Abstract
After birth, exposure to visual inputs modulates cortical development, inducing numerous changes in all of the components of the visual cortex. Most of the cortical changes thus induced occur during what is called the critical period. Astrocytes play an important role in the development, maintenance and plasticity of the cortex as well as in the structure and function of the vascular network. Visual deprivation induces a decrease in the astroglial population, whereas enhanced experience increases it. Exposure to an enriched environment has been shown to prevent the effects of dark-rearing in the visual cortex. Our purpose was to study the effects of an enriched environment on the density of astrocytes per reference surface at the visual cortex of dark-reared rats, in order to determine if enhanced experience is able to compensate the quantitative effects of visual deprivation and the role of physical exercise on the enrichment paradigm. Pregnant Sprague-Dawley rats were raised in one of the following rearing conditions: control rats with standard housing (12-h light/dark cycle); in total darkness for the dark-rearing experiments; and dark-rearing in conditions of enriched environment without and with physical exercise. The astrocytic density was estimated by immunohistochemistry for S-100β protein. Quantifications were performed in layer IV. The somatosensorial cortex barrel field was also studied as control. The volume of layer IV was stereologically calculated for each region, age and experimental condition. From the beginning of the critical period, astrocyte density was higher in control rats than in the enriched environment group without physical exercise, with densities of astrocytes around 20% higher at all of the different ages. In contrast, when the animals had access to voluntary exercise, densities were significantly higher than even the control rats. Our main result shows that strategies to apply environmental enrichment should always consider the incorporation of physical exercise, even for sensorial areas such as the visual area, where complex enriched experience by itself is not enough to compensate the effects of visual deprivation.
Keywords: astroglia, dark-rearing, environmental enrichment, S-100β, visual cortex, wheel running
Introduction
The postnatal development of the visual cortex is modulated by experience, which shapes functional and cortical architecture. Experience-mediated changes are at a maximum during a predetermined time window called the critical period (Berardi et al. 2000; Hensch, 2005), which in the rat visual cortex is between the third and fifth postnatal week with a peak at the fourth week (Fagiolini et al. 1994). Some authors have studied the effects of the increase and/or deprivation of visual experience on the neuronal (Bennett et al. 1964; Cancedda et al. 2004), genetic (Rampon et al. 2000), vascular (Black et al. 1987; Sirevaag et al. 1988; Argandoña & Lafuente, 1996, 2000; Argandoña et al. 2005; Bengoetxea et al. 2008) and astroglial (Corvetti et al. 2003, 2006) components of the cortex (see reviews by Markham & Greenough, 2004; Spires & Hannan, 2005).
Environmental enrichment was described as the combination of complex inanimate and social stimulation (Rosenzweig et al. 1978), has been shown to be connected with anatomical changes (review by Rosenzweig & Bennett, 1996) and neurogenesis (review by van Praag et al. 2000), and is widely proposed as a neuroprotective solution in neurodegenerative diseases (see reviews by Will et al. 2004; Nithianantharajah & Hannan, 2006; Laviola et al. 2008). Studies include diseases such as Alzheimer's (Jankowsky et al. 2005), Parkinson's (Bezard et al. 2003), Huntington's (Nithianantharajah et al. 2008), traumatic brain injury (Hamm et al. 1996; Hoffman et al. 2008; Ortuzar et al. 2009) and stroke (Briones et al. 2006). In experimental models, some authors have described how exposure to enriched environments prevents the effects of dark-rearing on the rat visual cortex (Bartoletti et al. 2004).
Astrocytes play a major role in the development of the cortex, as they are involved in the establishment of the neuronal networks and the maturation of the blood–brain barrier. Previous reports have shown that whereas visual deprivation induces a decrease in the astroglial population (Müller, 1990; Argandoña et al. 2003), enriched experience increases the density of astrocytes in the visual cortex (Sirevaag & Greenough, 1987; Sirevaag & Greenough, 1991).
Our purpose was to study the effects of an enriched environment and the role of physical exercise in the astroglial population in the visual cortex of dark-reared rats, in order to determine if the recovery of electrical properties and the closure of the critical period described by other authors can be correlated to quantitative effects on the astrocytic population.
Materials and methods
Animals
Four pregnant Sprague-Dawley rats were raised in one of the following rearing conditions: (a) control rats with standard housing (12-h light/dark cycle); (b) in total darkness for the dark-rearing experiments (DR); (c) dark-rearing in conditions of enriched environment consisting of a large cage (720 × 550 × 300 mm) furnished with toys and differently shaped objects (shelters, tunnels) that were changed every 2 days (with 12-h light/dark cycle) (DR-EE); and (d) dark-rearing in conditions of enriched environment with physical exercise, with unlimited access to a running wheel (DR-EE-Ex).
For dark-reared groups, pregnant rats were placed in a dark room at the beginning of pregnancy. Litters were born in complete darkness and weaned at postnatal day (P)21. Daily care was performed under dim red light.
