Abstract
The development of limb cartilage involves complex signalling pathways allowing the formation of distinct segments of cartilage that are maintained in the fully developed joint. In this study, we investigated the Notch signalling pathway and its role in cartilage development. The differential distribution of the Notch signalling family of receptors and their corresponding ligands in developing avian (gallus gallus) cartilage revealed expression of Notch 1, Delta 1, Jagged 1 and Jagged 2 in all limb mesenchyme cells at the early stages of cartilage anlagen development, which were subsequently restricted to the developing cartilage element. Expression of both Notch 1 and Jagged 1 became increasingly restricted to the surface cartilage once joint cavity formation had occurred. Delta 1 and Jagged 1 were restricted to a layer of cells underneath the surface cartilage and were also observed in the hypertrophic chondrocytes, where Notch 1 expression was evident in stage 40–44 limbs. Notch 2, Notch 3 and Notch 4 were not evident in early stage limbs but were present after cavitation, although expression was lost in late stage limbs (stage 40–44). We also demonstrated that inhibition of the Notch pathway leads to altered Notch receptor expression, disrupting cartilage differentiation. From these data it is clear that Notch signalling is a necessary and critical factor in regulating cell fate decisions allowing controlled chondrogenesis, elongation and subsequent maintenance of limb cartilage.
Keywords: cartilage development, gallus gallus, Notch signalling
Introduction
Research has already established that precisely controlled processes are required for joint development with a role for differential matrix synthesis and mechanical forces as major contributors (Archer et al. 1994; Edwards, 1994; Pitsillides et al. 1995; Dowthwaite et al. 2003). Alterations in gene expression and in the synthesis and degradation of signalling molecules, such as growth factors and their receptors, have also been shown to be necessary (Edwards, 1994; Vortkamp et al. 1996; Khan et al. 2007). More recently, it has become evident that the Notch signalling family contributes significantly to cartilage development and subsequently to formation of the adult joint.
The mammalian Notch genes encode for a family of transmembrane signalling receptors, Notch 1, Notch 2, Notch 3 and Notch 4 (Weinmaster et al. 1992; del Amo et al. 1993; Uyttendaele et al. 1996; Logeat et al. 1998), and their corresponding ligands, Delta 1, Delta 3 and Delta 4 (Bettenhausen et al. 1995; Dunwoodie et al. 1997; Shutter et al. 2000) and Jagged 1 and Jagged 2 (Lindsell et al. 1995; Lanford et al. 1999). For Notch signalling activation, the inactive Notch receptor migrates from the Golgi to the plasma membrane once subfragment 1 cleavage has taken place (Logeat et al. 1998; Lai, 2004). The active Notch receptor can then bind to its ligand resulting in a conformational change allowing subfragment 2 cleavage via tumour necrosis factor-α converting enzyme to occur (Brou et al. 2000). The Notch extracellular domain is generated and remains attached to the inside of the plasma membrane. The Notch extracellular domain undergoes subfragment 3 cleavage by a γ-secretase complex to release the Notch intracellular domain. The Notch intracellular domain can migrate to the nucleus where it binds to the CSL proteins (CBF-1/RBP-Jκ/Su(H)/Lag-2) (De Strooper et al. 1999; Iso et al. 2003). This group of transcription factors is required for the repression and activation of Notch, allowing a direct and immediate effect on gene expression (Greenwald & Rubin, 1992; Kimble & Simpson, 1997).
Research has already implicated the Notch pathway in numerous cell fate decisions throughout development and its role in limb development, and in particular of chondrogenesis, has recently become more evident (Austin et al. 1995; Conlon et al. 1995; Dornseifer et al. 1997; Henrique et al. 1997; Robey, 1997; Jiang et al. 1998; Artavanis-Tsakonas et al. 1999). In early limb development Notch signalling at the limb bud apex results in the formation of specialized cells at the apical ectodermal ridge that generate signals organizing outgrowth of the vertebrate limb bud (Laufer et al. 1997; Rodriguez-Esteban et al. 1997; Vargesson et al. 1998; Henderson et al. 2001). In the later stages of cartilage development, the importance of the Notch 2/Delta 1 pathway in regulating the progression of pre-hypertrophic chondrocytes to hypertrophic chondrocytes and, thus, controlling limb elongation was highlighted in work by Crowe et al. (1999). In this study, misexpression of Delta 1 resulted in limbs of reduced size that lacked hypertrophic chondrocytes. Recent morphological work, examining the distribution of the Notch family members and ligands in embryonic and post-natal mouse hind-limbs, highlighted the complex Notch signalling interactions involved in articular cartilage formation (Hayes et al. 2003). In the embryonic mouse limb, Notch 1 was restricted to the surface zone cells but, at maturity, Notch 1 was present only in the pre-hypertrophic and hypertrophic regions. Work by Dowthwaite et al. (2004) also demonstrated the expression of Notch 1 on surface zone progenitor cells of bovine articular cartilage. The observation that Notch 1 expression was a necessary factor required for colony formation indicates that Notch 1 is involved in maintaining cells in a chondroprogenitor state, regulating the proliferation and differentiation of surface zone cells and thus implicating Notch in cell fate decisions and in setting up tissue boundaries.
