Abstract
Background
Treatment of hyperlipidemia produces functional and structural improvements in atherosclerotic vessels. However, the effects of treating hyperlipidemia on the structure and function of the aortic valve has been controversial, and any effects could be confounded by pleiotropic effects of hypolipidemic treatment. The goal of this study was to determine whether reducing elevated plasma lipid levels with a “genetic switch” in Reversa mice (Ldlr−/−/Apob100/100/Mttpfl/fl/Mx1Cre+/+) reduces oxidative stress, reduces proosteogenic signaling, and retards the progression of aortic valve disease.
Methods and Results
After 6 months of hypercholesterolemia, Reversa mice exhibited increases in superoxide, lipid deposition, myofibroblast activation, calcium deposition, and pro-osteogenic protein expression in the aortic valve. Maximum aortic valve cusp separation, as judged by echocardiography, was not altered. During an additional 6 months of hypercholesterolemia, superoxide levels, valvular lipid deposition, and myofibroblast activation remained elevated. Furthermore, calcium deposition and pro-osteogenic gene expression became more pronounced and the aortic cusp separation decreased from 0.85 ± 0.04 to 0.70 ± 0.04 mm (mean ± SE; p < 0.05). Rapid normalization of cholesterol levels at 6 months of age (by inducing expression of Cre recombinase) normalized aortic valve superoxide levels, decreased myofibroblast activation, reduced valvular calcium burden, suppressed pro-osteogenic signaling cascades, and prevented the reductions in aortic valve cusp separation.
Conclusions
Collectively, these data indicate that reducing plasma lipid levels by genetic inactivation of the mttp gene in hypercholesterolemic mice with early aortic valve disease normalizes oxidative stress, reduces pro-osteogenic signaling, and halts the progression of aortic valve stenosis.
Keywords: free radicals, hypercholesterolemia, aortic valve stenosis, calcification
INTRODUCTION
Replacement of the aortic valve is the primary treatment for patients with symptomatic calcific aortic valve stenosis, and is the second most common thoracic surgery procedure in the United States 1. Risk factors for the development of aortic valve stenosis are similar to those of atherosclerosis and include older age 2, male sex, hypertension, smoking, diabetes mellitus 1, 2, and hypercholesterolemia 2. Stenotic aortic valves resemble atherosclerotic lesions pathologically, and contain calcium 3, high levels of matrix-remodeling enzymes 4–6, reduced endothelial nitric oxide synthase levels 7, and increased oxidative stress 8–10. Both stenotic valves and atherosclerotic lesions contain a subpopulation of cells with osteoblast-like activity 11–14, suggesting that deposition of calcium in these lesions is an active process.
Reducing plasma cholesterol levels in humans and experimental animals slows the progression and/or reduces the size of atherosclerotic lesions 15, 16, reduces oxidative stress 17, and improves nitric oxide bioavailability 15, 18, all of which might be expected to improve aortic valve disease. However, increases in collagen content, plaque fibrosis, and calcium burden (which may remain unchanged or increase) during regression of atherosclerotic lesions 15, 19 could translate into increased aortic valve cusp stiffness and could be deleterious for aortic valve function. Thus, it has been difficult to predict whether aggressive lipid lowering would be beneficial for the aortic valve.
Initial reports from retrospective studies suggested that reducing plasma lipids with “statins” slowed the progression of aortic valve stenosis in humans 20, 21. Three recently completed prospective clinical trials [SALTIRE 22, RAAVE 23, and SEAS24], however, have yielded conflicting results regarding lipid-lowering therapy. Although the SALTIRE and SEAS trials strongly suggest that simvastatin (or combined simvastatin and ezetimibe treatment24) does not slow progression of aortic valve stenosis in patients with borderline-high cholesterol levels, patients with slightly higher blood lipid levels showed a modest benefit of rosuvastatin on the progression of aortic valve stenosis in the RAAVE trial. Though reconciling these conflicting results is difficult (slight differences in patient populations, differing pleiotropic effects of the “statins” 25, etc.), no study has determined whether initiation of lipid-lowering therapy in early stages of disease can halt or reverse the progression to hemodynamically significant aortic valve stenosis.
Progress in understanding mechanisms underlying the progression of aortic valve stenosis has been slowed by the lack of animal models. Hypercholesterolemic rabbits and single-allele knockout mice develop histological evidence of aortic valve sclerosis 12, 26, but rarely develop hemodynamically significant aortic valve stenosis 27. Recently, we reported that “apo-B100–only” low density lipoprotein receptor–deficient mice (Apob100/100/Ldlr–/–) have severe hypercholesterolemia and that approximately one-third develop severe aortic valve stenosis 10.
In the current study, we used Ldlr–/–/Apob100/100 mice that were also homozygous for a conditional knockout allele in microsomal triglyceride transfer protein (Mttp) and the interferon-inducible Mx1-Cre transgene (“Reversa mice”) 28. The Mttp gene plays a critical role in production of apolipoprotein B-containing lipoproteins, and loss of Mttp activity dramatically reduces secretion of ApoB-containing lipoproteins into the plasma 29. Thus, Cre-mediated inactivation Mttp in these mice allowed us to “switch off” the severe hypercholesterolemia and test the effects on aortic valve stenosis—avoiding off-target effects of statin drug therapy. We hypothesized that lowering lipid levels with this “genetic switch” in early stages of aortic valve disease would retard progression towards aortic valve stenosis.