Animals were anaesthetized with 6% chloral hydrate (performed under dim red light for the dark-reared groups). After anaesthesia, the animals were transcardially perfused with a fixative containing 4% paraformaldehyde in 0.1 m phosphate buffer. Perfusion was carried out with a pump at a constant pressure of 20 mmHg. Following perfusion, brains were stored overnight at 4 °C in fresh fixative. The following day, a thick block of occipital cortex containing the visual area and a thick block of medial cortex containing the primary somatosensory area were removed coronally with a rodent brain matrix (Electron Microscopic Sciences, USA), rinsed in cold phosphate-buffered saline for 4 h and embedded in paraffin. The block was serially cut with a microtome into sections of 5 µm and mounted on slides coated with 3-aminopropyltriethoxylane.
Immunohistochemistry
Sections were incubated with an anti-S-100β polyclonal antibody (Ref. S2644; working dilution 1 : 200; Sigma-Aldrich, USA) and biotinylated secondary antibodies were used (Vector, USA). The immunohistochemical reaction was revealed by the avidin–biotin complex using diaminobenzidyne as chromogen. Sections were finally dehydrated and covered. As control for S-100β, we used brains of rats and humans that had been subjected to trauma. Negative controls in which the primary antiserum was omitted were also included in each staining run. Parallel sections were processed for haematoxylin/eosin for the identification and localization of the primary visual cortex (V1) and the primary somatosensory cortex barrel field (S1BF).
Quantitative analysis
To quantify the changes during development and to compare groups from both situations (normal and dark-rearing), a blind morphometric study was performed where the person who measured the sections did not know the particular characteristics of each case (neither the age of the rats nor whether they belonged to dark-reared or control groups). To estimate the number of astrocytes per area, we counted the number of positive cell bodies present in an area delimited by a grid fixed in the eyepiece, excluding those intersected by both the X and Y axes. The grid was a square with sides measuring 250 µm and the total surface area was therefore 62 500 µm2. The grid was randomly placed on the V1 between cortical layers III and V, ensuring that layer IV was included. For each condition, measurements were also taken from a non-visual area, e.g. the S1BF on layer IV (see Fig. 1). The areas were selected with the aid of the atlas of Paxinos & Watson (1998). A total of 224 animals, of both sexes, aged between P21 and P63 were used (n = 8 per age and condition). Astrocytes were recognized by their morphological characteristics, i.e. S-100β-positive cell bodies with short positive cytoplasmic processes, and by their nuclear morphology. We did not count positive cells that did not fit the morphology of astrocytes.
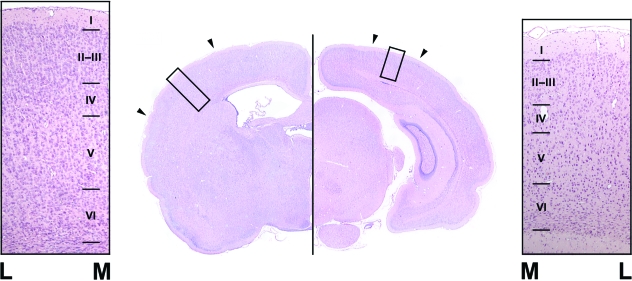
Fig. 1.
Photographs of haematoxylin/eosin-stained coronal sections containing the primary somatosensory cortex (left image) and the primary visual cortex (right image). Surrounding pictures show both cortices where interfaces between layers are indicated by lines (these lines serve as scale bars = 100 µm). M, medial side of the cortex; L, lateral side of the cortex.
In order to rule out the possibility that changes in astroglial density could be due to changes in volume, the whole volume of layer IV was calculated for each region (V1 and S1BF), age and condition using the Cavalieri method (Howard & Reed, 2005). Parallel haematoxylin/eosin-stained sections were examined with a computer coupled to an Olympus BX41 microscope equipped with a motorized stage, running the Mercator software (Explora Nova, La Rochelle, France). The anterior and posterior limits of both V1 and S1BF and the boundaries of layer IV were determined using the atlas of Paxinos & Watson (1998) aided by the cytoarchitectonic characteristics of V1 and S1BF (Fig. 1). Layer IV was demarcated on serially cut sections and the total volume was calculated by the Stereology Module incorporated into the Mercator image analysis software.
Measurements of each slice of the cortex were carried out on both the right and left hemispheres (eight fields were assessed on each hemisphere in each of the 10 slices taken per animal, i.e. 160 fields per animal) and the mean value per animal was calculated.
All statistical analyses were performed using SPSS statistical software (version 13.0 for Mac, SPSS, Inc., Chicago, IL, USA). Prior to analysis, data were examined for normal distribution using the Kolmogorov-Smirnov test and for homogeneity of variances using Levene's test. The effects of the age period, experimental condition and their interaction were determined by two-way anovawith posthoc analysis (posthoc tests used the Bonferroni correction for equal variances or Tamhane's T2 correction for unequal variances). In order to explore the effects of experimental conditions in greater depth, differences between them within age periods were evaluated using the one-way anovaanalysis with the aforementioned posthoc studies. Data are described as mean ± SEM. Significance was declared at P < 0.05.
All animal experiments were carried out in accordance with the European Community Council Directive of 24 November 1986 (86/609/EEC).
Results
The main result is the lack of significant differences in astroglial density in the visual cortex between the dark-reared groups without a running wheel, both of which were significantly lower than control rats. In contrast, when the environmental enrichment paradigm included voluntary exercise, values were significantly higher than even those for control rats.
Dark-rearing impacted the volume of visual cortex layer IV, which was significantly larger in controls than in all dark-reared groups. There were no differences between the dark-reared groups. In the somatosensory cortex, the volume of layer IV increased in dark-reared groups.