For our study, we decided to use an avian model, an ideal candidate for following the expression patterns of Notch receptors and Notch ligands during cartilage development from early mesenchymal condensations through to joint formation. The expression of the Notch receptors and ligands in specific regions of the cartilage anlagen suggest possible signalling boundaries and show that the Notch pathway is important in determining cell fate decisions.
Materials and methods
Tissue processing
Joint formation of the femoral tibiotarsus was studied between stages 28 and 44 (Hamburger & Hamilton, 1951). Limbs were fixed in 95% EtOH (Notch receptor analysis) or 4% paraformaldehyde (Notch ligand analysis) and processed for routine wax embedding. Late-stage limbs were decalcified in 10% ethylenediamine-tetra-acetic acid prior to wax embedding. Sections were cut at a thickness of 8 µm.
Immunohistochemistry
Sections were treated with appropriate 5% blocking serum to prevent non-specific binding of the secondary antibody before sections were immunolabelled with antibodies to Notch family members as follows: goat anti-human Notch 1 (C-20), rabbit anti-human Notch 2 (25-225), rabbit anti-mouse Notch 3 (M-134), rabbit anti-human Notch 4 (H-225), rabbit anti-human Delta 1 (H-265), rabbit anti-human Jagged 1 (H-114) and goat anti-rat Jagged 2 (R-19). All antibodies were obtained from Santa Cruz Biotech Inc. (Santa Cruz, CA, USA) and used at a concentration of 10 µg mL−1 in phosphate-buffered saline (PBS)/0.1% Tween overnight at 4 °C. The antibodies were localized using appropriate fluorescently labelled secondary antibodies at a concentration of 10 µg mL−1 (Sigma, Dorset, UK) diluted in PBS/10% chick sera for 30 min at room temperature (20 °C). Sections were mounted under Vectashield with propidium iodide (1 µg mL−1; VectaLabs, USA) and examined under fluorescent optics (Olympus BX61). Sections for Notch ligand expression were subjected to antigen unmasking treatment (VectorLabs, USA). Briefly, the solution was heated to boiling and the slides immersed for 2 min. Controls comprised sections incubated without primary antibody and incubation with either goat (for Notch 1 and Jagged 2) or rabbit (Notch 2–4, Delta 1 and Jagged 1; Sigma) immunoglobulins to test the specificity of both the primary and secondary antibodies (Supplementary Data Fig. 1).
Organ culture
White Leghorn fertile chick embryos (Henry Stewart, UK) were quickly decapitated at stage 34 according to Hamburger & Hamilton (1951) and the tibia removed. Tibiae were placed into Dulbecco's modified Eagle's medium (DMEM)/F12 containing 10% fetal calf serum, 1% gentamicin, 0.05 mg mL−1 L-ascorbate and 1 mg mL−1 glucose (DMEM/F12+) and incubated overnight. Tibiae were treated every 24 h with DMEM/F12+ containing the γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) (100 nm) or dimethylsulphoxide. At day 7, the medium was removed and the tibiae washed in PBS and fixed in 95% EtOH for 3 h. The samples were processed through an ascending series of alcohols, cleared in xylene and wax embedded. Sections were cut at 8 µm and used for subsequent immunohistochemistry or histological staining. For each treatment three tibiae were used and the experiment was repeated three times. All experimental procedures were performed in compliance with the guidelines for animal experimentation established by our institution which are in accordance with standard international laws.
Immunohistochemistry for Notch 1 in explanted tibia
Sections were dewaxed, dehydrated through xylene and a descending series of alcohols then washed in PBS for 5 min. Notch labelling was detected using the Vectastain ‘Universal Quick’ kit (Vectorlabs, UK) following the manufacturer's protocol. Notch antibody (C-20; Santa Cruz Biotech Int.) was used at a concentration of 10 µg mL−1 and appropriate controls were run in parallel. The 3,3′Diaminobenzidine (DAB) peroxidase substrate kit (Vectorlabs, UK) was used to visualize the labelling with eosin as a counterstain.
Alcian blue labelling
Sections of the tibiae were dewaxed, rehydrated through a descending series of alcohols and washed in PBS for 5 min. Alcian blue (pH 2.5) was applied for 30 min. Sections were thoroughly destained in running water, dehydrated and mounted in distyrene, plasticizer, xylene (DPX).
Results
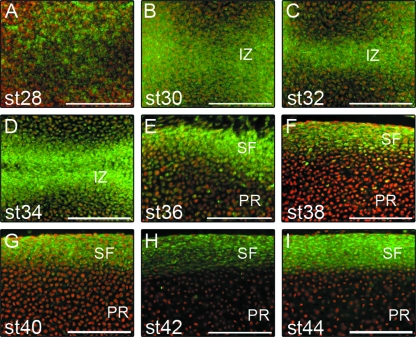
The four Notch receptors showed distinct distributions throughout development of the chick limb. Initially, at stage 28, Notch 1 label was widely observed in mesenchymal cells (Fig. 1A), whereas Notch 2 expression was not evident (data not shown). Immunopositive Notch 3 and Notch 4 label was not present in the developing cartilage anlagen. Notch 3, however, could be detected in developing blood vessels at the periphery of the condensing cartilage anlagen and positive Notch 4 label was observed in the developing musculature of the limb bud at stage 28 (data not shown). As limb outgrowth progressed, positive Notch 1 labelling was observed in the majority of cells within the developing cartilage anlagen (Fig. 1A), becoming highly concentrated within the interzone region of stage 30–34 limb buds (Fig. 1B–D). Notch 2, Notch 3 and Notch 4 were not present at stages 30–34.
