Abstract
Involvement of dopamine neurotransmission in human emotional processing is unclear but animal studies have indicated that it is critical for processing of fear response. In this experiment we examined dopaminergic involvement in the processing of human emotions. We used a novel dynamic molecular imaging technique to detect and map dopamine released during presentation of emotional stimuli. The technique exploited the competition between endogenously released dopamine and its ligand for receptor occupancy and involved dynamic voxel-wise measurement of the rate at which a dopamine receptor ligand (18F-Fallypride) was displaced from receptor sites during emotional processing. An increase in the rate indicated dopamine release. We found that the rate of ligand displacement increased significantly in the left amygdala, left medial temporal lobe (MTL) and left inferior frontal gyrus. The results provide the first direct evidence of dopaminergic modulation of human emotional processing and suggest that the modulation occurs at multiple levels of processing. This finding indicates that the neurocognitive models of human emotion should take into account dopaminergic effects, and that, there is a need to investigate whether manipulation of the dopaminergic system could be an alternate strategy for treatment of conditions in which emotional processing is impaired.
Animal studies have suggested that dopamine neurotransmission is critically involved in fear processing. In rodents intra-amygdalar infusions of antagonists of both dopamine D1 (Lamont and Kokkinidis, 1998) and D2 (Greba et al., 2001) receptors block expression of fear conditioning and the agonists improve conditioning and expression of fear (White and Viaud, 1991). Dopamine is involved in the processing not only in the amygdala but also in the frontal cortex. Thus, frontal infusions of the D1 receptor antagonists impair retention of learned fear conditioning (Runyan and Dash, 2004) and prevent expression of fear-related behaviors (Shah et al., 2004). Predictably, the agonists cause enhanced fear (Borowski and Kokkinidis, 1998). The agents that modulate the D2 receptors have opposite effects. Thus, the D2 antagonist metoclopramide induces higher and inappropriate fear response (Blackburn and Phillips, 1990) while the agonist quinpirole blocks the acquisition of fear conditioning and retrieval of emotional memories (Nader and LeDoux, 1999). In addition to the amygdala and frontal cortex, the hippocampus is also involved in fear processing and dopamine dependent amygdalo-hippocampal connectivity plays a critical role in the processing (Richardson et al., 2004).
It is unclear whether dopamine has similar involvement in human emotional processing. However, impairment of emotional expression in patients (e.g. Parkinson’s disease) with dysregulated dopamine neurotransmission (Lawrence et al., 2007) suggests that dopamine may have a role in the processing. This is further supported by the observation that dopamine receptor antagonist disrupts encoding of emotional stimuli in human volunteers (Mehta et al., 2005). While these observations are suggestive of dopaminergic involvement, there is no direct evidence. In the present study we examined dopaminergic activity in healthy volunteers during emotional processing.
MATERIALS AND METHODS
The study was conducted on native English speaking healthy young volunteers (n=8; mean age 24 years) of either sex (male=3). All volunteers were right-handed and had no history of a psychiatric or neurological disorder or that of drug dependence or addiction. After informed consents were obtained, volunteers were positioned in a positron emission tomography (PET) camera and given an intravenous bolus injection of a high affinity dopamine receptor ligand 18F-fallypride (5–8 mCi) at high specific activity (mean activity 6199 mCi/micromole). Immediately after the injection, cognitive task and PET data acquisition began. The data were acquired in 30 sec frames during the first 5 min and in 60 sec frames thereafter using ECAT EXACT HR+ PET camera operating in 3D mode.
The task consisted of a Control and a Test condition. In the Control, a list of emotionally neutral words (e.g., PARK, PENCIL) was shown and volunteers were asked to indicate the intensity of emotion elicited by each word in a scale of 1–3 (1=no emotion; 3=intense emotion) by pressing a corresponding key. After 25 min (Test condition), unbeknownst to volunteers, neutral words were replaced by words having emotional connotation (e.g., FIRE, BLOOD). The Test condition was administered for 20 min. In both conditions (Control and Test) stimuli were presented for 4500 msec and each stimulus was followed by a cross-mark (500 msec). A 15 sec break was allowed at the end of each 3-min block. The words used in the Control and Test conditions were carefully selected to ensure that they are commonly used in American English (Kucera and Francis, 1967) and elicit either neutral (Control condition) or negative (Test condition) emotion. Selection of stimuli used in the Control and Test conditions involved two steps. In the first step a list of neutral and emotional words was prepared by adapting stimuli from various sources (e.g., Wilhelm et al., 1996; Lang et al., 1999; Budson et al., 2006). In the second step we identified words that most frequently elicited either neutral or negative emotions in a pilot study. Further, we asked the volunteers to rate emotional arousal for each word to ensure that the stimuli presented in the Test condition elicited stronger emotions than those shown in the Control.