Immunoreactivity for S-100β was similar at all of the different ages and in all of the different areas that we studied, when strongly stained cell bodies and star-shaped processes were found in all cortical layers (Fig. 2). No morphological differences were found at any of the different ages between any of the different groups.
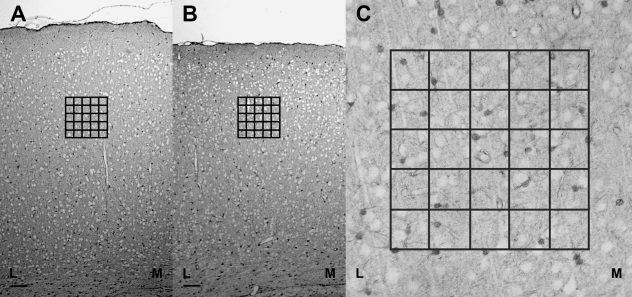
Fig. 2.
(A)S-100β positivity throughout the primary somatosensory cortex barrel field at postnatal day (P)28 in control rats and the placement of the counting grid in layer IV. Scale bar = 100 µm. (B) S-100β positivity throughout the primary visual cortex at P28 in control rats and the placement of the counting grid in layer IV. Scale bar = 100 µm. (C) Astroglial density was measured by counting the number of positive cells per area delimited by an overlying grid, excluding those intersected by both the X and Y axes. Grid side, 250 µm; grid area, 62 00 µm2; M, medial side of the cortex; L, lateral side of the cortex.
Astroglial density at each age
Primary visual cortex (Fig. 3)
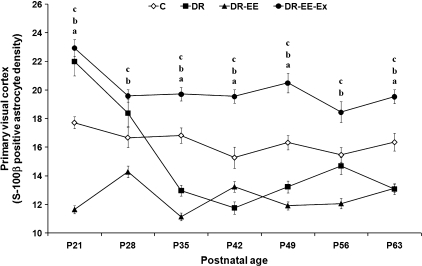
Fig. 3.
Comparison of average measurements between DR, DR-EE, DR-EE-Ex and control (C) groups at each of the ages considered. Horizontal axis shows the age of the animals. Vertical axis shows S-100β-positive astrocyte density per 62 500 µm2 of primary visual cortex (mean ± SEM). (a) C vs. DR significance; (b) C vs. DR-EE significance; (c) C vs. DR-EE-Ex significance (P ≤ 0.05) (one-way anovatest with posthoc correction). P, postnatal day.
At P21, the density of S-100β cells per reference surface was 34% lower in DR-EE groups compared with controls (11.6 vs. 17.7 astrocytes per 62 500 µm2), whereas it was respectively 24% and 29% higher in DR (22) and DR-EE-Ex (22.95). At P28, the astroglial density was 14% lower in DR-EE (14.3 vs. 16.7 astrocytes per 62 500 µm2) astrocytes per 62 500 µm2 and 17% and 10% higher in DR-EE-Ex (19.6) and DR (18.4). At P35, it was 23% lower in DR (13 vs. 16.8 astrocytes per 62 500 µm2), 33% lower in DR-EE (11.1) and 17% higher in DR-EE-Ex (19.7). At P42, it was 23% lower in DR (11.7 vs. 15.3 astrocytes per 62 500 µm2), 13% lower in DR-EE (13.2) and 27% higher in DR-EE-Ex (19.5). At P49, it was 19% lower in DR (13.2 vs. 16.3 astrocytes per 62 500 µm2), 27% lower in DR-EE (11.9) and 25% higher in DR-EE-Ex (20.5). At P56, it was 5% lower in DR (14.7 vs. 15.5 astrocytes per 62 500 µm2), 22% lower in DR-EE (12.1) and 19% higher in DR-EE-Ex (18.5). Finally, at P63, the astroglial density was 20% lower in DR (13.1 vs. 16.3 astrocytes per 62 500 µm2), 19% lower in DR-EE (13.1) and 19% higher in DR-EE-Ex (19.5).
Two-way anovarevealed a significant interaction between age and experimental condition (F = 20.1, df = 18, P < 0.000), as well as age (F = 23.9, df = 6, P < 0.000) and condition effects (F = 324.3, df = 3, P < 0.000). We therefore decided to perform a one-way anovaanalysis with corresponding posthoc tests to study the differences between experimental conditions at each age period. The statistical analysis showed that differences between DR-EE-Ex and controls were significant at all ages (P ≤ 0.05), as were differences between DR-EE and controls. Differences between DR and controls were significant at all ages except at P28 and P56. In addition, whereas differences between DR-EE and DR were significant at P21, P28, P35 and P56, they were not significant at P42, P49 and P63. The differences between DR-EE and DR-EE-Ex were significant at all ages up to P35 (Supporting Information).
Primary somatosensory cortex barrel field (Fig. 4)
Fig. 4.
Comparison of average measurements between DR, DR-EE, DR-EE-Ex and control (C) groups at each of the ages considered. Horizontal axis shows the age of the animals. Vertical axis shows S-100β-positive astrocyte density per 62 500 µm2 of primary somatosensory cortex barrel field (mean ± SEM). (a) C vs. DR significance; (b) C vs. DR-EE significance; (c) C vs. DR-EE-Ex significance (P ≤ 0.05) (one-way anovatest with posthoc correction). P, postnatal day.
