Abstract
We describe the ectopic ossification (EO) found in tendons of biglycan (Bgn), fibromodulin (Fmod) single and double Bgn/Fmod deficient (DKO) mice with aging. At 3 months, Fmod KO, Bgn KO and DKO displayed torn cruciate ligaments and EO in their quadriceps tendon, menisci and cruciate and patellar ligaments. The phenotype was the least severe in the Fmod KO, intermediate in the Bgn KO and the most severe in the DKO. This condition progressed with age in all 3 mouse strains and resulted in the development of large supernumerary sesmoid bones. To determine the role of exercise on the extent of EO, we subjected normal and DKO mice to treadmill exercise for 3 days a week for 4 weeks. The EO in moderately exercised DKO was decreased compared to unexercised DKO mice. Finally, DKO and Bgn KO mice tested using a rotarod showed they had a reduced ability to maintain their grip on a rotating cylinder compared to WT controls. In summary, we show: 1) a detailed description of EO formed by Bgn, Fmod or combined depletion, 2) the role of exercise in modulating EO, and 3) that Bgn and Fmod are critical in controlling motor function.
Keywords: extracellular matrix, tendon, ectopic ossification, treadmill, rotarod
Introduction
Musculoskeletal tissues have an elaborate and extensive extracellular matrix (ECM), whose precise function remains elusive. The ECM is composed of collagens, non-collagenous proteins (NCPs) and proteoglycans (PGs). So far, there are 23 types of collagen genes that have been identified. The collagenous proteins are made of three separate polypeptide chains intertwined to form triple alpha helical structures [Myllyharju and Kivirikko, 2001]. Each triple helix has different combinations of collagen chains depending on the collagen type [Myllyharju and Kivirikko, 2001]. Electron microscopy and biochemical analysis showed that the collagen triple helices align into fibers and that their structure is influenced by other proteins in the matrix including the NCPs and the PGs [Ameye and Young, 2002; Corsi et al., 2002]. The NCPs and PGs are numerous and heterogeneous and, in some cases, constitute families such as the SIBLINGs (Small integrin-binding ligand N-linked glycoproteins) [Bellahcene et al., 2008] or the SLRPs (Small Leucine-Rich Proteoglycans) [Ameye and Young, 2002; Schaefer and Iozzo, 2008]. Most ECM proteins undergo extensive post-translational modifications (PTMs) including glycolsylation, sulfation, phosphorylation, gamma carboxylation or transglutamination [Young, 2003]. In the case of PGs the PTMs are so extensive that they often constitute a greater molecular mass than the core proteins that they are attached to. Thus, the ECM is a complex and important part of the musculoskeletal system, whose functions at the tissue, cell and molecular levels are only now beginning to emerge.
This paper focuses on the role of Small Leucine-Rich Proteoglycans (SLRPs) in musculoskeletal tissues. The composition of the SRLP family has recently been reexamined and is now proposed to contain 17 members [Schaefer and Iozzo, 2008]. The family is divided into classes (I-V) based on their amino-acid sequence and intron-exon organization. Interestingly, several members reside side-by-side on chromosomes in in the genome often with three different classes in tandem (see Table 1). For example, the SLRPs decorin (class I), lumican, (class II), keratocan, (class II) and epiphican (class III) all reside one next to the other on human chromosome 12q. This type of gene arrangement has led to the speculation that the family arose by duplication of one or more of the SLRP genes during evolution [Ameye and Young, 2002].
Table 1. Chromosome Localization of the Human SLRPs.
Chromosome location of SLRPs in the human genome. Left column shows the chromosome number where the SLRPs are located. The second column indicates the SLRP class (I-V) of the gene. The third column lists the full name of the SLRP family member and the final column shows the gene nomenclature used for each SLRP in standard gene databases.
| Chromosome | Class | Protein | Gene |
|---|---|---|---|
| X | IV | nyctalopin | NYX |
| I | biglycan | BGN | |
| 1q32 | V | podocan | PODN |
| II | fibromodulin | FMOD | |
| II | PRELP | PRELP | |
| III | optican | OPTC | |
| 9q21-23 | I | ECM2 | ECM2 |
| I | asporin | ASPN | |
| II | osteoadherin | OMD | |
| III | osteoglycin/mimecan | OGN | |
| 11q | IV | tsukushi | TSKU |
| 12q | I | decorin | DCN |
| II | lumican | LUM | |
| II | keratocan | KERA | |
| III | epiphycan/PG-Lb/DSPG3 | EPYC | |
| 17q | IV | chondroadherin | CHAD |
| 19q | V | podocan-like protein 1 | PODNL1 |
Many SLRP family members are abundant in musculoskeletal tissues, including bone, cartilage, tendon, skin and muscle. In order to understand their function in vivo, mice were created that were unable to make one or more of the SLRPs by gene knockout technology. Our first endeavors focused on the SLRP biglycan (Bgn) a gene located on the X-chromosome whose expression is down-regulated in Turners' (XO) syndrome and up-regulated in Kleinfelter's syndrome (supernumery X). These are syndromes characterized by short and tall stature respectively [Geerkens et al., 1995]. Our initial experiments showed that Bgn deficient mice had mild age-related osteopenia (i.e., low bone mass) [Xu et al., 1998] due to defects in the number and differentiation capacity of osteogenic precursors [Chen et al., 2002; Chen et al., 2004]. The up-regulation of another highly related class I SLRP decorin (Dcn) in the absence of Bgn led us to propose that there was molecular compensation of one SLRP for another [Chen et al., 2004]. In order to unmask this, animals deficient in both Bgn and Dcn were generated and did indeed show functional compensation; these Bgn/Dcn KO mice had more severe osteopenia compared to Dcn KO mice which had normal bone mass [Corsi et al., 2002; Bi et al., 2005]. Despite this important finding, the overall health of the bgn/dcn KO mice was poor and they were unable to breed, making them a difficult model in which to analyze the role of these SLRPs in skeletal aging, and in particular in understanding their potential role in modulating the effects of environmental influences, such as exercise, on their behavior. For these reasons, we created mice unable to make Bgn and its molecular “cousin” fibromodulin (Fmod) [Svensson et al., 1999] (See Table 1). As predicted, the Bgn/Fmod double deficient mice (DKO) were not as severely affected as the Bgn/Dcn KO mice and despite their lower weight, were active and able to breed and produce offspring [Ameye et al., 2002]. This new animal model now allowed more flexibility and versatility in understanding the role of SLRPs during skeletal aging and when subjected to forced exercise activities, which is the topic of this paper.
