Abstract
Stroke is common in children with sickle cell anemia but is rarely attributed to the traditional causes of stroke identified in other children. We report an 11 year-old girl with sickle cell anemia who presented with severe headache and was found to have recurrent bilateral multifocal strokes in a cardioembolic pattern. Evaluation revealed the presence of a patent foramen ovale, antiphospholipid antibodies, and elevations in factor VIII and lipoprotein a. Sickle cell anemia is itself a hypercoaguable state with potential for increased right heart pressures, both of which predispose to paradoxical embolization via right-to-left intracardiac shunting of emboli causing stroke. This case suggests that the more traditional etiologies for pediatric stroke may also cause stroke in children with sickle cell anemia.
Introduction
Clinically overt stroke occurs in 8–10% of children with sickle cell anemia and evidence of silent stroke is detectible by MRI in up to 35%. [1] Traditionally, the diagnostic evaluation of stroke in affected patients ends with the identification of the abnormal hemoglobin. There are few identified risk factors, with previous stroke, severe anemia, acute chest syndrome, cerebral vasculopathy, and increased transcranial Doppler ultrasound velocity predisposing to recurrent stroke.[2] In children and young adults without sickle cell anemia, patent foramen ovale is an established risk factor for stroke via paradoxical embolization, whereby emboli from the venous circulation escape filtration by the lungs and pass from the right atrium directly to the left heart and on to the brain. [3,4] Coincident thrombophila and patent foramen ovale further increases the risk of stroke. [5] We report a girl with sickle cell anemia, antiphospholipid antibodies, elevations of factor VIII and lipoprotein a, and patent foramen ovale who presented with multiple recurrent strokes in a cardioembolic pattern suggesting a significant role for these alternative etiologies in her stroke.
Case Report
An 11 year old girl with homozygous sickle cell anemia (hemoglobin SS) presented with parvovirus-associated aplastic crisis. She had a recent history of cough, fever, diarrhea, as well as pain in her abdomen and lower back and mild headache. On admission, her hemoglobin concentration was 2.7 g/dl (baseline 8.3 g/dl), reticulocyte count was 0.1%, and polymerase chain reaction was positive for parvovirus B19. She was slowly transfused in multiple small aliquots to a hemoglobin concentration of 12.0 g/dl. Three days later, she developed a severe bilateral frontal headache with pounding quality with associated photophobia and phonophobia. There were no focal findings on her neurologic examination.
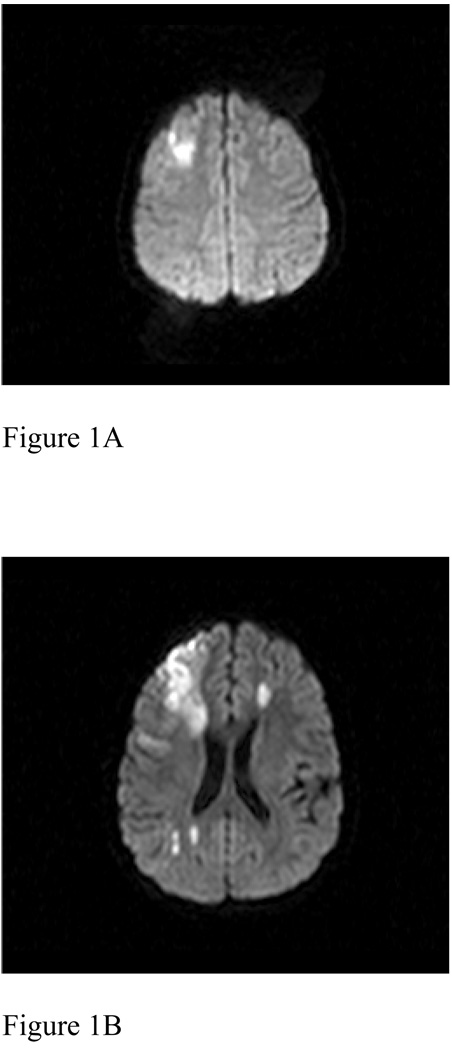
MRI identified a single area of restricted diffusion in the right superior frontal lobe with no evidence of hemorrhage (Figure 1A). She was treated for headache and was started on aspirin. Her headache resolved but recurred two days later. A repeat MRI at that time (Figure 1B) revealed enlargement of the area of restricted diffusion in the right frontal lobe with multiple additional areas of restricted diffusion in the deep left frontal white matter, as well as in the right periatrial and parietal occipital subcortical white matter and the right putamen. MRA revealed narrowing and irregularity of the supraclinoid internal carotid arteries and M1 segment of the right middle cerebral artery. There were no prior MRA or transcranial Doppler ultrasound studies. Patent foramen ovale was identified by noncontrasted transthoracic echocardiography. Antiphospholipid antibodies were detected, with marked elevation of anticardiolipin IgM and IgG as well as antiphosphatidylserine IgM and IgA antibodies. Further evaluation for hypercoagulability showed mildly elevated factor VIII activity (185%) and elevated lipoprotein a (83 mg/dl).