At P21, the density of S-100β cells per reference surface was 5% lower in DR groups compared with controls (12.6 vs. 13.2 astrocytes per 62 500 µm2), whereas it was respectively 43.7% and 57.8% higher in DR-EE (19) and DR-EE-Ex (20.9). At P28, the astroglial density was 10% higher in DR (13.1 vs. 11.9 astrocytes per 62 500 µm2) and 64% and 22% higher in DR-EE-Ex (19.6) and DR-EE (14.5). At P35, it was 12% higher in DR (13.8 vs. 12.3 astrocytes per 62 500 µm2), 23.4% higher in DR-EE (15.2) and 40% higher in DR-EE-Ex (17.2). At P42, it was 14% higher in DR (13 vs. 11.4 astrocytes per 62 500 µm2), 18% higher in DR-EE (13.4) and 57% higher in DR-EE-Ex (17.9). At P49, it was 16% higher in DR (13.1 vs. 11.3 astrocytes per 62 500 µm2), 25% higher in DR-EE (14) and 40% higher in DR-EE-Ex (15.9). At P56, it was 14% higher in DR (13.7 vs. 12 astrocytes per 62 500 µm2), 17% higher in DR-EE (14.1) and 36% higher in DR-EE-Ex (16.4). Finally, at P63, the astroglial density was 8% lower in DR (11.5 vs. 12.4 astrocytes per 62 500 µm2), 20% higher in DR-EE (15) and 23% higher in DR-EE-Ex (16.5).
Two-way anovarevealed a significant interaction between age and experimental condition (F = 8.9, df = 18, P < 0.000), as well as age (F = 21.1, df = 6, P < 0.000) and condition effects (F = 280, df = 3, P < 0.000). We therefore decided to perform a one-way anovaanalysis with corresponding posthoc tests to study the differences between experimental conditions at each age period. The statistical analysis showed that differences between DR-EE-Ex and controls were significant at all ages (P ≤ 0.05), as were differences between DR-EE and controls, except at P42. In contrast, differences between DR and controls were significant only at P35, P49 and P56. However, whereas differences between DR-EE and DR were significant at P21, P28, P35 and P63, they were not significant at P42, P49 and P56. The differences between DR-EE and DR-EE-Ex were significant at all ages (Supporting Information).
Volume of layer IV at each age
Primary visual cortex (Fig. 5)
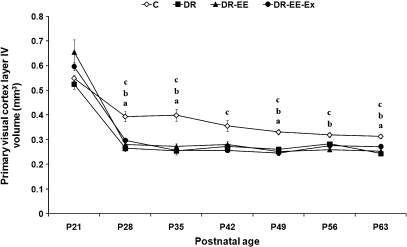
Fig. 5.
Comparison of average measurements between DR, DR-EE, DR-EE-Ex and control (C) groups at each of the ages considered. Horizontal axis shows the age of the animals. Vertical axis shows primary visual cortex layer IV volume (mm3) (mean ± SEM). (a) C vs. DR significance; (b) C vs. DR-EE significance; (c) C vs. DR-EE-Ex significance (P ≤ 0.05) (one-way anovatest with posthoc correction). P, postnatal day.
At P21, the volume of layer IV was 4% lower in DR (0.52 vs. 0.55 mm3), 20% higher in DR-EE (0.66) and 9% higher in DR-EE-Ex (0.6). At P28, it was 32% lower in DR (0.26 vs. 0.39 mm3), 29% lower in DR-EE (0.28) and 25% lower in DR-EE-Ex (0.3). At P35, it was 36% lower in DR (0.25 vs. 0.4 mm3), 31% lower in DR-EE (0.27) and 35% lower in DR-EE-Ex (0.26). At P42, it was 24% lower in DR (0.27 vs. 0.36 mm3), 21% lower in DR-EE (0.28) and 28% lower in DR-EE-Ex (0.26). At P49, it was 21% lower in DR (0.26 vs. 0.33 mm3), 24% lower in DR-EE (0.25) and 25% lower in DR-EE-Ex (0.24). At P56, it was 10% lower in DR (0.28 vs. 0.32 mm3), 18% lower in DR-EE (0.26) and 13% lower in DR-EE-Ex (0.27). Finally, at P63, the volume of layer IV was 22% lower in DR (0.24 vs. 0.31 mm3), 19% lower in DR-EE (0.25) and 13% lower in DR-EE-Ex (0.27).
Two-way anovarevealed a significant interaction between age and experimental condition (F = 5.55, df = 18, P < 0.000), as well as age (F = 210.18, df = 6, P < 0.000) and condition effects (F = 36.1, df = 3, P < 0.000). We therefore decided to perform a one-way anovaanalysis with corresponding posthoc tests to study the differences between experimental conditions at each age period. The statistical analysis showed that differences between dark-reared groups and controls were significant at all ages (P ≤ 0.05), except at P21 for all groups, at P42 for DR and DR-EE and at P56 for DR. No difference was found at any age between DR, DR-EE and DR-EE-Ex (Supporting Information).