Our paper is divided into three parts. The first part provides a detailed radiological and histological evaluation of the Bgn and Fmod single and double KO mice. Although we previously showed that there was ectopic tendon ossification in the Bgn and Fmod single and double-deficient mice using semi-quantitative radiographic scoring systems [Ameye et al., 2002], no detailed histological or radiographical description of the phenotype was provided. In this report, we document and compare in detail the histological and radiographical progression of ectopic tendon ossification in Bgn KO, Fmod KO and in mice with combined Bgn and Fmod deficiencies (DKO). In our previous studies we showed that forced intensive 5 days/week treadmill running increased the formation of ectopic ossifcation in DKO mice compared to static DKO mice [Ameye et al., 2002]. In the second part of the paper, we show that when mice were subjected to a reduced exercise regime (3 times a week) there was less ectopic ossification compared to unexercised mice, underscoring the fact that modification in the ECM can have different outcomes depending on the type of exercise applied. Considering the pathological features of the tendons and ligaments of the SLRP deficient mice, we then tested the ability of Bgn KO and DKO mice to perform on a rotarod as presented in the third part of the paper. These experiments revealed that 2 month-old DKO and 6-month old Bgn KO had severe impairment of motor function compared to control mice. This latter pilot data provides a foundation for future studies to evaluate the potential role of pain, hormone status, as well as muscle and neurological involvement in the motor defects found in the ECM compromised animals.
Methods
Generation of Bgn and fmod single and double deficient mice
All experiments were performed under an institutionally approved protocol for the use of animals in research (#NIDCR-IRP-98–058 and 01–151). Mice deficient in Bgn or Fmod were generated by gene targeting in embryonic stem cells as described previously [Xu et al., 1998; Svensson et al., 1999]. Heterozygous Bgn/Fmod-deficient mice were produced by breeding a homozygous Bgn-deficient female (Bgn-/-/Fmod+/+ or Bgn KO), with an Fmod-deficient male (Bgn+/0/Fmod-/- or Fmod KO),; Bgn males are designed as Bgn-/0 because the Bgn gene is located on the X chromosome and absent from the Y chromosome. F2 Bgn/Fmod double-deficient (male Bgn-/0/Fmod-/- and female Bgn-/-/Fmod-/-) mice were obtained by interbreeding F1 heterozygous Bgn/Fmod mice and will be referred to hereafter as Bgn/Fmod DKO mice (DKO). The background matched wild-type mice are referred to as WT and the single Bgn deficient mice as Bgn KO and Fmod deficient mice as Fmod KO.
Genotyping
All mice were genotyped for Bgn and Fmod alleles by PCR analysis as described previously [Ameye et al., 2002]. PCR products were resolved by electrophoresis through 1.8% agarose gels, yielding bands of 212 bp for the WT Bgn allele, 310 bp for the disrupted Bgn allele, 280 bp for the WT Fmod allele, and 603 bp for the disrupted Fmod allele.
Radiography
The legs of WT, Bgn KO, Fmod KO, and DKO animals were X-rayed using a MS-20 Specimen Radiography System (Faxitron X-ray Corporation, Wheeling, IL) at 90 s at 30 kV with XC-OMAT TL Kodak diagnostic films. X-rays were scanned using an Epson Expression 1680 scanner (Epson American, Inc., Long Beach, CA). For quantitation of the EO, the images were analyzed using ImageJ software (NIH, Bethesda, MD). Areas of ectopic ossification were traced using the Freehand Selections function and the area was measured in square pixels.
Histology
Knees were dissected and processed for histology using routine procedures described previously [Ameye et al., 2002]. In order to readily identify calcifying tissues at an early stage of formation, some knees from one-month old animals were processed in an undecalcified state and embedded in methyl methacrylate. From these blocks, 10 micrometer thick sections were cut and stained with Giemsa and von Kòssa.
The data presented in this paper were reproduced in at least 3 different animals of the same genotype at each age. Serial sections through the whole joints were obtained for each animal and observations were confirmed in different interspaced serial sections chosen to cover the whole joint. In this way, it was ensured that the reported observations were genuine and not local random abnormalities. In order to minimize experimental variability, the various genotypes were processed in parallel.
Rotarod Performance Test
WT, DKO and Bgn KO mice were selected after genotype confirmation and analyzed at 2 and 6 months of age respectively. Three male mice of each genotype were subject to rotarod (UGO Basile) analysis and weighed prior to testing. Mice were then acclimated by placing them on the rotating cylinder using the slowest speed settings (Figure 5A). For each analysis, an increased acceleration mode was used. When a mouse fell from the rod, a lever was depressed that stopped the clock showing the time in seconds that the mouse could hold onto the rotating drum (Figure 5).
Fig. 5.
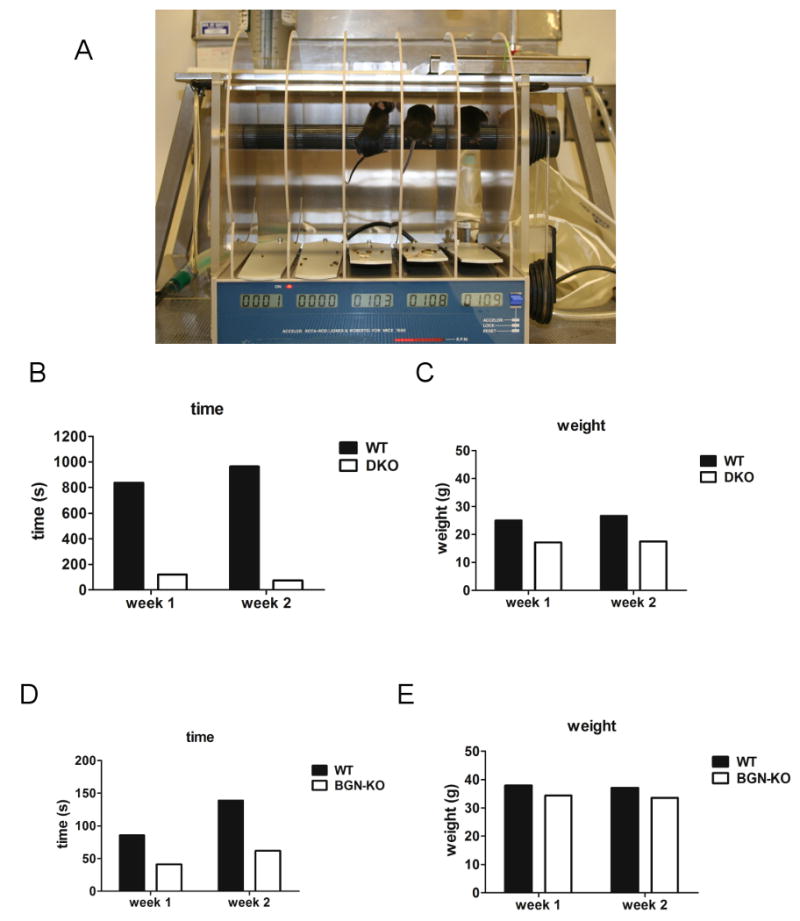
SLRP role in modulating motor skills as judged by rotarod performance. (A) WT, DKO and Bgn KO mice were tested for their ability to run on a rotating cylinder that accelerated its speed with time. Animals were tested at one and two weeks with similar results. Animals shown in (A) are WT mice. (B) DKO fell off the rotating rod sooner than the wild-type controls. The length of time was recorded in seconds (s). (C) Weights of the WT and DKO mice used in the rotarod experiment in grams (g). (D) Rotarod performance of WT and bgn KO mice. (E) Weights of WT and bgn KO mice at the time the rotarod measurements were taken. Note that older WT mice were much heavier than younger mice and fell off the rotarod quicker compared to younger WT mice.