METHODS
Animals
At 6–8 weeks of age, littermates were assigned to either “control”, “progression,” or “regression” groups. Control mice were given 4 injections of polyinosinic-polycytidylic acid (pI-pC, 225 µg, i.p.) at two-day intervals and maintained on a chow diet for 6 or 12 months. Progression mice were placed on a Western diet (Harlan Teklad #TD88137, 42% of calories from fat, 0.25% cholesterol) for 6 or 12 months. Regression mice were placed on a Western diet for 6 months, and then were given 4 injections of pI-pC (225 µg, i.p.), switched to a chow diet, and followed for an additional 6 months.
Measurement of blood lipids, oxidative stress, histology, immunohistochemistry, and aortic valve function
See detailed descriptions in online supplement.
Statistical analyses
All data are reported as mean ± SE. Significant differences between groups were detected using an analysis of variance, and Bonferroni-corrected t-tests were used for post hoc testing.
RESULTS
Plasma lipid levels
Total plasma cholesterol levels in 6- and 12-month-old pI-pC–treated mice on a chow diet (“control” groups) are reported in Table 1. Total plasma cholesterol levels in 6- and 12-month-old Reversa mice on a Western diet (“hypercholesterolemic/progression” groups) were significantly elevated versus control mice (see Table 1). Switching off Mttp expression after 6 months of hypercholesterolemia (“regression” group) reduced total plasma cholesterol levels by ∼75% (see Table 1).
Table 1.
Body weight, blood lipid, blood glucose, and insulin levels before and after normalization of blood lipids.
| Control |
Hypercholesterolemic |
“Reversed” |
|||
|---|---|---|---|---|---|
| 6mo |
12mo |
6mo |
12mo |
12mo |
|
| Body Weight (g) | 20.0 ± 0.9 | 20.8 ± 0.6 | 22.8 ± 0.9* | 24.7 ± 0.8* | 22.4 ± 1 |
| Plasma Cholesterol Levels (mg/dl) | 157 ± 20 | 145 ± 27 | 997 ± 87* | 828 ± 89* | 228 ± 30# |
| Whole Blood Glucose (mg/dl) | 193 ± 17 | 187 ± 14 | 217 ± 29 | 204 ± 13 | 180 ± 13 |
| Plasma insulin (ng/L) | 175 ± 13 | 283 ± 20 | 263 ± 18 | 478 ± 24* | 441 ± 45 |
denotes p < 0.05 versus the age-matched control group;
denotes p < 0.05 versus age-matched hypercholesterolemic groups. All measurements were obtained in non-fasting animals (n = 8–12 per group)
Whole blood glucose and plasma insulin levels
Whole blood glucose levels were not significantly changed by any treatment in 6- or 12-month-old month Reversa mice. Compared to control mice, however, plasma insulin levels were significantly increased in 6 and 12 month hypercholesterolemic mice (Table 1). Reducing cholesterol levels after 6 months of hypercholesterolemia did not reduce plasma insulin levels compared to 6 or 12 month old hypercholesterolemic mice (Table 1).
Valvular oxidative stress
In the hypercholesterolemic/progression groups, superoxide levels in the aortic valve were increased markedly after 6 months of hypercholesterolemia (compared to control mice) and remained elevated at 12 months (Figure 1). Reducing cholesterol levels after 6 months of hypercholesterolemia significantly reduced valvular superoxide levels at 12 months (Figure 1).
Figure 1.
Superoxide in aortic valve before and after normalization of blood lipids. A) and B) 6 and 12 month control; C) and D) 6 and 12months hypercholesterolemia; E) 12 month “reversed”; F) mean data for all mice (8–12 per group). Note that superoxide levels were markedly increased in both 6 and 12 month hypercholesterolemic animals, and were completely normalized by reduction of blood lipids in the “reversed” animals. Arrows highlight areas of positive staining in aortic valve tissue. *, p < 0.05 versus the time-matched control group; #, p< 0.05 versus 12 month hypercholesterolemic group.
Histological changes in the aortic valve
In control mice that were given pI-pC at 6–8 weeks of age, lipid deposition in the aortic valve was negligible at both 6 and 12 months. In contrast, lipid deposition was significantly increased in mice in the hypercholesterolemic/progression group at six months, and remained high at 12 months (Figure 2). In the regression group, normalizing blood lipids after 6 months of hypercholesterolemia significantly reduced valvular lipid content at 12 months (p < 0.05 versus the 12-month hypercholesterolemic group).
Figure 2.
Lipid (red staining) in aortic valve before and after normalization of blood lipids. A) and B) 6 and 12 month control; C) and D) 6 and 12months hypercholesterolemia; E) 12 month “reversed”; F) mean data for all mice (8–12 per group). Valvular lipid deposition was significantly increased in hypercholesterolemic mice at both 6 and 12 months. Arrows indicate aortic valve tissue. Normalizing blood lipids produced significant reductions in valvular lipid in “reversed” animals. *, p < 0.05 versus the time-matched control group; #, p< 0.05 versus 12 month hypercholesterolemic group.
In control mice, we rarely detected macrophages in the aortic valve at 6 and 12 months (Figure 3). Macrophage infiltration was significantly increased in the hypercholesterolemic/progression animals at both 6 and 12 months (Figure 3). Normalizing cholesterol levels after 6 months of hypercholesterolemia significantly reduced macrophage staining at the 12-month time point (Figure 3).
Figure 3.
