Abstract
The shape of the lumbar spine in the sagittal plane varies between individuals and as a result of postural changes but it is not known how the shape in different postures is related. Sagittal images of the lumbar spines of 24 male volunteers were acquired using a positional magnetic resonance scanner. The subjects were imaged lying supine, standing and sitting. An active shape model was used to characterize shape in terms of independent modes of variation. Two modes were identified that described the total (mode 1) and distribution (mode 2) of the curvature. The spinal shape was found to be intercorrelated between the three postures for both modes, suggesting that the lumbar spine has an element of shape that is partially maintained despite postural alterations. Mode 1 values indicated that the spine was straightest when standing and curviest when sitting. Mode 2 values indicated that the distribution in the curvature was most even when sitting and least even when lying supine. Systematic differences in the behaviour of the spine, when changing posture, were found that suggest that the shape of the spine may affect its biomechanics.
Keywords: active shape model, lordosis, lumbar spine, positional magnetic resonance imaging, posture
Introduction
The combination of rigid vertebral bodies interspersed by softer intervertebral discs makes the spine very flexible, allowing a wide range of postures to be adopted. Changes in the shape of the spine with postural alteration (Reuben et al. 1979; Dolan et al. 1988; Peterson et al. 1995; Wood et al. 1996; Lord et al. 1997) are of interest as they will affect the stresses and strains being experienced by the spinal tissues and the requirements of the supporting musculature. In sitting, for example, the natural lumbar curvature in the sagittal plane flattens with respect to the standing posture (Bridger et al. 1992) and it is thought that the resulting change in the stress experienced by the intervertebral discs may be a contributory factor for experiencing low back pain (Keegan, 1953). In lying supine the effects are more subtle and, although some studies have found the lumbar curvature to be reduced from the standing posture (Reuben et al. 1979; Wood et al. 1996), others have concluded that there is no difference (Chernukha et al. 1998; Andreasen et al. 2007).
As well as understanding how the spinal shape changes with posture, it is important to understand what factors are responsible for these changes in shape. Many previous studies have investigated factors such as the angle of the hip and knee joints and the tightness of the leg and trunk muscles (Bridger et al. 1992; McCarthy & Betz, 2000). However, there is some indication in the literature that factors intrinsic to the spine itself may play a role. Several studies have shown that the shape of the spine in the sagittal plane in normal healthy subjects in the standing posture exhibits considerable inter-subject variation (Keller et al. 2005; Roussouly et al. 2005; Meakin et al. 2008a,b). It has also been shown that subjects classified as having very little curvature in their lumbar spine (hypolordotic) sit with more flexion in their lumbar spine than control subjects and, conversely, subjects classified as having exaggerated curvatures (hyperlordotic) sit with more extension (Scannell & McGill, 2003). This suggests that the shape of an individual's spine in one posture may be related to the shape in another posture. The aim of the current study was therefore to determine the shape of the lumbar spine of normal healthy volunteers in three everyday postures (lying supine, standing and sitting) and to investigate the relationships between the shapes in these postures.
Materials and methods
Subjects
Magnetic resonance (MR) images of the lumbar spine from 24 male volunteers were used for this study. The images were part of a dataset that had been acquired for a previous study (Hirasawa et al. 2007). Approval from Grampian Research Ethics Committee (now North of Scotland Research Ethics Service) was obtained and all subjects gave their informed consent. None of the subjects reported any symptoms of low back pain and had only minor or no degenerative changes in their lumbar discs. The median age of the subjects was 26 (range 20–55) years.
Magnetic resonance imaging scanning
The images were acquired using a 0.6 T Upright™ positional MR imaging scanner (Fonar Corporation, Melville, NY, USA). Unlike a conventional MR scanner, the bed of a positional scanner is not fixed in the horizontal position but can be rotated so that the subject may be imaged in an upright position. Each subject was scanned in the morning in three postures: lying supine, standing upright and sitting. Prior to the supine scan, subjects were required to rest in the supine posture for 20 min. For scanning, a cushion was placed under the subject's knees so as to slightly flex their hips and knees. This technique, which is conventionally used in imaging investigations of the lumbar spine, has the effect of relaxing the psoas muscle and removing the force that it would otherwise place on the spine. For the standing and sitting scans the subjects were asked to adopt a relaxed neutral posture (i.e. not slumping or actively extending or flexing their lumbar spine). The subjects were allowed to rest their hands on a bar for comfort. In sitting, the hips and knees of the subjects were flexed at 90°. Scans were also acquired in the evening of the same day and used to determine the reliability of the lumbar spine shape in the standing posture.