Molecular Imaging
For mapping dopaminergic activity, we used a molecular imaging technique, which exploits the competition between an injected ligand and neurotransmitter for occupancy of the same receptor binding sites. Because of this competition, dopamine released during task performance, displaces its radiolabeled ligand from receptor sites. The displacement is detected by dynamically measuring the ligand concentration using a PET camera. We used this technique in earlier experiments to detect and map striatal dopamine released during performance of cognitive and behavioral tasks (Badgaiyan et al., 2003; Badgaiyan et al., 2007; Badgaiyan et al., 2008).
We had to modify the molecular imaging technique we used in earlier studies because this experiment required detection of dopaminergic activity in the brain areas located out side the striatum. Since dopamine receptor density is low in these areas, the ligand raclopride, used in our previous studies (Badgaiyan et al., 2003; Badgaiyan et al., 2007; Badgaiyan et al., 2008), cannot be employed because it does not bind or displace in detectable amounts in low-density areas (Badgaiyan et al., 2003). Therefore, in this experiment we used a high affinity dopamine receptor ligand fallypride, which binds in detectable amounts and displaces from receptor sites in extrastriatal areas in response to pharmacological (Mukherjee et al., 2002) and cognitive (Christian et al., 2006) challenges.
PET Data Analyses
The procedures used for analysis of PET data were essentially similar to those used in our earlier studies (Badgaiyan et al., 2003; Badgaiyan et al., 2007; Badgaiyan et al., 2008) and involved the following steps: The images were reconstructed as 128×128×63 element volumes using a standard three-dimensional filtered back projection algorithm with corrections for photon attenuation, random coincidences, scatter, and dead time. To minimize residual effects of head movements, images were registered to align each frame to a common orientation, using the following procedure: First, all frames were smoothed with a 5 mm FWHM Gaussian filter, then the variation in spatiotemporal distribution was corrected by registration of temporally adjacent frames, and finally, using a transformation matrix, all frames were aligned to a reference frame (the frame acquired at 25 min). Thereafter, a voxel-wise analysis of data was carried out on each subject using the kinetic model (discussed below) designed to detect transient changes in ligand displacement. Using this model, quantitative maps of kinetic parameters were generated for each volunteer. The data were then pooled to acquire cohort means and variances. This involved elastic registration of the sum of the image data of each subject, to a standard template, using the statistical parametric mapping software (SPM99; Wellcome Department of Imaging Neuroscience, London). The transformation parameters were then applied to the parametric images to pool data across volunteers. A voxel-wise t-map was then computed to localize voxels where increased rate of ligand displacement was observed after the task initiation (Test condition). Finally, time-activity curves were drawn for the voxels showing maximum ligand displacement. The cerebellum was used as a reference region (because of paucity of dopamine receptors in this region), and a time activity curve for this region was drawn to estimate the clearance rate of free and nonspecifically bound ligand.
The Kinetic Model
A detailed description of the kinetic model used to analyze the PET data can be found in our earlier publication (Alpert et al., 2003). This model is essentially a modified version of the simplified reference region model (SRRM), which accounts for time dependent changes in the ligand concentration (Friston et al., 1997). There was a need to modify the SRRM because it assumes a steady physiological state throughout the experiment. This assumption was not compatible with the experimental design that involves a change of task condition from Control to Test. The modified model therefore assumes that the steady state was not maintained. To eliminate the assumption we allowed the dissociation rate of ligand to change in response to an altered synaptic level of neurotransmitter. This was done by introducing a term γ·exp(−τ (t − T)) •ν(t − T) in the dissociation parameter of SRRM. In the modified model, γ represents the amplitude of ligand displacement, τ accounts for initial burst release of dopamine, t denotes the measurement time, T is the time of change in transmitter level, and ν is the unit step function. Using the least squares fitting procedure on a voxel-by-voxel basis, receptor binding parameters and γ are estimated. The null hypothesis assumes that the task did not elicit dopamine release and there was no change in the rate of ligand displacement (i.e., γ=0). This hypothesis was tested in each subject and the ‘γ’ values were pooled across subjects to acquire a cohort mean and variance. Additionally, we estimated the parameters that describe ligand transport and binding, and the time dependent effects elicited by the task. The solution of the differential equations describing the model for the instantaneous concentration history of the ligand has the following form:
where, CR is the concentration of radioligand in a region devoid of specific binding (reference), PET is the concentration of radioligand in a voxel with specific binding, R is the ratio of transport rates for the binding and reference regions, k2 describes the clearance of nonspecifically bound tracer from the voxel, and k2a includes information about dissociation from the receptor, γ represents the amplitude of transient effects, t denotes the measurement time, T is the task initiation time and ν(u-T) is the unit step function.