Figure 1.
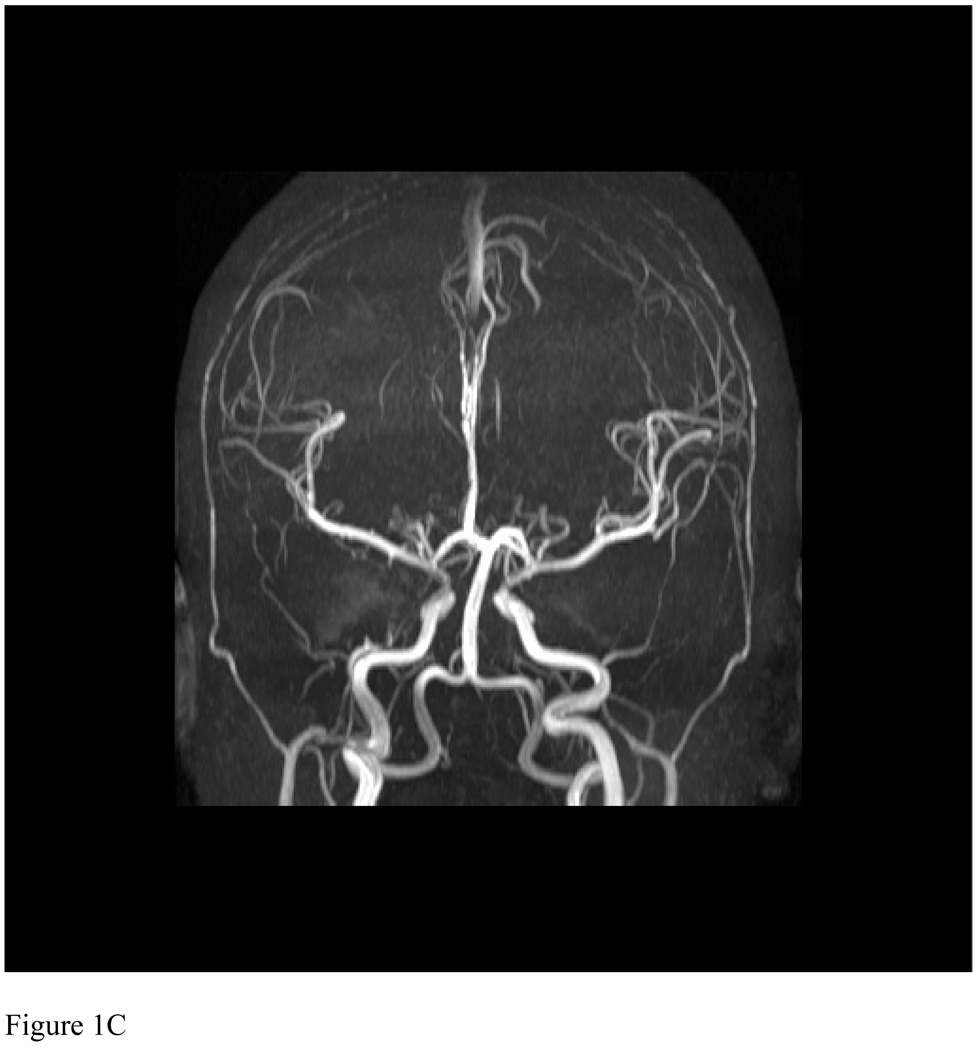
(A) Axial diffusion-weighted image (TR 4580.01/TE 90) revealed a single area of restricted diffusion in the right superior frontal lobe. (B) Axial diffusion-weighted image (TR 5129.89/TE 90) two days later revealed enlargement of the initial area with new areas of restricted diffusion in the periventricular, deep, and subcortical white matter of the left and right hemispheres. (C) 3-D time-of-flight MR Angiogram (TR 22.6872/TE 6.32305) from 2 months after presentation revealed no significant arteriopathy.