Fig. 1.
Differential expression of Notch 1 in the developing chick limb. (A) At stage 28, Notch 1 distribution was widespread throughout the mesenchyme of the developing cartilage anlagen. At stage 30 (B), stage 32 (C) and stage 34 (D), Notch 1 was distributed throughout the cartilage anlagen with increased intensity at the joint interzone (IZ). At stage 36 (E), stage 38 (F) and stage 40 (G), Notch 1 expression became increasingly restricted to the surface fibrocartilage (SF) but was also evident within cells of the proliferating region (PR). At stage 42 (H) and stage 44 (I), Notch 1 expression was restricted to the cartilage surface. Scale bars, 100 µm. st, stage.
Post-cavitation, weakly positive labelling for Notch 2, Notch 3 and Notch 4 was observed at the joint line and within the proliferating region of the cartilage epiphysis in stage 36–38 limbs (Fig. 2A–C). Positive Notch 1 label was seen less extensively within the proliferating region but remained concentrated in the surface fibrocartilage (Fig. 1E–G). Notch 1 labelling was also present in the pre-hypertrophic and hypertrophic regions of the cartilage anlagen by stage 38 (Fig. 2D–F). The pre-hypertrophic regions of stage 36 and 38 cartilage anlagen showed weak labelling of Notch 2, Notch 3 and Notch 4 (Fig. 2G–I). However, a more intense Notch 3 and Notch 4 label was evident in the hypertrophic regions (Fig. 2K,L) at the same stages (stage 36–38). Stage 42 and 44 limbs lost the Notch 1 labelling observed in the proliferating region of earlier staged limbs but showed extensive Notch 1 labelling in cells of the surface fibrocartilage (Fig. 1H,I). Notch 1 label was not present in the pre-hypertrophic region but chondrocytes within the hypertrophic region were positive for Notch 1 labelling. Notch 2, Notch 3 and Notch 4 were not present between stages 40 and 44 of cartilage development within the avian limb.
Fig. 2.
Differential expression of Notch 1–4 in the developing chick limb. At stage 38, Notch 2 (A), Notch 3 (B) and Notch 4 (C) were distributed in the surface fibrocartilage (SF) and weakly in the proliferating region (PR). (D) At stage 34, Notch 1 was distributed in the cells at the outer edge of the pre-hypertrophic region (arrowed). At stage 38 (E) and stage 44 (F), Notch 1 was observed in hypertrophic cells. Notch 2 (G), Notch 3 (H) and Notch 4 (I) expression were all present in the pre-hypertrophic region at stage 38. Notch 2 (J) was not present in the hypertrophic cells, whereas Notch 3 (K) and Notch 4 (L) were present at stage 38. Scale bars, 100 µm. N, Notch; st, stage.
The Notch ligand Delta 1 was observed throughout the stage 28 limb bud (Fig. 3A). Increasingly concentrated Delta 1 labelling was observed within this region during formation of the joint interzone. Subsequently, positive Delta 1 label was also observed in cells of the proliferating region at stage 30–34 (Fig. 3B–D). At stage 36–40, Delta 1 was expressed within cells of the surface fibrocartilage and in the proliferating region (Fig. 3E,G,I). No Delta 1 label was observed in pre-hypertrophic cells at stage 36 or 38, whereas Delta 1 labelling was abundant in cells of the hypertrophic regions of the cartilage anlagen at both stage 36 and 38 (Fig. 3F,H). At the late stages of cartilage development, Delta 1 labelling was observed in a layer of cells underneath the surface fibrocartilage (Fig. 3J,K) and was also expressed in a layer of cells between the proliferating region and the pre-hypertrophic region (Fig. 3L). Intense labelling of Delta 1 was also observed in the blood vessel walls within the proliferative region of the cartilage anlagen (Fig. 3J). No Delta 1 labelling was evident in cells of the hypertrophic region at stage 42 or 44.
Fig. 3.
Differential expression of Delta 1 in the developing chick limb. (A) At stage 28, Delta 1 expression was widespread in cells of the limb mesenchyme. At stage 30 (B), stage 32 (C) and stage 34 (D), Delta 1 expression was distributed throughout the cartilage anlagen with increasing intensity at the joint interzone (IZ). At stage 36 (E), stage 38 (G) and stage 40 (I), Delta 1 expression was evident in the surface fibrocartilage (SF) and within cells of the proliferating region (PR). Delta 1 was highly expressed in the hypertrophic regions of the stage 36 (F) and stage 40 (H) limb. At stage 42 (J) and stage 44 (K), Delta 1 expression was present in a layer of cells underneath the SF and in the blood vessel walls within the PR (black arrow). (L) At stage 44, Delta 1 expression was evident in cells partitioning the PR and pre-hypertrophic region (PH). Scale bars, 100 µm. st, stage.
