control rats. This finding, along with the present observations that AT2 receptors are localized to somatic membranes contacting glial processes, suggest that glial-derived AngII may affect neuronal signaling in brain regions associated with cardiovascular regulation (Fogarty and Matute, 2001).
Labeling for the AT2 receptor was also occasionally seen on plasmalemmal surfaces of dendrites near asymmetric (excitatory-type) or symmetric (inhibitory-type) synaptic contacts from unlabeled axon terminals. The extrasynaptic AT2 receptor distribution could reflect movement of the receptors along the membrane to and from sites within the synaptic junction, as has been well documented for other proteins (Triller and Choquet, 2005; Groc and Choquet, 2006). Therefore, we can not exclude the possibility that AT2 receptors in the PVN are activated by AngII that is released from presynaptic axon terminals and possibly independent of astrocytes.
AT2 receptor labeling in somatodendritic profiles containing VP
AT2 receptors and VP were co-expressed in somata and dendrites in the mouse PVN. These results in mouse are consistent with those reported previously by Shelat et al. (1998) showing a similar distribution of fluorescent immunolabeling for the AT2 receptor and VP in a subset of PVN neurons in the rat. The PVN is targeted by AngII-containing neurons from the SFO (Sawchenko and Swanson, 1983; Swanson and Sawchenko, 1983; Lind et al., 1984). Systemic administration of AngII produces c-fos activation in PVN neurons immunoreactive for VP (Dawson et al., 1998), and direct injection of AngII into the PVN increases plasma VP (Shoji et al., 1989). Additionally, local production of AngII in the brain tonically controls VP release (Schinke et al., 1999). In rat, it is known that VP-containing magnocellular neurons in the PVN project to the posterior pituitary gland, while the parvocellular neurons project to brainstem areas, such as the NTS, rostroventrolateral medulla, and preganglionic neurons located in the intermediolateral cell column of the spinal cord (Cechetto and Saper, 1988; Hallbeck et al., 2001; Benarroch, 2005). Release of VP from the posterior pituitary into the general circulation maintains body fluid balance by stimulating water reabsorption by the kidney (Bourque, 1998). In the NTS and spinal cord, VP is involved in regulating the baroreflex and sympathetic outflow (Gardiner and Bennett, 1986; Berecek and Swords, 1990; Toba et al., 1998; Raggenbass, 2001). Although the PVN subdivisions are less clearly defined in the mouse (Van Pett et al., 2000), the present ultrastructural findings indicate that AT2 receptors have a specific subcellular distribution in the PVN that is permitting for functional participation in the regulation of neuroendocrine and cardiovascular homeostatic functions. Moreover, the presence of AT2 receptors, but not VP, in axon terminals of the PVN suggest that dual labeled somata and dendrites belong to projection neurons.
AT2 receptor distribution in axonal profiles without VP
A few small axons and axon terminals in the PVN were immunolabeled for the AT2 receptor, but none of these contained VP. This is consistent with a primary involvement of AT2 receptors in modulating the postsynaptic excitability of PVN neurons and with the known extrinsic projections of VP containing neurons of the PVN (for review, see Benarroch, 2005). However, these AT2 receptor-labeled terminals apposed dendrites containing AT2 receptors, suggesting a potential presynaptic role in modulating the release of neurotransmitters that control the output from non-VP expressing neurons of the PVN. The transmitters within the AT2-containing axon terminals in the PVN are not known although their content of large dense core vesicles is consistent with storage of neuropeptides, such as AngII (Pickel and Chan, 1995; Guan et al., 2000; Huang et al., 2003).