RESULTS
There was a significant difference (p< 0.0001) in the intensity of emotion elicited by words included in the Control and Test conditions. Mean score (1=no emotion; 3=intense emotion) in the Control was 1.43±0.08 while it was 2.24±0.18 in the Test condition. There was no significant difference in the mean response time. It was 1435±102 and 1346±136 msec in the Control and Test conditions respectively. In the debriefing, volunteers indicated that many of the emotional (mean 27±5%) and a relatively small number of neutral (mean 8±4%) words elicited past memories.
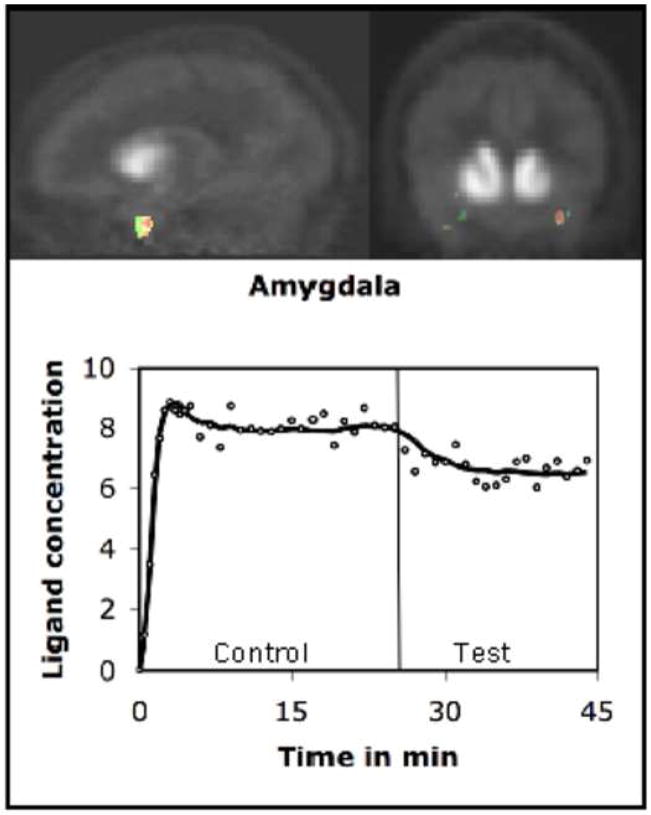
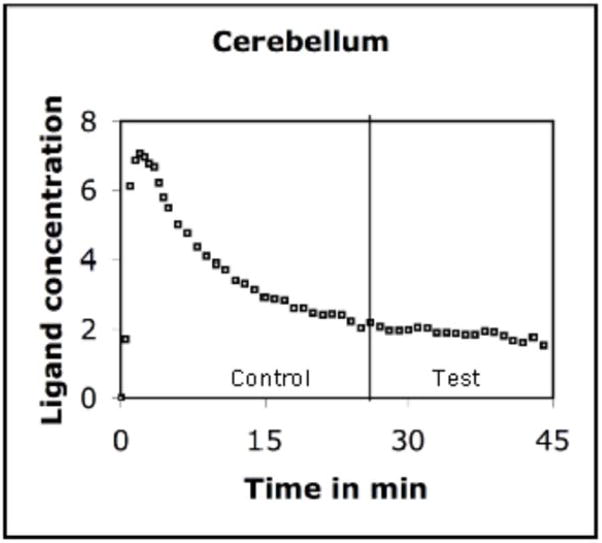
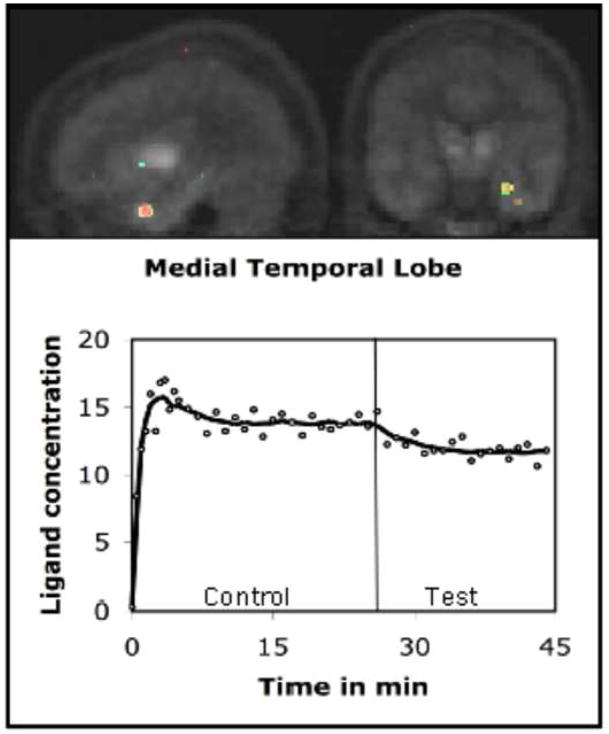
The PET data were analyzed using the kinetic model described above. The model uses receptor kinetic parameters for voxel-wise estimation of changes in the rate of ligand displacement during task performance. Analysis of the PET data indicated that the rate increased significantly in a number of brain regions during presentation of emotional words (Test condition). We computed a voxel-wise t-map of the difference in the rate measured in the Control and Test conditions. These maps were then pooled across volunteers for computation of group mean. The mean indicated a significant increase in the rate of ligand displacement in the left amygdala (t=3.46), left MTL (t=3.28), and left inferior frontal gyrus (t=3.52) in the Test condition. Approximate stereotactic (Talairach) coordinates of the points of maximum displacement were (x,y,z) −25, −6, −16 for amygdala; −29, −16, −12 for MTL and −40,20,12 for inferior frontal gyrus. We would however like to point out that the accuracy of these locations is constrained by relatively poor spatial localization of PET images. Further, we computed time activity curves using the data acquired in activated regions (Figures 1–3). The curve was also drawn for a reference region (cerebellum) where no significant task-induced change was observed (Figure 4).
FIGURE 1.