Jagged 1 was widely distributed in mesenchymal cells of the stage 28 limb bud (Fig. 4A). As joint formation proceeded (stage 30–34), Jagged 1 labelling became concentrated to the interzone region of the cartilage anlagen and was also observed in some cells of the proliferating region (Fig. 4B–D). In the stage 36–38 limb, post-cavitation, Jagged 1 labelling was observed in the surface fibrocartilage and within the proliferating region (Fig. 4E,F). Positive Jagged 1 label was observed in cells at the periphery beneath the perichondrium of the cartilage anlagen in the pre-hypertrophic region of stage 34–38 limbs (Fig. 4H,J,L). Extensive labelling of Jagged 1 was seen in the hypertrophic region in the stage 34–38 limb (Fig. 4I,K,M). By stage 40, Jagged 1 remained widely distributed in cells of the surface fibrocartilage and was observed in cells of the proliferating region (Fig. 4M). No Jagged 1 labelling was evident in cells of the pre-hypertrophic or hypertrophic regions of the cartilage anlagen at stage 40. In late stage limbs (stage 42–44) Jagged 1 labelling was restricted to a layer of cells underneath the surface fibrocartilage (Fig. 4N,O). Jagged 1 was not expressed in the pre-hypertrophic or hypertrophic regions of the cartilage anlagen at stage 42–44.
Fig. 4.
Differential expression of Jagged 1 in the developing chick limb. (A) At stage 28, Jagged 1 was abundant in cells of the developing cartilage anlagen. At stage 30 (B) and stage 32 (C), Jagged 1 expression was distributed throughout the cartilage anlagen with increased intensity at the joint interzone (IZ). At stage 34 (D), stage 36 (E), stage 38 (F) and stage 40 (G), Jagged 1 was highly expressed in the surface fibrocartilage (SF). Jagged 1 expression was also evident in cells at the edge of the pre-hypertrophic region (arrowed) at stage 34 (H), stage 36 (J) and stage 38 (L) limb. In cells of the hypertrophic region at stage 34 (I), stage 36 (K) and stage 38 (M) limb, Jagged 1 expression was present. At stage 42 (N) and stage 44 (O), Jagged 1 was expressed in a layer of cells under the SF. Scale bars, 100 µm. PR, proliferating region; st, stage.
Jagged 2 labelling was prominent throughout the limb mesenchyme at stage 28 (Fig. 5A), becoming concentrated at the interzone region at stage 30–34 (Fig. 5B–D). Weak label for Jagged 2 was also observed in cells of the proliferating region by stage 34. Post-cavitation (stage 36–38), abundant Jagged 2 labelling was observed in the surface fibrocartilage and was also evident sparsely in cells of the proliferating region (Fig. 5E,F). Jagged 2 labelling was not detected in the pre-hypertrophic or hypertrophic regions of the cartilage anlagen at stage 36–40. Jagged 2 labelling was, however, observed in a subset of cells at the side of the pre-hypertrophic region of the stage 36 anlagen (data not shown). Positive Jagged 2 labelling was restricted to the surface fibrocartilage in the stage 40, 42 and 44 limb (Fig. 5G–I) but no label was evident in the pre-hypertrophic or hypertrophic regions of the cartilage anlagen in these late-stage limbs.
Fig. 5.
Differential expression of Jagged 2 in the developing chick limb. (A) At stage 28, limb mesenchyme showed high expression of Jagged 2. At stage 30 (B), stage 32 (C) and stage 34 (D), Jagged 2 expression was distributed throughout the cartilage anlagen with increased intensity at the joint interzone (IZ). At stage 36 (E), stage 38 (F) and stage 40 (G), Jagged 2 expression becomes increasingly restricted to the surface fibrocartilage (SF) but is also evident in cells of the proliferating region (PR). At stage 42 (H) and stage 44 (I), Jagged 2 expression becomes restricted to the SF. Scale bars, 100 µm. st, stage.
Inhibition of Notch signalling
Initially, the length of the treated and untreated tibiae was measured after 7 days in culture and no difference in tibial length between treated and untreated tibiae was observed. On further analysis, tibiae cultured in control media showed evidence of chondrogenic differentiation throughout the proliferating flattened cell zone and pre-hypertrophic and hypertrophic zones of the cartilage anlagen, as highlighted by Alcian blue staining (Fig. 6A). It was also evident, as demonstrated on the high-power images, that explanted tibiae cultured in control media showed particularly high levels of Alcian blue staining in the surface fibrocartilage (Fig. 6C). The explanted tibiae cultured in media containing the Notch inhibitor DAPT showed chondrogenic differentiation in the pre-hypertrophic and hypertrophic regions (Fig. 6B). In these DAPT-treated explants, chondrogenic matrix was only evident at the very edge of the surface fibrocartilage (Fig. 6D). In the proliferating region there were far fewer cells staining for the presence of glycosaminoglycans within the matrix compared with control explants.
Fig. 6.