Macrophages (brown staining) in aortic valve before and after normalization of blood lipids. A) and B) 6 and 12 month control; C) and D) 6 and 12 months hypercholesterolemia; E) 12 month “reversed”; F) mean data for all animals studied (8–12 per group). Macrophage immunostaining was markedly increased in hypercholesterolemic mice at 6 and 12 months, and was almost completely eliminated by lowering blood lipid levels in “reversed” mice. Arrows indicate aortic valve tissue. *, p < 0.05 versus the time-matched control group; #, p< 0.05 versus 12 month hypercholesterolemic group.
In control mice, both valvular mineralization (Von Kossa, figure S1 online) and calcification (Alizarin Red, Figure 4) were negligible at the 6- and 12-month time points. In the hypercholesterolemic/progression mice, valvular mineralization and calcification were significantly elevated after 6 months of hypercholesterolemia, and increased further after 12 months of hypercholesterolemia (Figures S2 and 4). Reducing cholesterol levels with the genetic switch after 6 months of hypercholesterolemia prevented the increases in valvular mineralization and calcification at 12 months (p < 0.05 versus 12-month hypercholesteremic/progression mice, Figure S1 and 4).
Figure 4.
Calcification (red staining) of aortic valve before and after normalization of lipid levels. A) and B) 6 and 12 month control; C) and D) 6 and 12 months hypercholesterolemia; E) 12 month “reversed”; F) mean data for all animals studied (8–12 per group). Calcium burden was significantly increased in hypercholesterolemic mice at 6 and 12 months. Normalizing blood lipids produced significant reductions in valvular calcium burden in “reversed” mice. Arrows indicate aortic valve tissue. *, p < 0.05 versus the time-matched control group; #, p< 0.05 versus 12 month hypercholesterolemic group.
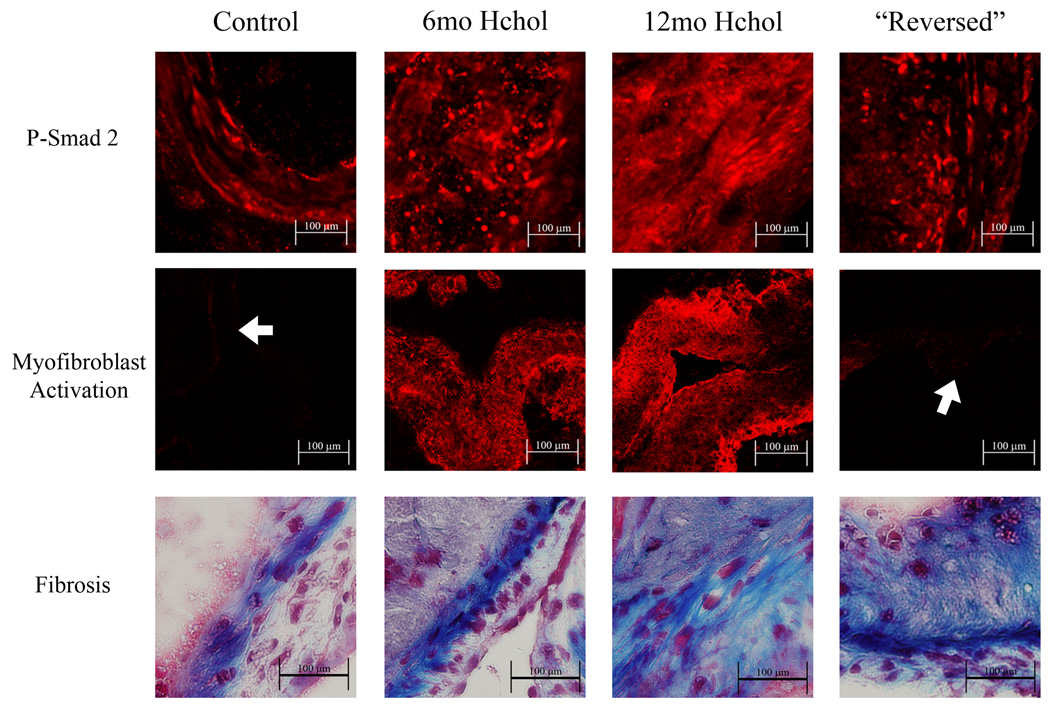
Pro-fibrotic signaling, myofibroblast activation, and fibrosis in the aortic valve
Levels of phospho-Smad2 were very low in valves from control mice (Figure 5). After 6 and 12 months of hypercholesterolemia, phospho-Smad2 was markedly increased (Figure 5). The increased phospho-Smad2 immunofluorescence was attenuated by reducing cholesterol levels after 6 months of hypercholesterolemia (Figure 5).
Figure 5.
Phospho-Smad2 immunofluorescence, alpha-smooth muscle actin immunofluorescence, and collagen (blue) staining and in the aortic valve before and after normalization of lipid levels. P-Smad2 levels were markedly increased in 6 and 12 month hypercholesterolemic mice. Normalizing blood lipids significantly reduced the amount of P-Smad2 immunofluorescence in “Reversed” mice. Alpha-smooth muscle actin immunofluorescence was increased in 6 and 12 month hypercholesterolemic mice, was virtually non-detectable in “Reversed” mice. Collagen deposition also progressively increased in hypercholesterolemic mice from 6 to 12 months, but was not reduced in “Reversed” mice. Arrows denote areas of aortic valve tissue when immunofluorescence is very faint.
Expression of smooth muscle α-actin was rarely detected in aortic valves from control mice at 6 or 12 months (Figure 5). Following both 6 and 12 months of hypercholesterolemia, there was significant expression of α-actin in the aortic valve (Figure 5). Normalizing cholesterol levels after 6 months of hypercholesterolemia virtually eliminated smooth muscle α-actin immunofluorescence in the aortic valve at 12 months (Figure 5).