Vascular distribution
We found VP and/or AT2 receptor-immunoreactive somata and dendrites with minimal astrocytic separation from the basal lamina beneath endothelial cells of microvessels in the mouse PVN. This observation is consistent with known vascular associations of VP-containing neurons in hypothalamic regions of other species (Duan and Ju, 1998; Riva et al., 1999). Moreover, the vascular associations of neurons containing VP and/or AT2 receptors correspond with the dense capillary network in the magnocellular subdivision of the PVN (Ambach and Palkovits, 1974; Sposito and Gross, 1987; Badaut et al., 2000). Although the functional significance of the close proximity between VP neurons and microvessels in the PVN is not clearly understood, blood vessels may potentially provide important sources of endothelial-derived neuromodulators, such as nitric oxide (NO), interactive with AngII in controlling VP release (Faraci and Heistad, 1991; Kadekaro and Summy-Long, 2000; Kadekaro, 2004). Alternatively, or in addition, VP neurons in the PVN may be receptive to plasma osmoregulation (Duan and Ju, 1998; Badaut et al., 2000). VP-containing neurons were commonly separated from microvessels in our study by thin glial leaflets. There is evidence for astrocytic involvement in the osmoregulation of hypothalamic neuroendocrine neurons. In the supraoptic nucleus, the release of taurine from glial cells is osmodependent (Deleuze et al., 1998), and VP secretion is altered by taurine activation of neuronal-localized glycine receptors (Hussy et al., 1997). Whether or not similar mechanisms exist in the PVN remain to be determined.
AT2 receptor labeling in glia
AT2 receptor immunolabeling was rarely found in glial processes of the PVN. Consistent with these findings, astrocytic cultures from rat hypothalamus express predominantly AT1, not AT2, receptors (Sumners et al., 1991; Wyse and Sernia, 1997). When AT2 receptor labeling of glia was observed in the present study, the immunoreactive glial processes were apposed to unlabeled axons and terminals. Depending on the brain region, astrocytes express different AngII receptor subtypes that are coupled to distinct signaling pathways (Tallant and Higson, 1997). Astrocytic AngII receptors are implicated in the regulation of local levels of angiotensinogen (Tallant and Higson, 1997; Dostal et al., 2000). Activation of astrocytic AngII receptors in vitro also results in the release of prostaglandins (Jaiswal et al., 1991), which are important mediators of cerebrovascular control (Iadecola and Nedergaard, 2007). Allen et al. (2006) have recently shown that increased glial expression of the AT1 receptor in the rostroventrolateral medulla, a brain region critical for sympathetic vasomotor tone, elevates blood pressure in conscious rats. The low AT2 receptor expression in PVN astrocytic processes suggests that glial AngII signaling in this brain region is primarily mediated by the AT1 or another AngII receptor subtype.
Acknowledgments
Grant Support: NIH; Grant numbers: MH40342, HL18974 to V.M. Pickel
LIST OF ABBREVIATIONS
- ABC
avidin-biotin peroxidase complex
- AngII
angiotensin II
- AT1
angiotensin type 1
- AT1A
angiotensin type 1A
- AT2
angiotensin type 2
- DAB
3,3′-diaminobenzidine
- GFP
green fluorescent protein
- IgG
immunoglobulin
- NO
nitric oxide
- NTS
nucleus of the solitary tract
- PBS
phosphate-buffered saline
- PVN
paraventricular nucleus
- SFO
subfornical organ
- TS
tris-buffered saline
- VP
vasopressin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem. 2001;276:39721–39726. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Young WS, Kiss A, Bathia A. Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin II. Neuroendocrinology. 1995;61:437–444. doi: 10.1159/000126866. [DOI] [PubMed] [Google Scholar]
- Ambach G, Palkovits M. Blood supply of the rat hypothalamus. II. Nucleus paraventricularis. Acta Morphol Acad Sci Hung. 1974;22:311–320. [PubMed] [Google Scholar]
