Abstract
Neuroligins (NLGs) are postsynaptic cell adhesion molecules that are thought to function in synaptogenesis. To investigate the role of NLGs on synaptic transmission once the synapse is formed, we transfected neuroligin-2(NLG2) in cultured mouse cerebellar granule cells (CGCs), and recorded GABAA (γ-aminobutyric acid) receptor mediated miniature postsynaptic currents (mISPCs). NLG2 transfected cells had mIPSCs with faster decay than matching GFP expressing controls at young culture ages (days in vitro, DIV 7-8). Down-regulation of NLG2 by the isoform specific shRNA-NLG2 resulted in an opposite effect. We and others have shown that the switch of α subunits of GABAA Rs from α2/3 to α1 underlies developmental speeding of the IPSC decay in various CNS regions, including the cerebellum. To assess whether the reduced decay time of mIPSCs by NLG2 is due to the recruitment of more α1 containing GABAARs at the synapses, we examined the prolongation of current decay by the zolpidem, which has been shown to preferentially enhance the activity of α1 subunit containing GABA channel. The application of zolpidem resulted in a significantly greater prolongation kinetics of synaptic currents in NLG2 over-expressing cells than control cells, suggesting that NLG2 over-expression accelerates synapse maturation by promoting incorporation of the α1 subunit-containing GABAARs at postsynaptic sites in immature cells. In addition, the effect of NLG2 on the speeding of decay time course of synaptic currents was abolished when we used CGC cultures from α1-/- mice. Lastly, to exclude the possibility that the fast decay of mIPSCs induced by NLG2 could be also due to the impacts of NLG2 on the GABA transient in synaptic cleft, we measured the sensitivity of mIPSCs to the fast-off competitive antagonists TPMPA. We found that TPMPA similarly inhibits mIPSCs in control and NLG2 over-expressing CGCs both at young age (DIV8) and old age (DIV14) of cultures. However, we confirm our previous finding of a greater inhibition of mIPSCs in young (DIV8) than more mature (DIV14) cultures. Together, our results suggest that NLG2 does not alter uniquantal GABA release, and the fast decay of mIPSC induced by NLG2 is due to the differential expression of postsynaptic GABAA receptor subtypes. Taken all together, we propose that NLG-2 plays important functional role in inhibitory synapse development and maturation.
Introduction
Normal functioning of the nervous system is fully dependent on the establishment of appropriate synaptic connections during development. Synapses are specialized junctions that are composed of a presynaptic structure containing synaptic vesicles clustered around active zone, and a postsynaptic compartment containing the neurotransmitter receptors. Synaptogenesis is a crucial process that involves two main steps. First, the initial physical contact is made between an axonal growth cone and a target cell. Secondly, specific recruitment of pre- and postsynaptic proteins makes the maturation of a functional synapse at the site of initial contact (1-3). Synaptically localized cell adhesion molecules (CAMs), which are encoded by large families of genes, are thought to be important in synaptogenesis by triggering initial trans-synaptic contacts through interacting the pre- and postsynaptic compartments, and by regulating the maturation of nascent synapses via mediating synaptic protein recruitment (3-7).
Recent findings on synaptogenesis suggested an important role of Neuroligins (NLGs), postsynaptic CAMs that are expressed on the surface of postsynaptic membranes at both excitatory and inhibitory synapses, in the formation of synapses and their maturation (8-17). NLGs interact with their presynaptic partners, neurexins, forming heterophillic trans-synaptic cell adhesion complex. Multiple genes have been found to encode NLGs. The rodent genome contains four NLG genes: Nlgn1, Nlgn2, Nlgn3 and Nlgn4 (18). Immunocytochemical studies showed that NLG-1 is primarily localized to the postsynaptic site of glutamatergic synapses, whereas NLG-2 is enriched at the GABAergic synapses (19, 20). Importantly, the expression level of NLGs increases over development with a 2-3 fold enhancement when adulthood is reached. Therefore, it is likely that NLGs play functional role at existing mature synapses besides triggering the formation of initial synaptic contacts.
The development of inhibitory synapses and γ-aminobutyric acid (GABA)-mediated synaptic inhibition is crucial in neural network formation and operations. Recent findings that highlight a role of NLG-2 in the GABAergic synapses come from the in vivo studies of NLG knockout mice, either lacking NLGs 1-3 or single NLG-2. A much severe loss of GABAergic synapses was revealed in NLG1-3 knockout model, while glutamatergic synapses remained largely unchanged. Furthermore, such a reduction of synapse numbers (15-20%) only found in the brainstem cells, but not in Hippocampal and Neocortical neurons of these mutant mice. Instead, they showed a severe dysfunction of synaptic transmission due to a severe loss of postsynaptic GABAA receptors and the scaffolding protein gephyrin (16). Further analysis of these knockout mice showed that NLGs are required for the synapse stabilization and strengthening through activity-dependent mechanisms (21). In addition, a very recent publication suggests a marked increase in anxiety-like behavior in NLG-2 knockout mice due to a selective impairment of GABAergic synaptic function (22). However, to date, the fundamental question remains: how does NLG-2 regulate inhibitory synaptic transmission at molecular level once the synapse is formed?
In the present study, we aim to answer this question by utilizing two culture systems, i.e. a co-culture between primary neurons and HEK293 cells expressing NLG-2 and recombinant GABAAR subunits and primary cerebellar granule cell (CGC) culture. We found that expression of NLG-2 dramatically promoted functional GABAergic synapse formation onto HEK293 cells in our co-culture system. The analysis of the impact of NLG-2 on spontaneous miniature GABAergic postsynaptic currents (mIPSCs) in cultured CGCs showed that NLG-2 facilitated inhibitory synapse formation and maturation by promoting incorporation of the α1 subunit-containing GABAARs at postsynaptic sites.
Results
NLG-2 was sufficient to induce GABAergic synapse formation in a co-culture between cultured CGCs and HEK293 cells expressing NLG-2 and GABAA receptors
We employed a co-culture system between CGCs and HEK293 cells to investigate the functional role of NLG-2 in GABAergic synapse assembly. This co-culture system allows us to determine the minimal requirements for artificial inhibitory synapse formation that faithfully reproduce the biophysical properties of endogenous GABAergic synapses (10, 17). Co-cultured CGCs were obtained from P7 transgenic mice that express enhanced green fluorescent protein (eGFP) driven by the GAD65 promoter (GAD65-eGFP). The availability of the GAD65-eGFP mice allows for direct visualization of GABAergic interneurons and the fine features of their axonal synaptic processes (23-25). At 6 days in vitro (DIV 6), we plated HEK293 cells transfected with NLG-2, GABAAR subunits (α1β2γ2, one of the largest populations of GABAARs in matured neurons), and pDsRed onto the cultured CGCs to assemble an artificial postsynaptic structure.