Treadmill Exercise Routine
Male and female WT and DKO mice that were 2 months of age were subjected to a treadmill running regimen using a model ECO-6M treadmill (Columbus Instruments, Columbus, OH) (termed “E” for exercised). Normal and mutant mice that were not subjected to the running regimen were used as a control (termed S for static). The animals ran 3 days a week for 4 weeks at a speed of 60 meters/minute for 30 minutes. The weight of the animals was measured once a week. After the 4-week period the animals were euthanized and the legs were collected and fixed in 4% paraformaldehyde for 3-4 days and stored in 70% ethanol at 4 C° until further analysis.
Results
Ectopic ossification of tendons and ligaments in knees from Bgn and/or Fmod deficient mice
In radiographs from 3 month-old WT knees, the only radio-dense structures observed besides the long bones (femur, tibia and fibula) were the anterior and/or the posterior part of the meniscus and two sesamoid bones (i.e., bones formed within tendons in regions that wrap around bony prominences): the patella located in the quadriceps tendon and the fabella located in the gastrocnemius tendon behind the knee (Fig 1A). Compared to WT, 3 month-old Fmod KO, Bgn KO and DKO mice developed ectopic radio-dense areas along the patellar ligament and the quadriceps tendon and larger radio-dense areas in and around the menisci (Fig. 1 B-D). The number and size of these radio-dense areas varied according to the genotype. They were less numerous and smallest in the Fmod KO (Fig 1B), were more numerous and larger in the Bgn KO (Fig 1C) and were even larger in Bgn/Fmod DKO (Fig 1D). At 6 and 9 months of age in WT tissues, a small radio-dense area developed in some animals along the quadriceps tendon (Fig. 1E and 1I), but the radio-dense area in the meniscus did not become larger.
Fig. 1.
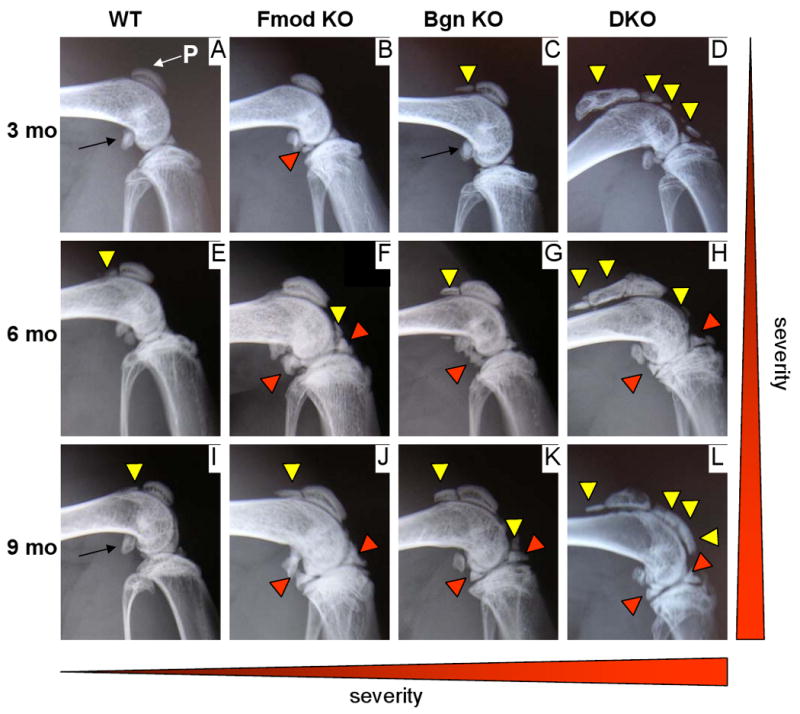
Radio-dense areas develop with age in biglycan and/or fibromodulin deficient knees. Sagittal radiographs from 3 (A-D), 6 (E-H) and 9 month-old (3 mo, 6 mo, 9 mo) WT, Fmod KO, Bgn KO and DKO. In comparison with WT, mutant mice display ectopic radio-dense areas along the patellar ligament and quadriceps tendons (yellow triangles). The radio-dense areas in the menisci are also larger (red triangles). The number and size of these radio-dense areas increase with age (vertical gradient on the right) and varies with the phenotype. At any age, they were more developed in the DKO knees than in both single KO knees and more developed in BgnKO knees than in Fmod KO knees (horizontal gradient at the bottom). Thin arrows point to the fabella, a sesamoid bone that in the mouse normally develops in the gastrocnemius tendon of the knee.
For each of the 3 SLRP-deficient genotypes, the size of the radio-dense areas around the meniscus, as well as the size and often the number of radio-dense areas in the patellar ligament and quadriceps tendon, increased progressively with age at 6 months (Fig 1 F-H) and 9 months (Fig 1 J-L). This age-related increase in severity was more pronounced in the Bgn KO than in the Fmod KO. At 9 months, the phenotype of the Bgn and Fmod KO were both less severe than the DKO phenotype (Fig. 1 J-L).
In order to characterize the nature and origin of these radio-dense areas in the SLRP-deficient mice, Von Kossà staining was performed on undecalcified sections from 3 month-old mice. With this method, mineralized tissues are stained in black and could easily be detected even at early stages of mineralization. At this age in the WT, the meniscus was partly mineralized, but this mineralization was restricted to its most inner part (Fig. 2A, asterisks near M). In comparison, at the same age, some Bgn KO displayed a damaged meniscus with a large ectopic bone with a well-developed bone marrow cavity (Fig. 2B) explaining the existence of larger radio-dense areas in and around the SLRP-deficient menisci compared to WT. Sections through the patellar ligament and quadriceps tendons of 3 month-old Bgn KO and DKO revealed that the radio-dense areas detected in these anatomical structures were supernumary patellae (SP) bones with well-developed bone marrow cavities (Fig 2 D). Early stages of formation of these ectopic sesamoid bones were observed in some sections (Fig 2D red arrow). At these early sites of mineralization, the tenocytes; i.e.. the cells of the tendons, lost their elongated fibroblast-like phenotype and acquired a rounded chondrocyte-like phenotype. This fibrocartilage tissue started to undergo mineralization by depositing mineral pericellularly. With time, the intercellular spaces became completely mineralized, but the mineralization remained confined to the fibrocartilage area within the tendon. The same mineralization process was observed in the cruciate ligament of 3-month-old Bgn KO and DKO (Fig 2 E-F and 3 B).
