Abstract
Background
Most estimates of short term and long term survival after hepatic resection of colorectal cancer metastases are derived from surgical case series. We used Medicare data to investigate operative mortality and long term survival in a national sample and looked at factors associated with survival.
Methods
We analyzed data for Medicare enrollees (age ≥ 65) admitted to hospital between January 1, 2000 and December 31, 2004 with a primary diagnosis of colorectal cancer with resection. We restricted the sample to those who subsequently underwent hepatic resection for liver metastases. We used the Medicare denominator file to determine operative mortality and long term survival and the factors that are associated with these outcomes.
Results
Of the 306061Medicare beneficiaries diagnosed with colorectal cancer, we identified 3957 patients who underwent hepatic resection for liver metastases. Crude 30-day mortality and 90-day mortality were 4.0% and 8.2%. The 5-year survival rate was 25.5%. Advancing age (HR=1.83; 95% CI 1.32, 2.53 for age 80 and old vs age 65–69), comorbid disease (HR=1.40; 95% CI 1.06, 1.85 for Charson 5+ vs Charlson 0) and synchronous colon/hepatic resection (HR=2.46; 95% CI 1.89, 3.20 for Synchronous vs Metachronous ) were associated with worse 90 day mortality. Likewise, long term mortality was also associated with age (HR =1.36; 95% CI 1.18, 1.56), comorbid disease (HR = 1.51; 95% CI 1.36, 1.69 and synchronous colon/hepatic resection (HR=1.37; 95% CI 1.24, 1.51 for synchronous vs metachronous)
Conclusion
In this national study, short and long term survival is worse than that reported in surgical case series. Subgroups at high risk for worse outcomes include the extreme elderly and those undergoing synchronous colon and hepatic resection.
Background
Colorectal cancer is the third most common cancer in men and women in the United States1. If diagnosed and resected at an early stage, the prognosis is excellent. However, once disease has extended outside the bowel, the prognosis worsens substantially. The liver is the most common site of metastases. If left untreated, 5-year survival is essentially nil.2 Therefore, medical and surgical strategies have been developed to treat advanced disease. Studies evaluating chemotherapy for metastatic colon cancer have generally been disappointing. For example, median survival was less than 1.5 years in two studies of aggressive chemotherapy regimens for advanced disease.3, 4 In contrast, for those with limited metastatic disease, reports from surgical series are much more favorable. A synthetic review comprising 19 case series evaluating hepatic resection for colorectal metastases, estimated five-year survival between 21–44%.5 In the two largest US studies of this operation, 5 year survival was between 33%6 and 38%.7 Furthermore, surgical mortality from hepatectomy is generally reported to be less than 5%.5, 8
Studies of surgical resection for hepatic metastases have a number of limitations. The data evaluating its effectiveness derives almost exclusively from case series generally drawn from large academic centers where patient selection or surgical expertise may be superior to what is found in many communities. Many of these series are single-center studies that report on a small number of patients, averaging 283 patients in ten US series.6, 7, 9–16 Finally, follow up in these studies is frequently limited or incomplete. For example, data on mortality may be obtained exclusively through chart reviews rather than supplementing with the use of death registries. All of these factors may lead to an overly optimistic estimate of the benefits derived from hepatic resection for metastatic colon cancer.
In fact, a recently published study using administrative data (SEER Medicare) did study hepatic resection in those with colorectal cancer metastases and found that outcomes achieved nationally may not be as good as those observed in surgical case series.17 However, that study was limited in a number of ways. First, many of the patients included in the study were diagnosed with cancer over a decade ago (1991–2001) and so the results may not reflect more current practice. Second, the study population was limited to those in the combined SEER-Medicare dataset (n=833) and so study of factors associated with early and late mortality was limited.
Therefore, we assessed survival after hepatic resection of colorectal cancer metastases in a large population-based national sample utilizing recent Medicare data. In addition to describing short and long term mortality in this population, we also examined important risk factors for these outcomes.