We next tested for presence of synaptic currents in these NLG-2 transfected HEK293 cells 24-48 hrs post-plating. As illustrated in Figure 1A, whole-cell patch clamp recordings with a holding potential at -60mV verified the existence of synaptic currents resembling endogenous neuron-neuron synaptic responses. Nearly 50% of HEK293 cells transfected with GABAAR subunits & NLG-2 showed spontaneous miniature inhibitory postsynaptic currents (mIPSCs) in the presence of TTX; in contrast, mIPSCs could only be detected in 9% HEK293 cells that were transfected with GABAAR subunits alone (Fig 1B). Furthermore, in control cells where mIPSCs could be detected, the frequency was significantly lower than that seen in NLG-2 transfected cells (data not shown). However, given the high sensitivity of GABAARs to GABA, the synaptic currents recorded from HEK293 cells may be generated by the GABA spillover from neuron-neuron synapses on neighboring CGCs. Therefore, it is possible that more HEK293 cells with synaptic currents induced by NLG-2 may be due to the higher surface expression level of GABAA receptors in presence of NLG-2 when compared to the control HEK293 cells in absence of NLG-2. To exclude this possibility, total GABAAR surface expression was determined by the evoked whole-cell current response to a focal application of a saturating concentration of GABA (1mM). We found the GABAAR surface expression was similar in HEK293 cells transfected with GABAAR subunits and NLG-2 or with GABAARs alone (Fig 1C, D). These currents were subsequently normalized to cell capacitance (which is proportional to cell surface area) to generate current density values (61.4 ± 4.8 pA/pF for 22 control cells and 59.7± 5.6 pA/pF in 29 NLG-2 transfected cells, Fig 1D). These data taken together suggested that NLG-2 promoted the initial steps in GABAergic synapse formation, presumably via trans-synaptic interactions with presynaptic neurexins expressed on axonal processes in contact with transfected HEK293 cells.
Figure 1. NLG-2 Was Sufficient To Induce GABAergic Synapse Formation.
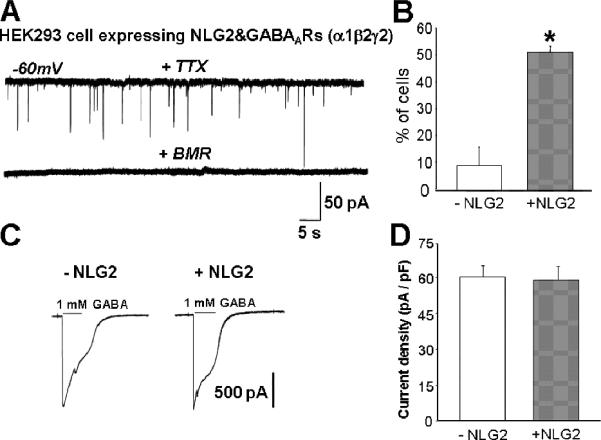
(A) Representative recordings of GABA-mIPSCs from a HEK293 cell transfected with NLG-2 & GABAAR subunits & pDsRed and co-cultured with CGCs. Perfusion with BMR (25μM) abolished all synaptic events. (B) Summary of percent of HEK293 cells transfected NLG-2&GABAARs &DsRed vs. GABAARs & DsRed in which mIPSCs could be detected (*p<0.01 vs. control, n>22 cells from four different co-culture preparations). (C) Evoked whole-cell current response to an application of a saturating concentration of GABA (1mM) in control (left) and NLG-2 expressing HEK293 cells (right).
(D) Summary of whole cell current density (pA/pF) in control (-NLG-2) and NLG-2 transfected (+NLG-2) HEK293 cells
To determine whether induction of synapses by NLG-2 was specific for α1β2γ2 subtype, we tested for the presence of mIPSCs in HEK293 cells transfected with α3β2γ2 in the co-culture system. We failed to detect synaptic currents in these HEK293 cells in presence of NLG-2. The possibility of lack of expression of α3 was ruled out by the detection of evoked whole-cell current response to an application of a saturating concentration of GABA (1mM, 56.2±6.1pA/pF, n=19). Because there is no biochemical evidence suggesting a direct interaction between NLG-2 and GABAA receptors (GABAARs), we therefore considered two possibilities for this negative result. First, via an interaction with an unknown NLG-2 binding protein expressed endogenously in HEK293 cells, NLG-2 may selectively recruit α1 subunit but not α3 subunits. Alternatively, NLG-2 alone may not be able to induce a precise apposition and tightly connected pre- and postsynaptic site that is required for α3β2γ2 GABAARs to produce synaptic responses, as GABAARs containing α3β2γ2 are much less sensitive to presynaptically released GABA compared to α1β2γ2 GABAARs (26-29).
NLG-2 increases frequency and peak amplitude, but reduces decay time of mIPSCs
Because NLG-2 specifically promoted the formation of artificial synapse in our co-culture system, we next investigated whether synaptic transmission was changed by altered NLG-2 levels in cultured cerebellar granule cells (CGCs). These cultures provide a homogeneous neuronal population from which the synaptic receptor pools can be well measured (30). With its small cell body size (only 3-4 μm diameter) and simple dendritic arborization, the CGC allows determination of synaptic current with much higher recording resolution due to good space clamping, when compared to other types of cultured neurons (31). We transfected CGCs with NLG-2 and GFP using a modification of calcium phosphate precipitation technique as described (32). GABA-mIPSCs were then recorded at DIV7-8 (48 hrs post-transfection) in the presence of TTX (0.5μM), NBQX (5 μM, AMPA receptor antagonist) and strychnine (0.5μM, glycine receptor antagonist) as described (30). Sample traces of GABA-mIPSCs recorded in cells expressing GFP alone, NLG-2 and GFP are illustrated in Fig. 2A. Over-expression of NLG-2 significantly increased the frequency of mIPSCs when compared to control GFP cells (Fig 2A, C), suggesting an increase in synaptic input terminals or pre-synaptic release probability. In addition, significant increase in the peak amplitude of mIPSCs were also observed in CGCs expressing NLG-2 (Fig 2A, D), indicating an increased number of synaptic GABAAR in the postsynaptic sites or a change of biophysical channel properties, such as single-channel conductance or mean single-channel open time. Of interest, the weighted time constant of decay (τw) was significantly faster in CGCs expressing NLG-2 when compared to that of control cells (Fig 2E, 35.6±1.8ms for control cells, 28.6±1.6ms for NLG-2, n>40), suggesting a greater incorporation of synaptic GABAARs with fast decay time component in NLG-2 transfected cells. Further analysis of decay curves by double exponential fitting allows to analyzing fast and slow components (τ fast and τ slow), i.e. the relative contributions of the fast or slow component to peak synaptic currents (Fig 2B; τ fast: 21±2ms for control GFP cells, 13±2ms for NLG-2 over-expressing cells; τ slow: 87±5ms for control cells, 79±8ms for NLG-2 cells). We found that only the fast component was significantly affected in NLG-2 expressing cells, while the slow component showed no significant changes compared to control cells. In addition, the difference of % of τ fast also showed significant increase in NLG-2 over-expressing CGCs (74±3% compared to that of control cells (61±3%). These results consistently suggest an increased incorporation of synaptic GABAARs with fast decay time constant in NLG-2 expressing CGCs.
Figure 2. NLG-2 Increases Frequency and Peak Amplitude, But Reduces Decay Time of mIPSCs.
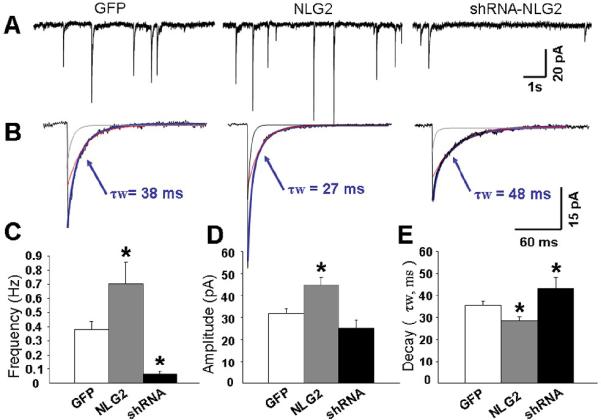
(A) Representative recordings of GABA-mIPSCs in the presence of TTX (0.5 μM), strychnine (0.5 μM) and NBQX (5 μM) from cultured CGCs at DIV 7 transfected with GFP (Left), NLG-2/GFP (Middle), or shRNA-NLG2/GFP (Right).