Fig. 2.
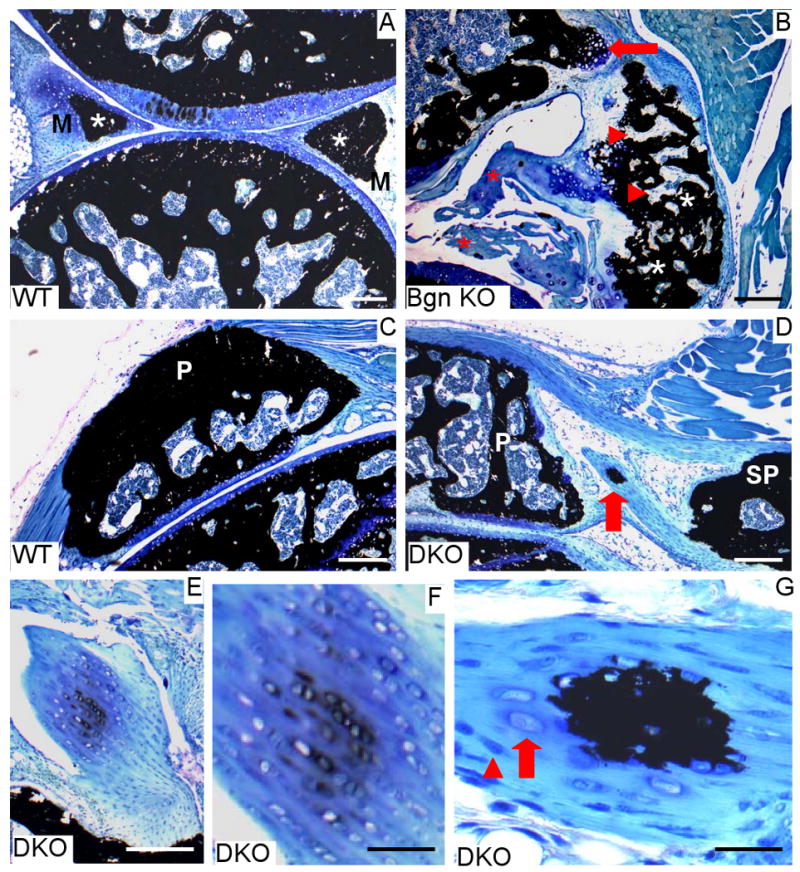
Ectopic ossification in three-month old biglycan single-deficient or biglycan/fibromodulin double-deficient knees. Knee sagittal histological sections from three month-old WT, Bgn KO and DKO. Von Kòssa staining with mineralized areas stained in black. (A) Knee from WT mouse. Note that the meniscus (M) is partly mineralized towards its most central part (asterisks). Bar= 100 μm. B) Knee from BGN-KO. A large ectopic bone with well-developed bone marrow area (triangles) is visible together with a tibial osteophyte in formation (arrow) and a damaged meniscus (asterisks). Bar= 100 μm. (C) Patella (P) in WT knee. Bar= 100 μm. (D) A large ectopic secondary patella (SP) is present along with the normal patella in this double-deficient knee. Note the presence of an early mineralization site in the tendon between the two patellae (arrow). Bar= 100 μm. (E) Cruciate ligament of double-deficient mice containing fibrocartilage undergoing mineralization. Bar= 100 μm. (F) Enlarged area of figure E. Bar= 30 μm. (G) Enlarged area of (D) depicting the early mineralization site in tendon. Note the presence of 2 morphologically distinct cell types: fibroblast-like (triangle) and chondrocyte-like (arrow). Bar= 15 μm.
Fig. 3.
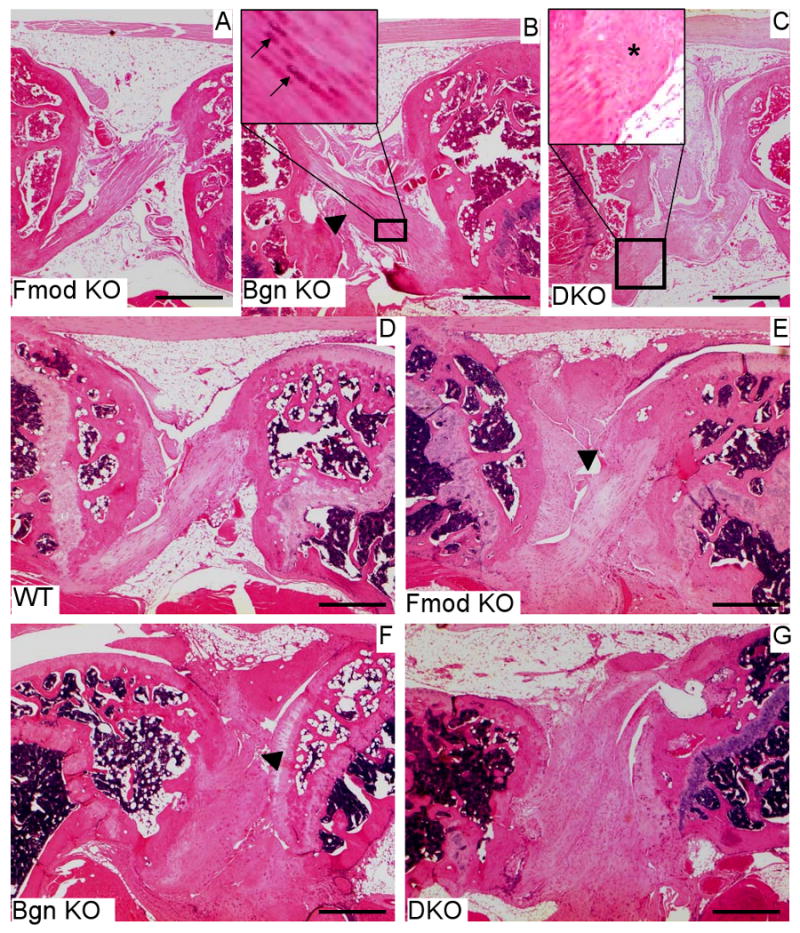
Impaired knee cruciate ligaments in 3 and 9-month old biglycan and/or fibromodulin deficient mice (A-C) Sagittal histological sections from 3 month-old knees. (A) Normal cruciate ligament in Fmod KO mice (B) Cruciate ligament in Bgn KO mice with local abnormal thickening (triangle). Some tenocytes display a chondrocyte-like phenotype (arrows in upper left panel). (C) Thickened cruciate ligament in double-deficient mice (DKO). Note the loss of the columnar arrangements of the tenocytes along the long tendon axis (asterisk in the upper left panel). (D-G) Knees from 9 month-old mice. (D) Cruciate ligament in WT mouse. (E) Slightly hypertrophic cruciate ligament in Fmod KO displaying a small partial severing (triangle). The columnar arrangement of the tenocytes along the long axis of the tendon is preserved. (F) Hypertrophic cruciate ligament in Bgn KO with almost complete separation and tearing (triangle). (G) Highly hypertrophic cruciate ligament in double deficient knee. All bars= 600 μm.