T2-weighted para-sagittal images were acquired using the following parameters: repetition time = 3262 ms; echo time = 140 ms; number of averages = 2. The image acquisition time was 3 min and 16 s and the median time between each scan was 15 min. Eleven slices were obtained, each with a thickness of 4.5 mm and a gap of 0.5 mm. A 30 cm field of view was used with an acquisition matrix of 256 × 200. The data were subsequently reformatted onto a 256 × 256 matrix for image processing. The slice closest to the mid-sagittal plane of the spine (as defined by observing the spinal canal to be wider than in adjacent slices) was selected from the scans and converted to JPG format.
Shape modelling
The shape and changes in shape of the lumbar spine were categorized from the MR images using an active shape model (ASM). Shape modelling is an image-processing method that may be used to locate and characterize an object in a series of images (Cootes & Taylor, 2004) and has been shown to be a reliable method for characterizing the lumbar spine (Meakin et al. 2008a).
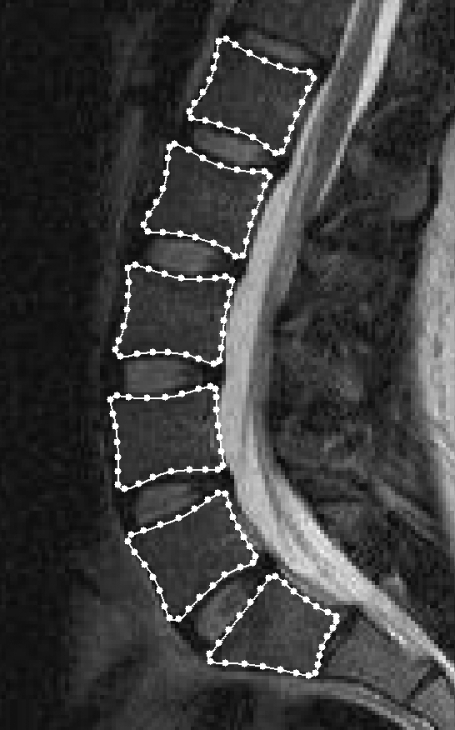
The model was created using the Active Appearance Modelling software tools from the University of Manchester, UK (http://www.isbe.man.ac.uk/∼bim/software/am_tools_doc/index.html). The model was defined by placing landmark points around the periphery of each vertebral body from L1 to S1 (Fig. 1). The same number of landmark points (28 per vertebral body, 168 in total) was used for each image and each point always referred to the same anatomical feature. After all of the 72 images had been marked (by one observer), the software aligned each set of points into a common co-ordinate frame by scaling, translating and rotating; this means that size differences and rigid body movements were removed from the model. The software then calculated the average position of the points (to give the average spine shape) and used principal component analysis to analyse the variation in their position. Principal component analysis is a statistical analysis method that can be used to reduce the dimensionality of a data set by identifying new, independent variables that describe patterns of variation. In the ASM, the new variables are called ‘modes of variation’ and are ordered such that the first one is the most important, describing the largest proportion of variance in shape, and the second and subsequent modes account for decreasing proportions of variance. The actual shape of the object in an image can thus be described using a linear combination of the modes of variation. This allows the shape to be quantified (using the coefficients of the modes of variation) in an efficient manner (using only the first, more important, modes). In the ASM, the values of the coefficients were assigned to each image and then transformed so that, for each mode of variation, the mean value was zero and the SD was unity. Two models were created, one using the images of the subjects in three different postures and another that also included the repeated scans in the standing posture.
Fig. 1.