(B) Averages of mIPSCs recorded from an individual neuron with superimposed exponential curves (gray line represents fast component, red line represents slow component) and an indication of the weighted decay time constant (τw, blue line) from the double exponential fitting of these currents.
(C,D,E) Bar graphs summarizing data derived from the comparison in NLG-2 over-expressing, shRNA-NLG2 expressing CGCs to control GFP expressing cells for frequency (C), amplitude (D) and decay (E) of mIPSCs. Note the significant acceleration together with the increase in frequency and amplitude of mIPSCs in NLG-2 transfect cells (* p<0.05 vs. GFP, values are based on the average n>40 from at least 3 separate cultures).
Because our results on basal GABAergic synaptic transmission were obtained by over-expressing NLG-2 in cultured CGCs, it raises the possibility that the findings were only due to NLG-2 overexpression. To address the role of endogenous NLG-2, we examined whether knockdown of NLG-2 levels had the opposite effect on GABAAR mediated mIPSCs. Using the same protocol as described above, we transfected CGCs with small-hairpin RNAs (shRNA) directed against the rodent NLG-2 at DIV5. Knock-down of NLG-2 expression is strictly isoform specific by using NLG-2-shRNAs, a kind gift from Dr. Peter Scheiffle (13). When NLG-2-shRNAs were transfected into cultured CGCs, we observed a dramatic reduction in the occurrence (0.06 ± 0.02Hz, nearly 50% of cells recorded without mIPSCs) of GABAergic mIPSCs 48-72 hrs after transfection, suggesting an essential function of NLG-2 in inhibitory synapse formation. However, in those cells that exhibited miniature IPSCs, we found that suppression of NLG-2 did not significantly change their synaptic current peak amplitudes (Fig 2D). It is likely due to the presence of the residual NLG-2 levels (< 10% as reported by Chih et al., 13) that escaped the down-regulation by sh-RNA NLG-2. As an alternative, there may be other postsynaptic molecules that promote the recruitment of postsynaptic GABAAR, and postsynaptic expression of GABAA receptors could be independent of synaptic NLG-2. Consistent with our prediction, the decay time course of mIPSCs in NLG-2-shRNAs expressing CGCs (Fig 2 B, E) was significantly slower than that of control GFP cells.
NLG-2 enhances the sensitivity of Zolpidem on the decay of GABAergic mIPSCs
We and others have shown that the α1 subunit of GABAARs are highly expressed in adult CGCs and is the predominant α subunit in the mature rodent cerebellum. The switch of α subunits of GABAA receptors from α2/3 to α1 is the main cause underlying developmental speeding of the IPSC decay. (30, 33-37). The α1 subunit produces currents with fast decay kinetics and confers sensitivity to the imidazopyridine, zolpidem (30, 37). To assess whether the reduced decay time of mIPSCs by NLG-2 was due to the recruitment of more α1 containing GABAARs at the synapses, we examined the prolongation of current decay by application of zolpidem (100 nM), which has been shown to preferentially enhance chloride currents at α1 subunit containing receptor subtypes (38). As reported previously (39), the effect of Zolpidem on mIPSCs amplitude relates to the degree of occupancy of postsynaptic GABAA receptors. Therefore, we limited our analysis to the effect of Zolpidem on the duration of mIPSCs. Indeed, the application of Zolpidem resulted in a significantly greater decay prolongation of synaptic currents in neurons transfected with NLG-2 than control GFP cells at DIV 7-8, supporting the hypothesis that NLG-2 over-expression promoted the incorporation of α1 subunit-containing GABAARs at postsynaptic sites (Fig 3). To further confirm this effect of endogenous NLG-2, suppression of NLG-2 expression was induced by shRNA-NLG2 transfection. As expected, the application of Zolpidem showed significantly less prolongation of the decay time compared to that of the control CGCs (Fig 3 A, B, C). However, when we examined the prolongation effect of Zolpidem on the decay of mIPSCs in cells at mature culture ages (DIV12-14), we did not observe significant changes between NLG-2 expressing cells and control GFP cells (increased τw: 96 ± 14 % for NLG-2 transfected cells; 83±8 % for control cells, Fig 3D). However, we were unable to collect enough miniature spontaneous events required for decay time constant analysis in those cells with knock down of endogenous NLG-2 at this mature culture age, since most of the cells recorded overexressing shRNA showed no or very few mIPSCs. Together, these results suggested that NLG-2 accelerated the decay of mIPSCs by promoting incorporation of the a1 subunit-containing GABAARs at postsynaptic sites but only in immature cells.
Figure 3. NLG-2 Enhances the Sensitivity of Zolpidem on the Decay of GABAergic mIPSCs.
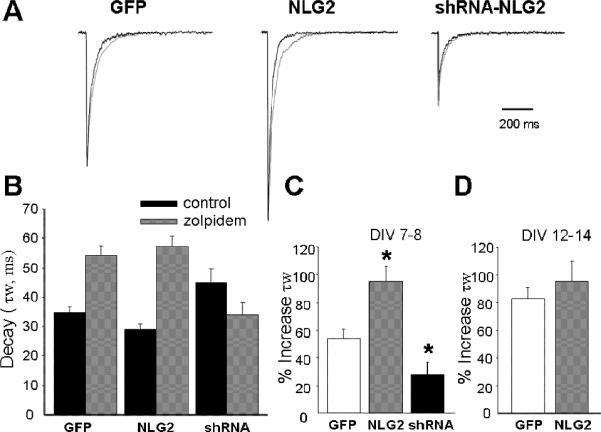
(A) Representative superimposed averages of mIPSCs recorded at DIV 7-8 before (black trace) and during (gray trace) local application of 200 nM Zolpidem in cultured CGCs expressing GFP, NLG-2 and shRNA-NLG2. (Current peaks in each pair of traces are normalized to those recorded during Zolpidem application.) (B) Summary of actual mean τw values of CGCs expressing GFP, NLG-2 and shRNA-NLG2 before (control, black bar) and during (gray bar) application of Zolpidem. (C, D) Bar graphs summarizing the average percent increase in the τw of mIPSCs by Zolpidem application (% increase τw) at two different culture ages, i.e. DIV 7-8 (C) and DIV 12 -14 (D) * p<0.05, vs control GFP expressing cells.
While the significant prolongation effect of Zolpidem on GABAergic mIPSCs in NLG-2 overexpressing cells strongly support the incorporation of α1 expression at postsynaptic sites, we noticed that the decay time of mIPSCs in NLG-2 expressing CGCs at DIV 7-8 was nearly 2 times slower than that of mature CGCs at DIV 12-14 (τw, 18.1 ± 1.5 ms), when α1-containing GABAARs α1β2γ2 become predominant. In addition, we compared the decay time of mIPSCs in CGCs expressing NLG-2 to that seen for currents recorded in outside-out excised patches expressing α1β2γ2 exposed to a rapid application of GABA, a method which produces decay kinetics similar to spontaneous IPSCs (40-44). mIPSCs decay in CGCs expressing NLG-2 was about 3 times slower than the decay time of the currents from outside-out excised patches in HEK293. We, therefore, speculated that the decrease in decay time constant of mIPSCs induced by NLG-2 CGCs could not solely be due to the expression of α1β2γ2 receptors. Thus we examined the decay time of mIPSCs at DIV 7-8 in α1β2γ2 over-expressing CGCs. Indeed, the comparison of the decay time of mIPSCs between NLG-2 and α1β2γ2 overexpressing CGCs revealed significant faster decay in α1β2γ2 over-expressing cells (15.6 ± 1.8 ms). Consistent with our prediction, the effect of Zolpidem on current decay in α1β2γ2 over-expressing CGCs exhibited greater prolongation of decay time (increased τw: 114.8 ± 27.2%) than that of NLG-2 expressing CGCs (increased τw: 96.1 ± 14.4 %).