- Ambuhl P, Felix D, Imboden H, Khosla MC, Ferrario CM. Effects of angiotensin analogues and angiotensin receptor antagonists on paraventricular neurones. Regul Pept. 1992;38:111–120. doi: 10.1016/0167-0115(92)90049-z. [DOI] [PubMed] [Google Scholar]
- Armando I, Volpi S, Aguilera G, Saavedra JM. Angiotensin II AT1 receptor blockade prevents the hypothalamic corticotropin-releasing factor response to isolation stress. Brain Res. 2007;1142:92–99. doi: 10.1016/j.brainres.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5:1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Badaut J, Nehlig A, Verbavatz J, Stoeckel M, Freund-Mercier MJ, Lasbennes F. Hypervascularization in the magnocellular nuclei of the rat hypothalamus: relationship with the distribution of aquaporin-4 and markers of energy metabolism. J Neuroendocrinol. 2000;12:960–969. doi: 10.1046/j.1365-2826.2000.00539.x. [DOI] [PubMed] [Google Scholar]
- Bains JS, Potyok A, Ferguson AV. Angiotensin II actions in paraventricular nucleus: functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res. 1992;599:223–229. doi: 10.1016/0006-8993(92)90395-p. [DOI] [PubMed] [Google Scholar]
- Barker TA, Massett MP, Korshunov VA, Mohan AM, Kennedy AJ, Berk BC. Angiotensin II type 2 receptor expression after vascular injury: differing effects of angiotensin-converting enzyme inhibition and angiotensin receptor blockade. Hypertension. 2006;48:942–949. doi: 10.1161/01.HYP.0000241061.51003.b7. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Paraventricular nucleus, stress response, and cardiovascular disease. Clin Auton Res. 2005;15:254–263. doi: 10.1007/s10286-005-0290-7. [DOI] [PubMed] [Google Scholar]
- Berecek KH, Swords BH. Central role for vasopressin in cardiovascular regulation and the pathogenesis of hypertension. Hypertension. 1990;16:213–224. doi: 10.1161/01.hyp.16.3.213. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Advances in brain vasopressin. Vol. 119. Amsterdam: Elsevier Science B.V.; 1998. Osmoregulation of vasopressin neurons: a synergy of intrinsic and synaptic processes; pp. 59–76. [DOI] [PubMed] [Google Scholar]
- Camara AK, Osborn J. Central AT1 and AT2 receptors mediate chronic intracerebroventricular angiotensin II-induced drinking in rats fed high sodium chloride diet from weaning. Acta Physiol Scand. 2001;171:195–201. doi: 10.1046/j.1365-201x.2001.00790.x. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J Comp Neurol. 1988;272:579–604. doi: 10.1002/cne.902720410. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol. 2005;32:419–425. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- Dawson CA, Jhamandas JH, Krukoff TL. Activation by systemic angiotensin II of neurochemically identified neurons in rat hypothalamic paraventricular nucleus. J Neuroendocrinol. 1998;10:453–459. doi: 10.1046/j.1365-2826.1998.00225.x. [DOI] [PubMed] [Google Scholar]
- de Wardener HE. The hypothalamus and hypertension. Physiol Rev. 2001;81:1599–1658. doi: 10.1152/physrev.2001.81.4.1599. [DOI] [PubMed] [Google Scholar]
- Deleuze C, Duvoid A, Hussy N. Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. J Physiol. 1998;507 (Pt 2):463–471. doi: 10.1111/j.1469-7793.1998.463bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto M, Bieche I, Deshayes F, Vacher S, Nouet S, Collura V, Seitz I, Louis S, Pineau P, Amsellem-Ouazana D, Couraud PO, Strosberg AD, Stoppa-Lyonnet D, Lidereau R, Nahmias C. Structural organization and expression of human MTUS1, a candidate 8p22 tumor suppressor gene encoding a family of angiotensin II AT2 receptor-interacting proteins, ATIP. Gene. 2006;380:127–136. doi: 10.1016/j.gene.2006.05.021. [DOI] [PubMed] [Google Scholar]
- do-Prado MH, Camargo GM, Renzi A, Saad WA, Luiz AC, Queiroz RC, Camargo LA. Paraventricular nucleus administration of DuP753 or PD123319 inhibits the effects of angiotensin on water and sodium intake. Braz J Med Biol Res. 1996;29:1499–1502. [PubMed] [Google Scholar]