In control mice, collagen staining was restricted to a narrow band in the fibrosa of the valve. After 6 and 12 months of hypercholesterolemia, collagen deposition extended beyond the fibrosa layer and was found in association with lipid-laden plaques (Figure 5). The increased collagen staining was not detectably altered by reducing cholesterol levels after 6 months of hypercholesterolemia.
Pro-calcific signaling in the valve
Phospho-Smad1/5/8 levels were relatively low in control mice at 6 and 12 months, and were dramatically increased in hypercholesterolemic mice at 6 and 12 months (Figure 6). Phospho-Smad1/5/8 immunofluorescence was markedly reduced by normalizing cholesterol levels after 6 months of hypercholesterolemia (Figure 6).
Figure 6.
Pro-calcific proteins in the aortic valve before and after normalization of lipid levels. P-Smad1/5/8 was markedly increased in 6 and 12 month hypercholesterolemic animals, and was substantially reduced in the “reversed” group. Immunofluorescence of the pro-osteogenic genes Msx2, CBFA1, and Osterix was increased in both 6 and 12 month hypercholesterolemic animals, and markedly reduced by normalizing blood lipid levels. Arrows denote aortic valve tissue when fluorescence is very faint, or point to valvular tissue when non-valvular tissue is present in the micrograph.
Immunofluorescence of Msx2, CBFA1, and Osterix were relatively low in control animals at 6 and 12 months (Figure 6). Immunofluorescence of all three pro-osteogenic genes was modest in hypercholesterolemic animals at 6 months, but was markedly increased in hypercholesterolemic mice at 12 months (Figure 6). Increases in Msx2, CBFA1, and Osterix immunofluorescence were nearly abolished by normalizing cholesterol levels at 6 months (Figure 6).
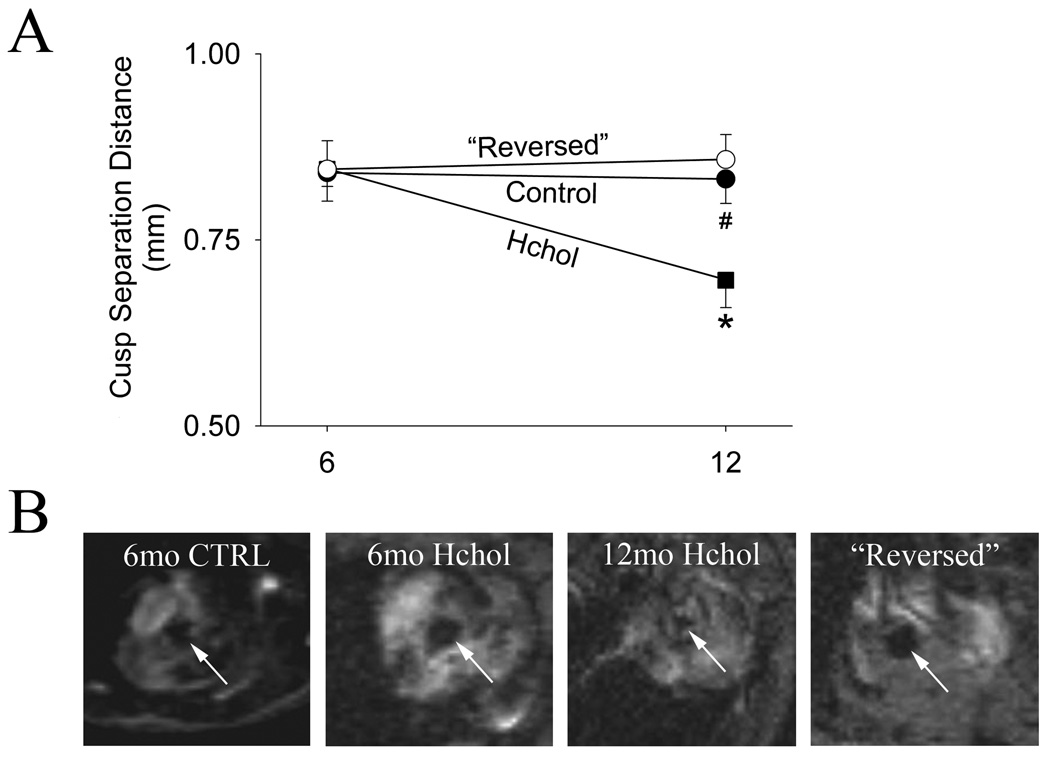
Aortic valve function
In control mice, aortic valve cusp separation distance averaged 0.84 ± 0.04 mm at 6 months and 0.83 ± 0.03 mm at 12 months (Figure 7). Aortic valve cusp separation distance did not differ significantly between control and hypercholesterolemic mice at 6 months. Cusp separation distance decreased significantly from 6 to 12 months in the hypercholesterolemic group (Figure 7), and the prevalence of hemodynamically significant aortic valve stenosis increased from 14 to 50% (see online supplement, Figure S2). Reducing cholesterol levels with the genetic switch at 6 months of age completely prevented the reductions in aortic valve cusp separation (see Figure 7).
Figure 7.