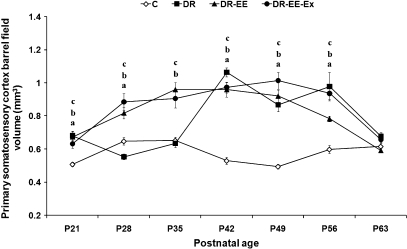
Primary somatosensory cortex barrel field (Fig. 6)
Fig. 6.
Comparison of average measurements between DR, DR-EE, DR-EE-Ex and control (C) groups at each of the ages considered. Horizontal axis shows the age of the animals. Vertical axis shows primary somatosensory cortex barrel field volume (mm3) (mean ± SEM). (a) C vs. DR significance; (b) C vs. DR-EE significance; (c) C vs. DR-EE-Ex significance (P ≤ 0.05) (one-way anovatest with posthoc correction). P, postnatal day.
At P21, the volume of layer IV was 34% higher in DR (0.68 vs. 0.51 mm3), 32% higher in DR-EE (0.67) and 24% higher in DR-EE-Ex (0.63). At P28, it was 14% lower in DR (0.55 vs. 0.65 mm3), 26% higher in DR-EE (0.82) and 36% higher in DR-EE-Ex (0.88). At P35, it was 2% lower in DR (0.64 vs. 0.65 mm3), 47% higher in DR-EE (0.96) and 38% higher in DR-EE-Ex (0.91). At P42, it was 101% higher in DR (1.1 vs. 0.53 mm3), 81% higher in DR-EE (0.96) and 84% higher in DR-EE-Ex (0.97). At P49, it was 76% higher in DR (0.87 vs. 0.5 mm3), 86% higher in DR-EE (0.92) and 92% higher in DR-EE-Ex (0.95). At P56, it was 64% higher in DR (0.98 vs. 0.6 mm3), 31% higher in DR-EE (0.78) and 56% higher in DR-EE-Ex (0.94). Finally, at P63, the volume of layer IV was 10% higher in DR (0.68 vs. 0.62 mm3), 4% lower in DR-EE (0.59) and 6% higher in DR-EE-Ex (0.66).
Two-way anovarevealed a significant interaction between age and experimental condition (F = 11.65, df = 18, P < 0.000), as well as age (F = 29.49, df = 6, P < 0.000) and condition effects (F = 81.8, df = 3, P < 0.000). We therefore decided to perform a one-way anovaanalysis with corresponding posthoc tests to study the differences between experimental conditions at each age period. The statistical analysis showed that differences between dark-reared groups and controls were significant at all ages (P ≤ 0.05), except at P63 for all groups and at P35 for DR. However, whereas differences between DR-EE and DR were significant at P28 and P35, they were not significant at all other ages. The differences between DR and DR-EE-Ex showed a similar pattern. The differences between DR-EE and DR-EE-Ex were not significant at any age, except for a slight difference at P56 (Supporting Information).
Developmental value trends
Primary visual cortex
In controls, the number of S-100β-positive cells per measured surface (62 500 µm2) remained almost constant with a maximum difference of 10% between P21 and P42. A similar pattern was observed in DR-EE rats but with lower figures at all ages. This group showed a maximum difference of 28% between P28 and P35. In contrast, data for DR-EE-Ex rats had homogeneous values at all ages (the maximum difference was 24% between P21 and P56). These values were higher than in controls at all of the studied ages. The main variability of values was achieved in the DR group, as a 47% decrease was found from P21 to P42. Whereas up to P28 values were higher than in controls, from then on they were more homogeneous and lower than in controls. In controls, the volume of layer IV decreased by 27% from P21 to P28 remaining constant from then on, with a maximum difference of 20% between P35 and P63. A similar pattern was observed in dark-reared groups, with values dropping by 50% from P21 to P28 and remaining constant up to P63, at around 25–35% lower than in controls.
Primary somatosensory cortex barrel field
In controls, the number of S-100β-positive cells per measured surface (62 500 µm2) remained almost constant with a maximum difference of 14% between P21 and P49. A similar pattern was observed in DR rats, with slightly higher figures at all ages except at P21 and P63, when they were lower. Whereas the maximum difference against controls was 16% at P49, the maximum difference within the DR group was between P35 and P63 (16%). For the DR-EE group, maximum values were at P21 and decreased thereafter remaining more or less stable at 18–24% higher than in controls. The largest drop was between P21 and P28 (23%), for the rest of the values apart from P21, the maximum difference was between P35 and P42 (12%). The main difference against controls was measured in the DR-EE-Ex group, where maximum values were at P21, 66% higher than in controls. Afterwards, there was a slight decrease, although not as dramatic as in the DR-EE group (5% between P21 and P28), and differences against controls varied from 64% at P28 to 33% at P63. The main difference within the group was between P21 and P49 (23%). In controls, the volume of layer IV remained constant with a maximum difference of 25% between P35 and P42. A similar pattern was observed in DR rats up to P35, with a maximum difference of 34% at P21 and a minimum of 2% at P35. From this age, the volume increased dramatically (60%) and it was 100% higher than in controls at P42, 76% higher at P49, 63% higher at P56 and only 10% at P63. The volume observed for DR-EE and DR-EE-Ex followed a similar pattern. It increased from P21 to P42 (54%), remained constant up to P56 and then decreased between P56 and P63 (30% in DR-EE-Ex and 24% in DR-EE). The volume was similar for the three dark-reared groups from P42.