The abnormalitites of the cruciate ligaments in the SLRP-deficient mice were not limited to their tendency to mineralize. Three month-old Fmod KO or Bgn KO cruciate ligaments maintained a normal parallel alignment of the columns of tenocytes and tendon fibers (Fig. 3 A), although Bgn deficient cruciate ligaments were slightly hypertrophic (Fig. 3 B inset). In the DKO ligaments, this hypertrophy was more pronounced, the tenocytes locally lost their regular columnar organization and in other places, the tendon fibers lost their lateral cohesion and were wavy (Fig 3 C inset). By 9 months, the hypertrophy and disorganization of the cruciate ligaments were observed in the 3 SLRP-deficient genotypes. The abnormalitites continued to be more pronounced in the DKO than in the single deficient mice (Fig E-G). In addition partial and/or total tears were observed in the 3 SLRP-deficient genotypes (Fig. 3 E-F) when by comparison, 9 month-old WT cruciate ligaments maintained a normal organization except for some local limited hypertrophic thickening (Fig 3 D).
Moderate Forced treadmill showed decreased Ectopic Ossification in DKO
Two month-old male and female WT and DKO mice were identified and subject to forced treadmill running 30 minutes three times a week, (Monday, Wednesday, and Friday) for a duration of 4 weeks. Upon completion of the running regime, mice were euthanized and legs were dissected and fixed for further processing by faxitron X-ray analysis. X-rays were then scanned and the area of ectopic ossification was traced and quantitated (See Figure 4A for typical X-ray used for the scans). Neither exercised (E) nor static (S) WT mice displayed any discernible ectopic ossification. In contrast to both male (M) and female (F) WT mice the DKO mice had substantial ectopic ossification with the males being greater than the females (Figure 4B). In DKO mice subject to moderate exercise (E) both males and females had reduced ectopic ossification compared to static mice (S). All animals increased in weight from 1 week to 4 weeks where the females were slightly lighter than the males for either genotype. Male and female DKO mice were lighter than the WT animals (4C and 5C) and the weights of any of the animals tested did not change appreciably with the exercise routine (Figure 4C).
Fig. 4.
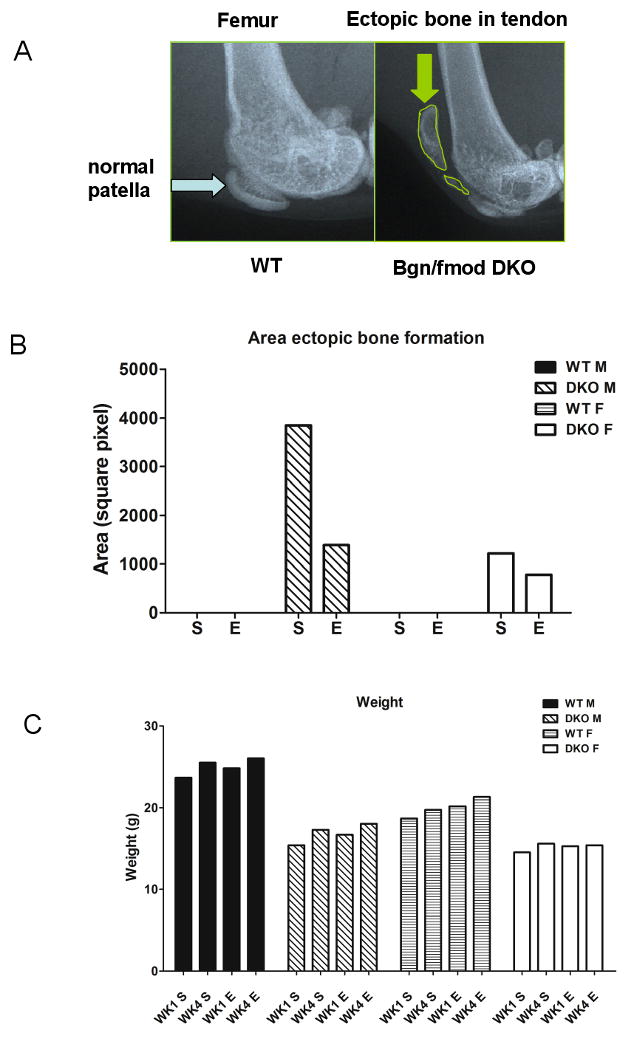
Ectopic ossification in normal and DKO mice subjected to moderate forced treadmill exercise. (A) X-ray of 3 month-old WT mice showing normal patella (blue arrow) compared to DKO which had rampant ectopic ossification (EO). The area of ectopic ossifcation was traced (see green arrow) and quantitated using NIH image J. (B) The relative amount of ectopic ossification in male (M) and female (F) mice subjected to exercise (E) or not (S=static). Male mice had more EO compared to females but both had decreased ectopic ossification after the forced moderate treadmill exercise regime. (C) Weights of WT and DKO male (M) and female (F) mice at week 1 and week 4 before and after exercise
DKO mice have impaired performance on a rotarod
Considering the abundant ectopic ossification, compromised tendon strength [Ameye et al., 2002], tears in miniscus and osteoarthritis [Ameye et al., 2002] found in the bgn/fmod KO mice, we predicted that they would have deficiencies in their motor skills due to one or more of these pathologies. To test this theory, we measured the amount of time the normal and SLRP deficient mice could maintain themselves on the rotating cylinder of the rotarod (Figure 5A). For this test, we used young 2-month old DKO mice and older 6-month old Bgn KO mice and tested them during two weeks. Observing the behaviour of the 2 month-old WT compared to age matech DKO it was clear that the normal animals tended to try to turn backwards on the rod compared to the DKO, who seemed to use all their energy to keep themselves facing forward on the wheel. One of the DKO mice used for the study had long nails on its paw and appeared unable to groom due to inability to flex the knee joints. We have previously shown that DKO have a compromised gait compared to WT controls. Our rotarod analysis showed that both male and female (not shown) DKO mice fell off much quicker compared to normal mice (Figure 5B) and that this defect was not likely due to differences in weight considering the DKO KO mice were lighter than the WT controls. Differences in rotarod performance were also found in 6 month-old bgn KO mice compared to WT mice (Figure 5C) which also did not seem due to excess weight (Figure 5D) in the Bgn KO compared to WT controls. WT mice were less agile and heavier with age (Figure 5C, 5E).