Methods
Cohort Selection
Using Medicare’s 100% national MedPAR files, all part B entitled, non HMO patients admitted to an acute care hospital between January 1, 2000 and December 31, 2004 with a first time primary diagnosis of colorectal cancer with resection (ICD 9 diagnosis codes 153, 154 and procedure codes 48.4–48.69 or 45.7–45.8) were identified. We identified those with simultaneously diagnosed or subsequent development of liver metastases (ICD 9 197) who underwent hepatic resection (ICD 9 50.22 and 50.3) between January 1, 2000 through December 31st 2004. The hepatic resection cohort consisted of patients with both the diagnostic code for liver metastases AND one of the two surgical codes for hepatic resection.
Measures and Outcomes
Information on patient demographics (age, sex and race) was obtained from the national denominator file supplied by CMS. A modified (colon cancer removed) Charlson score was calculated using the comorbidities coded at the time of the initial colonic resection. Type of resection (partial hepatectomy vs lobectomy) was drawn directly from ICD9 coding. Timing of the colonic and hepatic resections was defined as either synchronous or metachronous. Resections were considered synchronous if both operations (ie. colonic and hepatic resection) were performed during the initial hospitalization, and metachronous if performed during separate hospitalizations. Metachronous resections were further sub-classified between those performed within 1 year of initial primary resection (but not during the initial hospitalization) and those occurring 1 year after the initial primary resection.
The main outcome was survival after hepatic resection using the Medicare Denominator file to identify deaths through Dec 31, 2005.
Statistical Analysis
Crude mortality at 30 and 90 days was computed overall, by age group and timing of resection. Long term mortality was estimated using Kaplan Meier survival curves, overall and by age group to adjust for censoring due to mortality.18 Cox proportional hazards regression were used to estimate the effects of patient characteristics (age, sex, race), comorbidity, timing and type of surgery on 90-day and long-term mortality, respectively.19 Age was categorized in four groups (65–69, 70–74, 75–79, 80+ years), race as black versus non-black, and Charlson comorbidity as 0, 1, 2, 3–4 and 5+ comorbidities. All analyses were performed using the SAS system version 9.1. All statistical tests were performed at the 5% level of significance and were two-sided.
Results
We identified 306,061Medicare beneficiaries who were diagnosed and surgically treated for colorectal cancer. From this group, we identified 3957 individuals who underwent resection of the liver for metastatic disease. The characteristics of this cohort are reported in table 1. Of those undergoing hepatic resection, 40% were 75 years or older, 45% were female and 7.1% were black. Approximately 32% underwent synchronous resection (i.e. same hospitalization) of their colon cancer and metastatic liver lesion with the rest undergoing metachronous (i.e. not same hospitalization) resection. Amongst the 2668 patients undergoing metachronous resection, 40% occurred within the first year after the colon resection and 60% were a year or more after the colon resection.
Table 1.