Discussion
The present study shows that enriched environments have no effects on the decrease of the astroglial population of the visual cortex induced by visual deprivation, if they do not include physical exercise. Visual deprivation occurs with an increase of the astroglial density in the somatosensory cortex that can be interpreted as a compensatory mechanism. The effects of physical exercise are similar in both areas, suggesting a general effect all over the cortex. Although all dark-reared groups had lower volumes than controls, the lack of differences in volume between dark-rearing conditions means that differences in astroglial density cannot be due to variations in volume.
The effects of environmental enrichment have been widely studied since the work of Krech et al. (1960). Although most of the environmental paradigms include the use of a running wheel in the enrichment paradigm, its use to study specific visual enrichment can mask results, as exercise by itself can increase the secretion of growth factors such as brain-derived neurotrophic factor (Cotman et al. 2007) or insulin-like growth factor (Trejo et al. 2001). In a recent work, we demonstrated the visual exclusiveness of the environmental enrichment without exercise, as it was unable to compensate the decrease in vascular density and vascular endothelial growth factor secretion in dark-reared rats (Bengoetxea et al. 2008). In contrast, some authors have described the lack of usefulness of physical exercise in behavioral models that use enriched environments, as results do not differ when a running wheel is included in the environmental enrichment paradigm (Pietropaolo et al. 2006, 2008).
Our results in controls (Fig. 5) show a decrease in the visual cortex layer IV volume at the beginning of the critical period that could be linked to the decrease in the neuronal, vascular and astroglial densities previously reported (Argandoña & Lafuente, 2000; Argandoña et al. 2003, 2005; Heck et al. 2008). Dark-reared groups show a similar pattern but at a significantly lower level that mirrors the lower astroglial, vascular and neuronal densities previously found in dark-reared rats. Although there is an increase in astroglial density when exercise is included, it does not show changes in volume. Our results agree with previous findings that showed a similar volume of layer IV in the visual cortex of control young adult rats (Yates et al. 2008).
Results on the somatosensory cortex show that environmental enrichment in dark-reared animals (Fig. 4), even without physical exercise, induces a higher astroglial density that could be explained as a compensation for the lower activity in the visual cortex induced by light deprivation (Rauschecker et al. 1992). It must be taken into account that the critical period for the somatosensory cortex is already closed at P21, when the first data were examined. In addition, physical exercise increases the number of astrocytes compared with those produced by environmental enrichment alone. The increase of astroglial density in both studied areas suggests a general effect all over the cortex. Some authors have described an experience-mediated up-regulation of growth factors such as brain-derived neurotrophic factor or insulin-like growth factor, supporting this hypothesis for a global brain effect (Vaynman & Gomez-Pinilla, 2005; Trejo et al. 2008). Indeed, some authors have described that wheel-running induces microglial proliferation, whereas environmental enrichment stimulates astroglial proliferation (Ehninger & Kempermann, 2003).
Previous evidence also indicates that physical activity induces astrocytic proliferation in adult brain areas that undergo plasticity during motoric behaviour (Li et al. 2005) and reduced numbers of glial cells in areas related to olfactory behaviour (Ang et al. 2004). Therefore, the relative role played by exercise alone when in combination with environmental enrichment in sensorial areas has yet to be determined.
Our results on the volume of visual cortex layer IV show a similar pattern (Fig. 5), as animals reared in EE with or without exercise show a greater volume from the beginning of the critical period for the visual cortex (P21), due to the aforementioned compensation. As the critical period for the somatosensory cortex occurs earlier (in the first week of life), the effects of EE are noticeable right from the beginning of the study. In contrast, effects on DR animals are produced only after P21, when the lack of visual experience requires compensation by the somatosensory cortex.
Another point of interest is the differences between groups at the first stages of the critical period, when astroglial density in the DR group is greater than in controls (Fig. 3). Previous research has shown that environmental enrichment can accelerate the beginning of the critical period, which has been described as manifesting a decrease in the expression of astroglial markers such as glial fibrillary acidic protein or S-100 (Corvetti et al. 2003, 2006). Although numbers are similar in DR and DR-EE-Ex animals, at levels higher than age-matched controls, the underlying reason could be completely different, as the figures for DR could be due to immaturity of the cortex due to retardation at the beginning of the critical period and those for DR-EE-Ex to the general effects of exercise. In contrast, EE in DR animals prior to the critical period could be a factor in the recovery of the starting point of the critical period before the consequences of visual deprivation. Nevertheless, once the critical period reaches its peak in physiological conditions (P35), density is always lower in dark-reared animals not submitted to voluntary physical exercise. The fact that animals are weaned at P21 with a change from liquid to solid diet could also explain certain variations in the data, as some authors have found some metabolic changes at this age due to the change of feeding by weaning (Nehlig et al. 1989; Argandoña et al. 2003).
Concluding remarks
Our main result shows that strategies to apply environmental enrichment should always consider the incorporation of physical exercise, even for sensorial areas such as the visual area, where complex environmental enrichment is not enough by itself to offset the effects of visual deprivation on a key player in the cerebral cortex such as the astroglial population. In addition, physical exercise enhances the effect of environmental enrichment in the somatosensory cortex, where enrichment alone can affect the astroglial population.