Discussion
The first part of this study documents the mineralization and degenerative changes that occur in the meniscus and cruciate ligaments of mice deficient in biglycan, fibromodulin or both. Except for the structural defects taking place in Fmod KO cruciate ligaments [Gill et al., 2002], these structural changes in the menisci of Bgn KO, Fmod KO and the DKO have not been reported previously. Taken together, this study clearly demonstrates that the Fmod and Bgn single deficiency leads to an overall impairment soft tissues in the knee joints (meniscus, ligament and tendon) in addition to the destruction of articular cartilage previously reported [Ameye et al., 2002]. Compared to Fmod deficiency, Bgn deficiency resulted, by 3 months of age, in a significantly more severe impairment of the soft tissues as assessed by the extent of ectopic ossification. This differential impairment could be due to a functional difference between the 2 SLRPs or due to a difference in SLRP composition between these tissues. By nine months, the Bgn KO and Fmod KO displayed similar levels of severity in ectopic ossification. Compared to the single KO mice, the DKO mice developed more severe ectopic ossification. Thus, the combined deficiency in Bgn and Fmod led to an earlier onset and/or more rapid progression of the ectopic ossification. The more severe phenotype in the DKO mice indicates than the effects of each single deficiency add upon each other and suggest that both Bgn and Fmod play a distinct role in the maintenance of tendon/ligament homeostasis.
Previous studies from our lab showed that tendon contains a small population of cells with stem/progenitor cell features [Bi et al., 2007]. These cells could be isolated from either mouse or human tendons and were clonogenic, multipotent and self-renewing all characteristics of progenitor cells. When young mice were treated with a pulse of BrdU and then analyzed after 24 weeks the tendon tissue still contained a small amount of BrdU positive cells (also called label retaining cells or LTR), indicating that they were slowly cycling, which is another feature of stem/osteoprogenitor cells [Bi et al., 2007]. Interestingly, the LTR cells were embedded deep within the ECM, indicating that tendon may have a different kind of “niche” for its stem/projector cells, made up primarily of ECM components [Bi et al., 2007]. Our findings that Bgn KO, Fbn KO and DKO mice all develop pathological ectopic ossfication in tendons further supports the concept that components of the ECM such as the SLRPs could have important roles in controlling tendon stem/progenitor cell fate and function, in this case, causing them to form ectopic bone.
The mechanisms that control the function of tendon stem/progenitor cell fate may be related to growth factor modulation. Several SLRP family members have been shown to bind to TGF-beta, so we speculated that a dysfunction in the TGF-beta family member BMP-2, could be one basis for the EO found in the DKO mice. BMP (bone morphogenic protein) is a growth factor that promotes bone formation and ectopic ossification. To test this theory, a series of experiments was performed showing that DKO mice had increased BMP-2 signalling as judged by increased SMAD-1 phosphorylation, increased SMAD-4 translocation to the nucleus and increased activation of a BMP responsive promoter in cells from the mutant mice compared to wild type controls [Bi et al., 2007]. Our current working theory is that tendons deplete of bgn or fmod fail to bind and regulate BMP. The outcome of this unregulated BMP activity is that the stem/progenitor cells have more stimulation by BMP causing them to form pathological bone instead of normal tendon (Fig 6). A similar mechanism has recently been proposed to occur in connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP), a disease characterized by massive ossification of numerous soft tissues including muscles, tendons and ligaments [Billings et al., 2008]. Interestingly other mouse models acquire ectopic ossification in their tendons/ligaments including the St/ort mouse of unknown etiology [Mason et al., 2001] and transgenic mice overproducing BMP-2 [Kan et al., 2004; Rittenberg et al., 2005]. It is tempting to speculate that such models are “phenocopies”, sharing a common feature of having abnormal extra-cellular matrices that alter tendon stem/progenitor cell function.
Fig. 6.
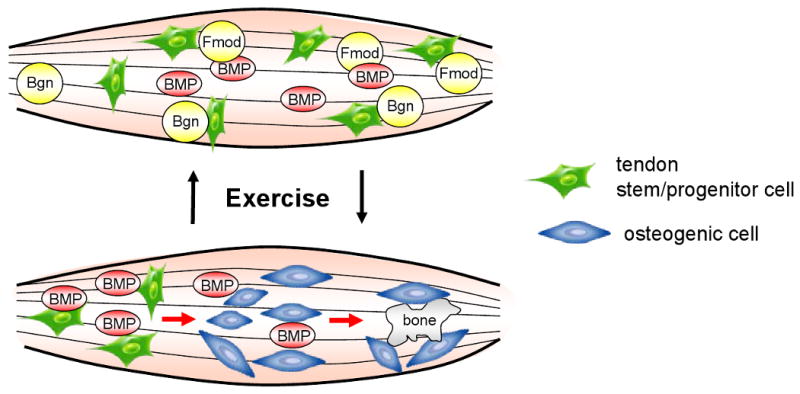
Proposed model showing the role of ECM proteoglycans bgn and fmod in controlling the fate of tendon stem/progenitor cells (TSPC). In this model Bgn and Fmod bind to growth factors such as BMP. This growth factor binding is lost when bgn or fmod is depleted and can affect the differentiation fate of TSPC by stimulating the BMP signalling pathway. In this model, the increased BMP activity causes TSPC to differentiate into osteoblasts and form bone. The normal ECM-rich niche controls this BMP activity so that TSPC can differentiate into tenocytes that form tendon. Forced treadmill exercise can either increase or decrease the differentiation of TSPC towards bone or tendon in a mechanism depending on the exercise regime.
An interesting point raised by these studies is whether or not individuals with any genetic pre-disposition to tendon weakness caused by deficiencies in their ECM can be “rescued” by exercise. When Bgn deficient mice were subjected to treadmill running and the bones were examined structurally by uCT, or functionally by biomechanical testing, they gained both mass and strength comparable to the WT mice levels prior to exercise [Wallace et al., 2006]. This suggests that it may be possible to overcome skeletal abnormalities caused by ECM deficiencies. However, our current studies point to the possibility that the type of exercise that is used could be critical to the outcome. Specifically, DKO mice with a 5 day/week daily treadmill exercise regime acquired more EO [Ameye et al., 2002], while those that exercised every other day had less EO. These data suggest that the ECM can react differently depending on the nature and duration of the exercise. It must also be noted here that the age of the mice used for the intensive exercise was 1 month and for the moderate exercise it was 2 months introducing yet another factor that could contribute to ECM and TSPC response to exercise. The molecular bases for these differential responses are not clear however it is tempting to speculate that their foundation is based not only in altered cell and growth factor function but also in altered tissue strength and structure. It is also possible that the type of ECM defect may be critical to the response to exercise. Support of this concept is based on studies using mice with mutations in type II collagen. Mice with one allele of the type II collagen gene inactivated benefited by voluntary running and had less osteoarthritis [Lapvetelainen et al., 2002], while transgenic mice with a deletion mutant (which formed a defective type II collagen chain) had more osteoarthritis by the same voluntary running regime [Lapvetelainen et al., 2001]. These findings underscore the fact that different forms of exercise could lead to either positive or negative effects on musculoskeletal system depending on the nature of the ECM being challenged.