Dopamine was released in the amygdala during emotional processing. The figure shows t-maps of the rate of ligand (18F-Fallypride) displacement before and after task initiation. The maps were superimposed on the mean PET images and represent changes across volunteers. The time-activity curve shows the concentration history (circles) and least square fits (solid line) for the ligand in the activated region of a volunteer. There was a significant increase in the rate of ligand displacement after task was initiated (vertical line). The ligand concentration is expressed as kBq/cc.
FIGURE 3.
The areas of the inferior frontal gyrus where dopamine was released during emotional processing. The time activity curve suggests significant decrease in the ligand concentration (expressed as kBq/cc) after task initiation (vertical line).
FIGURE 4.
There was no change in the rate of ligand displacement after task initiation in the cerebellum, which was used as a reference region because of paucity of dopamine receptors in this area. The curve indicates that nonspecific binding of the ligand was not affected by the task. The ligand concentration is expressed as kBq/cc.
DISCUSSION
The findings of this experiment have significant theoretical and clinical implications. The experiment provides for the first time, a direct evidence of dopaminergic involvement in the processing of human emotions. The observation of increased dopaminergic activity in the amygdala, MTL and inferior frontal gyrus is consistent with the findings of previous structural and functional studies that have implicated these areas in human emotional processing (Gur et al., 2002).
The results suggest that dopamine modulates human emotional processing at multiple levels in some of the same areas that process fear response in laboratory animals (Greba et al., 2001). These areas include the amygdala, MTL and frontal cortex. Further, the observation of dopamine release in all three structures that communicate extensively during emotional processing (LaBar and Cabeza, 2006), indicate that the communication between these structures could be mediated by dopamine neurotransmission. Since these communications are critical for activation of motivational and mnemonic components of emotion (LeDoux, 1993; Packard and Wingard, 2004; Phelps, 2004; Richardson et al., 2004; Peleg-Raibstein et al., 2005; LaBar and Cabeza, 2006), dopamine appears to have an important role in human emotional processing.