Aortic valve function examined by echocardiography (A) and MRI (B) before and after normalization of lipid levels. A) Leaflet separation distance derived from echocardiography during progression and regression of aortic valve disease from 6 to 12 months. Samples of echocardiographic images are provided in online supplementary Figure S3. B) Systolic short-axis magnetic resonance images acquired in the plane of the aortic valve, using a “white blood” pulse sequence. Blood velocity through the valve is sufficient to cause blood signal dephasing, rendering blood-filled voxels black (arrows) and providing contrast with valve tissue and surrounding cardiac structures Valve areas measured off-line by electronic planimetry: 6mo CTRL = 0.09 mm2, 6mo Hchol = 1.0 mm2, 12mo Hchol = 0.05 mm2, “Reversed” = 1.19 mm2). Arrows point towards the aortic valve orifice.
Qualitatively similar changes in aortic valve function were observed by magnetic resonance imaging in a small subset of mice, and significant reductions in aortic valve orifice area were observed in some of the hypercholesterolemic mice (Figure 7). Reductions in aortic valve orifice area were markedly attenuated by normalizing cholesterol levels at 6 months (Figure 7).
DISCUSSION
The major novel findings of this study are: (1) in hypercholesterolemic mice, superoxide is increased and myofibroblasts are activated in early stages of valve disease, preceding stenosis of the aortic valve; (2) after 6 months of hypercholesterolemia, normalization of cholesterol levels with a “genetic switch” decreases oxidative stress, myofibroblast activation, and pro-osteogenic signaling in the aortic valve; and (3) normalization of cholesterol levels in mice with early aortic valve disease halts the progression of valve calcification and prevents adverse changes in cusp mobility.
Oxidative stress during progression and regression of aortic valve disease
There were marked increases in superoxide levels in hypercholesterolemic mice with early aortic valve disease (i.e., after 6 months of hypercholesterolemia). Previous work from our laboratory and other groups has revealed that oxidative stress is increased in both humans 8, 9 and mice 10 with aortic valve stenosis. The current data suggest that increases in oxidative stress occurs well before development of aortic valve stenosis, and is not merely an epiphenomenon related to hemodynamically significant valve stenosis. These data are reminiscent of observations in mouse models of atherosclerosis, where increases in oxidative stress may play a significant role in the initiation of atherosclerotic plaques 30. Furthermore, recent reports indicate that oxidative stress may play a key role in bone morphogenetic protein signaling pathways 31, 32 and pro-calcific gene expression 33, 34, and several groups have demonstrated that increases in oxidative stress can accelerate the rate of inorganic phosphate-mediated calcification in cultured cells 8, 35, 36.
After 12 months of hypercholesterolemia, we found that superoxide levels did not increase significantly above those observed at 6 months, despite progressive reductions in aortic valve opening. This dissociation between hemodynamic disturbances and superoxide levels supports the hypothesis that oxidative stress is not merely an epiphenomenon of severe aortic valve stenosis but instead may play an important role in amplifying pro-calcific gene expression in early stages of the disease.
Reduction of cholesterol levels reverses pro-osteogenic changes in the aortic valve
After 6 months of hyperlipidemia, we observed substantial valvular lipid deposition and macrophage infiltration, similar to changes that have been reported in atherosclerotic plaques 37. Dietary and pharmacological interventions in hypercholesterolemic animals significantly reduce lipid content and macrophage burden in atherosclerotic lesions in arteries 38. The only studies that have examined effects of lipid lowering in aortic valve disease in experimental animals have examined effects of statins on the earliest stages of initiation and progression of the disease 7, 12. Our study, however, shows a clear dissociation between valvular lipid levels and leaflet restriction in more advanced stages of the disease, which suggests that valvular lipid content per se is not a key determinant of reductions in aortic valve function. To our knowledge, this is the first study to show that reducing cholesterol levels after development of early pathological changes in the aortic valve results in reduced valvular lipid content and inflammatory cell infiltrate.
We also observed marked increases in myofibroblast activation in early aortic valve disease, as judged by increased numbers of smooth muscle α-actin–positive cells in the valve. Activated valvular myofibroblasts may play not only increase valvular collagen content but also could have the potential to differentiate to an osteoblast-like phenotype 39. Studies of calcifying vascular smooth muscle in vitro demonstrated that loss of smooth muscle α-actin expression is an important event during differentiation to osteoblast-like cells 35, 40, 41. Data from humans with aortic valve stenosis, however, suggest that some osteoblast-like cells may retain their contractile properties even in advanced stages of the disease 42. The data reported here also suggest that there is concomitant upregulation of smooth muscle α-actin and osteogenic genes in mouse valves. Additional work is needed to clarify differences in gene expression during differentiation of these cell types (i.e., valvular myofibroblasts versus vascular smooth muscle cells).
We observed significant increases in valvular cusp mineralization (Von Kossa) and calcium deposition (Alizarin Red) in hypercholesterolemic mice at both 6 and 12 months. These changes were associated with increases in phospho-Smad1/5/8, which are typically associated with bone morphogenetic protein (BMP) signaling. In 12-month hypercholesterolemic animals, we also observed substantial increases in immunofluorescence of Msx2, CBFA1, and Osterix, which are associated with differentiation of cells to an osteoblast-like phenotype 43.
After normalization of cholesterol levels, we observed marked reductions in phospho-Smad1/5/8, Msx2, and Osterix immunofluorescence, along with corresponding reductions in valvular calcium. These reductions in valvular calcium are somewhat surprising, as calcium burden is typically not reduced in regression studies that examined calcified atherosclerotic plaques 15, 44. Several groups, however, have identified osteoclast-like cells in the aortic valve (which are less common in atherosclerotic plaques), that may promote a micro-environment that is conducive to resorption of bone-like matrices during regression of valve disease.
