Abstract
Background and Purpose
Lesion volume measured on MRI has been used as an objective surrogate marker for outcome in clinical trials. However, lesion volumes vary over time because of edema and tissue loss. This study aims to determine if lesion volumes measured at 30 and 90 days after ictus significantly differ.
Methods
We performed a retrospective study of 18 patients who had acute (<24 hours) DWI and follow-up fluid-attenuated inversion recovery imaging at 5, 30, and 90 days. Two expert readers segmented lesions and the mean volumes of both reads were used in all statistical analyses.
Results
Patient age was 65.8 (SD, 13.7) years and median NIHSS at baseline was 11.5. Inter-rater variability for lesion volume measurements was 3.7 (5.8) mL. Acute DWI volume was 19.3 (17.3) mL. Fluid-attenuated inversion recovery volumes for 5, 30, and 90 days were 34.3 (23.5), 18.6 (14.0), and 15.9 (13.8) mL, respectively. These volumes differed significantly (P<0.001). Linear regression revealed a strong correlation (r= 0.96; P<0.001) between lesion volumes at 30 and 90 days with a slope that did not vary significantly from 1.0 (P= 0.448).
Conclusions
Lesions continue to evolve between 5 and 90 days, but by 30 days lesion volume approaches final infarct volume. While clinical response is the most meaningful outcome measure, our findings suggest that lesion volumes measured at 30 days may provide a sufficient approximation for final infarct volume for use in early phase clinical trials.
Keywords: final infarct volume, fluid-attenuated inversion recovery, lesion volume evolution, magnetic resonance imaging, stroke
Lesion volume measured on MRI provides an objective, quantitative measurement of stroke severity1 and has been used as a surrogate of clinical outcome in therapeutic trials.2–5 DWI is used to detect ischemia during the acute period;6 T2-weighted fluid-attenuated inversion recovery (FLAIR) imaging can likewise be used to visualize vasogenic edema and infarction in the subacute and chronic stages.7 Standards of lesion measurement have been produced,8 and acute lesion evolution in various settings has been investigated extensively.9–14 Less extensive, however, is the investigation of lesion volume during the chronic stage despite the use of final infarct volume as an outcome measure. There is no consensus of when final occurs, although 30 days2,3 and 90 days4,5 have been used as imaging end points in clinical trials. Earlier outcome times minimize both loss to follow-up and the occurrence of confounding adverse events unrelated to the acute intervention. The purpose of this study is to determine if lesion volume measured at 90 days could be approximated at 5 or 30 days, thereby reducing time to imaging outcome while remaining rigorous in a definition of final infarct volume.
Patients and Methods
Patients
This is a retrospective analysis of patients who consented to a natural history protocol between June 2000 and January 2006. Inclusion criteria required an imaging-confirmed diagnosis of ischemic stroke that affected the anterior circulation, a baseline lesion volume >2 mL on DWI obtained within 24 hours of time last known well, and successful follow-up FLAIR imaging at ≈5, 30, and 90 days. Patients scanned at all time points were more likely to have received thrombolytic therapy. Patients with evidence of acute hemorrhage were excluded.
Image Analysis
Images were blinded to patient identifiers and time point. Lesions were traced according to the segmentation method described elsewhere8 by 2 expert readers (M.R.G. and M.L.) using MIPAV (Medical Image Processing, Analysis, and Visualization, BIRSS; NIH, Bethesda, Md). Acute lesions were identified from trace-weighted, diffusion-weighted images. Lesions on follow-up were identified from FLAIR images. Typical imaging parameters were as follows: DWI: b=0, 1000 sec/mm2, TR/TE ≈6000/72 ms; FLAIR: TR/TE ≈9000/140 ms; TI ≈2200 ms; FOV=22 cm; matrix=256×128×40; NEX=1; and resolution=0.85×1.7×3.5, where TR indicates repetition time; TE, echo time; TI, inversion time; FOV, field of view; NEX, number of acquisitions.
Statistical Analysis
Values are reported as mean (SD [range]) unless otherwise noted. Normality was tested using the Shapiro-Wilk and Kolmogorov-Smirnov tests. The difference in lesion volumes vs time was investigated using paired t tests and Wilcoxon signed-rank tests for normally distributed and non-normally distributed data, respectively. The relationship between lesion volumes vs time was assessed using linear regression. P<0.05 was considered statistically significant.