Baseline Characteristics of the hepatic resection cohort
| Covariate | Frequency N (%) | Crude Mortality at 90 days N (%) |
|---|---|---|
| Age | ||
| 65–69 | 1073 (27.1) | 58 (5.4) |
| 70–74 | 1300 (32.8) | 87 (6.7) |
| 75–79 | 970 (24.5) | 84 (8.7) |
| 80 and over | 614 (15.5%) | 97 (15.8) |
| Sex | ||
| Male | 2177 (55.0) | 187 (8.6) |
| Female | 1780 (45.0) | 139 (7.8) |
| Race | ||
| Non-black | 3676 (92.9) | 308 (8.4) |
| Black | 281 (7.1) | 18 (6.4) |
| Charlson Comorbidity Score | ||
| 0 | 1929 (48.8) | 123 (6.4) |
| 1 | 345 (8.7) | 22 (6.4) |
| 2 | 470 (11.9) | 37 (7.9) |
| 3–4 | 198 (5.0) | 18 (9.1) |
| 5+ | 1015 (25.6) | 126 (12.4) |
| Type of Resection | ||
| Partial hepatectomy | 2763 (69.8) | 207 (7.5) |
| Hepatic lobectomy | 1194 (30.2) | 119 (10.0) |
| Timing of Hepatic Resection | ||
| Synchronous | 1289 (32.6) | 171(13.3) |
| Metachronous 1–12 mos | 1078 (27.2) | 52 (4.8) |
| Metachronous 12+ mos | 1590 (40.2) | 103 (6.5) |
Short term mortality
Crude thirty day mortality in this cohort was 4.0%; and by 90 days, mortality had doubled to 8.2%. Table 1 reports the crude 90-day mortality according to important risk factors. For those aged 80 or older, 90-day mortality was 15.8% compared to 5.4% in those aged 65–69. In univariate anlaysis, timing of resection was also an important factor predicting early mortality. At 90 days, mortality for those undergoing synchronous resection (13.3%) was more than twice that of those undergoing metachronous resection (5.8%). Since some patients with synchronous lesions (i.e. detected at the time of their primary colorectal cancer) might undergo delayed resection (after their first hospitalization with primary resection), we subclassified metachronous resection into those occurring more or less than one year from their primary colon resection. The 90 day mortality of synchronous resection was significantly greater than metachronous resection either during the first year after primary resection (4.8%) or one year after the primary resection (6.5%).
In multivariate analysis (table 2), advanced age and the timing of the hepatic resection remained significant predictors of early (90 day) mortality. In addition, hepatic lobectomy was also associated with higher 90 day mortality compared to partial hepatectomy (HR = 1.96, 95% CI = 1.54– 2.49). Severe co-morbid illness also independently predicted 90 day mortality. Specifically, those with the highest Charlson score were 40% more likely to have died at 90 days compared to those in the lowest Charlson group (HR= 1.40; 95% CI 1.06–1.85).
Table 2.
Results of multivariable regression model on short (90 day) and long term mortality
| Covariate | 90 Days Hazard Ratio (95% Confidence Interval) |
Overall Hazard Ratio (95% Confidence Interval) |
|---|---|---|
| Age | ||
| 65–69 | Reference | Reference |
| 70–74 | 0.96 (0.72, 1.29) | 0.94 (0.85, 1.04) |
| 75–79 | 1.19 (0.88, 1.59 ) | 1.17 (1.05, 1.30) |
| 80 and over | 1.83 (1.32, 2.53) | 1.35 (1.18, 1.55) |
| Sex | ||
| Male | Reference | Reference |
| Female | 0.84 (0.68, 1.05) | 1.04 (0.96, 1.13) |
| Race | ||
| Non-black | Reference | Reference |
| Black | 0.71 (0.44, 1.15) | 1.04 (0.89, 1.22) |
| Charlson Comorbidity Score | ||
| 0 | Reference | Reference |
| 1 | 1.01 (0.64, 1.59) | 1.11 (0.95, 1.29) |
| 2 | 1.21 (0.84, 1.75) | 1.23 (1.07, 1.40) |
| 3–4 | 1.45 (0.88, 2.39) | 1.11 (0.91, 1.36) |
| 5+ | 1.40 (1.06, 1.85) | 1.51 (1.36, 1.69) |
| Type of Resection | ||
| Partial hepatectomy | Reference | Reference |
| Hepatic lobectomy | 1.96 (1.542, 2.49) | 1.09 (0.99, 1.20) |
| Timing of Hepatic Resection | ||
| Metachronous | Reference | Reference |
| Synchronous | 2.46 (1.89, 3.20) | 1.37 (1.24, 151) |
Long term mortality
Overall, 5 years after hepatic resection, 25.47%(se=0.01) remained alive. (figure 1). Figure 2 shows the long term survival of the hepatic resection cohort by age group. Five year survival ranged from 29.3%(se=0.02) in the 65–69 year old group to 17.1%(0.02) in the 80 year old and older cohort. Survival was significantly different across these age strata (p<.001).