The results are consistent with the neurocognitive models that have implicated the amygdala, MTL and frontal cortex in emotional processing (Yancey and Phelps, 2001; Phelps, 2004; LaBar and Cabeza, 2006). Based on the data acquired in this experiment, these models can be further extended to include the role of dopamine neurotransmission in the processing. Further, our findings of dopaminergic modulation of human emotion explain why the processing of emotional expressions is impaired in Parkinson’s (Lawrence et al., 2007) and schizophrenic (Laviolette, 2007) patients who have dysregulated dopamine neurotransmission. In addition, this experiment provides empirical data to support the hypothesis that dopaminergic agents (cocaine and amphetamine) induce addictive behavior by activating the emotional memory system in the amygdala, frontal and hippocampal areas (Nestler and Carlezon, 2006)
In animals, dopamine is known to influence consolidation and retrieval of emotional memories (White and Viaud, 1991; Lamont and Kokkinidis, 1998; Greba et al., 2001). It may have a similar role in the human brain but in the present experiment it is difficult to identify mnemonic component that elicited dopamine release because separation of the encoding and retrieval processes in human cognitive experiments is difficult (Buckner et al., 2001).
The results indicate that human and animal emotional processing involves the same brain areas and neurotransmission but differ in lateralization. The dopaminergic effect in animals is not lateralized, but it appears to be lateralized in the human brain. In this experiment we observed dopamine release only in the left hemisphere. fMRI experiments have also reported lateralized (mostly left) activation and it has been observed that after damage to the left amygdala, emotional encoding function is transferred to the left hippocampus, and not to the right amygdala (Richardson et al., 2004). These studies also support left lateralization of human emotional processing. Since, there is little consensus on the suggestion that the lateralization is based on the gender (left lateralization in female) or valence (left lateralization for positive valence) (Hamann and Canli, 2004), the reason for lateralized activity is unclear. It probably reflects hemispheric functional specialization of the human brain.
Our results suggest that dopamine may have significant modulatory effect on human emotional processing. It not only modulates activities of the areas that process emotions but regulates the amygdala-hippocampal and amygdala-frontal connectivities, which are important components of the processing (LeDoux, 1993; Packard and Wingard, 2004; Phelps, 2004; Richardson et al., 2004; Peleg-Raibstein et al., 2005; LaBar and Cabeza, 2006). The data indicate that neurocognitive models of human emotional processing should account for dopaminergic effects and that there is a need to investigate the role of dopaminergic agents as an alternate strategy for treatment of conditions (like posttraumatic stress disorder) in which emotional processing is dysregulated.
FIGURE 2.
The figure (t-map) shows the left medial temporal lobe (MTL) area where dopamine was released during emotional processing. The time-activity curve depicts the concentration history of the ligand in the activated MTL region, which included the hippocampus and parahippocampus. The ligand concentration (expressed as kBq/cc) reduced significantly in this area after task initiation (vertical line).
Acknowledgments
NIH (1R21MH073624 and 1R21MH079435), Dana Foundation, and Shriners Hospital for Children.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alpert NM, Badgaiyan RD, Livini E, Fischman AJ. A novel method for noninvasive detection of neuromodulatory changes in specific neurotransmitter systems. NeuroImage. 2003;19:1049–1060. doi: 10.1016/s1053-8119(03)00186-1. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Striatal dopamine release during unrewarded motor task in human volunteers. Neuroreport. 2003;14:1421–1424. doi: 10.1097/00001756-200308060-00003. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Striatal dopamine release in sequential learning. NeuroImage. 2007;38:549–556. doi: 10.1016/j.neuroimage.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Explicit Motor Memory Activates the Striatal Dopamine System. NeuroReport. 2008;19:409–412. doi: 10.1097/WNR.0b013e3282f6435f. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Phillips AG. Enhancement of freezing behaviour by metoclopramide: implications for neuroleptic-induced avoidance deficits. Pharmacol Biochem Behav. 1990;35:685–691. doi: 10.1016/0091-3057(90)90308-5. [DOI] [PubMed] [Google Scholar]
- Borowski TB, Kokkinidis L. The effects of cocaine, amphetamine, and the dopamine D1 receptor agonist SKF 38393 on fear extinction as measured with potentiated startle: implications for psychomotor stimulant psychosis. Behav Neurosci. 1998;112:952–965. doi: 10.1037//0735-7044.112.4.952. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME, Sheridan MA. Encoding processes during retrieval tasks. J Cogn Neurosci. 2001;13:406–15. doi: 10.1162/08989290151137430. [DOI] [PubMed] [Google Scholar]