Magnetic resonance image of the lumbar spine in the sagittal plane with 168 landmark points placed around the vertebral bodies from L1 to S1. These points were used to define the active shape model and were placed in consistent positions for all 72 images.
Statistical analysis
The effect of posture was tested using repeated-measures anova with Sidak post-hoc comparisons. The relationship between the spinal shapes in the different postures was tested using Pearson's correlation coefficient. Measures of agreement plots were also used to test for systematic effects in the relationships (Bland & Altman, 1986). To ensure that the data met the assumptions underlying the statistical tests, they were tested using the Kolmogorov-Smirnov test of normality and the Mauchly test of sphericity. Reliability was assessed by determining the intra-class correlation coefficient (two-way random effects model, absolute agreement, single measures) and measurement error [2.77 × the within-subject SD as calculated using one-way anova (Bland & Altman, 1996)]. For all tests, a probability of 5% or less was taken to indicate statistical significance.
Results
The first two modes of variation (M1 and M2) that were determined by the shape model were observed to relate to the shape of the lumbar spine (Fig. 2). These two modes accounted for 91% of the total variance in the shape (M1, 86%; M2, 5%). The vertebral body centroid angles given in Fig. 2 demonstrate that M1 corresponds to variation in the total lumbar curvature (lordosis), whereas M2 corresponds to variation in the distribution of the curvature with minimal differences in the total (< 1°). Higher modes, each of which accounted for no more than 2% of the total variance, were not related to the lumbar spine shape. They are likely to be attributable to other factors such as the shape of the vertebral bodies, the disc space between the bones, or noise.
Fig. 2.
Modes of variation from the active shape model. The average shape is shown in the centre [mode 1 (M1) = 0; mode 2 (M2) = 0] together with the effects of varying M1 and M2 by 2 SDs (sd). The total (φtotal) and segmental (φ2,3,4,5) vertebral body centroid angles are given to assist with interpretation; these are the angles made by the lines connecting adjacent vertebral body centroids (Chen, 1999).
The values for M1 and M2 for the 24 subjects in each posture are shown in Fig. 3 together with the mean and SD. Within each mode, the values in the three postures were found to be significantly intercorrelated with Pearson correlation coefficients of at least 0.6 (P < 0.001).
Fig. 3.
The values for mode 1 (M1) and mode 2 (M2) for each subject in the three postures. The means (SD) for the 24 subjects are also shown for each posture.
The values for M1 in the sitting posture were higher than in the standing [sit–stand: 95% confidence interval (CI), 1.4–2.0, P < 0.001] and supine (sit–supine: 95% CI, 1.2–1.8, P < 0.001) postures, corresponding to the spine being straightest in sitting. No consistent difference was found between the values for M1 in the standing and supine postures (P = 0.2). However, a measures of agreement plot (Fig. 4) showed that there was a systematic effect where the difference in M1 between the two postures was significantly correlated to the average. This corresponds to low M1 values in the supine posture becoming lower in the standing posture (i.e. curvy shapes becoming curvier) and vice versa for high M1 values (straighter shapes becoming straighter). A similar statistically significant relationship was also found for the change in M1 between the sitting and lying postures.
Fig. 4.
Measures of agreement plots for the standing and supine postures. The difference in the values for each subject in the two postures is plotted against the average. The Pearson correlation coefficient, R, was statistically significant for both modes (P < 0.01). M1, mode 1; M2, mode 2.
The values for M2 in the sitting posture were found to be lower than in the supine posture (supine–sit: 95% CI, 0.19–0.90, P = 0.002), corresponding to the spine being more even in sitting. No other consistent differences were found for M2. However, as with M1, there was a statistically significant systematic relationship between the difference and the average values of M2 in the supine and standing posture (Fig. 4).
The intra-class correlation coefficients (M1, 0.96; M2, 0.92) showed that the reliability of the results, estimated from the images of the subjects in the standing posture in the morning and evening, was excellent. The measurement errors were also calculated from this repeated data to be 0.07 (M1) and 0.29 (M2). This equates to relative errors (percentage of the full range of values for the standing posture) of 2% and 7%.