Figure 1.
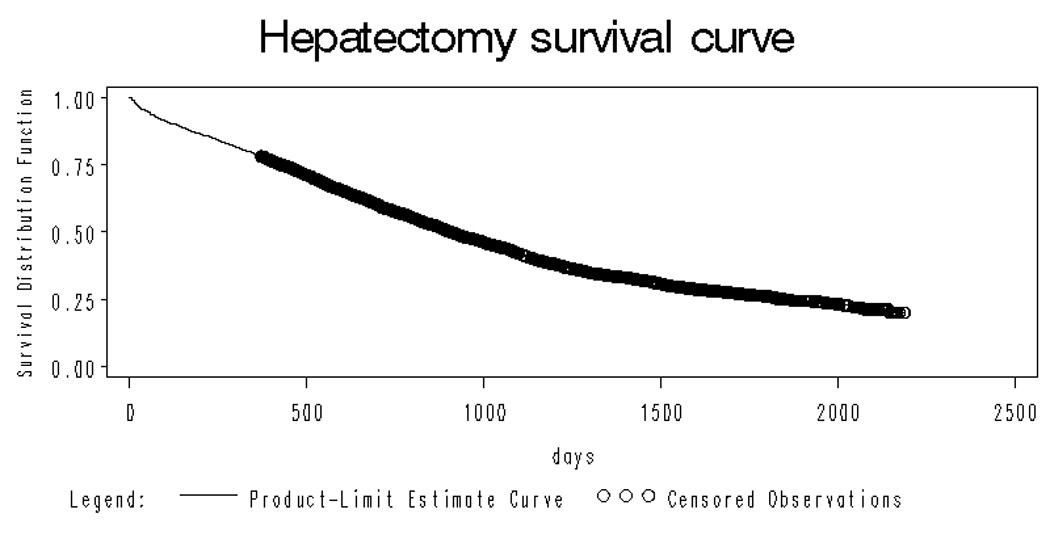
Overall survival curve for the entire hepatic resection cohort
Figure 2.
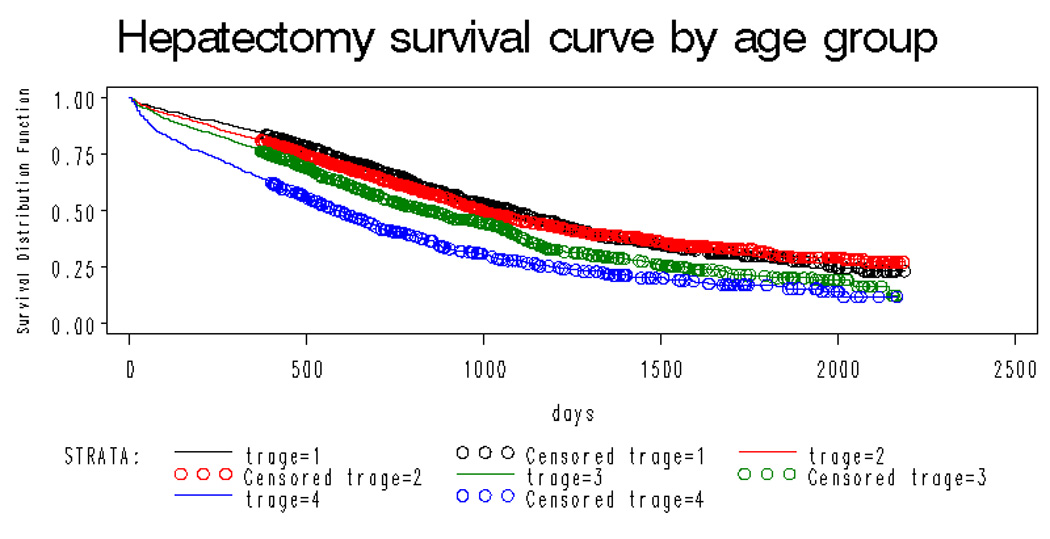
Overall survival curves for the entire hepatic resection cohort by age (black line age 65–69; red line age 70–74; green line age 75–79; blue line age 80+) showing significant difference across strata of age (p<.001)
The results of the multivariate long-term survival analysis are shown in table 2. The factors most predictive of long term mortality were increasing age (HR= 1.36; 95% CI 1.18, 1.55) for age 80+ vs 65–69), comorbid disease(HR = 1.51; 95% CI 1.36, 1.69) for highest vs lowest Charlson score) and synchronous hepatic resection (HR= 1.37; 95% CI 1.24, 1.51) synchronous vs metachronous). While type of resection (hepatic lobectomy vs partial hepatectomy) was associated with short term survival, it was not associated with long term survival (HR=1.09 95% CI 0.99, 1.20).