Acknowledgments
This work was financially supported by 9/212.327-15887 (University of the Basque Country), IT/460/07 and SAIOTEK (Basque Government).
Supplemental material
Additional Supporting Information may be found in the online version of this article.
Table S1 S-100β-positive astrocyte density per 62 500 μm2 of primary visual cortex (V1) for control (C), DR, DR-EE and DR-EE-Ex groups at each of the ages considered (mean number of S-100β-positive cells per 62 500 μm2 of V1 ± SEM; percentage of difference and statistical significance, P value, F value and degrees of freedom) (one-way anova test with posthoc correction).
Table S2 S-100β-positive astrocyte density per 62 500 μm2 of primary somatosensory cortex barrel field (S1BF) for control (C), DR, DR-EE and DR-EE-Ex groups at each of the ages considered (mean number of S-100β-positive cells per 62 500 μm2 of primary visual cortex ± SEM; percentage of difference and statistical significance, P value, F value and degrees of freedom) (one-way anova test with posthoc correction).
Table S3 Primary visual cortex (V1) layer IV volume (mm3) for control (C), DR, DR-EE and DR-EE-Ex groups at each of the ages considered [V1 layer IV volume (mm3) ± SEM; percentage of difference and statistical significance, P value, F value and degrees of freedom] (one-way anova test with posthoc correction).
Table S4 Primary somatosensory cortex barrel field (S1BF) volume (mm3) for control (C), DR, DR-EE and DR-EE-Ex groups at each of the ages considered [S1BF volume (mm3) ± SEM; percentage of difference and statistical significance, P value, F value and degrees of freedom) (one-way anova test with posthoc correction).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Ang ET, Wong PT, Moochhala S, Ng YK. Cytokine changes in the horizontal diagonal band of Broca in the septum after running and stroke: a correlation to glial activation. Neuroscience. 2004;129:337–347. doi: 10.1016/j.neuroscience.2004.06.087. [DOI] [PubMed] [Google Scholar]
- Argandoña EG, Lafuente JV. Effects of dark-rearing on the vascularization of the developmental rat visual cortex. Brain Res. 1996;732:43–51. doi: 10.1016/0006-8993(96)00485-4. [DOI] [PubMed] [Google Scholar]
- Argandoña EG, Lafuente JV. Influence of visual experience deprivation on the postnatal development of the microvascular bed in layer IV of the rat visual cortex. Brain Res. 2000;855:137–142. doi: 10.1016/s0006-8993(99)02361-6. [DOI] [PubMed] [Google Scholar]
- Argandoña EG, Rossi ML, Lafuente JV. Visual deprivation effects on the s100β positive astrocytic population in the developing rat visual cortex: a quantitative study. Dev Brain Res. 2003;141:63–69. doi: 10.1016/s0165-3806(02)00643-0. [DOI] [PubMed] [Google Scholar]
- Argandoña EG, Bengoetxea H, Lafuente JV. Lack of experience-mediated differences in the immunohistochemical expression of blood-brain barrier markers (EBA and GluT-1) during the postnatal development of the rat visual cortex. Dev Brain Res. 2005;156:158–166. doi: 10.1016/j.devbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Bartoletti A, Medini P, Berardi N, Maffei L. Environmental enrichment prevents effects of dark-rearing in the rat visual cortex. Nat Neurosci. 2004;7:215–216. doi: 10.1038/nn1201. [DOI] [PubMed] [Google Scholar]
- Bengoetxea H, Argandoña EG, Lafuente JV. Effects of visual experience on vascular endothelial growth factor expression during the postnatal development of the rat visual cortex. Cereb Cortex. 2008;18:1630–1639. doi: 10.1093/cercor/bhm190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett EL, Diamond MC, Krech D, Rosenzweig MR. Chemical and anatomical plasticity brain. Science. 1964;146:610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Bezard E, Dovero S, Belin D, et al. Enriched environment confers resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and cocaine: involvement of dopamine transporter and trophic factors. J Neurosci. 2003;23:10999–11007. doi: 10.1523/JNEUROSCI.23-35-10999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Sirevaag AM, Greenough WT. Complex experience promotes capillary formation in young rat visual cortex. Neurosci Lett. 1987;83:351–355. doi: 10.1016/0304-3940(87)90113-3. [DOI] [PubMed] [Google Scholar]
- Briones TL, Woods J, Wadowska M, Rogozinska M. Amelioration of cognitive impairment and changes in microtubule-associated protein 2 after transient global cerebral ischemia are influenced by complex environment experience. Behav Brain Res. 2006;168:261–271. doi: 10.1016/j.bbr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Cancedda L, Putignano E, Sale A, Viegi A, Berardi N, Maffei L. Acceleration of visual system development by environmental enrichment. J Neurosci. 2004;24:4840–4848. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvetti L, Capsoni S, Cattaneo A, Domenici L. Postnatal development of GFAP in mouse visual cortex is not affected by light deprivation. Glia. 2003;41:404–414. doi: 10.1002/glia.10194. [DOI] [PubMed] [Google Scholar]
- Corvetti L, Aztiria E, Domenici L. Reduction of GFAP induced by long dark rearing is not restricted to visual cortex. Brain Res. 2006;1067:146–153. doi: 10.1016/j.brainres.2005.10.072. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex. 2003;13:845–851. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Temple MD, O'Dell DM, Pike BR, Lyeth BG. Exposure to environmental complexity promotes recovery of cognitive function after traumatic brain injury. J Neurotrauma. 1996;13:41–47. doi: 10.1089/neu.1996.13.41. [DOI] [PubMed] [Google Scholar]