Stage 34 tibia, explanted and cultured for 7 days in control media or media containing the Notch inhibitor N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT). Alcian blue stain was present in the surface region (SF), proliferating region (PR), pre-hypertrophic region and hypertrophic region of the cartilage anlagen in control (A) and treated (B) tibiae. (C) The control tibiae showed an intense Alcian blue staining in the SF and PR. (D) The explanted tibiae cultured in media containing DAPT showed a very thin region of Alcian blue staining at the surface fibrocartilage and a small number of cells within the PR showed Alcian blue staining (arrowed). Explanted tibiae were labelled for Notch 1 receptor expression. (E) Notch 1 receptor expression was observed in a high number of cells in the SF and PR of the control tibia. (F) The tibia cultured in DAPT media showed a small amount of Notch 1 receptor expression in the PR (arrowed). The hypertrophic regions of tibiae cultured in control media and DAPT media show similar levels of Notch 1 receptor expression (G, H). Scale bars, 100 µm.
On further investigation, the explanted tibiae cultured in control media were labelled for Notch 1 expression. This revealed a high number of cells in the proliferative region positive for Notch 1 receptor expression (Fig. 6E). The DAPT-treated tibiae also showed Notch 1 labelling in the proliferative region but far fewer cells were labelled compared with the control tibiae and no Notch 1 labelling was present within the surface fibrocartilage (Fig. 6F). Notch 1 labelling was present in the hypertrophic region of the control and treated explanted tibia. There was no difference in the number of cells labelled for Notch 1 expression in the hypertrophic region in the control and treated explants (Fig. 6G,H).
Discussion
This study has fully documented the diverse spatio-temporal expression of Notch receptors and their corresponding ligands during cartilage development of the avian limb (Fig. 7). Expression of Notch 1, Delta 1, Jagged 1 and Jagged 2 in limb mesenchyme at an early stage (stage 28–32) of cartilage development suggests that Notch signalling is involved in controlling cell fate decisions. Previous research has already shown that Delta 1 and Jagged 1 are often coexpressed, signalling via the Notch pathway to determine cell fates, and it is plausible that a similar signalling pathway occurs within the interzone region (Eddison et al. 2000; Brooker et al. 2006). Here, Notch 1 and its corresponding ligands could be necessary for initial cell fate decisions involved in joint cavitation including cell differentiation, cell proliferation and the setting up of cell boundaries.
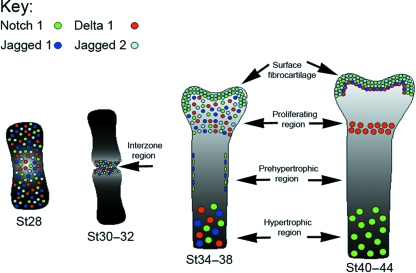
Fig. 7.
Summary of Notch receptor and ligand expression in the developing chick cartilage anlagen. At stage 28, Notch 1, Delta 1, Jagged 1 and Jagged 2 were present in the limb mesenchyme. At stage 30–32, Notch 1, Delta 1, Jagged 1 and Jagged 2 were widespread in the interzone region. At stage 34–38, Notch 1 and Jagged 2 were present in the surface fibrocartilage. Notch 1, Delta 1, Jagged 1 and Jagged 2 were present in the proliferating and hypertrophic region at stage 34–38. At stage 40–44, Notch 1 and Jagged 2 were present in the surface fibrocartilage and Delta 1 and Jagged 1 were expressed in cells underneath the Notch 1/Jagged 2-expressing cells of the surface region. Notch 1 was present in the hypertrophic region at stage 40–44.
Post-cavitation, the cartilage epiphysis at stage 34–38 showed expression of all four Notch receptors and three of their corresponding ligands within the proliferating region. The presence of Notch and its receptors within the proliferating region suggests that Notch signalling has a role in regulating cell differentiation and possibly cell proliferation by dictating when and how many cells enter into pre-hypertrophy while still maintaining a proliferating cell population. Indeed, it has already been established that Notch signalling is important in regulating the timing of cell differentiation in work by Crowe et al. (1999). This work demonstrated that Delta 1 expression was necessary for determining when pre-hypertrophic cells mature to hypertrophic cells. In our study we demonstrate that Delta 1 is expressed in the hypertrophic region of stage 36–38 limbs but that, once the limbs reach stage 44, Delta 1 expression is lost within the main body of the hypertrophic cells, only remaining on a thin interface of cells between the proliferating and pre-hypertrophic regions. This would again support a role for Notch signalling in regulating maturation of the cartilage as expression of Notch receptors and Notch ligands alters stage by stage. The expression of Delta 1 between the proliferating and pre-hypertrophic cells at this late stage (stage 44) could also suggest that Notch is needed to maintain chondrocyte maturation, and thus limb elongation, in the adult joint.
At present, we can only speculate a role for Notch 4 in cartilage development. Previous research has suggested that Notch 4 could be responsible for preventing angiogenesis and vascular ingression in endothelial cells (Leong et al. 2002). In cartilage maturation, vascularization of the cartilage anlagen begins the process of endochondral ossification, allowing osteoclast and osteoblast invasion, removing hypertrophic cartilage extracellular matrix and replacing it with bone extracellular matrix (Carlevaro et al. 2000). The expression of Notch 4 in the hypertrophic chondrocytes of the stage 34–38 limbs could be involved in preventing vascularization, as Notch 4 expression is lost by stage 44, consistent with initial cartilage erosion.