- Dostal DE, Booz GW, Baker KM. Regulation of angiotensinogen gene expression and protein in neonatal rat cardiac fibroblasts by glucocorticoid and beta-adrenergic stimulation. Basic Res Cardiol. 2000;95:485–490. doi: 10.1007/s003950070025. [DOI] [PubMed] [Google Scholar]
- Duan X, Ju G. The organization of chemically characterized afferents to the perivascular neuronal groups of the hypothalamic magnocellular neurosecretory system in the rat. Brain Res Bull. 1998;46:409–415. doi: 10.1016/s0361-9230(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Regulation of cerebral blood vessels by humoral and endothelium-dependent mechanisms. Update on humoral regulation of vascular tone. Hypertension. 1991;17:917–922. doi: 10.1161/01.hyp.17.6.917. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Day TA, Renaud LP. Subfornical organ efferents influence the excitability of neurohypophyseal and tuberoinfundibular paraventricular nucleus neurons in the rat. Neuroendocrinology. 1984a;39:423–428. doi: 10.1159/000124015. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Day TA, Renaud LP. Subfornical organ stimulation excites paraventricular neurons projecting to dorsal medulla. Am J Physiol. 1984b;247:R1088–1092. doi: 10.1152/ajpregu.1984.247.6.R1088. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Kasting NW. Electrical stimulation in subfornical organ increases plasma vasopressin concentrations in the conscious rat. Am J Physiol. 1986;251:R425–428. doi: 10.1152/ajpregu.1986.251.2.R425. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Kasting NW. Angiotensin acts at the subfornical organ to increase plasma oxytocin concentrations in the rat. Regul Pept. 1988;23:343–352. doi: 10.1016/0167-0115(88)90235-2. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Washburn DL. Angiotensin II: a peptidergic neurotransmitter in central autonomic pathways. Prog Neurobiol. 1998;54:169–192. doi: 10.1016/s0301-0082(97)00065-8. [DOI] [PubMed] [Google Scholar]
- Fogarty DJ, Matute C. Angiotensin receptor-like immunoreactivity in adult brain white matter astrocytes and oligodendrocytes. Glia. 2001;35:131–146. doi: 10.1002/glia.1078. [DOI] [PubMed] [Google Scholar]
- Gallinat S, Busche S, Raizada MK, Sumners C. The angiotensin II type 2 receptor: an enigma with multiple variations. Am J Physiol Endocrinol Metab. 2000;278:E357–374. doi: 10.1152/ajpendo.2000.278.3.E357. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Bennett T. Endogenous vasopressin and baroreflex mechanisms. Brain Res. 1986;396:317–334. doi: 10.1016/0165-0173(86)90003-2. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Huang J, Speth RC, Iadecola C, Pickel VM. Angiotensin II AT-1A receptor immunolabeling in rat medial nucleus tractus solitarius neurons: subcellular targeting and relationships with catecholamines. Neuroscience. 2005;130:713–723. doi: 10.1016/j.neuroscience.2004.08.057. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D. AMPA and NMDA glutamate receptor trafficking: multiple roads for reaching and leaving the synapse. Cell Tissue Res. 2006;326:423–438. doi: 10.1007/s00441-006-0254-9. [DOI] [PubMed] [Google Scholar]
- Gross V, Obst M, Luft FC. Insights into angiotensin II receptor function through AT2 receptor knockout mice. Acta Physiol Scand. 2004;181:487–494. doi: 10.1111/j.1365-201X.2004.01322.x. [DOI] [PubMed] [Google Scholar]
- Guan JL, Wang QP, Shioda S. Observation of the ultrastructure and synaptic relationships of angiotensin II-like immunoreactive neurons in the rat area postrema. Synapse. 2000;38:231–237. doi: 10.1002/1098-2396(20001201)38:3<231::AID-SYN1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Hallbeck M, Larhammar D, Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J Comp Neurol. 2001;433:222–238. doi: 10.1002/cne.1137. [DOI] [PubMed] [Google Scholar]