Results
Patients
The total number of patients with all 4 imaging time points (acute, 5, 30, and 90 days) was 45. Of these, 16 patients had lesions that were <2 mL on acute DWI, 7 did not have lesions in anterior circulation, and 4 showed imaging evidence of acute hemorrhage. Eighteen patients met all inclusion/exclusion criteria and were included in further analysis. Mean patient age was 65.8 (13.7) years. Median (SD) NIHSS at baseline was 11.5 (6.7). Modified Rankin score was available at both 30 and 90 days for 15 of 18 patients; 7 of 15 and 11 of 15 were functionally independent (modified Rankin score <2) at 30 and 90 days, respectively. Eleven patients (61.1%) were male. The mean time from last known well until the acute DWI scan was 7.4 (6.7 [0.8–22.2]) hours. The mean times until 5-, 30-, and 90-day scans were 4.9 (0.7 [2.5–5.5]), 32.0 (3.4 [27.6–42.2]), and 95.0 (8.1 [76.5–108.5]) days, respectively.
Lesion Analysis
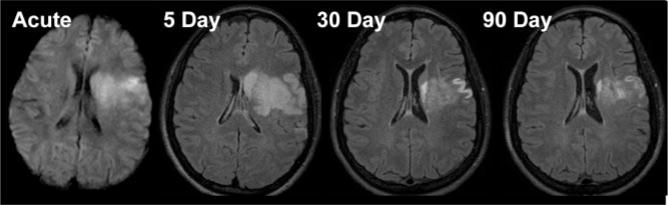
Inter-rater variability between the 2 readers for this study was 3.7 (5.8) mL. Mean lesion volume for the acute DWI was 19.3 (17.3) mL. Lesion volumes on follow-up FLAIR were 34.3 (23.5), 18.6 (14.0), and 15.9 (13.8) mL, for 5, 30, and 90 days, respectively. Lesion volumes expanded an average of 15.0 mL from the acute to 5-day period, decreased 15.7 mL in the 5- to 30-day period, with an additional 2.7 mL decrease from 30 to 90 days. These volumes (acute to 5, 5–30, 5–90, and 30–90 days) differed significantly (P<0.01). There was no significant difference between acute to 30 (P=0.744) or acute to 90 days (P=0.064). Lesion volume evolution for a representative stroke patient from acute DWI to 90-day FLAIR is shown in Figure 1.
Figure 1.
Acute DWI and follow-up FLAIR images at 5, 30, and 90 days in a representative stroke patient. Lesion volume increased from the acute to 5-day time point. This increase may be attributed to the formation of vasogenic edema in the subacute stage. The resolution of this edema is accompanied by tissue loss during the chronic stage, with decreasing lesion volume at 30 and 90 days. Whereas lesion volume measured at all time points differed significantly (P<0.01), the decrease in lesion volume between 30 and 90 days was in the range of inter-reader variability.
The results for the corresponding linear regression may be found in Figure 2. A strong correlation was found between all time points: r2=0.81 (P<0.001), 0.76 (P<0.001), and 0.93 (P<0.001), for 5 to 30, 5 to 90, and 30 to 90 days, respectively. The regression of 30- to 90-day volumes had a slope of 0.95, which did not differ significantly from a slope of 1.0 (P= 0.448). The regression of 5-day to 30-day and 5-day to 90-day volumes had slopes that differed significantly from a slope of 1.0: 0.54 and 0.51, respectively (P<0.001 for both). The intercept for all regression lines did not differ from zero (P= 0.93, 0.57, and 0.27 for 5 to 30, 5 to 90, and 30 to 90 days, respectively). An association between change in lesion volume and change in modified Rankin score between 30 and 90 days was not observed in these data.
Figure 2.
Regression lines of lesion volumes plotted between time points as compared to an ideal line. The 30-day volumes plotted against 5-day volumes (A). The 90-day volumes plotted against 5-day volumes (B). The 90-day volumes plotted against 30-day volumes (C). All 3 lines in (A), (B), and (C) were well correlated (r2= 0.81, 0.76, and 0.93, respectively). Slopes and standard errors were calculated for t statistics to obtain probability values. The line between 30- and 90-day volumes in (C) did not vary from a slope of 1.0 (P= 0.448), whereas the lines in (A) and (B) did (P<0.001 for both). Calculations similar to the slope calculations were performed on the intercepts to test whether they varied significantly from zero, and none did; P=0.93, 0.57, and 0.27 for A, B, and C, respectively.