- Budson AE, Todman RW, Chong H, Adams EH, Kensinger EA, Krangel TS, Wright CI. False recognition of emotional word lists in aging and Alzheimer disease. Cogn Behav Neurol. 2006;19:71–78. doi: 10.1097/01.wnn.0000213905.49525.d0. [DOI] [PubMed] [Google Scholar]
- Christian BT, Lehrer DS, Shi B, Narayanan TK, Strohmeyer PS, Buchsbaum MS, Mantil JC. Measuring dopamine neuromodulation in the thalamus: using [F-18]fallypride PET to study dopamine release during a spatial attention task. Neuroimage. 2006;31:139–152. doi: 10.1016/j.neuroimage.2005.11.052. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Malizia AL, Wilson S, Cunningham VJ, Jones T, Nutt DJ. Analysis of dynamic radioligand displacement or “activation” studies. J Cereb Blood Flow Metab. 1997;17:80–93. doi: 10.1097/00004647-199701000-00011. [DOI] [PubMed] [Google Scholar]
- Greba Q, Gifkins A, Kokkinidis L. Inhibition of amygdaloid dopamine D2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res. 2001;899:218–226. doi: 10.1016/s0006-8993(01)02243-0. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–87. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, Kohler C, Alsop D, Maldjian J, Ragland JD, Gur RC. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- Hamann S, Canli T. Individual differences in emotion processing. Curr Opin Neurobiol. 2004;14:233–238. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present-day American English. Brown University Press; Providence, Rhode Island: 1967. [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lamont EW, Kokkinidis L. Infusion of the dopamine D1 receptor antagonist SCH 23390 into the amygdala blocks fear expression in a potentiated startle paradigm. Brain Res. 1998;795:128–136. doi: 10.1016/s0006-8993(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings 1999 [Google Scholar]
- Laviolette SR. Dopamine modulation of emotional processing in cortical and subcortical neural circuits: evidence for a final common pathway in schizophrenia? Schizophr Bull. 2007;33:971–981. doi: 10.1093/schbul/sbm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AD, Goerendt IK, Brooks DJ. Impaired recognition of facial expressions of anger in Parkinson’s disease patients acutely withdrawn from dopamine replacement therapy. Neuropsychologia. 2007;45:65–74. doi: 10.1016/j.neuropsychologia.2006.04.016. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory systems in the brain. Behav Brain Res. 1993;58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Hinton EC, Montgomery AJ, Bantick RA, Grasby PM. Sulpiride and mnemonic function: effects of a dopamine D2 receptor antagonist on working memory, emotional memory and long-term memory in healthy volunteers. J Psychopharmacol. 2005;19:29–38. doi: 10.1177/0269881105048889. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, Mantil J. Brain imaging of 18F-fallypride in normal volunteers: Blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- Nader K, LeDoux JE. Inhibition of the mesoamygdala dopaminergic pathway impairs the retrieval of conditioned fear associations. Behav Neurosci. 1999;113:891–901. doi: 10.1037//0735-7044.113.5.891. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WAJ. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Packard MG, Wingard JC. Amygdala and “emotional” modulation of the relative use of multiple memory systems. Neurobiol Learn Mem. 2004;82:243–252. doi: 10.1016/j.nlm.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Pezze MA, Ferger B, Zhang WN, Murphy CA, Feldon J, Bast T. Activation of dopaminergic neurotransmission in the medial prefrontal cortex by N-methyl-d-aspartate stimulation of the ventral hippocampus in rats. Neuroscience. 2005;132:219–232. doi: 10.1016/j.neuroscience.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Dash PK. Intra-medial prefrontal administration of SCH-23390 attenuates ERK phosphorylation and long-term memory for trace fear conditioning in rats. Neurobiol Learn Mem. 2004;82:65–70. doi: 10.1016/j.nlm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Shah AA, Sjovold T, Treit D. Selective antagonism of medial prefrontal cortex D4 receptors decreases fear-related behaviour in rats. Eur J Neurosci. 2004;19:3393–3397. doi: 10.1111/j.0953-816X.2004.03447.x. [DOI] [PubMed] [Google Scholar]
- White NM, Viaud M. Localized intracaudate dopamine D2 receptor activation during the post- training period improves memory for visual or olfactory conditioned emotional responses in rats. Behav Neural Biol. 1991;55:255–69. doi: 10.1016/0163-1047(91)90609-t. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, McNally RJ, Baer L, Florin I. Directed forgetting in obsessive-compulsive disorder. Behav Res Ther. 1996;34:633–641. doi: 10.1016/0005-7967(96)00040-x. [DOI] [PubMed] [Google Scholar]
- Yancey SW, Phelps EA. Functional neuroimaging and episodic memory: a perspective. J Clin Exp Neuropsychol. 2001;23:32–48. doi: 10.1076/jcen.23.1.32.1220. [DOI] [PubMed] [Google Scholar]