Discussion
The main aim of our study was determine the shape of the lumbar spine, in the sagittal plane, of normal healthy subjects in the supine, standing and sitting postures, and to investigate the relationship between the shapes in these three postures. Active shape modelling was used to characterize the shape and, as in our previous work on the standing posture alone (Meakin et al. 2008a), was found to describe the lumbar spine efficiently using two modes of variation, one for the total curvature and one for the distribution of curvature. Qualitatively the modes were similar to those found by the model of the standing posture (Meakin et al. 2008a) but, because of the additional postures included in the current mode, had a different mean shape and described different proportions of the total variance.
Our study showed that lumbar spine shape varies considerably between individuals in all three postures and that the shape in one posture is related to that in the other two. Previous studies demonstrated the variability in the shape of the spine but mostly only considered one posture (e.g. Keller et al. 2005; Roussouly et al. 2005; Meakin et al. 2008a,b). Scannell & McGill (2003) showed that subjects with extremely small or large lumbar curvatures in standing had similarly exaggerated curvatures in sitting but did not quantify the relationship. Our results suggest that the lumbar spine of an individual has an element of intrinsic shape that is partially maintained throughout postural changes. The idea of an individual having a unique spinal shape has been alluded to before (Stagnara et al. 1982) but has not previously been shown to influence a range of different postures. We cannot be certain if this would be true in more extreme postures where the lumbar spine is fully flexed or extended, or in other parts of the spine. However, the correlations between various measures of shape in the lumbar, thoracic and cervical regions, which have been determined by other authors (e.g. Berthonnaud et al. 2005), suggest that the shape of the whole spine may be characteristic of the individual.
The curvature of the spine in the three postures was similar to that found in previous studies (Reuben et al. 1979; Dolan et al. 1988; Wood et al. 1996; Lord et al. 1997; Andreasen et al. 2007). The distribution of the curvature in different postures has not been explicitly investigated before, although examination of the results of Wood et al. (1996) suggests that, like us, they might have found the curvature to be more evenly distributed in standing compared with supine.
The difference in lumbar spine shape in standing and lying supine was, on average, small and not significant. However, the effect of changing between these two postures was found to differ for different shaped spines; the curvature increased for curvier spines and decreased for straighter spines. Similarly divergent behaviour was found in a previous study that investigated load bearing in the upright posture and attributed the divergence to either a shape-dependent buckling mechanism or a shape-dependent muscle recruitment strategy (Meakin et al. 2008b). In the current study, standing upright from the supine posture is analogous to load bearing in the upright postures as it involves transferring the weight of the body above L1 [estimated to be 39% of total body weight (Duval-Beaupère & Robain, 1987)] from the bed of the scanner to the lumbar spine.
A number of factors may be hypothesized to underpin the wide inter-subject variation in lumbar spine shape and the partial preservation of this shape between postures. In the sagittal plane the curvature is dictated by the wedged shape of the vertebral bodies and intervertebral discs (Masharawi et al. 2008), the pelvic incidence (defined as the inclination of the sacral end-plate with respect to a line joining the midpoint of the end-plate to the axis of the hip joints) (Legaye, 2007), and the orientation of the pelvis about the hip joints (Day et al. 1984). Vertebral body wedging and pelvic incidence both relate to bone morphology and can be considered to change little over time in adults (Grados et al. 1999; Peleg et al. 2007; Masharawi et al. 2008). In contrast, intervertebral disc wedging and pelvic orientation may be altered to allow postural changes to take place and are controlled by balancing the forces of body weight and muscle action (McCarthy & Betz, 2000; Scannell & McGill, 2003; Kim et al. 2006). Even these, however, are likely to influence spinal shape as the lumbar discs are wedged even when unloaded (Pooni et al. 1986). Although factors such as body weight distribution and muscle tone (Scannell & McGill, 2003) can modify spinal shape, some of the above factors may be genetically determined. Recent studies have shown that spinal shape has a familial correlation that is strongest for the most closely related subjects (Dryden et al. 2007) and that the range of motion of the lumbar spine has a substantial genetic influence (Battié et al. 2008).