She had a recurrence of severe headache two weeks later with repeat MRI demonstrating new areas of infarction in the bilateral caudate and right putamen (not shown). She was anticoagulated with heparin and transitioned to therapy with warfarin, given her ongoing recurrent stroke, patent foramen ovale, and hypercoaguable state. Aspirin therapy was continued and chronic transfusion therapy, the starndard prevention for recurrent stroke in sickle cell disease was initiated. Follow up MRI two months later (not shown) revealed areas of increased T2 signal in fluid attenuated inversion recovery images in areas corresponding to the previous areas of restricted diffusion but no evidence of further stroke. Follow-up MRA showed widely patient arteries (Figure 1C), demonstrating that she did not have a significant sickle vasculopathy and that the vascular abnormalities observed at the time of acute stroke were transient. Antiphospholipid antibody titers returned to normal levels by 12 weeks and warfarin was discontinued 18 months later. She continues on verapamil for migraine prophylaxis as well as aspirin and chronic transfusions. She has had no further evidence of recurrent stroke for 4 years.
Discussion
Our patient had multiple risk factors for stroke: Sickle cell anemia itself, possible sickle cerebral vasculopathy, a severe anemic event, transfusion, and multiple thrombophilic abnormalities including transient antiphospholipid antibodies, elevated factor VIII, and elevated lipoprotein a. Antiphospholipid antibodies have been reported in sickle cell anemia [6] and following parvovirus infection. [7] Elevated factor VIII has been demonstrated to increase risk of venous and arterial thrombotic disease in African-Americans and elevated lipoprotein a has been identified as a risk factor for stroke in children. Her hypercoaguable state and an underlying sickle vasculopathy could have led to multiple embolic events; however, the normal appearing cerebral vasculature on MRA two months after presentation argues against the presence of a significant irreversible sickle vasculopathy. Her presentation with recurrent, multifocal, bilateral infarcts was more consistent with a cardiac source. We suspect patent foramen ovale may have played a causal role by the mechanism of paradoxical embolization of thrombi resulting from her hypercoagulable state. A significant association between patent foramen ovale and stroke was demonstrated in a meta-analysis of case-control studies of young adult patients ≤ 55 years of age, with patent foramen ovale noted in 17.8% (95% CI: 14.3%–21.3%) of the combined control group and in 40.2% (95% CI: 36.2%–44.2%) of patients with stroke. [3] The role of paradoxical embolization in stroke and treatment options for patent foramen ovale is the subject of much recent controversy. [8]
There are several physiologic features of sickle cell anemia that may serve to predispose to stroke by paradoxical embolization. First, the disorder is, in itself, a hypercoagulable state. Old and new thrombi are observed in the pulmonary vasculature in postmortem studies of patients with sickle cell anemia, illustrating the effective pulmonary filter. Patients have high levels of thrombin, abnormal activation of fibrinolysis, decreased levels of anticoagulant proteins, and platelet activation. [9] Second, right-to-left shunting is favored by the pathophysiologic changes secondary to anemia itself and to pulmonary hypertension, which may affect up to 46% of children with sickle cell anemia. [10] Right heart pressures can also increase in the setting of acute chest syndrome, a condition associated with stroke. [11] During vaso-occlusive crisis, immobility and dehydration favor formation of deep venous thrombi, a potential source of paradoxical emboli, and pulmonary artery pressures increase, favoring right-to-left shunting. [12]
Studies of fat embolization syndrome in sickle cell anemia are also supportive of a role of paradoxical embolization in stroke. Multiple scattered punctate MRI abnormalities, consistent with embolic phenomenon were reported with fat embolization syndrome following vaso-occlusive crisis. [13] Neurologic symptoms, including focal signs and focal lesions on MRI, were noted in half of the patients with fat emboli detected by bronchoscopy in patients with vaso-occlusive crisis and acute chest syndrome, while none of the patients without pulmonary fat emboli had neurologic symptoms. [14] The heart was not examined in these studies, but the only route for fat emboli to the brain is via right-to-left shunting in the heart or lungs.