Finally, we observed marked increases in valvular fibrosis in hypercholesterolemic mice at 6 and 12 months. Valvular fibrosis was associated with increases in immunofluorescence for phospho-Smad2, which is generally coupled to transforming growth factor (TGF)-β signaling 45. Interestingly, TGF-β is markedly increased in patients with severe aortic valve stenosis 46, and has also been implicated as a contributor to calcification of valvular interstitial cells in vitro 46, 47. Increased TGF-β signaling has also been reported in atherosclerotic plaques, where it may promote collagen synthesis and formation of a fibrous cap 37.
Following the normalization of cholesterol levels, we observed reductions in both phospho-Smad2 levels and myofibroblast activation. Reducing blood lipids did not, however, result in reductions in valve fibrosis. Similar observations have been made during the early stages of regression in atherosclerotic monkeys, where reductions in lipid content were associated with increased collagen content 15. These increases in plaque collagen content can persist even after long-term regression, and could serve an important role in stabilizing plaques. Our data suggest, however, that increases in valvular fibrosis—at least to the extent observed in the present study—are not likely to be a primary determinant of the severity of aortic valve stenosis induced by hypercholesterolemia. Additionally, our data suggest that lipid lowering therapy is not likely to slow the progression of valve disease when valvular fibrosis and/or leaflet fusion are the primary causes of cusp restriction.
Reducing cholesterol levels halts the progression of aortic valve stenosis
The major finding of this study was that normalizing cholesterol levels with a “genetic switch” halts the progression of aortic valve stenosis in hypercholesterolemic mice. Clinical trials examining the effect of statin therapy on progression of aortic valve stenosis have yielded conflicting results 22–24, and retrospective studies suggest a poor correlation between LDL levels and hemodynamic progression of aortic valve stenosis 48. Further complicating matters is the fact that animal models rarely manifest progression to hemodynamically significant aortic valve stenosis (transvalvular gradient >20 mm Hg) 7, 12, 26, 27. Thus to our knowledge, this is the first study to demonstrate that lowering blood lipids in the presence of early changes in the aortic valve prevents reductions in cusp mobility and valve orifice area.
Our findings contrast with some recent reports from human trials which demonstrated no beneficial effect of lipid lowering therapy on progression of aortic valve stenosis. These studies initiated lipid lowering therapy after moderate to severe aortic valve stenosis was present. In the present investigation, we reduced blood lipids by >60% at a time that preceded any detectable impairment in valve function. Collectively, these data suggest that intervening in the early stages of the disease holds the most promise for finding that lipid lowering slows the progression of aortic valve disease in humans.
Determining mechanisms underlying the beneficial effects of HMG-CoA reductase inhibitors (“statins”) is difficult due to “off-target” or pleiotropic effects of these drugs 25. We chose not to administer statins for two reasons. First, depending upon the dosage and compound used, statins may not reduce cholesterol levels in Ldlr-deficient mice 49. Second, our goal was to examine effects of lowering cholesterol per se, independent of pleiotropic effects of drugs. By inactivating Mttp, we avoided any confounding pharmacological effects of statins and were able to show that lipid lowering is responsible for attenuating pro-osteogenic signaling and retarding the progression of aortic valve disease.
Limitations
An important limitation in our study design is that lipid lowering was initiated at 6 months, which represents an early stage of valve disease. However, pro-oxidative, pro-inflammatory, and pro-calcific signaling pathways were already activated at that time. Whether normalization of lipid levels would stop progression or induce the regression of more advanced disease is not clear. Effectiveness of lipid lowering on progression of advanced aortic valve stenosis would likely depend on the ability of this intervention to change macrophage emigration, lipid resorption, osteoblast-like cell activity, and osteoclast-like cell recruitment and activity within the valve.
Due to a limited tissue availability in mouse aortic valves, we were not able to assess DNA binding activity of the pro-osteogenic factors reported in this study. Our immunohistochemical measurements of pro-osteogenic protein levels, however, may actually underestimate the amount of pro-osteogenic activity as data from cell culture experiments have demonstrated that increased binding nuclear binding of CBFA1 can occur in the absence of changes in protein levels.
Conclusions
Reduction of blood lipids in a hypercholesterolemic mouse model of aortic valve stenosis reduces oxidative stress, lipid and calcium burden in the valve, and attenuates pro-osteogenic signaling pathways. We speculate that reducing blood lipids in hypercholesterolemic humans with early aortic valve disease could elicit similar changes and slow the rate of disease progression.
ACKNOWLEDGMENTS
The authors thank Katherine Walters for staining tissue sections and developing immunohistochemistry protocols, Lauren Castaneda for assistance with histological imaging and data analysis, and Samantha Ryan for assistance with management of the mouse colony.
FUNDING SOURCES
Studies were supported by NIH grants HL-092235, HL-62984, NS-24621, RR-017369, funds provided by the VA Medical Service, and a Carver Research Program of Excellence.
Footnotes
DISCLOSURES
None.