NLG-2 does not accelerate current decay in cultured CGCs from α1-/- mice
Given the results above, it is possible that, in addition to α1 subunit, the NLG-2 might also affect other synaptic α subunits. The changes in mIPSC decay seen with altered expression of NLG-2 might also be due to a regulation of other synaptic a subunits, such as α6, α2 or α3 that are abundantly expressed in α1 -/- CGCs. We then examined whether the reduced decay of mIPSCs is fully due to the recruitment of more α1 containing GABAARs at the synapses using cultured CGCs from α1 -/- mice (37). As illustrated in Figure 4, over-expression of NLG-2 significantly increased the frequency of mIPSCs when compared to that of control GFP expressing α1-/-cells, suggesting the effect of NLG-2 on promoting synaptic input terminals or pre-synaptic release probability was not affected by the loss of postsynaptic α1 subunit. Synaptic current decay time analysis showed no significant changes between NLG-2 over-expression cells and control cells (Fig 4E), suggesting synaptic α1 subunit expression accounted for the acceleration in the decay time of mIPSCs induced by NLG-2 in wild type of CGCs. As expected, due to the absence of α1 subunit in this mutant mouse line, we did not observe significant changes in the prolongation of current decay induced by 200nM Zolpidem compared to that of control α1 -/- CGCs (Fig 4F). Interestingly, a significant increase in the peak amplitude of mIPSCs was also observed in α1 -/- CGCs expressing NLG-2 (Fig 4 A, D), though less efficiently than it did in the wild-type CGCs. These results indicated that, besides α1 containing GABAARs, NLG-2 was able to recruit other type of GABAARs, albeit less efficiently than it did for α1-containing GABAA receptors, to postsynaptic sites. Therefore, we speculate that, besides α1 subunits, NLG-2 exhibit non-selective recruitment of synaptic enriched α2 and/or α3 containing GABAA receptors in α1-/- CGCs. Indeed, the application of Zolpidem at higher concentration (500 nM), which enhance not only current responses of α1 but also current responses mediated by α2/α3 containing GABAA receptors, resulted in a significantly greater prolongation kinetics in NLG-2 expressing α1-/- CGCs than that in control GFP expressing cells (% increase τw; 44.1 ± 7.1 for control group; 66.0 ± 8.2 for NLG-2 transfected cells), indicating the incorporation of α2 or α3 expression induced by NLG-2.
Figure 4. NLG-2 Does Not Accelerate Current Decay in Cultured CGCs from α1-/- mice.
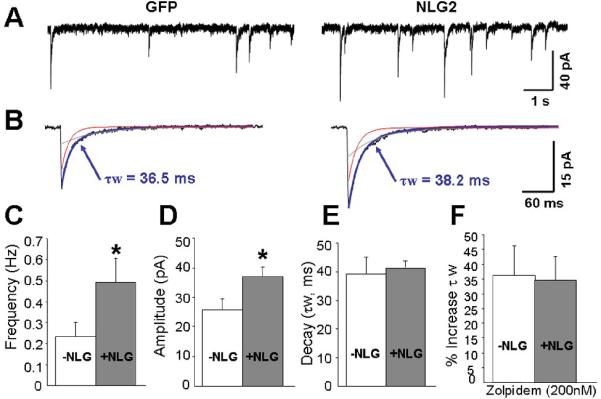
(A) Examples of GABA-mIPSCs recorded in cultured CGCs at DIV 7-8 from α1-/- mice in the presence of TTX, strychnine and NBQX. The CGCs were either transfected with GFP (Left) or NLG-2/GFP (Right). (B) Averages of mIPSCs recorded from an individual neuron with superimposed exponential curves (τ fast in red, τ slow in gray) and an indication of the weighted decay time constant (τw in blue) from the exponential fitting of these currents.
(C,D, E) Bar graphs summarizing the average frequency (C), peak amplitude (D), decay time constant (E) of mIPSCs.
(F) Summary of data derived from the comparison of control GFP and NLG-2 over-expressing CGCs for the percent increase τw by Zolpidem (200 nM) application (* p<0.05, vs control GFP expressing cells).
NLG-2 has no effect on the inhibition of GABAergic mIPSCs by TPMPA
The appropriate synapses development requires highly coordinated tran-synaptic structure and function of presynaptic and postsynaptic components. Given the bidirectional nature of NLG-2, it is possible that the fast decay of mIPSCs induced by NLG-2 could be also due to the impacts of NLG- 2 on the GABA transient in synaptic cleft. To exclude the possibility, we next focused on examining the fast-off competitive antagonist TPMPA (200 μM) on GABAergic mIPSCs in cultured CGCs with altered NLG-2 levels. It is known that, in the presence of quickly dissociate competitive antagonist, the amount of mIPSC block strongly depends on the strength of the presynaptic GABA release, because antagonists and GABA compete for the same binding site. Of note, a prerequisite for an efficient displacement of competitive antagonist by synaptic agonist is that the dissociation time constant of the antagonist is comparable with the duration time of the GABA transient in synaptic cleft. TPMPA was found to be a suitable tool to reveal differences in the GABA transient in synaptic cleft due to its fast dissociation time constant of ~0.46ms (45-47). In this set of studies, GABAergic mIPSCs were recorded in absence and presence of TPMPA at two different culture ages, i.e. DIV7-8 and DIV12-14. We found that TPMPA similarly inhibited mIPSCs in control GFP and NLG-2 over-expressing CGCs both at DIV7-8 and DIV12-14 cultures (Fig 5). However, consistent with previous work (47), we did observe the differential inhibition of mIPSCs between young (DIV7-8) and old (DIV12-14) cultures (Fig 5A, B), suggesting a stronger presynaptic GABA release in young neurons. Together, these results may suggest that NLG-2 does not alter uniquantal GABA release, and the fast decay of mIPSC induced by NLG-2 was due to the differential expression of postsynaptic GABAA receptor subtypes.
Figure 5. NLG-2 Has No Effect on the Inhibition of GABAergic mIPSCs by TPMPA.
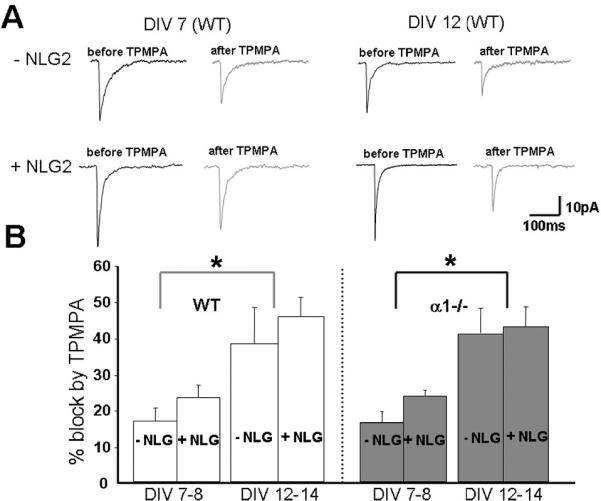
(A) Examples of the average GABA-mIPSCs recorded from control (-NLG2) and NLG-2 transfected WT CGCs at DIV7 (left) and DIV12 cell (right) before (black trace) and in the presence of TPMPA (200μM, gray trace).