The wide variation in the shape of the lumbar spine has both biomechanical and clinical implications. The amount of curvature has previously been predicted to affect the relative proportion of shear and compressive stresses and strains in the spinal tissues (Aspden, 1989; Shirazi-Adl & Parnianpour, 1999; Shirazi-Adl et al. 2002) and the evenness of the curvature has also been found to relate to the incidence of pathologies such spondylolisthesis (Jackson et al. 2003; Labelle et al. 2004) and disc degeneration (Farfan et al. 1972). An individual's spinal shape may thus make them more susceptible to suffering injury and developing particular pathologies. Variation in how the spinal shape changes during postural alteration may also be important as this will also affect the tissue stresses and strains. Scannell & McGill (2003) have suggested that subjects with straight lumbar spines have a greater risk of tissue damage when sitting than subjects with more curvature. Most biomechanical models that aim to understand the stresses in the spine and mechanisms of injury do not take shape variation into account and may therefore be missing important information.
The images that we used in our study were restricted to those from male subjects. Within the literature there is mixed evidence as to whether there are differences in lumbar shape between the sexes (Grados et al. 1999; Nourbakhsh et al. 2001; Mac-Thiong et al. 2004; Dryden et al. 2007) and so we cannot be certain that the results of our study are applicable to both sexes.
The reliability of our results will be contingent on the consistency in spinal shape on two occasions and the consistency in measuring this shape. We have previously determined the inter- and intra-observer reliability on a single set of images and found shape modelling to be very reliable (Meakin et al. 2008a). In the current study we measured images of subjects in the standing posture acquired in the morning and evening of the same day. Although the effect of diurnal variation in disc height means that the two sets of images were not acquired under identical conditions, the excellent agreement between them suggests that the results of our study are reliable.
Acknowledgments
We thank Dr Y. Hirasawa and Mrs B. MacLennan (research radiographer) for acquiring the MR images.
References
- Andreasen ML, Langhoff L, Jensen TW, Albert HB. Reproduction of the lumbar lordosis: a comparison of standing radiographs versus supine magnetic resonance imaging obtained with straightened lower extremities. J Manip Physiol Ther. 2007;30:26–30. doi: 10.1016/j.jmpt.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Aspden RM. The spine as an arch. A new mathematical model. Spine. 1989;14:266–274. doi: 10.1097/00007632-198903000-00005. [DOI] [PubMed] [Google Scholar]
- Battié MC, Levalahti E, Videman T, Burton K, Kaprio J. Heritability of lumbar flexibility and the role of disc degeneration and body weight. J Appl Physiol. 2008;104:379–385. doi: 10.1152/japplphysiol.01009.2007. [DOI] [PubMed] [Google Scholar]
- Berthonnaud E, Dimnet J, Roussouly P, Labelle H. Analysis of the sagittal balance of the spine and pelvis using shape and orientation parameters. J Spinal Disord Tech. 2005;18:40–47. doi: 10.1097/01.bsd.0000117542.88865.77. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;I:307–310. [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measurement error. BMJ. 1996;313:744. doi: 10.1136/bmj.313.7059.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger RS, Orkin D, Henneberg M. A quantitative investigation of lumbar and pelvic postures in standing and sitting: Interrelationships with body position and hip muscle length. Int J Ind Ergon. 1992;9:235–244. [Google Scholar]