In this paper we show that both single and double SLRP KO mice have tears in their ligaments a feature not observed in the WT mice. We previously showed that tendons from the DKO mice had reduced stiffness indicating that they were biomechanically compromised. We speculate that the loss of tissue strength and integrity could be due to faulty collagen fibril structure, a characteristic of numerous SLRP deficient tissues [Ameye and Young, 2002] including tendon, bone, skin [Corsi et al., 2002], dentin [Goldberg et al., 2005; Goldberg et al., 2006], and periodontal ligament [Matheson et al., 2005]. The profound influence of Bgn and Fmod in regulating the functions of tendon is not surprising considering how abundant their expression is in developing [Lechner et al., 2006] and post-natal tendon tissue [Bi et al., 2007].
In addition to defects in tendon, the Bgn, Fmod and DKO mutant lines all develop early onset osteoarthritis (OA) [Ameye et al., 2002]. The extent and temporal appearance of the OA is more severe in the DKO compared to either the single Bgn or Fmod KO and was observed as early as one month of age in the knee (data not shown). We previously proposed that tendon weakness causes instability of the joint that can then contribute to the OA phenotype. Since numerous musculoskeletal tissues are affected when Bgn and Fmod are depleted we predicted they would have defective motor skills. Both single Bgn KO and DKO mice had reduced rotarod performance compared to age and sex matched WT mice. At this point, we do not know precisely which tissue is responsible for the reduction in motor capacity in SLRP KO mice. Clearly EO of tendon and OA in cartilage could be important factors, however, it is also possible that muscle or even neurological function is also involved. In this regard, it should be noted that Bgn binds to several members of the dystrophin-associated protein complex (DAPC), a structure that links the cytoskeleton to the ECM and is critical for muscle cell survival. Bgn's functions in muscle appear to be multifaceted and include binding and regulating the DAPC members alpha-dystroglycan [Bowe et al., 2000], alpha and gamma sarcoglycan [Rafii et al., 2006], dystrobrevin and syntrophin [Mercado et al., 2006]. The expression and localization of alpha-dystrobrevin-1 and 2 and alpha and beta 1 syntrophin in the sarcolemma was lower in the Bgn KO and interestingly could be “rescued” by injecting recombinant Bgn into the muscle [Mercado et al., 2006]. This latter observation suggests the possibility that the muscular dystrophy-like phenotype described in the Bgn KO mice as well as its other musculoskeletal abnormalities such as reduced motor skills could be corrected by protein therapy using one or more recombinant SRLPs.
Ectopic ossification has been found in human tendons subject to trauma. One report describes extensive post-traumatic ossification of patellar tendon that was not symptomatic until 40 years after the injury [Matsumoto et al., 1999]. This suggests that the damaged tissues retained a “memory” of previous trauma possibly related to the fact that tendon turnover is slow. In this regard one wonders if the SLRP deficient tendons have “microdamage’ in their ECM that is not visible by conventional histology and that it can exert its effects over time or when the tissue is challenged. A similar mechanism was previously proposed in equine tendons by Smith and colleagues [Smith et al., 2002], who additionally suggested that tendon type, site and age could all be influencing factors. A pre-existing damaged ECM could, in turn, lead to greater sensitivity to force (or trauma) and subsequently increased susceptibility to damage resulting in a “vicious cycle”. In this context, it should be pointed out that tendon may respond to external forces by changing the composition of the ECM itself. For example, when mechanosensitive scaffolds were used to apply pressure to tendon graphs, numerous ECM components were up-regulated including the fibrocartilage related proteins aggrecan, type II collagen and TGF-beta 3. Indeed, a recent review of the literature revealed that there were over 20 distinct changes in collagenous, non-collagenous and proteoglycan components in the ECM in tendinopathies [Xu and Murrell, 2008].
There are many biological questions that remain about the etiology of the EO in SLRP deficient tendons. Male and female Bgn KO mice show differences in their bone phenotype [Nielsen et al., 2003; Wallace et al., 2006] and in this paper we show males and females differ in their susceptibility to EO. What, then, is the role of gender and hormone status in this pathology? Analysis of patients with x-linked hypophosphatemic rickets (osteomalacia), which is more severe in females showed that 69% of the subjects had substantial calcification of tendon and ligament insertions forming ectopic structures that appeared to be similar to lamellar bone [Polisson et al., 1985]. Could phosphate metabolism or other up or down-stream related factors be involved in the EO formation in SLRP deficiency? Other questions remain about the cell and tissue causes of the motor function abnormalities in SLRP deficiency. Bgn expression is up-regulated in the ECM of brain tissue after CNS injury [Stichel et al., 1995]. Is the CNS somehow involved in controlling coordination and grip through the action of the SLRPs? Would additional tests uncover these functions including beam walking, tail hanging or grid walking? Are memory, learning or visual discrimination involved? Finally, is the reduced motor function and inability of mutant mice to hold onto the rotating rod related to pain? These and other mechanistic questions await further investigation and may shed light on the development of new ways to ameliorate pathological ossification or to improve defective motor function.