- Hein L, Barsh GS, Pratt RE, Dzau VJ, Kobilka BK. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature. 1995;377:744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- Hernandez Schulman I, Zhou MS, Raij L. Cross-talk between angiotensin II receptor types 1 and 2: potential role in vascular remodeling in humans. Hypertension. 2007;49:270–271. doi: 10.1161/01.HYP.0000253966.21795.d3. [DOI] [PubMed] [Google Scholar]
- Hogarty DC, Speakman EA, Puig V, Phillips MI. The role of angiotensin, AT1 and AT2 receptors in the pressor, drinking and vasopressin responses to central angiotensin. Brain Res. 1992;586:289–294. doi: 10.1016/0006-8993(92)91638-u. [DOI] [PubMed] [Google Scholar]
- Hohle S, Spitznagel H, Rascher W, Culman J, Unger T. Angiotensin AT1 receptor-mediated vasopressin release and drinking are potentiated by an AT2 receptor antagonist. Eur J Pharmacol. 1995;275:277–282. doi: 10.1016/0014-2999(95)00005-6. [DOI] [PubMed] [Google Scholar]
- Huang J, Hara Y, Anrather J, Speth RC, Iadecola C, Pickel VM. Angiotensin II subtype 1A (AT1A) receptors in the rat sensory vagal complex: subcellular localization and association with endogenous angiotensin. Neuroscience. 2003;122:21–36. doi: 10.1016/s0306-4522(03)00606-7. [DOI] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Pantaloni A, Desarmenien MG, Moos F. Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. J Physiol. 1997;502 (Pt 3):609–621. doi: 10.1111/j.1469-7793.1997.609bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo S, Yamada C, Takezawa T, Urano A. Expression of provasopressin gene during ontogeny in the hypothalamus of developing mice. Neuroscience. 1992;46:241–250. doi: 10.1016/0306-4522(92)90024-v. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BL, Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- Inagami T, Kambayashi Y, Ichiki T, Tsuzuki S, Eguchi S, Yamakawa T. Angiotensin receptors: molecular biology and signalling. Clin Exp Pharmacol Physiol. 1999;26:544–549. doi: 10.1046/j.1440-1681.1999.03086.x. [DOI] [PubMed] [Google Scholar]
- Jaiswal N, Tallant EA, Diz DI, Khosla MC, Ferrario CM. Subtype 2 angiotensin receptors mediate prostaglandin synthesis in human astrocytes. Hypertension. 1991;17:1115–1120. doi: 10.1161/01.hyp.17.6.1115. [DOI] [PubMed] [Google Scholar]
- Jensen LL, Harding JW, Wright JW. Role of paraventricular nucleus in control of blood pressure and drinking in rats. Am J Physiol. 1992;262:F1068–1075. doi: 10.1152/ajprenal.1992.262.6.F1068. [DOI] [PubMed] [Google Scholar]
- Johren O, Imboden H, Hauser W, Maye I, Sanvitto GL, Saavedra JM. Localization of angiotensin-converting enzyme, angiotensin II, angiotensin II receptor subtypes, and vasopressin in the mouse hypothalamus. Brain Res. 1997;757:218–227. doi: 10.1016/s0006-8993(97)00220-5. [DOI] [PubMed] [Google Scholar]
- Kadekaro M. Nitric oxide modulation of the hypothalamo-neurohypophyseal system. Braz J Med Biol Res. 2004;37:441–450. doi: 10.1590/s0100-879x2004000400001. [DOI] [PubMed] [Google Scholar]
- Kadekaro M, Summy-Long JY. Centrally produced nitric oxide and the regulaton of body fluid and blood pressure homeostases. Clin Exp Pharmacol Physiol. 2000;27:450–459. doi: 10.1046/j.1440-1681.2000.03264.x. [DOI] [PubMed] [Google Scholar]
- Keil LC, Summy-Long J, Severs WB. Release of vasopressin by angiotensin II. Endocrinology. 1975;96:1063–1065. doi: 10.1210/endo-96-4-1063. [DOI] [PubMed] [Google Scholar]
- Knepel W, Nutto D, Meyer DK. Effect of transection of subfornical organ efferent projections on vasopressin release induced by angiotensin or isoprenaline in the rat. Brain Res. 1982;248:180–184. doi: 10.1016/0006-8993(82)91161-1. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Kim KS, Yang EK, Lee JH, Lee EJ, Park JS, Kim HJ. Effect of brain angiotensin II AT1, AT2, and cholinergic receptor antagonism on drinking in water-deprived rats. Regul Pept. 1996;66:41–46. doi: 10.1016/0167-0115(96)00063-8. [DOI] [PubMed] [Google Scholar]
- Lenkei Z, Corvol P, Llorens-Cortes C. Comparative expression of vasopressin and angiotensin type-1 receptor mRNA in rat hypothalamic nuclei: a double in situ hybridization study. Brain Res Mol Brain Res. 1995;34:135–142. doi: 10.1016/0169-328x(95)00160-t. [DOI] [PubMed] [Google Scholar]