- Chen YL. Vertebral centroid measurement of lumbar lordosis compared with the Cobb technique. Spine. 1999;24:1786–1790. doi: 10.1097/00007632-199909010-00007. [DOI] [PubMed] [Google Scholar]
- Chernukha KV, Daffner RH, Reigel DH. Lumbar lordosis measurement, a new method versus Cobb technique. Spine. 1998;23:74–79. doi: 10.1097/00007632-199801010-00016. [DOI] [PubMed] [Google Scholar]
- Cootes TF, Taylor CJ. Anatomical statistical models and their role in feature extraction. Br J Radiol. 2004;77:S133–S139. doi: 10.1259/bjr/20343922. [DOI] [PubMed] [Google Scholar]
- Day JW, Smidt GL, Lehmann T. Effect of pelvic tilt on standing posture. Phys Ther. 1984;64:510–516. doi: 10.1093/ptj/64.4.510. [DOI] [PubMed] [Google Scholar]
- Dolan P, Adams MA, Hutton WC. Commonly adopted postures and their effect on the lumbar spine. Spine. 1988;13:197–201. doi: 10.1097/00007632-198802000-00012. [DOI] [PubMed] [Google Scholar]
- Dryden IL, Oxborrow N, Dickson R. Familial relationships of normal spine shape. Statist Med. 2007;27:1993–2003. doi: 10.1002/sim.3162. [DOI] [PubMed] [Google Scholar]
- Duval-Beaupère G, Robain G. Visualization on full spine radiographs of the anatomical connections of the centres of the segmental body mass supported by each vertebra and measured in vivo. Int Orthop. 1987;11:261–269. doi: 10.1007/BF00271459. [DOI] [PubMed] [Google Scholar]
- Farfan HF, Huberdeau RM, Dubow HI. Lumbar intervertebral disc degeneration: the influence of geometrical features on the pattern of disc degeneration – a post mortem study. J Bone Joint Surg Am. 1972;54:492–510. [PubMed] [Google Scholar]
- Grados F, Fardellone P, Benammar M, Muller C, Roux C, Sebert JL. Influence of age and sex on vertebral shape indices assessed by radiographic morphometry. Osteoporos Int. 1999;10:450–455. doi: 10.1007/s001980050253. [DOI] [PubMed] [Google Scholar]
- Hirasawa Y, Bashir WA, Smith FW, Magnusson ML, Pope MH, Takahashi K. Postural changes of the dural sac in the lumbar spines of symptomatic individuals using positional stand-up magnetic resonance imaging. Spine. 2007;32:E136–E140. doi: 10.1097/01.brs.0000255202.94153.ca. [DOI] [PubMed] [Google Scholar]
- Jackson RP, Phipps T, Hales C, Surber J. Pelvic lordosis and alignment in spondylolisthesis. Spine. 2003;28:151–160. doi: 10.1097/00007632-200301150-00011. [DOI] [PubMed] [Google Scholar]
- Keegan JJ. Alterations of the lumbar curve related to posture and seating. J Bone Joint Surg Am. 1953;35:589–603. [PubMed] [Google Scholar]
- Keller TS, Colloca CJ, Harrison DE, Harrison DD, Janik TJ. Influence of spine morphology on intervertebral disc loads and stresses in asymptomatic adults: implications for the ideal spine. Spine J. 2005;5:297–309. doi: 10.1016/j.spinee.2004.10.050. [DOI] [PubMed] [Google Scholar]
- Kim H-J, Chung S, Kim S, et al. Influences of trunk muscle on lumbar lordosis and sacral angle. Eur Spine J. 2006;15:409–414. doi: 10.1007/s00586-005-0976-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle H, Roussouly P, Berthonnaud E, et al. Spondylolisthesis, pelvic incidence, and spinopelvic balance: a correlation study. Spine. 2004;29:2049–2054. doi: 10.1097/01.brs.0000138279.53439.cc. [DOI] [PubMed] [Google Scholar]
- Legaye J. The femoro-sacral posterior angle: anatomical sagittal pelvic parameter usable with dome-shaped sacrum. Eur Spine J. 2007;16:219–225. doi: 10.1007/s00586-006-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord MJ, Small JM, Dinsay JM, Watkins RG. Lumbar lordosis: effects of sitting and standing. Spine. 1997;22:2571–2574. doi: 10.1097/00007632-199711010-00020. [DOI] [PubMed] [Google Scholar]