Discussion
The natural history of patients suffering from hepatic metastases from colorectal cancer has been well described. Wagner and colleagues2 reported the history of 252 patients with unresected disease. While those with solitary lesions fared better than those with widespread disease, essentially all died within 5 years. To improve mortality, surgeons have attempted resection of limited hepatic metastases. Early surgical reports of long term survival after this procedure were met with some skepticism.20 However, case series demonstrating long term survival with this intervention have continued to emerge and hepatic resection is now standard therapy for those with isolated liver metastases. In fact, US studies focusing on the use of this technique in experienced centers limited to the last fifteen years suggest that 5 year survival approaching 60% can be achieved.21, 22 Some groups have even reported long term 'cure' with this approach.23
Our population-based study suggests that the benefit from resection may be less than previously described by others. Five-year survival of 25.5% in Medicare beneficiaries is the lowest reported of any US study to date and is significantly lower than the results reported in the two largest US studies. The larger of the two studies was a multicenter trial that included 607 subjects and reported a 5 year survival of 33%.6 A single center report evaluating outcome in 466 patients determined 5-year survival to be 38%.7 Some of the difference may be explained by the fact that many surgical series report long term results only for those with negative margins at the time of resection. Margin status has been demonstrated to be an important factor in predicting long term survival.22 We are reporting survival after operation irrespective of postoperative margin status.
Another important finding of our study is that the early mortality risk associated with hepatic resection may be somewhat greater than previously thought. The 30-day mortality of 4.2% is higher than the 0–3.8% reported in many US studies. 7, 11–15 By 90 days, overall mortality nearly doubles to 8.3%. While these deaths may not be directly attributable to the resection (i.e. “surgical mortality”), such an estimate is useful to a patient considering the risks involved in this surgery. In fact, some other recent studies of hepatic resection have also employed the 90 day outcome measure because of the delayed nature of some of the complications (e.g. progressive liver failure) that occur directly related to this surgery.24, 25 While our results demonstrate a somewhat higher ‘early’ mortality than seen in most other US studies, they are quite consistent with another older US study using administrative data. Wade and colleagues9 utilized the VA patient treatment file and identified 161 male Veterans who underwent hepatic resection for colorectal cancer metastases. In that study 30 day and 90 day mortality were 4% and 8% respectively, nearly identical to ours. A 4.3% 30 day mortality was also observed by Cummings et al when using a cohort undergoing hepatic resection derived from SEER-Medicare data.17
This analysis highlights the higher risk of this operation in those of advancing age and with metastatic disease identified at the time of their initial colorectal cancer diagnosis. These findings have not been uniformly confirmed by other studies. At least five US studies7, 14, 16, 26, 27 found no significant association between age and outcome. Only one recent US study that focused exclusively on patients undergoing hepatic resection for colorectal metastases 15 found an effect similar to ours. Another very large (n=1059) surgical series of patients undergoing hepatic resection for various indications (nearly all malignant) has been reported.24 The indication for hepatic resection was colorectal cancer metastases in half the cases. Age was also an independent predictor of mortality in that study. It is important to note that when considering our analysis of age, our cohort is limited to those eligible for Medicare (age 65 or older). . However, the average age in the five studies that showed no association of mortality with age was greater than 60 and thus not substantially different from our cohort. It is possible that the outcomes of older subjects are better in these centers because the criteria for selection for operation may be more stringent than that which occurs more commonly in the community.