Acknowledgments
This research was supported in part by the Intramural Program of the National Institutes of Dental Research, National Institutes of Health
References
- Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. Faseb J. 2002;16(7):673–680. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12(9):107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- Bellahcene A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer. 2008;8(3):212–226. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- Bi Y, Stuelten CH, Kilts T, Wadhwa S, Iozzo RV, Robey PG, Chen XD, Young MF. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280(34):30481–30489. doi: 10.1074/jbc.M500573200. [DOI] [PubMed] [Google Scholar]
- Billings PC, Fiori JL, Bentwood JL, O'Connell MP, Jiao X, Nussbaum B, Caron RJ, Shore EM, Kaplan FS. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP) J Bone Miner Res. 2008;23(3):305–313. doi: 10.1359/JBMR.071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe MA, Mendis DB, Fallon JR. The small leucine-rich repeat proteoglycan biglycan binds to alpha-dystroglycan and is upregulated in dystrophic muscle. J Cell Biol. 2000;148(4):801–810. doi: 10.1083/jcb.148.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XD, Fisher LW, Robey PG, Young MF. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. Faseb J. 2004;18(9):948–958. doi: 10.1096/fj.03-0899com. [DOI] [PubMed] [Google Scholar]
- Chen XD, Shi S, Xu T, Robey PG, Young MF. Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J Bone Miner Res. 2002;17(2):331–340. doi: 10.1359/jbmr.2002.17.2.331. [DOI] [PubMed] [Google Scholar]
- Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17(7):1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- Geerkens C, Vetter U, Just W, Fedarko NS, Fisher LW, Young MF, Termine JD, Robey PG, Wohrle D, Vogel W. The X-chromosomal human biglycan gene BGN is subject to X inactivation but is transcribed like an X-Y homologous gene. Hum Genet. 1995;96(1):44–52. doi: 10.1007/BF00214185. [DOI] [PubMed] [Google Scholar]
- Gill MR, Oldberg A, Reinholt FP. Fibromodulin-null murine knee joints display increased incidences of osteoarthritis and alterations in tissue biochemistry. Osteoarthritis Cartilage. 2002;10(10):751–757. doi: 10.1053/joca.2002.0527. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Septier D, Oldberg A, Young MF, Ameye LG. Fibromodulin-deficient mice display impaired collagen fibrillogenesis in predentin as well as altered dentin mineralization and enamel formation. J Histochem Cytochem. 2006;54(5):525–537. doi: 10.1369/jhc.5A6650.2005. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Septier D, Rapoport O, Iozzo RV, Young MF, Ameye LG. Targeted disruption of two small leucine-rich proteoglycans, biglycan and decorin, excerpts divergent effects on enamel and dentin formation. Calcif Tissue Int. 2005;77(5):297–310. doi: 10.1007/s00223-005-0026-7. [DOI] [PubMed] [Google Scholar]
- Kan L, Hu M, Gomes WA, Kessler JA. Transgenic mice overexpressing BMP4 develop a fibrodysplasia ossificans progressiva (FOP)-like phenotype. Am J Pathol. 2004;165(4):1107–1115. doi: 10.1016/S0002-9440(10)63372-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapvetelainen T, Hyttinen M, Lindblom J, Langsjo TK, Sironen R, Li SW, Arita M, Prockop DJ, Puustjarvi K, Helminen HJ. More knee joint osteoarthritis (OA) in mice after inactivation of one allele of type II procollagen gene but less OA after lifelong voluntary wheel running exercise. Osteoarthritis Cartilage. 2001;9(2):152–160. doi: 10.1053/joca.2000.0370. [DOI] [PubMed] [Google Scholar]
- Lapvetelainen T, Hyttinen MM, Saamanen AM, Langsjo T, Sahlman J, Felszeghy S, Vuorio E, Helminen HJ. Lifelong voluntary joint loading increases osteoarthritis in mice housing a deletion mutation in type II procollagen gene, and slightly also in non-transgenic mice. Ann Rheum Dis. 2002;61(9):810–817. doi: 10.1136/ard.61.9.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner BE, Lim JH, Mercado ML, Fallon JR. Developmental regulation of biglycan expression in muscle and tendon. Muscle Nerve. 2006;34(3):347–355. doi: 10.1002/mus.20596. [DOI] [PubMed] [Google Scholar]
- Mason RM, Chambers MG, Flannelly J, Gaffen JD, Dudhia J, Bayliss MT. The STR/ort mouse and its use as a model of osteoarthritis. Osteoarthritis Cartilage. 2001;9(2):85–91. doi: 10.1053/joca.2000.0363. [DOI] [PubMed] [Google Scholar]
- Matheson S, Larjava H, Hakkinen L. Distinctive localization and function for lumican, fibromodulin and decorin to regulate collagen fibril organization in periodontal tissues. J Periodontal Res. 2005;40(4):312–324. doi: 10.1111/j.1600-0765.2005.00800.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Kawakubo M, Otani T, Fujikawa K. Extensive post-traumatic ossification of the patellar tendon. A report of two cases. J Bone Joint Surg Br. 1999;81(1):34–36. doi: 10.1302/0301-620x.81b1.9074. [DOI] [PubMed] [Google Scholar]
- Mercado ML, Amenta AR, Hagiwara H, Rafii MS, Lechner BE, Owens RT, McQuillan DJ, Froehner SC, Fallon JR. Biglycan regulates the expression and sarcolemmal localization of dystrobrevin, syntrophin, and nNOS. Faseb J. 2006;20(10):1724–1726. doi: 10.1096/fj.05-5124fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases. Ann Med. 2001;33(1):7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- Nielsen KL, Allen MR, Bloomfield SA, Andersen TL, Chen XD, Poulsen HS, Young MF, Heegaard AM. Biglycan deficiency interferes with ovariectomy-induced bone loss. J Bone Miner Res. 2003;18(12):2152–2158. doi: 10.1359/jbmr.2003.18.12.2152. [DOI] [PubMed] [Google Scholar]
- Polisson RP, Martinez S, Khoury M, Harrell RM, Lyles KW, Friedman N, Harrelson JM, Reisner E, Drezner MK. Calcification of entheses associated with X-linked hypophosphatemic osteomalacia. N Engl J Med. 1985;313(1):1–6. doi: 10.1056/NEJM198507043130101. [DOI] [PubMed] [Google Scholar]
- Rafii MS, Hagiwara H, Mercado ML, Seo NS, Xu T, Dugan T, Owens RT, Hook M, McQuillan DJ, Young MF, Fallon JR. Biglycan binds to alpha- and gamma-sarcoglycan and regulates their expression during development. J Cell Physiol. 2006;209(2):439–447. doi: 10.1002/jcp.20740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg B, Partridge E, Baker G, Clokie C, Zohar R, Dennis JW, Tenenbaum HC. Regulation of BMP-induced ectopic bone formation by Ahsg. J Orthop Res. 2005;23(3):653–662. doi: 10.1016/j.orthres.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283(31):21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RK, Birch HL, Goodman S, Heinegard D, Goodship AE. The influence of ageing and exercise on tendon growth and degeneration--hypotheses for the initiation and prevention of strain-induced tendinopathies. Comp Biochem Physiol A Mol Integr Physiol. 2002;133(4):1039–1050. doi: 10.1016/s1095-6433(02)00148-4. [DOI] [PubMed] [Google Scholar]
- Stichel CC, Kappler J, Junghans U, Koops A, Kresse H, Muller HW. Differential expression of the small chondroitin/dermatan sulfate proteoglycans decorin and biglycan after injury of the adult rat brain. Brain Res. 1995;704(2):263–274. doi: 10.1016/0006-8993(95)01131-5. [DOI] [PubMed] [Google Scholar]
- Svensson L, Aszodi A, Reinholt FP, Fassler R, Heinegard D, Oldberg A. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274(14):9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Rajachar RM, Chen XD, Shi S, Allen MR, Bloomfield SA, Les CM, Robey PG, Young MF, Kohn DH. The mechanical phenotype of biglycan-deficient mice is bone- and gender-specific. Bone. 2006;39(1):106–116. doi: 10.1016/j.bone.2005.12.081. [DOI] [PubMed] [Google Scholar]
- Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard AM, Sommer B, Satomura K, Dominguez P, Zhao C, Kulkarni AB, Robey PG, Young MF. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20(1):78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- Xu Y, Murrell GA. The basic science of tendinopathy. Clin Orthop Relat Res. 2008;466(7):1528–1538. doi: 10.1007/s11999-008-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MF. Bone matrix proteins: their function, regulation, and relationship to osteoporosis. Osteoporos Int. 2003;14 3:S35–42. doi: 10.1007/s00198-002-1342-7. [DOI] [PubMed] [Google Scholar]