- Li Z, Ferguson AV. Angiotensin II responsiveness of rat paraventricular and subfornical organ neurons in vitro. Neuroscience. 1993a;55:197–207. doi: 10.1016/0306-4522(93)90466-s. [DOI] [PubMed] [Google Scholar]
- Li Z, Ferguson AV. Subfornical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol. 1993b;265:R302–309. doi: 10.1152/ajpregu.1993.265.2.R302. [DOI] [PubMed] [Google Scholar]
- Li Z, Iwai M, Wu L, Shiuchi T, Jinno T, Cui TX, Horiuchi M. Role of AT2 receptor in the brain in regulation of blood pressure and water intake. Am J Physiol Heart Circ Physiol. 2003;284:H116–121. doi: 10.1152/ajpheart.00515.2002. [DOI] [PubMed] [Google Scholar]
- Lind RW, Swanson LW, Ganten D. Angiotensin II immunoreactive pathways in the central nervous system of the rat: evidence for a projection from the subfornical organ to the paraventricular nucleus of the hypothalamus. Clin Exp Hypertens A. 1984;6:1915–1920. doi: 10.3109/10641968409046101. [DOI] [PubMed] [Google Scholar]
- Lippoldt A, Bunnemann B, Ueki A, Rosen L, Cintra A, Hasselrot U, Metzger R, Hilgenfeldt U, Brosnihan B, Ganten D, et al. On the plasticity of the cerebellar renin-angiotensin system: localization of components and effects of mechanical perturbation. Brain Res. 1994;668:144–159. doi: 10.1016/0006-8993(94)90520-7. [DOI] [PubMed] [Google Scholar]
- Milsted A, Barna BP, Ransohoff RM, Brosnihan KB, Ferrario CM. Astrocyte cultures derived from human brain tissue express angiotensinogen mRNA. Proc Natl Acad Sci U S A. 1990;87:5720–5723. doi: 10.1073/pnas.87.15.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miselis RR. The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural network subserving water balance. Brain Res. 1981;230:1–23. doi: 10.1016/0006-8993(81)90388-7. [DOI] [PubMed] [Google Scholar]
- Miselis RR, Shapiro RE, Hand PJ. Subfornical organ efferents to neural systems for control of body water. Science. 1979;205:1022–1025. doi: 10.1126/science.472723. [DOI] [PubMed] [Google Scholar]
- Nio Y, Matsubara H, Murasawa S, Kanasaki M, Inada M. Regulation of gene transcription of angiotensin II receptor subtypes in myocardial infarction. J Clin Invest. 1995;95:46–54. doi: 10.1172/JCI117675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouet S, Amzallag N, Li JM, Louis S, Seitz I, Cui TX, Alleaume AM, Di Benedetto M, Boden C, Masson M, Strosberg AD, Horiuchi M, Couraud PO, Nahmias C. Trans-inactivation of receptor tyrosine kinases by novel angiotensin II AT2 receptor-interacting protein, ATIP. J Biol Chem. 2004;279:28989–28997. doi: 10.1074/jbc.M403880200. [DOI] [PubMed] [Google Scholar]
- Ohkubo N, Matsubara H, Nozawa Y, Mori Y, Murasawa S, Kijima K, Maruyama K, Masaki H, Tsutumi Y, Shibazaki Y, Iwasaka T, Inada M. Angiotensin type 2 receptors are reexpressed by cardiac fibroblasts from failing myopathic hamster hearts and inhibit cell growth and fibrillar collagen metabolism. Circulation. 1997;96:3954–3962. doi: 10.1161/01.cir.96.11.3954. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ, editors. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Peters A, Palay SL, Webster H. The fine structure of the nervous system. New York: Oxford University Press; 1991. [Google Scholar]
- Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep. 2006;8:232–241. doi: 10.1007/s11906-006-0056-1. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J. Co-localization of angiotensin II and gamma-aminobutyric acid in axon terminals in the rat subfornical organ. Neurosci Lett. 1995;193:89–92. doi: 10.1016/0304-3940(95)11673-k. [DOI] [PubMed] [Google Scholar]
- Portillo F, Carrasco M, Vallo JJ. Separate populations of neurons within the paraventricular hypothalamic nucleus of the rat project to vagal and thoracic autonomic preganglionic levels and express c-Fos protein induced by lithium chloride. J Chem Neuroanat. 1998;14:95–102. doi: 10.1016/s0891-0618(97)10022-9. [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Identification of an efferent projection from the paraventricular nucleus of the hypothalamus terminating close to spinally projecting rostral ventrolateral medullary neurons. Neuroscience. 1999;88:949–957. doi: 10.1016/s0306-4522(98)00255-3. [DOI] [PubMed] [Google Scholar]