Clinical Perspective
Hypercholesterolemia is a major risk factor for calcific aortic valve stenosis. Calcium deposition and ossification of the aortic valve cusps appears to be an active biological process rather than a passive precipitation of calcium. However, clinical trials of lipid lowering therapy on the progression of moderate–severe aortic valve stenosis have yielded disappointing results. In this study, we examined aortic valve stenosis in a severely hypercholesterolemic mouse model in which the hyperlipidemia could be eliminated with a genetic switch. In the severely hypercholesterolemic mice, we observed progressive aortic valve stenosis and increases in both oxidative stress and calcium deposition in the aortic valve. We also observed evidence of TGF-β and bone morphogenetic signaling. Switching off the hypercholesterolemia during early stages of valve disease (i.e., before any reductions in the opening of the aortic cusps) completely prevented the development of aortic stenosis and also reduced oxidative stress, proosteogenic signaling, and valvular calcium burden. These data suggest that calcification of the aortic valve during hypercholesterolemia is an active process, and aggressive lipid lowering therapy—when initiated very early in the course of disease—can prevent the development of aortic valve stenosis.
REFERENCES
- 1.Bonow RO, Carabello B, de Leon AC, Edmunds LH, Jr, Fedderly BJ, Freed MD, Gaasch WH, McKay CR, Nishimura RA, O'Gara PT, O'Rourke RA, Rahimtoola SH, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A, Jr, Gibbons RJ, Russell RO, Ryan TJ, Smith SC., Jr ACC/AHA Guidelines for the Management of Patients With Valvular Heart Disease. Executive Summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Valvular Heart Disease) J Heart Valve Dis. 1998;7:672–707. [PubMed] [Google Scholar]
- 2.Messika-Zeitoun D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Nkomo VT, Breen JF, Maalouf J, Scott C, Tajik AJ, Enriquez-Sarano M. Aortic valve calcification: determinants and progression in the population. Arterioscler Thromb Vasc Biol. 2007;27:642–648. doi: 10.1161/01.ATV.0000255952.47980.c2. [DOI] [PubMed] [Google Scholar]
- 3.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 4.Jian B, Jones PL, Li Q, Mohler ER, 3rd, Schoen FJ, Levy RJ. Matrix metalloproteinase-2 is associated with tenascin-C in calcific aortic stenosis. Am J Pathol. 2001;159:321–327. doi: 10.1016/S0002-9440(10)61698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaden JJ, Vocke DC, Fischer CS, Grobholz R, Brueckmann M, Vahl CF, Hagl S, Haase KK, Dempfle CE, Borggrefe M. Expression and activity of matrix metalloproteinase-2 in calcific aortic stenosis. Z Kardiol. 2004;93:124–130. doi: 10.1007/s00392-004-1021-0. [DOI] [PubMed] [Google Scholar]
- 6.Satta J, Oiva J, Salo T, Eriksen H, Ohtonen P, Biancari F, Juvonen TS, Soini Y. Evidence for an altered balance between matrix metalloproteinase-9 and its inhibitors in calcific aortic stenosis. Ann Thorac Surg. 2003;76:681–688. doi: 10.1016/s0003-4975(03)00529-0. discussion 688. [DOI] [PubMed] [Google Scholar]
- 7.Rajamannan NM, Subramaniam M, Stock SR, Stone NJ, Springett M, Ignatiev KI, McConnell JP, Singh RJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits calcification and enhances nitric oxide synthase production in the hypercholesterolaemic aortic valve. Heart. 2005;91:806–810. doi: 10.1136/hrt.2003.029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J, Jr, Pomerantzeff PM, Laurindo FR. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol. 2008;28:463–470. doi: 10.1161/ATVBAHA.107.156745. [DOI] [PubMed] [Google Scholar]
- 9.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva RA, Heistad DD. Dysregulation of anti-oxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation. 2006;114:2065–2069. doi: 10.1161/CIRCULATIONAHA.106.634139. [DOI] [PubMed] [Google Scholar]
- 11.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2660–2665. doi: 10.1161/01.cir.0000017435.87463.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K, Demer LL. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–2510. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 14.Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation. 2002;105:650–655. doi: 10.1161/hc0502.102969. [DOI] [PubMed] [Google Scholar]
- 15.Benzuly KH, Padgett RC, Kaul S, Piegors DJ, Armstrong ML, Heistad DD. Functional improvement precedes structural regression of atherosclerosis. Circulation. 1994;89:1810–1818. doi: 10.1161/01.cir.89.4.1810. [DOI] [PubMed] [Google Scholar]
- 16.Sipahi I, Nicholls SJ, Tuzcu EM, Nissen SE. Coronary atherosclerosis can regress with very intensive statin therapy. Cleve Clin J Med. 2006;73:937–944. doi: 10.3949/ccjm.73.10.937. [DOI] [PubMed] [Google Scholar]
- 17.Hathaway CA, Heistad DD, Piegors DJ, Miller FJ., Jr Regression of atherosclerosis in monkeys reduces vascular superoxide levels. Circ Res. 2002;90:277–283. doi: 10.1161/hh0302.104724. [DOI] [PubMed] [Google Scholar]
- 18.Harrison DG, Armstrong ML, Freiman PC, Heistad DD. Restoration of endothelium-dependent relaxation by dietary treatment of atherosclerosis. J Clin Invest. 1987;80:1808–1811. doi: 10.1172/JCI113276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corti R, Osende JI, Fallon JT, Fuster V, Mizsei G, Jneid H, Wright SD, Chaplin WF, Badimon JJ. The selective peroxisomal proliferator-activated receptor-gamma agonist has an additive effect on plaque regression in combination with simvastatin in experimental atherosclerosis: in vivo study by high-resolution magnetic resonance imaging. J Am Coll Cardiol. 2004;43:464–473. doi: 10.1016/j.jacc.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 20.Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation. 2001;104:2205–2209. doi: 10.1161/hc4301.098249. [DOI] [PubMed] [Google Scholar]