In mammalian articular cartilage, which grows appositionally, Notch 1 was expressed in surface zone chondrocytes in both bovine and murine cartilage tissues and it is interesting to note that the same expression pattern is observed in chick fibrocartilage (Hayes et al. 2001, 2003; Dowthwaite et al. 2002). In addition, Dowthwaite et al. (2004) demonstrated that Notch signal inhibition in surface zone cells reduced clonality, concluding that Notch 1 is a requirement for a chondroprogenitor population – a population that has the ability for unlimited self-renewal and plasticity. As such, the chondroprogenitor population would be necessary for the appositional growth of the cartilage. However, the expression of Notch 1 itself is not a marker for such a population. It is very likely therefore that Notch 1 expression is needed to maintain the distinct zonal architecture of the cartilage anlagen by regulating cartilage differentiation and maturation, which involves the differential expression of Notch ligands. Accordingly, Delta 1 and Jagged 1 expression were both observed in the layer of cells underneath the surface fibrocartilage. The observed high Notch 1 expression in surface fibrocartilage and the presence of Notch ligands in neighbouring cells could indicate that this expression pattern may function to maintain a precursor cell population within the surface region but at the same time allow a controlled number of cells to differentiate into chondrocytes and enter terminal differentiation. Indeed, we did observe Delta 1 at the proliferating region to the pre-hypertrophic interface. Here, regulation of differentiation will be critical in determining the length of the limb. As the pre-hypertrophic cells mature they become hypertrophic chondrocytes, which are replaced by osteoblasts and bone matrix – the result being controlled elongation of the limb.
The tibiae cultured in control media for 7 days showed a very high number of cells that expressed Notch 1 receptor in the proliferative region. This result is similar to the stage 38–40 in-ovo tibiae, corresponding to the stage 34 tibiae being in culture for 7 days and thus the Notch signalling pathway being maintained. When the tibiae were cultured in media containing the Notch inhibitor DAPT, however, reduced Notch 1 expression was observed compared with controls. The loss of Notch 1 receptor expression in Notch-inhibited tibiae could equate to the loss of chondrogenic matrix observed in the surface fibrocartilage of these tibiae. These data would seem to suggest that loss of Notch 1 expression results in the cells maintaining an undifferentiated phenotype and thus not undergoing chondrogenic differentiation. In contrast, it has been shown that abolition of Notch signalling is needed to allow full chondrogenesis to occur in human bone marrow stromal cells, demonstrating that Notch signalling is not conserved between species (Oldershaw et al. 2008). It was interesting to note that cells within the pre-hypertrophic and hypertrophic regions of the treated tibiae showed no changes in differentiation, possibly suggesting that, once cell fate has been determined, Notch inhibition does not affect future limb growth. When interpreting this data, however, it needs to be taken into consideration that the Notch inhibitor DAPT might not fully penetrate the tibiae, so continued Notch inhibition would not be maintained throughout the cartilage anlagen.
It is clear that expression of the Notch receptors in combination with their corresponding ligands is part of the very complex and precise signalling pathway that occurs during development of the cartilage anlagen. There are other factors, however, that also influence Notch signalling and need to be taken into consideration. For example, the Fringe family of glycosylases can modify Notch to lessen its susceptibility to activation by Jagged and presence of the active intracellular adaptor protein Numb results in cells that are unresponsive to Notch activation (Frise et al. 1996; Guo et al. 1996; Hicks et al. 2000; Haines & Irvine, 2003). Other proteins, such as Mind Bomb, a ubiquitin ligase that targets the intracellular domain of the Notch ligand preventing the ligand from activating Notch receptors, could also be present during cartilage development (Jiang et al. 1996; Itoh et al. 2003). The coexpression of Notch receptors is another mechanism that the cell is able to utilize to regulate Notch signalling events, for example, Notch 2 has previously been shown to down-regulate Notch 1 and Notch 3 activity (Shimizu et al. 2002).
This study adds to previous research indicating that the Notch signalling pathway is a necessary component of many developmental systems. Notch signalling interactions in developing avian cartilage allow growth and maintenance of the limb through highly controlled cell signalling pathways, of which the differential distribution of Notch receptors and Notch ligands are essential. We conclude that it is very plausible that Notch signalling maintains cells in an undifferentiated state until an appropriate time when they are able to respond to inductive cues and differentiate into particular cell types. At present, it is too early to deduce exact mechanisms of Notch signalling in the developing cartilage as a great deal of further work will be needed. This study, however, will allow for a greater understanding of future in-vivo research into chondrocyte differentiation and the Notch signalling pathway.
Acknowledgments
We gratefully acknowledge the financial support of the Arthritis Research Council.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Data
Control data for immunohistochemical methods (Fig. 1). Low-magnification images for Notch 1, Delta 1, Jagged 1 and Jagged 2 describe further the expression of Notch receptors and ligands in the developing cartilage anlagen (Fig. 2).