We also found a marked difference in outcome between those operated on for synchronous as opposed to metachronous metastases. Results from older case series were inconsistent in this area with some investigators finding increased risk for those with synchronous disease7, 14, 26, 28, 29 and others not.12, 15, 16, 27 While controversy in this area remains, most recent work suggests that synchronous resection can be considered30–34, but only in well selected patient populations. For example, age34 and extent of metastatic burden (and thus extent of hepatic resection required)30, 31, 33, 34 appear to be important factors increasing the risk of early morbidity and mortality from synchronous resection. Our work suggests that if such careful selection is required for these patients, it may not be regularly occurring on a national basis.
Our study’s main strength is its size and national representativeness. It is the largest study of hepatectomy performed to date and represents a complete national sample of individuals over age 65 undergoing operation from 2000–2004, excluding only those who were enrolled in HMOs, not part B entitled or received their operation in the VA. Most case series have emanated from single centers. The experience at these centers may well not reflect the broader national experience of patients undergoing resection.
Our study has a number of limitations. First, these results reflect only the experience of those greater than 65 years of age. Therefore, the generalizablity of our results to a younger cohort cannot be assessed. However, more than two-thirds of incident colorectal cancers occur in this age group.35 A second limitation is that we could only utilize information available in administrative data, and could not control for other important clinical variables such as positive tumor margins or hepatic tumor size.7 Because tumor margins cannot be predicted preoperatively, however, our study may provide more accurate estimates of the average risk experience of patients facing a decision about whether to attempt resection. Another potential limitation when utilizing administrative data relates to the accuracy of the coding. However, our analysis derives from the diagnostic codes for cancer and various surgical operations. Prior work suggests that coding for these items are quite good with sensitivities and specificities both greater than 90%.36 Third, while our study reports true risk associated with undergoing this operation, it does not compare the effectiveness of this therapy to other approaches for metastatic colorectal cancer (e.g. non operative). Given the lack of trials assessing the effectiveness of this intervention, this limitation applies to all studies in this area. Finally, we are describing the results after hepatic resection for the ‘average’ patient undergoing the procedure on a national level. It is possible that certain centers, because of patient selection and surgical expertise, perform substantially better than the results shown in this analysis. In fact, given the well documented association between volume and outcome for colorectal cancer resection37, 38 and hepatic resection39 this seems likely. However, our results are generalizable to the majority of patients who undergo this procedure and are not at the small number of centers with particular expertise in performing this operation.
In summary, estimates for survival following hepatic resection for metastatic colorectal derived from single center series may be overly optimistic. Based on our work a more reasonable 5 year survival for all comers is 25%. . The surgical risk of undergoing this intervention may be greater than estimated in previous case series. Specifically, conventional ’30 day mortality’ rates may not accurately reflect the early mortality experience by patients undergoing this operation. By 90 days, overall mortality was 8% and exceeded 10% in certain subgroups (>80; synchronous resection). Finally, advancing age and synchronous resection predict worse long term survival.
Some have suggested that randomized trials would not be appropriate to test the efficacy of surgical resection of isolated metastatic disease.7 However, our work suggests that it may be possible to identify some groups that incur the most up front risk from this operation while deriving the least long term benefit. For example, those over age 80 may be one such group. Trials directly comparing less invasive therapies with surgical resection of metastatses in term of effectiveness and quality of life in these populations might be considered. Finally, it appears that results achieved with surgical resection of colorectal metastases nationally may not be as good as that accomplished at select surgical centers with expertise in this area. Further work to better understand the major drivers of this observed disparity (e.g. patient selection) and programs to improve outcomes nationally are warranted.