- Raggenbass M. Vasopressin- and oxytocin-induced activity in the central nervous system: electrophysiological studies using in-vitro systems. Prog Neurobiol. 2001;64:307–326. doi: 10.1016/s0301-0082(00)00064-2. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Flanagan-Cato LM, Yee DK, Ma LY, Sakai RR, Fluharty SJ. Immunohistochemical mapping of angiotensin type 2 (AT2) receptors in rat brain. Brain Res. 1994;662:45–59. doi: 10.1016/0006-8993(94)90794-3. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Theveniau M, Yang XD, Siemens IR, Yee DK, Reisine T, Fluharty SJ. Development of polyclonal antibodies against angiotensin type 2 receptors. Proc Natl Acad Sci U S A. 1993;90:7956–7960. doi: 10.1073/pnas.90.17.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva C, Eggli P, Felix D, Mosimann R, Imboden H. Hypothalamic accessory nuclei and their relation to the angiotensinergic and vasopressinergic systems. Regul Pept. 1999;83:129–133. doi: 10.1016/s0167-0115(99)00062-2. [DOI] [PubMed] [Google Scholar]
- Rollenhagen A, Lubke JH. The morphology of excitatory central synapses: from structure to function. Cell Tissue Res. 2006;326:221–237. doi: 10.1007/s00441-006-0288-z. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Fregly MJ. Brain angiotensin AT-2 receptor antagonism and water intake. Brain Res Bull. 1993;32:391–394. doi: 10.1016/0361-9230(93)90205-p. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. A method for tracing biochemically defined pathways in the central nervous system using combined fluorescence retrograde transport and immunohistochemical techniques. Brain Res. 1981;210:31–51. doi: 10.1016/0006-8993(81)90882-9. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization and biochemical specificity of afferent projections to the paraventricular and supraoptic nuclei. Prog Brain Res. 1983;60:19–29. doi: 10.1016/S0079-6123(08)64371-X. [DOI] [PubMed] [Google Scholar]
- Schinke M, Baltatu O, Bohm M, Peters J, Rascher W, Bricca G, Lippoldt A, Ganten D, Bader M. Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen. Proc Natl Acad Sci U S A. 1999;96:3975–3980. doi: 10.1073/pnas.96.7.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonemann MD, Ryan AK, McEvilly RJ, O’Connell SM, Arias CA, Kalla KA, Li P, Sawchenko PE, Rosenfeld MG. Development and survival of the endocrine hypothalamus and posterior pituitary gland requires the neuronal POU domain factor Brn-2. Genes Dev. 1995;9:3122–3135. doi: 10.1101/gad.9.24.3122. [DOI] [PubMed] [Google Scholar]
- Schumacker PT. Angiotensin II signaling in the brain: compartmentalization of redox signaling? Circ Res. 2002;91:982–984. doi: 10.1161/01.res.0000045655.34731.b6. [DOI] [PubMed] [Google Scholar]
- Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res. 1998;801:239–243. doi: 10.1016/s0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- Shoji M, Share L, Crofton JT. Effect on vasopressin release of microinjection of angiotensin II into the paraventricular nucleus of conscious rats. Neuroendocrinology. 1989;50:327–333. doi: 10.1159/000125241. [DOI] [PubMed] [Google Scholar]
- Smith PM, Bains JS, Ferguson AV. Long duration pressor responses following activation of subfornical organ neurons in rats are the result of increased circulating vasopressin. Neurosci Lett. 1997;233:81–84. doi: 10.1016/s0304-3940(97)00626-5. [DOI] [PubMed] [Google Scholar]
- Sposito NM, Gross PM. Morphometry of individual capillary beds in the hypothalamo-neurohypophysial system of rats. Brain Res. 1987;403:375–379. doi: 10.1016/0006-8993(87)90079-5. [DOI] [PubMed] [Google Scholar]