(B) Summary of the results obtained using WT CGCs (white columns) and α1-/- cultured CGCs (gray columns) at two different DIVs (indicated above columns, a dotted line was used to separate the left four columns (DIV7-8) from the right four ones (DIV 12-14)). Columns represent the percentage of the mIPSC peak amplitude inhibited by TPMPA (*p<0.05 indicates significant difference between two culture ages under the same conditions).
Because the inhibition of mIPSCs induced by TPMPA critically depends on its postsynaptic GABAAR binding and unbinding kinetics, it raised the possibility that the lack of differential block of mIPSCs by TPMPA in control CGCs and NLG-2 over-expressing cells was due to differential expression of α1 containing postsynaptic GABAARs induced by NLG-2. To test this possibility, mIPSCs were recorded in CGCs culture from α1 -/- mice, and the sensitivity of mIPSCs to TPMPA was compared in control GFP cells and NLG-2 over-expressing α1 -/- cells. Again, the block of mIPSCs by TPMPA in control and NLG-2 over-expressing cells at the same culture period is similar, but a stronger block of mIPSCs by TPMPA was also observed at DIV 12-14 when compared to that of young age cultures (DIV7-8, Fig 5). These results together demonstrated that NLG-2 did not regulate GABA transient in synaptic cleft, and further support the pre-synaptic origin of the differential sensitivity to TPMPA at differential culture ages.
The intracellular domains of NLG-2 are responsible for recruiting α1 subunit containing GABAARs at postsynaptic sites
Lastly, we examined whether the intracellular tail of NLG-2 was required for recruiting of α1 containing GABAA receptors. To be able to achieve this goal, it is prerequisite to have a NLG-2 mutant with removal of the cytoplasmic tail. A FLAG tagged mutant construct with removal of last 70 amino acid residues at C-terminus of NLG-2 (NLG2ΔC) was kindly provided by Dr. Yutaka Hata (Tokyo Medical and Dental University, it was named pFLAG mNL2 (658-766) in its original publication, 48). Considering the possibility of mis-sorting or defects in surface transport of this mutant proteins (due to mis-folding of the truncation), we first expressed this mutant in HEK293 cells together with GFP. Surface labeling using anti-FLAG M2 antibody (1:500, Mouse, Sigma) and Cy3-conjugated secondary antibody (1:1000, Jackson ImmunoResearch Laboratories, West Grove, PA) showed clear expression of NLG2ΔC on the cell surface (Fig 6A). We then examined the effect of NLG2ΔC on GABAergic mIPSCs by transfection in cultured CGCs at DIV7-8. We found no significant change in the occurrence of mIPSCs when compared to that of control group. Due to the lack of N-terminus neurexin binding domain in this truncation construct, it is reasonable to see the no change of mIPSCs frequency(Fig 6B). As expected, the peak amplitude and decay time constant of mIPSCs in the NLG2ΔC over-expressing CGCs did not show significant changes from that seen in control GFP expressing CGCs (Fig 6 C, D). Again, no significant changes in the prolongation of current decay induced by Zolpidem were observed in NLG2ΔC and control GFP over-expressing CGCs (data not shown). Taken together, these results suggested that the cytoplasmic tail of NLG-2 might be essential for the recruitment of α1-containg-GABAA receptors.
Figure 6. The Intracellular Domains of NLG-2 Are Responsible for Recruiting α1 Subunit Containing GABAARs at Synapses.
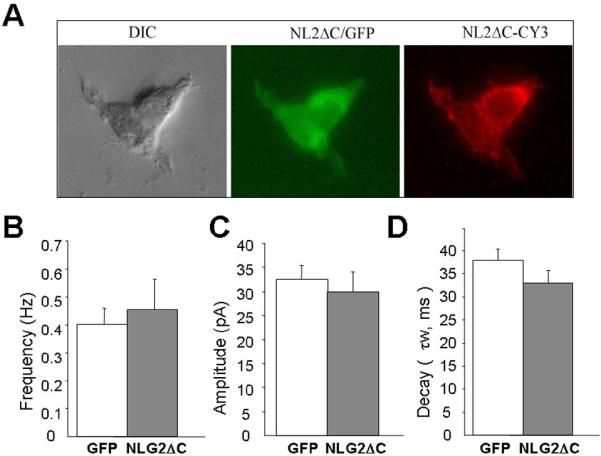
(A) Example microphotographs demonstrating surface expression of NLG-2ΔC mutant with removal of cytoplasmic tail (anti FLAG-CY3, red fluorescence) in HEK293 cells transfected with NLG-2ΔC (658-766) and GFP (green fluorescence).
(B,C,D) Summary of the averages of frequency (B) peak amplitude (C) and decay (D) of the GABA-mIPSCs in cultured CGCs expressing GFP only or NLG-2 mutant with removal of C-terminus. Data derived from >20 cells in 4 sets of cultures.
Discussion
Is NLG-2 indispensable for initiation of synapse formation?
In present study, we clearly demonstrated a functional role of NLG-2 in reconstituting GABAergic synapses in the co-culture system as a previous reported by Dong et al. (17). Over-expression of NLG-2 and GABAA receptor subunits in co-cultured HEK293 cells allowed us to detect robust spontaneous miniature IPSCs from these cells but not in the control group only expressing GABAA receptors. Consistently, over-expression of NLG-2 in cultured CGCs greatly increased the occurrence of mIPSCs, whereas knockdown of NLG-2 expression by shRNA-NLG2 leaded to a robust reduction in mini frequency. Similar effects were also observed in cultured HPCs by altered expression levels of NLG-2 (9, 11, 13, 15). However, a recent study by Varoqueaux et al. (16) argued that neuroligins may function in synaptic function and maturation rather than in the induction phase of synaptogenesis, since the synapse density appeared to be normal in both NLG 1- 2 double and NLG 1-3 triple knockout mice in most of the brain regions examined. A slight reduction of synapse numbers (15-20%) only found in the brainstem cells of these mutant mice. Therefore, the prominent synaptogenic effects of NLG-2 seen in vitro culture systems seem not completely aligned with that of in vivo. In fact, it is known that global knockout model is prone to compensatory effects from other functionally redundant genes. Besides Neuroligins, a number of so-called synaptogenic cell adhesion molecules, such as SynCAMs and netrin-G2 ligand (NGL-2), and integrins, have all been identified to be capable of inducing synapse formation when expressed in non-neuronal cells (49-51). Therefore, it is entirely possible that knocking out one family of these synaptogenic cell adhesion molecules will not strongly affect synapse formation, as other functionally overlapped cell adhesion molecules can still establish the synaptic contacts as compensation. Indeed, SynCAM1 knock-out mice (52) showed no identifiable effect on the formation of synapses and nervous system function including reflexes, motor control and anxiety (unpublished studies in Dr. Philip Washbourn's lab), suggesting there may be a functional overlap between SynCAM family members or other trans-synaptic cell adhesion molecules. Nevertheless, future studies with the generation of transgenic mice that expressing an inhibitory construct specifically for NLG-2 with an inducible promoter certainly will allow us to determine whether these molecules have a role in the formation of synapse in vivo. On the other hand, the possibility cannot be excluded that the initiation of artificial synapse formation in CGCs-HEK co-culture system and the increase in mIPSCs frequency which mainly depends on synapse numbers, induced by NLG-2 may be secondary to the differences in synaptic efficacy and stabilization rather than to the interference with genuine synaptogenic effect of NLG-2. Numerous studies have reported such activity-dependent homeostatic regulation of synapse densities. In our case, functionally advantaged (or disadvantaged) CGCs with altered expression of NLG-2 could maintain their synapses more (or less) efficiently when competing with other un-transfected neurons in culture. However, both in vitro and in vivo analysis revealed an important role of NLG-2 on the recruitment of postsynaptic GABAA receptors during development. Unlike glutamatergic synapses where contain multiple CAMs and postsynaptic signaling molecules interacting scaffolding proteins (3-5, 7), the molecular interactions that control the GABAergic postsynaptic assembly are thought to be more limited. As a consequence, the compensation for recruitment of postsynaptic receptors due to the absence of trans-synaptic NLG-Neurexin system in GABAergic synapses is likely less efficient than in glutamatergic synapses. Indeed, a more prominent defect in GABAergic/glycinergic synaptic transmissions was found in the brain stem of the NLG knockout mice due to a severe loss of postsynaptic GABAA receptors (16).