Acknowledgments
Dr Robertson’s work is supported by a VA HSR&D Career Development Award. Financial support was provided by grants from the Robert Wood Johnson Foundation, the National Institutes of Health (Grant Number CA52192). The funding organizations had no role in the design, collection, analysis, interpretation of the data, or preparation of the manuscript
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007 Jan–Feb;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Wagner JS, Adson MA, Van Heerden JA, Adson MH, Ilstrup DM. The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann Surg. 1984 May;199(5):502–508. doi: 10.1097/00000658-198405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saltz LB, Cox JV, Blanke C, et al. Irinotecan Study Group. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000 Sep 28;343(13):905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 4.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000 Mar 25;355(9209):1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 5.Beard SM, Holmes M, Price C, Majeed AW. Hepatic resection for colorectal liver metastases: A cost-effectiveness analysis. Ann Surg. 2000 Dec;232(6):763–776. doi: 10.1097/00000658-200012000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes KS, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986 Aug;100(2):278–284. [PubMed] [Google Scholar]
- 7.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997 Mar;15(3):938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 8.Nordlinger B, Rougier P. Liver metastases from colorectal cancer: the turning point. J Clin Oncol. 2002 Mar 15;20(6):1442–1445. doi: 10.1200/JCO.2002.20.6.1442. [DOI] [PubMed] [Google Scholar]
- 9.Wade TP, Virgo KS, Li MJ, Callander PW, Longo WE, Johnson FE. Outcomes after detection of metastatic carcinoma of the colon and rectum in a national hospital system. J Am Coll Surg. 1996 Apr;182(4):353–361. [PubMed] [Google Scholar]
- 10.Steele G, Jr, Bleday R, Mayer RJ, Lindblad A, Petrelli N, Weaver D. A prospective evaluation of hepatic resection for colorectal carcinoma metastases to the liver: Gastrointestinal Tumor Study Group Protocol 6584. J Clin Oncol. 1991 Jul;9(7):1105–1112. doi: 10.1200/JCO.1991.9.7.1105. [DOI] [PubMed] [Google Scholar]
- 11.Bakalakos EA, Kim JA, Young DC, Martin EW., Jr Determinants of survival following hepatic resection for metastatic colorectal cancer. World J Surg. 1998 Apr;22(4):399–404. doi: 10.1007/s002689900404. discussion 404-395. [DOI] [PubMed] [Google Scholar]
- 12.Rosen CB, Nagorney DM, Taswell HF, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992 Oct;216(4):493–504. doi: 10.1097/00000658-199210000-00012. discussion 504-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuhrman GM, Curley SA, Hohn DC, Roh MS. Improved survival after resection of colorectal liver metastases. Ann Surg Oncol. 1995 Nov;2(6):537–541. doi: 10.1007/BF02307088. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins LT, Millikan KW, Bines SD, Staren ED, Doolas A. Hepatic resection for metastatic colorectal cancer. Am Surg. 1997 Jul;63(7):605–610. [PubMed] [Google Scholar]
- 15.Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994 Oct;116(4):703–710. discussion 710-701. [PMC free article] [PubMed] [Google Scholar]
- 16.Cady B, Stone MD, McDermott WV, Jr, et al. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg. 1992 May;127(5):561–568. doi: 10.1001/archsurg.1992.01420050085011. discussion 568–569. [DOI] [PubMed] [Google Scholar]
- 17.Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007 Feb 15;109(4):718–726. doi: 10.1002/cncr.22448. [DOI] [PubMed] [Google Scholar]
- 18.Cox DR, Oakes D. Analysis of Survival Data. New York: Chapman and Hall; 1984. [Google Scholar]