Discussion
The results of this study suggest that lesion volume measured at 30 days, but not at 5 days, may be an acceptable approximation of 90-day lesion volume. Although lesion volume was slightly larger at 30 vs 90 days, regression of 30-to 90-day volume indicated a strong relationship with a slope of 0.95 that did not differ significantly from 1.0. On average, there was a 5% decrease in lesion volume between 30 and 90 days. This volume difference was in the range of inter-reader variability8 and unlikely to be of clinical significance. We conclude that 30 days is a reliable time point for measurement of final infarct volume.
Infarct volume can serve as a surrogate of clinical outcome in stroke trials.10 Meaningful assessment of final infarct volume must therefore be made after a time sufficiently advanced through the course of lesion evolution to remove the confounding effects of edema. Between the acute and subacute stage, edema increases lesion volume, peaking at ≈3 to 8 days after ictus, after which it begins to subside.10,15 Loss of lesion volume at the chronic stage is attributable not only to resolution of edema but also to timely reperfusion of salvageable tissue, sulcal atrophy, ventricular enlargement, and hypodense cavities.9,16,17 Our study demonstrates that lesion volume decreases by a significant factor between the subacute and chronic stages between 5 and 30 days.
Even though final infarct volume is overestimated at 5 days, there was a strong correlation with final infarct volume. If the regression of 5-day to final volume remains strong and robust in a larger sample, a 5-day measurement may potentially serve as an end point assessed while the patient is still in the hospital.
The data set involved in this study does have limitations. The sample size was small, and this was largely attributable to the difficulty in obtaining follow-up scans. Those patients who returned for scans at 90 days are likely patients who experienced less severe strokes. Larger samples with NIHSS more typical for stroke trials will be required to confirm the observations of this study. Additionally, reperfusion (spontaneous or therapeutic) is an additional cause of lesion volume dynamics between the acute and chronic course of a stroke; our imaging regimen did not gather perfusion data or incorporate it into this analysis.
The data presented herein indicate that 30 days after ictus is a sufficient time to wait in assessing final infarct volume. This provides practical guidance for selecting a time point to assess infarct volume in clinical trials. It also provides justification for comparing or pooling data from existing clinical trials using either 30 days2,3 or 90 days4,5 as an end point into a single cohort. Measuring lesion volume provides a useful quantitative measurement of stroke severity, but it cannot replace clinical outcome. Clinical scales provide an assessment more meaningful to the patient, relatives, and the costs of stroke to a health care system. They may also provide data for a discussion of neuroplasticity as a result of reorganization and rehabilitation. As a strong and tested surrogate, however, lesion volume assessment at 30 days balances 2 important factors that make it a preferable outcome time point by reducing loss to follow-up relative to a 90-day assessment while providing a good estimate of final infarct volume.
Acknowledgments
The authors are grateful to the patients for consenting to participate in this study and to the NIH/Suburban Hospital Stroke Team and Suburban Hospital, Bethesda, Maryland, for patient care.
Sources of Funding
The Division of Intramural Research of the National Institutes of Health and the National Institute of Neurological Disorders and Stroke supported this research.
Footnotes
Part of this work was presented at the International Stroke Conference, San Francisco, Calif, 2007.
Disclosures
None.