Does NLG-2 specifically recruit α1 containing GABAA receptors to postsynaptic sites?
Our experiments clearly demonstrated that the reduced decay of GABAergic mIPSCs induced by NLG-2 is due to promoted incorporation of synaptic α1 containing GABAA receptors. This is supported by the significantly prolonged deactivation of mIPSCs in NLG-2 over-expressing CGCs and by application of Zolpidem (200 nM), at a concentration shown to preferentially enhance Cl- currents of α1 containing GABAA receptors. Furthermore, cultured CGCs with knockdown of endogenous NLG-2 exhibited significantly slower decay time course and less prolongation in presence of Zolpidem when compared with control CGCs, suggesting a reduced recruitment of synaptic α1 subunits. No significant changes in decay time of mIPSCs and its sensitivity to Zolpidem were observed in cultured CGCs transfected with NLG-2 from α1-/- mice. These results further support the hypothesis that fast decay of mIPSCs induced by NLG-2 requires synaptic α1 expression. Lastly, in our co-culture system, the presence of mIPSCs can only be detected in HEK293 cells expressing NLG-2 and α1β2γ2 subunit combination but not α3β2γ2, indicating the possibility that NLG2 may selectively recruit mature α1 subunit but not immature α3 subunits. Given these results above, we believe that enhanced expression of synaptic α1 subunits accounts for the acceleration in decay time of mIPSCs induced by NLG-2, and NLG-2 has a preferential recruitment of α1 subunits compared to other synaptic enriched α subunits.
However, it is possible that, in addition to α1 subunit, the NLG-2 might also regulate the incorporation of other synaptic α subunits. As illustrated in Figure 4D, over-expression of NLG-2 significantly increased the peak amplitude of mIPSCs in α1 -/- CGCs, though less efficiently than in wild-type CGCs, indicating that NLG-2 is able to recruit other type of GABAARs less efficiently. In addition, the mIPSCs' decay time with NLG-2 over-expression was slower than that of mIPSCs recorded from CGCs in mature age cultures, α1β2γ2 over-expressing CGCs and GABA currents from outside-out patch expressing α1β2γ2 and. We and others have shown increased expression level of α6 in CGCs along development, and also demonstrated that α6/α1 containing GABAARs have a fast decay comparable to α1 (53-55). Therefore, an increased α6 could possibly contribute to the fast decay of mIPSCs induced by over-expression of NLG-2. However, a moderate increase (though did not reach significant change, Fig 4E) in the decay time of mIPSCs seen from NLG-2 transfected α1-/- CGCs seem negate this possibility. Therefore, we speculate that, besides α1 subunits, NLG-2 exhibit non-selective recruitment of synaptic enriched α2 and/or α3 containing GABAA receptors in α1-/- CGCs. Indeed, the application of Zolpidem at higher concentration (500 nM) resulted in a significantly greater prolongation kinetics in NLG-2 expressing α1-/- CGCs than that in control GFP expressing cells, indicating the incorporation of α2 or α3 expression induced by NLG-2. To define which synaptic α subunits that are regulated by NLG-2, further biochemical and immunocytochemical studies will be needed due to the lack of α2 or α3 containing receptor modulators. Together, these results may provide an indication that the effect of NLG-2 is on a global regulation of inhibitory synapse development rather than on the recruitment of a specific subunit at postsynaptic sites.
Does NLG-2 have impacts on pre-synaptic release probability?
Our analysis of TPMPA sensitivity clearly demonstrated that NLG-2 had no effect on the GABA transient in synaptic cleft, and supported the postsynaptic origin of the acceleration of synaptic current decay induced by NLG-2. This result did not fully exclude the possibility that NLG-2 may regulate other presynaptic functions, such as release probability. Given the trans-synaptic nature of NLG-2, it is entirely possible that, NLG-2 plays an important role in shaping presynaptic function by interacting with its presynaptic partner- neurexin. In fact, recent studies demonstrated a general and essential role for neurexin in Ca2+ triggered presynaptic neurotransmitter release (56-58). Over-expression of NLG-2 in our cultured CGCs induced significant greater occurrence of mIPSCs, while knock down of NLG-2 resulted in a significant reduction (about 6 times less) miniature frequency of mIPSCs compared to that of control group (Fig 2), suggesting either a change in synaptic numbers or a change in presynaptic release probability. Indeed, a recent finding showed the retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-NLG1 at excitatory synapses (59). As a parallel from studies of the excitatory synapse, it will be very important to further examine the presynaptic release probability, such as paired pulse facilitation (PPF) or depression (PPD), and the success or failure rate of synaptic transmission, with altered expression levels of NLG-2 in microisland CGC cultures (60) or a more physiological preparation by using NLG-2 selectively knock-out mice (21).
Does C-terminus of NLG-2 fully responsible for the acceleration of mIPSCs?
Our experiments demonstrated that NLG-2 mutant with partial removal of the cytoplasmic tail was still able to induce the enhancement of frequency of mIPSCs but with less potent effect than that of full-length NLG-2. No significant changes were observed in the peak amplitude and decay kinetics in NLG-2 ΔC when compared with control GFP expressing cells (Fig 6). These results suggested that GABAA receptors could be primarily recruited to synapses independent from the C-terminus of NLG-2, but the recruitment of matured subunits of GABAA receptors required the intracellular domain of NLG-2, possibly via assembly of postsynaptic scaffolding proteins. As an alternative, it is likely that the synaptic localization of NLG-2 could be independent of its C-terminus. Further immnuocytochemical studies that allow us to visualize the localization of NLG-2 needed to perform to confirm this speculation. Our findings are reminiscent of the pioneer work from Peter Scheiffele's group demonstrating the respective contribution of the intracellular and extracelluar domains of NLG-1 to postsynaptic differentiation (13). It is known that GABAA receptor trafficking, expression and clustering at synapses could be regulated by a number of scaffolding proteins, such as gephyrin, collybistin, GABARAP, dystrophin-dystroglycan complex, and cytoskeleton proteins (61, 62). An interesting recent study showed that synaptic scaffolding molecule (S-SCAM), consisting of multiple PDZ domains, a guanylate kinase (GK) domain and two WW domains (interacting with proline-rich seqeunces) interacts with NLG-2. It can form a tripartite complex with NLG-2 and β-dystroglycan at GABAergic synapses (48). Unlike the abundant knowledge about postsynaptic apparatus at excitatory synapses, much less is known about the molecular interaction at inhibitory postsynaptic structure. Because no biochemical evidence has so far been reported for a direct interaction between NLG-2 and GABAA receptors (11), future studies are necessary to find out what postsynaptic adaptor proteins mediate the association of NLG-2 and GABAA receptors. Such work will be essential to define the intracellular domains that are responsible for the effect of NLG-2 on postsynaptic differentiation.