- Steckelings UM, Kaschina E, Unger T. The AT2 receptor--a matter of love and hate. Peptides. 2005;26:1401–1409. doi: 10.1016/j.peptides.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Hawelu-Johnson CL, Guyenet PG, Lynch KR. Astrocytes synthesize angiotensinogen in brain. Science. 1988;242:1444–1446. doi: 10.1126/science.3201232. [DOI] [PubMed] [Google Scholar]
- Sumners C, Tang W, Zelezna B, Raizada MK. Angiotensin II receptor subtypes are coupled with distinct signal-transduction mechanisms in neurons and astrocytes from rat brain. Proc Natl Acad Sci USA. 1991;88:7567–7571. doi: 10.1073/pnas.88.17.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tallant EA, Higson JT. Angiotensin II activates distinct signal transduction pathways in astrocytes isolated from neonatal rat brain. Glia. 1997;19:333–342. [PubMed] [Google Scholar]
- Tanaka J, Kaba H, Saito H, Seto K. Efferent pathways from the region of the subfornical organ to hypothalamic paraventricular nucleus: an electrophysiological study in the rat. Exp Brain Res. 1986;62:509–514. doi: 10.1007/BF00236029. [DOI] [PubMed] [Google Scholar]
- Toba K, Ohta M, Kimura T, Nagano K, Ito S, Ouchi Y. Role of brain vasopressin in regulation of blood pressure. Prog Brain Res. 1998;119:337–349. doi: 10.1016/s0079-6123(08)61579-4. [DOI] [PubMed] [Google Scholar]
- Toney GM, Porter JP. Functional roles of brain AT1 and AT2 receptors in the central angiotensin II pressor response in conscious young spontaneously hypertensive rats. Brain Res Dev Brain Res. 1993;71:193–199. doi: 10.1016/0165-3806(93)90171-6. [DOI] [PubMed] [Google Scholar]
- Triller A, Choquet D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move! Trends Neurosci. 2005;28:133–139. doi: 10.1016/j.tins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Veltmar A, Culman J, Qadri F, Rascher W, Unger T. Involvement of adrenergic and angiotensinergic receptors in the paraventricular nucleus in the angiotensin II-induced vasopressin release. J Pharmacol Exp Ther. 1992;263:1253–1260. [PubMed] [Google Scholar]
- Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, Iadecola C. Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension. 2006;48:482–489. doi: 10.1161/01.HYP.0000236647.55200.07. [DOI] [PubMed] [Google Scholar]
- Wang G, Anrather J, Huang J, Speth RC, Pickel VM, Iadecola C. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius. J Neurosci. 2004;24:5516–5524. doi: 10.1523/JNEUROSCI.1176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural immunocytochemical localization of mu opioid receptors and Leu5-enkephalin in the patch compartment of the rat caudate-putamen nucleus. J Comp Neurol. 1996;375:659–674. doi: 10.1002/(SICI)1096-9861(19961125)375:4<659::AID-CNE7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. J Comp Neurol. 2002;442:392–404. doi: 10.1002/cne.10086. [DOI] [PubMed] [Google Scholar]
- Wright JW, Roberts KA, Stubley LA, Hanesworth JM, Harding JW. Hypothalamic angiotensin release in response to AII or glutamic acid stimulation of the SFO in rats. Brain Res Bull. 1993;31:649–654. doi: 10.1016/0361-9230(93)90136-y. [DOI] [PubMed] [Google Scholar]
- Wruck CJ, Funke-Kaiser H, Pufe T, Kusserow H, Menk M, Schefe JH, Kruse ML, Stoll M, Unger T. Regulation of transport of the angiotensin AT2 receptor by a novel membrane-associated Golgi protein. Arterioscler Thromb Vasc Biol. 2005;25:57–64. doi: 10.1161/01.ATV.0000150662.51436.14. [DOI] [PubMed] [Google Scholar]
- Wyse B, Sernia C. Growth hormone regulates AT-1a angiotensin receptors in astrocytes. Endocrinology. 1997;138:4176–4180. doi: 10.1210/endo.138.10.5430. [DOI] [PubMed] [Google Scholar]
- Yayama K, Hiyoshi H, Imazu D, Okamoto H. Angiotensin II stimulates endothelial NO synthase phosphorylation in thoracic aorta of mice with abdominal aortic banding via type 2 receptor. Hypertension. 2006;48:958–964. doi: 10.1161/01.HYP.0000244108.30909.27. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension. 2005;45:717–723. doi: 10.1161/01.HYP.0000153463.22621.5e. [DOI] [PubMed] [Google Scholar]