- 19.Harrell FE. Regression modeling strategies:with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 20.Silen W. Hepatic resection for metastases from colorectal carcinoma is of dubious value. Arch Surg. 1989 Sep;124(9):1021–1022. doi: 10.1001/archsurg.1989.01410090027004. [DOI] [PubMed] [Google Scholar]
- 21.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002 Jun;235(6):759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005 May;241(5):715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007 Oct 10;25(29):4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 24.Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007 May;204(5):854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. discussion 862-854. [DOI] [PubMed] [Google Scholar]
- 25.Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006 May 1;24(13):2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 26.Harmon KE, Ryan JA, Jr, Biehl TR, Lee FT. Benefits and safety of hepatic resection for colorectal metastases. Am J Surg. 1999 May;177(5):402–404. doi: 10.1016/s0002-9610(99)00070-7. [DOI] [PubMed] [Google Scholar]
- 27.Wanebo HJ, Chu QD, Vezeridis MP, Soderberg C. Patient selection for hepatic resection of colorectal metastases. Arch Surg. 1996 Mar;131(3):322–329. doi: 10.1001/archsurg.1996.01430150100019. [DOI] [PubMed] [Google Scholar]
- 28.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995 Jan–Feb;19(1):59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 29.Sugawara Y, Yamamoto J, Yamasaki S, Shimada K, Kosuge T, Makuuchi M. Estimating the prognosis of hepatic resection in patients with metastatic liver tumors from colorectal cancer with special concern for the timing of hepatectomy. Surgery. 2001 Apr;129(4):408–413. doi: 10.1067/msy.2001.112001. [DOI] [PubMed] [Google Scholar]
- 30.Bolton JS, Fuhrman GM. Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg. 2000 May;231(5):743–751. doi: 10.1097/00000658-200005000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capussotti L, Vigano L, Ferrero A, Lo Tesoriere R, Ribero D, Polastri R. Timing of resection of liver metastases synchronous to colorectal tumor: proposal of prognosis-based decisional model. Ann Surg Oncol. 2007 Mar;14(3):1143–1150. doi: 10.1245/s10434-006-9284-5. [DOI] [PubMed] [Google Scholar]
- 32.de Santibanes E, Lassalle FB, McCormack L, et al. Simultaneous colorectal and hepatic resections for colorectal cancer: postoperative and longterm outcomes. J Am Coll Surg. 2002 Aug;195(2):196–202. doi: 10.1016/s1072-7515(02)01235-8. [DOI] [PubMed] [Google Scholar]
- 33.Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007 Dec;14(12):3481–3491. doi: 10.1245/s10434-007-9522-5. [DOI] [PubMed] [Google Scholar]
- 34.Thelen A, Jonas S, Benckert C, et al. Simultaneous versus staged liver resection of synchronous liver metastases from colorectal cancer. Int J Colorectal Dis. 2007 Oct;22(10):1269–1276. doi: 10.1007/s00384-007-0286-y. [DOI] [PubMed] [Google Scholar]
- 35.Baranovsky A, Myers MH. Cancer incidence and survival in patients 65 years of age and older. CA Cancer J Clin. 1986 Jan–Feb;36(1):26–41. doi: 10.3322/canjclin.36.1.26. [DOI] [PubMed] [Google Scholar]
- 36.Fisher ES, Whaley FS, Krushat WM, et al. The accuracy of Medicare's hospital claims data: progress has been made, but problems remain. Am J Public Health. 1992 Feb;82(2):243–248. doi: 10.2105/ajph.82.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrag D, Panageas KS, Riedel E, et al. Surgeon volume compared to hospital volume as a predictor of outcome following primary colon cancer resection. J Surg Oncol. 2003 Jun;83(2):68–78. doi: 10.1002/jso.10244. discussion 78-69. [DOI] [PubMed] [Google Scholar]
- 38.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002 Apr 11;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 39.Choti MA, Bowman HM, Pitt HA, et al. Should hepatic resections be performed at high-volume referral centers? J Gastrointest Surg. 1998 Jan–Feb;2(1):11–20. doi: 10.1016/s1091-255x(98)80098-x. [DOI] [PubMed] [Google Scholar]