Materials and Methods
Cerebellar granule cell culture and transfection
Primary cultures of cerebellar granule neurons were prepared from postnatal day 5-7 mice. The generation of GAD65-eGFP mice is described in our previous works (63, this mouse line was derived from Dr. Szabó's laboratory, Brian Robertson (University of Leeds, Leeds, UK)). Mouse pups were sacrificed by decapitation in agreement with the guidelines of the Georgetown University Animal Care and Use Committee. The cerebella were then removed, dissociated with trypsin (0.25 mg/ml, Sigma, St. Louis, MO) and plated in 35 mm Nunc dishes at a density of 1.1×106 cells/ml on glass coverslips (Fisher Scientific, Pittsburgh, PA) coated with poly-D-lysine (10 μg/ml, Sigma). The cells were cultured in basal Eagle's medium supplemented with 10% bovine calf serum, 2 mM glutamine, and 100 μg/ml gentamycin (all from Invitrogen Corporation Carlsbad, CA), and maintained at 37°C in 5% CO2. The final concentration of KCl in the culture medium was adjusted to 25 mM. At days in vitro (DIV) 5 the medium was replaced with low (5 mM) K+ medium (MEM supplemented with 5 mg/ml glucose, 0.1 mg/ml transferrin, 0.025 mg/ml insulin, 2 mM glutamine, 20 μg/ml gentamicin (Invitrogen) and cytosine arabinofuranoside 10 μM, Sigma) as previously described (64, 65) to facilitate the formation of synaptic network between cerebellar granule cells and GABAergic interneurons in culture. Primary cultures of mouse CGCs were transfected at DIV5 using a modification of the calcium phosphate precipitation technique (66). Briefly, a glass coverslip with CGCs was transferred to a well in a 4-well plate with 500μl transfection medium composed of MEM medium (Cat #11575-032, Invitrogen, Carlsbad, CA) with pH adjusted to 7.90 by 5 M NaOH. Then 30 μl of DNA/Ca2+ mixture containing cDNAs and 2 mM CaCl2 were added and incubated for 30 min at room temperature. After two washes with the transfection medium, the original culture medium was returned and neurons were maintained at 37 °C in 5% CO2. pDsRed plasmid (Clontech, Palo Alto, CA) was also transfected to allow visualization of successfully transfected cells. Each coverslip was transfected with 3 μg plasmids in a total. The NLG-2, shRNA-NLG-2 constructs are described previously in Chih et al. (13) and α1, β2 and γ2 plasmid constructs in our previous work (43)
Co-culture between CGCs and HEK293 cells
Primary cultures of CGCs were prepared from GAD-65eGFP mice. The generation of GAD-65eGFP mice is described in our previous work (Fiszman et al., 2005). At Div5-6, HEK293 cells that were transfected with NLG-2, GABAAR subunits α1β2γ2, and pDsRed were plated onto the cultured CGCs to assemble an artificial postsynaptic structure as we previously described (10).
Electrophysiology
Coverslips with CGCs were placed on the stage of an inverted microscope (TM2000, Nikon) equipped with fluorescent and phase-contrast optics. All recordings were performed at room temperature (24-26°C) from neurons maintained for 7 to 14 days in vitro. Extra-cellular solution contained (in mM): NaCl (145), KCl (5), MgCl2 (1), CaCl2 (1), HEPES (5), glucose (5), sucrose (20) phenol red (0.25mg/l) and D-serine (10 μM) (all from Sigma) adjusted to pH 7.4 with NaOH, was continuously perfused. Osmolarity was adjusted to 315 mOsms with sucrose. Electrodes were pulled in two stages on a vertical pipette puller to a resistance of 5-8 MΩ, from borosilicate glass capillaries (Wiretrol II, Drummond, Broomall, PA), and filled with KCl-based internal solution containing (in mM): K-Cl (145), HEPES (10), ATP.Mg (5), GTP.Na (0.2), and EGTA (10), adjusted to pH 7.2 with KOH. Whole-cell voltage-clamp recordings from CGCs were made at -60 mV and performed at room temperature using an Axopatch 200 or an Axopatch-1D amplifier (Axon Instruments, Union City, CA). A transient current response to a hyperpolarizing 10 mV pulse was used to assess access resistance and capacitance throughout the recordings, and recordings with >20% changes were discarded. Currents were filtered at 2 kHz with an 8-pole low-pass Bessel filter (Frequency Devices, Haverhill, MA), digitized at 5-10 kHz using an IBM-compatible microcomputer equipped with Digidata 1322A data acquisition board and pCLAMP9 software (both from Axon Instruments).
The mIPSCs were isolated by application of TTX (0.5 μM), NBQX (5 μM), and strychnine (0.5 μM). All detected events were used for event frequency analysis, but superimposing events were eliminated for the amplitude, rise time, and decay kinetic analysis. GABA-mIPSCs were identified using a semi-automated threshold based mini detection software [Mini Analysis; Synaptosoft, Fort Lee, NJ (www.synaptosoft.com)] and were visually confirmed. The decay of GABA-mIPSCs was fitted using Clampfit 9.2 (Axon Instruments) from averages of more than 50 events selected with Mini Analysis in each cell studied. The decay phase of currents was fitted using a simplex algorithm for least squares exponential fitting routine with a double exponential equation of the form I(t)=I1*exp(-t/τ1)+I2*exp(-t/τ2), where Ix is the peak current amplitude of a decay component and τx is the corresponding decay time constant. To allow for easier comparison of decay times between experimental conditions, the two decay time components were combined into a weighted time constant τw = [I1/(I1+I2)] * τ1 + [I2/(I1+I2)] * τ2. All data are expressed as mean ± standard error of the mean, p values represent the results of Analysis of variance (ANOVA) for multiple comparisons or two-tailed unpaired Student's t tests.
Immunocytochemistry (Surface Staining)
Cultured HEK293 cells were washed twice with extracellular recording solution (ECS). Coverslips were then blocked for 1 hr at room temperature using 10% BSA (Sigma) in PBS followed by incubation of the primary antibody in PBS containing 3% BSA for 1 hr at room temperature. The coverslips were then fixed for 20-30mins with 4% Sucrose /4% Paraformaldehyde in PBS follwed by 3 washes in PBS. The second primary antibody of interest was then incubated in PBS containing 3% BSA, and 0.02% sodium azide for 30-60mins. After incubation, the cells were treated with three, five minute washes in PBS and subsequently mounted using ProLong Anti-fade Kit.
Imaging and analysis
Stained cells were imaged on an Axioskop FS microscope (Zeiss, Germany) equipped with a 63x, 0.9 NA, Achroplan, water-immersion objective or with a Nikon E600 microscope (Nikon, Japan) equipped with a 60x, 1.0 N.A. objective. Digital images were acquired with a CFW-1310 (Scion Corporation, Frederick, MD), 10-bit (1,024 gray scale intensity level) CCD digital camera, 1360 × 1024 pixel array. For a given antibody stain, images were acquired using identical parameters. 8 bit images were analyzed blindly with MetaMorph (Universal Imaging, Downingtown, PA) after background subtraction of camera noise, and flatfield division to level the intensity. Images were pseudocolored for presentation with Adobe Photoshop 7.0 (Adobe, San Jose, CA). Antibodypositive clusters were defined as clusters of fluorescence that were at least twice the background fluorescence of the image. The cluster size limitation was set by the auto-region selection (0.5×0.5 μm) by using MetaMorph software.
Acknowledgments
We thank Dr. Peter Scheiffele and Dr. Yutaka Hata for providing shRNA NLG-2 and NLG-2ΔC constructs, respectively.
Funding. This work was supported by National Institutes of Health Grants (MH64797) to Dr. Stefano Vicini.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests. The authors have declared that no competing interest exists.