Supplementary Data Fig. 1 Sections of chick hind-limb at stage 32 (A), stage 36 (B), stage 40 (C) and stage 44 (D) were immunolabelled as described in Materials and methods with the primary antibody replaced by goat immunoglobulins. Sections of chick hind-limb tissue at stage 38 (E), stage 38 pre-hypertrophic region (F), stage 42 (G) and stage 44 hypertrophic region (H) were immunolabelled as described in Materials and methods with the primary antibody replaced by rabbit immunoglobulins. Positive controls, in chick tissue, to show the specificity of the antibodies for Notch 1 (I) (muscle), Delta 1 (J) (epidermis), Jagged 1 (K) (epidermis) and Jagged 2 (L) (epidermis). Scale bars, 50 μm. SF, surface fibrocartilage; PR, proliferating region.
Supplementary Data Fig. 2 Differential expression of Notch 1 receptor and Notch ligands in developing chick limbs. Notch 1 expression was present in the interzone region (IZ) at stage 32 (A) and in the surface fibrocartilage (SF) at stage 36 (B) and stage 42 (C). Delta 1 expression was widespread in the stage 28 limb (D). Delta 1 was expressed in the IZ at stage 34 (E) and in the SF at stage 38 (F). Jagged 1 expression was present in the IZ at stage 32 (G) in the SF and in the proliferative region at stage 36 (H) and stage 40 (I). Jagged 2 expression was widespread in the IZ at stage 34 (J). Jagged 2 expression was present at the SF at stage 36 (K) and stage 38 (H). Scale bars, 100 μm.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Archer CW, Morrison H, Pitsillides AA. Cellular aspects of the development of diarthrodial joints and articular cartilage. J Anat. 1994;184(Pt 3):447–456. [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Austin CP, Feldman DE, Ida JA, Jr, Cepko CL. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995;121:3637–3650. doi: 10.1242/dev.121.11.3637. [DOI] [PubMed] [Google Scholar]
- Bettenhausen B, Hrabe de Angelis M, Simon D, Guenet JL, Gossler A. Transient and restricted expression during mouse embryogenesis of Dll1, a murine gene closely related to Drosophila Delta. Development. 1995;121:2407–2418. doi: 10.1242/dev.121.8.2407. [DOI] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Brou C, Logeat F, Gupta N, et al. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- Carlevaro MF, Cermelli S, Cancedda R, Descalzi Cancedda F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci. 2000;113(Pt 1):59–69. doi: 10.1242/jcs.113.1.59. [DOI] [PubMed] [Google Scholar]
- Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- Crowe R, Zikherman J, Niswander L. Delta-1 negatively regulates the transition from prehypertrophic to hypertrophic chondrocytes during cartilage formation. Development. 1999;126:987–998. doi: 10.1242/dev.126.5.987. [DOI] [PubMed] [Google Scholar]
- del Amo FF, Gendron-Maguire M, Swiatek PJ, Jenkins NA, Copeland NG, Gridley T. Cloning, analysis, and chromosomal localization of Notch-1, a mouse homolog of Drosophila Notch. Genomics. 1993;15:259–264. doi: 10.1006/geno.1993.1055. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Dornseifer P, Takke C, Campos-Ortega JA. Overexpression of a zebrafish homologue of the Drosophila neurogenic gene Delta perturbs differentiation of primary neurons and somite development. Mech Dev. 1997;63:159–171. doi: 10.1016/s0925-4773(97)00037-3. [DOI] [PubMed] [Google Scholar]
- Dowthwaite G, Bowyer S, Bishop J, Redman S, Archer CW. Orthopaedic Research Society. Dallas, Texas: Wiley; 2002. The identification and characterisation of articular cartilage progenitor cells. In. Transactions, Vol 27. [Google Scholar]
- Dowthwaite GP, Bishop JC, Redman SN, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- Dowthwaite GP, Flannery CR, Flannelly J, Lewthwaite JC, Archer CW, Pitsillides AA. A mechanism underlying the movement requirement for synovial joint cavitation. Matrix Biol. 2003;22:311–322. doi: 10.1016/s0945-053x(03)00037-4. [DOI] [PubMed] [Google Scholar]
- Dunwoodie SL, Henrique D, Harrison SM, Beddington RS. Mouse Dll3: a novel divergent Delta gene which may complement the function of other Delta homologues during early pattern formation in the mouse embryo. Development. 1997;124:3065–3076. doi: 10.1242/dev.124.16.3065. [DOI] [PubMed] [Google Scholar]
- Eddison M, Le Roux I, Lewis J. Notch signaling in the development of the inner ear: lessons from Drosophila. Proc Natl Acad Sci USA. 2000;97:11692–11699. doi: 10.1073/pnas.97.22.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JC. Cell biology, physiology and pathology. Ann Rheum Dis. 1994;54:389–391. doi: 10.1136/ard.54.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frise E, Knoblich JA, Younger-Shepherd S, Jan LY, Jan YN. The Drosophila Numb protein inhibits signaling of the Notch receptor during cell-cell interaction in sensory organ lineage. Proc Natl Acad Sci USA. 1996;93:11925–11932. doi: 10.1073/pnas.93.21.11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I, Rubin GM. Making a difference: the role of cell-cell interactions in establishing separate identities for equivalent cells. Cell. 1992;68:271–281. doi: 10.1016/0092-8674(92)90470-w. [DOI] [PubMed] [Google Scholar]
- Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron. 1996;17:27–41. doi: 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- Haines N, Irvine KD. Glycosylation regulates Notch signalling. Nat Rev Mol Cell Biol. 2003;4:786–797. doi: 10.1038/nrm1228. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hayes AJ, Dowthwaite GP, Webster SV, Archer CW. The distribution of Notch receptors and their ligands during articular cartilage development. J Anat. 2003;202:495–502. doi: 10.1046/j.1469-7580.2003.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl) 2001;203:469–479. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- Henderson AM, Wang SJ, Taylor AC, Aitkenhead M, Hughes CC. The basic helix-loop-helix transcription factor HESR1 regulates endothelial cell tube formation. J Biol Chem. 2001;276:6169–6176. doi: 10.1074/jbc.M008506200. [DOI] [PubMed] [Google Scholar]
- Henrique D, Hirsinger E, Adam J, et al. Maintenance of neuroepithelial progenitor cells by Delta-Notch signalling in the embryonic chick retina. Curr Biol. 1997;7:661–670. doi: 10.1016/s0960-9822(06)00293-4. [DOI] [PubMed] [Google Scholar]
- Hicks C, Johnston SH, diSibio G, Collazo A, Vogt TF, Weinmaster G. Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat Cell Biol. 2000;2:515–520. doi: 10.1038/35019553. [DOI] [PubMed] [Google Scholar]
- Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Jiang R, Lan Y, Chapman HD, et al. Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice. Genes Dev. 1998;12:1046–1057. doi: 10.1101/gad.12.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YJ, Brand M, Heisenberg CP, et al. Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio. Development. 1996;123:205–216. doi: 10.1242/dev.123.1.205. [DOI] [PubMed] [Google Scholar]
- Khan IM, Redman SN, Williams R, Dowthwaite GP, Oldfield SF, Archer CW. The development of synovial joints. Curr Top Dev Biol. 2007;79:1–36. doi: 10.1016/S0070-2153(06)79001-9. [DOI] [PubMed] [Google Scholar]
- Kimble J, Simpson P. The LIN-12/Notch signaling pathway and its regulation. Annu Rev Cell Dev Biol. 1997;13:333–361. doi: 10.1146/annurev.cellbio.13.1.333. [DOI] [PubMed] [Google Scholar]
- Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, et al. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Laufer E, Dahn R, Orozco OE, et al. Expression of Radical fringe in limb-bud ectoderm regulates apical ectodermal ridge formation. Nature. 1997;386:366–373. doi: 10.1038/386366a0. [DOI] [PubMed] [Google Scholar]
- Leong KG, Hu X, Li L, et al. Activated Notch4 inhibits angiogenesis: role of beta 1-integrin activation. Mol Cell Biol. 2002;22:2830–2841. doi: 10.1128/MCB.22.8.2830-2841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsell CE, Shawber CJ, Boulter J, Weinmaster G. Jagged: a mammalian ligand that activates Notch1. Cell. 1995;80:909–917. doi: 10.1016/0092-8674(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Logeat F, Bessia C, Brou C, et al. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci USA. 1998;95:8108–8112. doi: 10.1073/pnas.95.14.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldershaw RA, Tew SR, Russell AM, et al. Notch signaling through Jagged-1 is necessary to initiate chondrogenesis in human bone marrow stromal cells but must be switched off to complete chondrogenesis. Stem Cells. 2008;26:666–674. doi: 10.1634/stemcells.2007-0806. [DOI] [PubMed] [Google Scholar]
- Pitsillides AA, Archer CW, Prehm P, Bayliss MT, Edwards JC. Alterations in hyaluronan synthesis during developing joint cavitation. J Histochem Cytochem. 1995;43:263–273. doi: 10.1177/43.3.7868856. [DOI] [PubMed] [Google Scholar]
- Robey E. Notch in vertebrates. Curr Opin Genet Dev. 1997;7:551–557. doi: 10.1016/s0959-437x(97)80085-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Esteban C, Schwabe JW, De La Pena J, Foys B, Eshelman B, Belmonte JC. Radical fringe positions the apical ectodermal ridge at the dorsoventral boundary of the vertebrate limb. Nature. 1997;386:360–366. doi: 10.1038/386360a0. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Chiba S, Saito T, Kumano K, Hamada Y, Hirai H. Functional diversity among Notch1, Notch2, and Notch3 receptors. Biochem Biophys Res Commun. 2002;291:775–779. doi: 10.1006/bbrc.2002.6528. [DOI] [PubMed] [Google Scholar]
- Shutter JR, Scully S, Fan W, et al. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 2000;14:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- Uyttendaele H, Marazzi G, Wu G, Yan Q, Sassoon D, Kitajewski J. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development. 1996;122:2251–2259. doi: 10.1242/dev.122.7.2251. [DOI] [PubMed] [Google Scholar]
- Vargesson N, Patel K, Lewis J, Tickle C. Expression patterns of Notch1, Serrate1, Serrate2 and Delta1 in tissues of the developing chick limb. Mech Dev. 1998;77:197–199. doi: 10.1016/s0925-4773(98)00138-5. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Weinmaster G, Roberts VJ, Lemke G. Notch2: a second mammalian Notch gene. Development. 1992;116:931–941. doi: 10.1242/dev.116.4.931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.