- Heck N, Golbs A, Riedemann T, Sun JJ, Lessmann V, Luhmann HJ. Activity-dependent regulation of neuronal apoptosis in neonatal mouse. Cereb Cortex. 2008;18:1335–1349. doi: 10.1093/cercor/bhm165. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Malena RR, Westergom BP, et al. Environmental enrichment-mediated functional improvement after experimental traumatic brain injury is contingent on task-specific neurobehavioral experience. Neurosci Lett. 2008;431:226–230. doi: 10.1016/j.neulet.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology, Three-Dimensional Measurement in Microscopy. 2nd edn. Oxford: Garland Science/Bios Scientific Publishers; 2005. [Google Scholar]
- Jankowsky JL, Melnikova T, Fadale DJ, et al. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer's disease. J Neurosci. 2005;25:5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krech D, Rosenzweig MR, Bennett EL. Effects of environmental complexity and training on brain chemistry. J Comp Physiol Psychol. 1960;53:509–519. doi: 10.1037/h0045402. [DOI] [PubMed] [Google Scholar]
- Laviola G, Hannan AJ, Macri S, Solinas M, Jaber M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol Dis. 2008;31:159–168. doi: 10.1016/j.nbd.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Li J, Ding YH, Rafols JA, Lai Q, McAllister II JP, Ding Y. Increased astrocyte proliferation in rats after running exercise. Neurosci Lett. 2005;386:160–164. doi: 10.1016/j.neulet.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CM. Dark-rearing retards the maturation of astrocytes in restricted layers of cat visual cortex. Glia. 1990;3:487–494. doi: 10.1002/glia.440030607. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Pereira de Vasconcelos A, Boyet S. Postnatal changes in local cerebral blood flow measured by the quantitative autoradiographic [14C]iodoantipyrine technique in freely moving rats. J Cereb Blood Flow Metab. 1989;9:579–588. doi: 10.1038/jcbfm.1989.83. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Barkus C, Murphy M, Hannan AJ. Gene–environment interactions modulating cognitive function and molecular correlates of synaptic plasticity in Huntington's disease transgenic mice. Neurobiol Dis. 2008;29:490–504. doi: 10.1016/j.nbd.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Ortuzar N, Argandoña EG, Bengoetxea H, Leis O, Bulnes S, Lafuente JV. Effects of VEGF administration or neutralization in the BBB of developing rat brain. Acta Neurochir Suppl. 2009;106 doi: 10.1007/978-3-211-98811-4_9. in press. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Pietropaolo S, Feldon J, Alleva E, Cirulli F, Yee BK. The role of voluntary exercise in enriched rearing: a behavioral analysis. Behav Neurosci. 2006;120:787–803. doi: 10.1037/0735-7044.120.4.787. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Sun Y, Li R, Brana C, Feldon J, Yee BK. The impact of voluntary exercise on mental health in rodents: a neuroplasticity perspective. Behav Brain Res. 2008;192:42–60. doi: 10.1016/j.bbr.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Rampon C, Jiang CH, Dong H, et al. Effects of environmental enrichment on gene expression in the brain. Proc Natl Acad Sci USA. 2000;97:12880–12884. doi: 10.1073/pnas.97.23.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Korte M, Egert U. Crossmodal changes in the somatosensory vibrissa/barrel system of visually deprived animals. Proc Natl Acad Sci USA. 1992;89:5063–5067. doi: 10.1073/pnas.89.11.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Res. 1978;153:563–576. doi: 10.1016/0006-8993(78)90340-2. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. Plasticity of GFAP-immunoreactive astrocyte size and number in visual cortex of rats reared in complex environments. Brain Res. 1991;540:273–278. doi: 10.1016/0006-8993(91)90517-y. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. III. Neuronal and glial nuclei, boutons, dendrites, and capillaries. Brain Res. 1987;424:320–322. doi: 10.1016/0006-8993(87)91477-6. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Black JE, Shafron D, Greenough WT. Direct evidence that complex experience increases capillary branching and surface area in visual cortex of young rats. Brain Res. 1988;471:299–304. doi: 10.1016/0165-3806(88)90107-1. [DOI] [PubMed] [Google Scholar]
- Spires TL, Hannan AJ. Nature, nurture and neurology: gene–environment interactions in neurodegenerative disease. FEBS Anniversary Prize Lecture delivered on 27 June 2004 at the 29th FEBS Congress in Warsaw. FEBS J. 2005;272:2347–2361. doi: 10.1111/j.1742-4658.2005.04677.x. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martín MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990–2002) Prog Neurobiol. 2004;72:167–182. doi: 10.1016/j.pneurobio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Yates MA, Markham JA, Anderson SE, Morris JR, Juraska JM. Regional variability in age-related loss of neurons from the primary visual cortex and medial prefrontal cortex of male and female rats. Brain Res. 2008;1218:1–12. doi: 10.1016/j.brainres.2008.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.