- 21.Shavelle DM, Takasu J, Budoff MJ, Mao S, Zhao XQ, O'Brien KD. HMG CoA reductase inhibitor (statin) and aortic valve calcium. Lancet. 2002;359(9312):1125–1126. doi: 10.1016/S0140-6736(02)08161-8. [DOI] [PubMed] [Google Scholar]
- 22.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 23.Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Goncalves F, Rajamannan NM. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–561. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 25.Yildirir A, Muderrisoglu H. Non-lipid effects of statins: emerging new indications. Curr Vasc Pharmacol. 2004;2:309–318. doi: 10.2174/1570161043385475. [DOI] [PubMed] [Google Scholar]
- 26.Drolet MC, Roussel E, Deshaies Y, Couet J, Arsenault M. A high fat/high carbohydrate diet induces aortic valve disease in C57BL/6J mice. J Am Coll Cardiol. 2006;47:850–855. doi: 10.1016/j.jacc.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka K, Sata M, Fukuda D, Suematsu Y, Motomura N, Takamoto S, Hirata Y, Nagai R. Age-associated aortic stenosis in apolipoprotein E-deficient mice. J Am Coll Cardiol. 2005;46:134–141. doi: 10.1016/j.jacc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 28.Lieu HD, Withycombe SK, Walker Q, Rong JX, Walzem RL, Wong JS, Hamilton RL, Fisher EA, Young SG. Eliminating atherogenesis in mice by switching off hepatic lipoprotein secretion. Circulation. 2003;107:1315–1321. doi: 10.1161/01.cir.0000054781.50889.0c. [DOI] [PubMed] [Google Scholar]
- 29.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 30.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Csiszar A, Labinskyy N, Jo H, Ballabh P, Ungvari ZI. Differential Pro-inflammatory and Pro-oxidant Effects of Bone Morphogenetic Protein-4 in Coronary and Pulmonary Arterial Endothelial Cells. Am J Physiol Heart Circ Physiol. 2008;295:569–577. doi: 10.1152/ajpheart.00180.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, Behrmann A, Towler DA. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci. 2007;1117:40–50. doi: 10.1196/annals.1402.075. [DOI] [PubMed] [Google Scholar]
- 33.Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou ZP, Zeng WS, Cheng BL, Luo SQ. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J Biol Chem. 2005;280:17497–17506. doi: 10.1074/jbc.M409332200. [DOI] [PubMed] [Google Scholar]
- 34.Pang M, Martinez AF, Fernandez I, Balkan W, Troen BR. AP-1 stimulates the cathepsin K promoter in RAW 264.7 cells. Gene. 2007;403:151–158. doi: 10.1016/j.gene.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 37.Libby P. The molecular mechanisms of the thrombotic complications of atherosclerosis. J Intern Med. 2008;263:517–527. doi: 10.1111/j.1365-2796.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5:91–102. doi: 10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
- 39.Osman L, Chester AH, Sarathchandra P, Latif N, Meng W, Taylor PM, Yacoub MH. A novel role of the sympatho-adrenergic system in regulating valve calcification. Circulation. 2007;116:282–287. doi: 10.1161/CIRCULATIONAHA.106.681072. [DOI] [PubMed] [Google Scholar]
- 40.Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka T, Sato H, Doi H, Yoshida CA, Shimizu T, Matsui H, Yamazaki M, Akiyama H, Kawai-Kowase K, Iso T, Komori T, Arai M, Kurabayashi M. Runx2 represses myocardin-mediated differentiation and facilitates osteogenic conversion of vascular smooth muscle cells. Mol Cell Biol. 2008;28:1147–1160. doi: 10.1128/MCB.01771-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008;473:98–105. doi: 10.1016/j.abb.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 44.Stary HC. The development of calcium deposits in atherosclerotic lesions and their persistence after lipid regression. Am J Cardiol. 2001;88:16E–19E. doi: 10.1016/s0002-9149(01)01713-1. [DOI] [PubMed] [Google Scholar]
- 45.Euler-Taimor G, Heger J. The complex pattern of SMAD signaling in the cardiovascular system. Cardiovasc Res. 2006;69:15–25. doi: 10.1016/j.cardiores.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Jian B, Narula N, Li QY, Mohler ER, 3rd, Levy RJ. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003;75:457–465. doi: 10.1016/s0003-4975(02)04312-6. discussion 465-456. [DOI] [PubMed] [Google Scholar]
- 47.Helske S, Syvaranta S, Kupari M, Lappalainen J, Laine M, Lommi J, Turto H, Mayranpaa M, Werkkala K, Kovanen PT, Lindstedt KA. Possible role for mast cell-derived cathepsin G in the adverse remodelling of stenotic aortic valves. Eur Heart J. 2006;27:1495–1504. doi: 10.1093/eurheartj/ehi706. [DOI] [PubMed] [Google Scholar]
- 48.Rosenhek R, Rader F, Loho N, Gabriel H, Heger M, Klaar U, Schemper M, Binder T, Maurer G, Baumgartner H. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110:1291–1295. doi: 10.1161/01.CIR.0000140723.15274.53. [DOI] [PubMed] [Google Scholar]
- 49.Chen Z, Fukutomi T, Zago AC, Ehlers R, Detmers PA, Wright SD, Rogers C, Simon DI. Simvastatin reduces neointimal thickening in low-density lipoprotein receptor-deficient mice after experimental angioplasty without changing plasma lipids. Circulation. 2002;106:20–23. doi: 10.1161/01.cir.0000022843.76104.01. [DOI] [PubMed] [Google Scholar]