- Mac-Thiong JM, Berthonnaud E, Dimar JR, 2nd, Betz RR, Labelle H. Sagittal alignment of the spine and pelvis during growth. Spine. 2004;29:1642–1647. doi: 10.1097/01.brs.0000132312.78469.7b. [DOI] [PubMed] [Google Scholar]
- Masharawi Y, Salame K, Mirovsky Y, et al. Vertebral body shape variation in the thoracic and lumbar spine: characterization of its asymmetry and wedging. Clin Anat. 2008;21:46–54. doi: 10.1002/ca.20532. [DOI] [PubMed] [Google Scholar]
- McCarthy JJ, Betz RR. The relationship between tight hamstrings and lumbar hypolordosis in children with cerebral palsy. Spine. 2000;25:211–213. doi: 10.1097/00007632-200001150-00011. [DOI] [PubMed] [Google Scholar]
- Meakin JR, Gregory JS, Smith FW, Gilbert FJ, Aspden RM. Characterising the shape of the lumbar spine using an active shape model: reliability and precision of the method. Spine. 2008a;33:807–813. doi: 10.1097/BRS.0b013e31816949e6. [DOI] [PubMed] [Google Scholar]
- Meakin JR, Smith FW, Gilbert FJ, Aspden RM. The effect of axial load on the sagittal plane curvature of the upright human spine in vivo. J Biomech. 2008b;41:2850–2854. doi: 10.1016/j.jbiomech.2008.06.035. [DOI] [PubMed] [Google Scholar]
- Nourbakhsh MR, Moussavi SJ, Salavati M. Effects of lifestyle and work-related physical activity on the degree of lumbar lordosis and chronic low back pain in a Middle East population. J Spinal Disord. 2001;14:283–292. doi: 10.1097/00002517-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Peleg S, Dar G, Medlej B, et al. Orientation of the human sacrum: anthropological perspectives and methodological approaches. Am J Phys Anthrop. 2007;133:967–977. doi: 10.1002/ajpa.20599. [DOI] [PubMed] [Google Scholar]
- Peterson MD, Nelson LM, McManus AC, Jackson RP. The effect of operative position on lumbar lordosis. A radiographic study of patients under anesthesia in the prone and 90–90 positions. Spine. 1995;20:1419–1424. [PubMed] [Google Scholar]
- Pooni JS, Hukins DWL, Harris PF. Comparison of the structure of the human intervertebral discs in the cervical, thoracic and lumbar regions of the spine. Surg Rad Anat. 1986;8:175–182. doi: 10.1007/BF02427846. [DOI] [PubMed] [Google Scholar]
- Reuben JD, Brown RH, Nash CL, Brower EM. In vivo effects of axial loading on healthy, adolescent spines. Clin Orthop Relat Res. 1979;139:17–27. [PubMed] [Google Scholar]
- Roussouly P, Gollogly S, Berthonnaud E, Dimnet J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine. 2005;30:346–353. doi: 10.1097/01.brs.0000152379.54463.65. [DOI] [PubMed] [Google Scholar]
- Scannell JP, McGill SM. Lumbar posture – should it, and can it, be modified? A study of passive tissue stiffness and lumbar position during activities of daily living. Phys Ther. 2003;83:907–917. [PubMed] [Google Scholar]
- Shirazi-Adl A, Parnianpour M. Transactions of the 45th annual meeting of the Orthopaedic Research Society. Anaheim, CA.: 1999. Pelvic tilt and lordosis control spinal postural response in compression; p. 1012. [Google Scholar]
- Shirazi-Adl A, Sadouk S, Parnianpour M, Pop D, El-Rich M. Muscle force evaluation and role of posture in human lumbar spine under compression. Eur Spine J. 2002;11:519–526. doi: 10.1007/s00586-002-0397-7. [DOI] [PubMed] [Google Scholar]
- Stagnara P, De Mauroy JC, Dran G, et al. Reciprocal angulation of vertebral bodies in a sagittal plane: approach to references for the evaluation of kyphosis and lordosis. Spine. 1982;7:335–342. doi: 10.1097/00007632-198207000-00003. [DOI] [PubMed] [Google Scholar]
- Wood KB, Kos P, Schendel M, Persson K. Effect of patient position on the sagittal-plane profile of the thoracolumbar spine. J Spinal Disord. 1996;9:165–169. [PubMed] [Google Scholar]