Our case illustrates that children with sickle cell anemia and stroke may have other traditional stroke risk factors in addition to their hemoglobinopathy. The consensus recommendation of the Paediatric Stroke Working Group [15] is to include evaluation for other hypercoagulable conditions as well as transthoracic echocardiogram in the diagnostic evaluation of children with sickle cell anemia and stroke. In these children, recognition of these alternative risk factors could alter the management and potentially reduce recurrence or aid in primary prevention of stroke.
Acknowledgements
The authors were supported by the NHLBI Comprehensive Sickle Cell Center Program U54 HL 70588, the North and Central Texas Clinical and Translational Science Initiative from the NIH KL2 RR024983, and MMD by the First American Real Estate Information Services, Inc. The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steen RG, Emudianughe T, Hankins GM, Wynn LW, Wang WC, Xiong X, Helton KJ. Brain imaging findings in pediatric patients with sickle cell disease. Radiology. 2003;228:216–225. doi: 10.1148/radiol.2281020943. [DOI] [PubMed] [Google Scholar]
- 2.Quinn CT, Miller ST. Risk Factors and prediction of outcomes in children and adolescents who have sickle cell anemia. Hematol Oncol Clin N Am. 2004;18:1339–1354. doi: 10.1016/j.hoc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology. 2000;55:1172–1179. doi: 10.1212/wnl.55.8.1172. [DOI] [PubMed] [Google Scholar]
- 4.Benedik MP, Zaletel M, Megli NP, Podnar T. Patent foramen ovale and unexplained ischemic cerebrovascular events in children. Catheter Cardiovasc Interv. 2007;70:999–1007. doi: 10.1002/ccd.21305. [DOI] [PubMed] [Google Scholar]
- 5.Hassell KL. Hematologic Ramifications of patent foramen ovale--role of hypercoagulable state. Cardiol Clin. 2005;23:65–71. doi: 10.1016/j.ccl.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Westerman MP, Green D, Gilman-Sachs A, Beaman K, Freels S, Boggio L, Allen S, Zuckerman L, Schlegel R, Williamson P. Antiphospholipid antibodies, proteins C and S, and coagulation changes in sickle cell disease. J Lab Clin Med. 1999;134:352–362. doi: 10.1016/s0022-2143(99)90149-x. [DOI] [PubMed] [Google Scholar]
- 7.Uthman IW, Gharavi AE. Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31:256–263. doi: 10.1053/sarh.2002.28303. [DOI] [PubMed] [Google Scholar]
- 8.Thaler DE, Saver JL. Cryptogenic stroke and patent foramen ovale. Current Opinion in Cardiology. 2008;23:537–544. doi: 10.1097/HCO.0b013e32831311bd. [DOI] [PubMed] [Google Scholar]
- 9.Ataga KI, Orriner ER. Hypercoagulability in sickle cell disease: a curious paradox. Am. J. Med. 2003;115:721–728. doi: 10.1016/j.amjmed.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Caboot JB, Allen JL. Pulmonary complications of sickle cell disease in children. Current Opinion in Pediatrics. 2008;20:279–287. doi: 10.1097/MOP.0b013e3282ff62c4. [DOI] [PubMed] [Google Scholar]
- 11.Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW. and the Cooperative Study of Sickle Cell Disease. Cerebrovascular accidents in sickle cell disease: Rates and Risk Factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 12.Machado RF, Mack AK, Martyr S, Barnett C, MacArthur P, Sachdev V, Ernst I, Hunter LA, Coles WA, Nichols JP, Kato GJ, Gladwin MT. Severity of pulmonary hypertension during vaso-occlusive pain crisis and exercise in patients with sickle cell disease. Br J Haematol. 2007;136:319–325. doi: 10.1111/j.1365-2141.2006.06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton DP, Ferriero DM, Mentzer WC. Nontraumatic fat embolism syndrome in sickle cell anemia. Pediatr Neurol. 1995;12:77–80. doi: 10.1016/0887-8994(94)00108-e. [DOI] [PubMed] [Google Scholar]
- 14.Vichinsky E, Williams R, Das M, Earles AN, Lewis N, Adler A, McQuitty J. Pulmonary fat embolism: a distinct cause of severe acute chest syndrome in sickle cell anemia. Blood. 1994;83:3107–3112. [PubMed] [Google Scholar]
- 15.Paediatric Stroke Working Group. Stroke in childhood: clinical guidelines for diagnosis, management and rehabilitation. 2004 [cited 2008 May 20] Available from www.rcplondon.ac.uk/pubs/books/childstroke/