References
- 1.Lovblad KO, Baird AE, Schlaug G, Benfield A, Siewert B, Voetsch B, Connor A, Burzynski C, Edelman RR, Warach S. Ischemic lesion volumes in acute stroke by diffusion-weighted magnetic resonance imaging correlate with clinical outcome. Ann Neurol. 1997;42:164 –170. doi: 10.1002/ana.410420206. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, Fischer M, Furlan A, Kaste M, Lees KR, Soehngen M, Warach S. The desmoteplase in acute ischemic stroke trial (DIAS): A phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 3.Furlan AJ, Eyding D, Albers GW, Al-Rawi Y, Lees KR, Rowley HA, Sachara C, Soehngen M, Warach S, Hacke W. Dose escalation of desmoteplase for acute ischemic stroke (DEDAS): Evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37:1227–1231. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- 4.Warach S, Pettigrew LC, Dashe JF, Pullicino P, Lefkowitz DM, Sabounjian L, Harnett K, Schwiderski U, Gammans R. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 investigators. Ann Neurol. 2000;48:713–722. [PubMed] [Google Scholar]
- 5.Warach S, Kaufman D, Chiu D, Devlin T, Luby M, Rashid A, Clayton L, Kaste M, Lees KR, Sacco R, Fisher M. Effect of the glycine antagonist gavestinel on cerebral infarcts in acute stroke patients, a randomized placebo-controlled trial: The gain MRI substudy. Cerebrovasc Dis. 2006;21:106 –111. doi: 10.1159/000090208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol. 1995;37:231–241. doi: 10.1002/ana.410370214. [DOI] [PubMed] [Google Scholar]
- 7.Brant-Zawadzki M, Atkinson D, Detrick M, Bradley WG, Scidmore G. Fluid-attenuated inversion recovery (flair) for assessment of cerebral infarction. Initial clinical experience in 50 patients. Stroke. 1996;27:1187–1191. doi: 10.1161/01.str.27.7.1187. [DOI] [PubMed] [Google Scholar]
- 8.Luby M, Bykowski JL, Schellinger PD, Merino JG, Warach S. Intra- and interrater reliability of ischemic lesion volume measurements on diffusion-weighted, mean transit time and fluid-attenuated inversion recovery MRI. Stroke. 2006;37:2951–2956. doi: 10.1161/01.STR.0000249416.77132.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritzl A, Meisel S, Wittsack HJ, Fink GR, Siebler M, Modder U, Seitz RJ. Development of brain infarct volume as assessed by magnetic resonance imaging (MRI): Follow-up of diffusion-weighted MRI lesions. J Magnetic Resonance Imaging. 2004;20:201–207. doi: 10.1002/jmri.20096. [DOI] [PubMed] [Google Scholar]
- 10.Lansberg MG, O’Brien MW, Tong DC, Moseley ME, Albers GW. Evolution of cerebral infarct volume assessed by diffusion-weighted magnetic resonance imaging. Arch Neurol. 2001;58:613–617. doi: 10.1001/archneur.58.4.613. [DOI] [PubMed] [Google Scholar]
- 11.Pialat JB, Wiart M, Nighoghossian N, Adeleine P, Derex L, Hermier M, Froment JC, Berthezene Y. Evolution of lesion volume in acute stroke treated by intravenous t-pa. J Magnetic Resonance Imaging. 2005;22:23–28. doi: 10.1002/jmri.20363. [DOI] [PubMed] [Google Scholar]
- 12.Schellinger PD, Jansen O, Fiebach JB, Heiland S, Steiner T, Schwab S, Pohlers O, Ryssel H, Sartor K, Hacke W. Monitoring intravenous recombinant tissue plasminogen activator thrombolysis for acute ischemic stroke with diffusion and perfusion MRI. Stroke. 2000;31:1318 –1328. doi: 10.1161/01.str.31.6.1318. [DOI] [PubMed] [Google Scholar]
- 13.Delgado-Mederos R, Rovira A, Alvarez-Sabin J, Ribo M, Munuera J, Rubiera M, Santamarina E, Maisterra O, Delgado P, Montaner J, Molina CA. Speed of t-PA-induced clot lysis predicts DWI lesion evolution in acute stroke. Stroke. 2007;38:955–960. doi: 10.1161/01.STR.0000257977.32525.6e. [DOI] [PubMed] [Google Scholar]
- 14.Schwamm LH, Koroshetz WJ, Sorensen AG, Wang B, Copen WA, Budzik R, Rordorf G, Buonanno FS, Schaefer PW, Gonzalez RG. Time course of lesion development in patients with acute stroke: Serial diffusion- and hemodynamic-weighted magnetic resonance imaging. Stroke. 1998;29:2268–2276. doi: 10.1161/01.str.29.11.2268. [DOI] [PubMed] [Google Scholar]
- 15.Beaulieu C, de Crespigny A, Tong DC, Moseley ME, Albers GW, Marks MP. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: Evolution of lesion volume and correlation with clinical outcome. Ann Neurol. 1999;46:568–578. doi: 10.1002/1531-8249(199910)46:4<568::aid-ana4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 17.Saver JL. Time is brain-quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]