Reference Cited
- 1.Brose N. Synaptic cell adhesion proteins and synaptogenesis in the mammalian central nervous system. Naturwissenschaften. 1999;86:516–524. doi: 10.1007/s001140050666. [DOI] [PubMed] [Google Scholar]
- 2.Serafini T. Finding a partner in a crowd: neuronal diversity and synaptogenesis. Cell. 1999;98:133–136. doi: 10.1016/s0092-8674(00)81008-9. [DOI] [PubMed] [Google Scholar]
- 3.Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 4.Garner CC, Zhai RG, Gundelfinger ED, Ziv NE. Molecular mechanisms of CNS synaptogenesis. Trends Neurosci. 2002;25:243–251. doi: 10.1016/s0166-2236(02)02152-5. [DOI] [PubMed] [Google Scholar]
- 5.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Sheng M. Some assembly required: the development of neuronal synapses. Nat Rev Mol Cell Biol. 2003;4:833–841. doi: 10.1038/nrm1242. [DOI] [PubMed] [Google Scholar]
- 7.Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annu Rev Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- 8.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 9.Dean C, Scholl FG, Choih J, DeMaria S, Berger J, et al. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu Z, Washbourne P, Ortinski P, Vicini S. Functional excitatory synapses in HEK293 cells expressing neuroligin and glutamate receptors. J. Neurophysiol. 2003;90:3950–3957. doi: 10.1152/jn.00647.2003. [DOI] [PubMed] [Google Scholar]
- 11.Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci U S A. 2004;10:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- 14.Chubykin AA, Liu X, Comoletti D, Tsigelny I, Taylor P, et al. Dissection of synapse induction by neuroligins: effect of a neuroligin mutation associated with autism. J Biol Chem. 2005;280:22365–22374. doi: 10.1074/jbc.M410723200. [DOI] [PubMed] [Google Scholar]
- 15.Levinson JN, Chery N, Huang K, Wong TP, Gerrow K, et al. Neuroligins mediate excitatory and inhibitory synapse formation: involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J Biol Chem. 2005;280:17312–17319. doi: 10.1074/jbc.M413812200. [DOI] [PubMed] [Google Scholar]
- 16.Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, et al. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–54. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Dong N, Qi J, Chen G. Molecular reconstitution of functional GABAergic synapses with expression of neuroligin-2 and GABAA receptors. Mol Cell Neurosci. 2007;35:14–23. doi: 10.1016/j.mcn.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, et al. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- 19.Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–56. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- 21.Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2 Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blundell J, Tabuchi K, Bolliger MF, Blaiss CA, Brose N, et al. Genes Brain Behav. 2008. Increased Anxiety-like Behavior in Mice Lacking the Inhibitory Synapse Cell Adhesion Molecule Neuroligin 2. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brager DH, Luther PW, Erdelyi F, Szabo G, Alger BE. Regulation of exocytosis from single visualized GABAergic boutons in hippocampal slices. J Neurosci. 2003;23:10475–10486. doi: 10.1523/JNEUROSCI.23-33-10475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galarreta M, Erdélyi F, Szabó G, Hestrin S. Electrical coupling among irregular-spiking GABAergic interneurons expressing cannabinoid receptors. J Neurosci. 2004;24:9770–9778. doi: 10.1523/JNEUROSCI.3027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Benedito G, Sugress K, Erdélyi F, Szabó G, Molnár Z, et al. Preferential origin and layer destination of GAD65-eGFP cortical interneurons. Cereb Cortex. 2004;14:1122–1133. doi: 10.1093/cercor/bhh072. [DOI] [PubMed] [Google Scholar]
- 26.Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verdoorn TA. Formation of heteromeric gamma-aminobutyric acid type A receptors containing two different alpha subunits. Mol Pharmacol. 1994;45:475–480. [PubMed] [Google Scholar]
- 28.Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABAA receptor channels are dependent on alpha-subunit isoform. Biophys J. 1997;73:2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClellan AM, Twyman RE. Receptor system response kinetics reveal functional subtypes of native murine and recombinant human GABAA receptors. J Physiol. 1999;515:711–27. doi: 10.1111/j.1469-7793.1999.711ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortinski PI, Lu C, Takagaki K, Fu Z, Vicini S. Expression of distinct alpha subunits of GABAA receptor regulates inhibitory synaptic strength. J Neurophysiol. 2004;92:1718–27. doi: 10.1152/jn.00243.2004. [DOI] [PubMed] [Google Scholar]
- 31.Silver RA, Traynelis SF, Cull-Candy SG. Rapid-time-course miniature and evoked excitatory currents at cerebellar synapses in situ. Nature. 1992;355:163–166. doi: 10.1038/355163a0. [DOI] [PubMed] [Google Scholar]
- 32.Fu Z, Logan SM, Vicini S. Deletion of the NR2A subunit prevents developmental changes of NMDA-mEPSCs in cultured mouse cerebellar granule neurones. J Physiol. 2005;563:867–881. doi: 10.1113/jphysiol.2004.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1010. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nusser Z, Roberts JDB, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J Neurosci. 1995;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, et al. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pritchett DB, Seeburg PH. γ-aminobutyric acidA receptor 5-subunit creates novel type II benzodiazepine receptor pharmacology. J Neurochem. 1990;54:1802–1804. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 39.Perrais D, Ropert N. Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses. J Neurosci. 1999;19:578–588. doi: 10.1523/JNEUROSCI.19-02-00578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maconochie DJ, Zempel JM, Steinbach JH. How quickly can GABAA receptors open? Neuron. 1994;12:61–71. doi: 10.1016/0896-6273(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 41.Puia G, Costa E, Vicini S. Functional diversity of GABA-activated Cl- currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron. 1994;12:117–126. doi: 10.1016/0896-6273(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 42.Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 43.Tia S, Wang JF, Kotchabhakdi N, Vicini S. Distinct deactivation and desensitization kinetics of recombinant GABAA receptors. Neuropharmacology. 1996b;35:1375–1382. doi: 10.1016/s0028-3908(96)00018-4. [DOI] [PubMed] [Google Scholar]
- 44.Barberis A, Mozrzymas JW, Ortinski PI, Vicini S. Desensitization and binding properties determine distinct α1β2γ2 and α3β2γ2 GABAA receptor-channel kinetic behavior. Eur J Neurosci. 2007;25:2726–2740. doi: 10.1111/j.1460-9568.2007.05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ragozzino D, Woodward RM, Murata Y, Eusebi F, Overman LE, et al. Design and in vitro pharmacology of a selective gamma-aminobutyric acidC receptor antagonist. Mol Pharmacol. 1996;50(4):1024–1030. [PubMed] [Google Scholar]
- 46.Jones MV, Jonas P, Sahara Y, Westbrook GL. Microscopic kinetics and energetics distinguish GABAA receptor agonists from antagonists. Biophys J. 2001;81:2660–2670. doi: 10.1016/S0006-3495(01)75909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barberis A, Lu C, Vicini S, Mozrzymas JW. Developmental Changes of GABA Synaptic Transient in Cerebellar Granule Cells. Molecular pharmacology. 2005;67:1221–1228. doi: 10.1124/mol.104.006437. [DOI] [PubMed] [Google Scholar]
- 48.Sumita K, Sato Y, Iida J, Kawata A, Hamano M, et al. Synaptic scaffolding molecule (S-SCAM) membrane-associated guanylate kinase with inverted organization (MAGI)-2 is associated with cell adhesion molecules at inhibitory synapses in rat hippocampal neurons. J Neurochem. 2007;100:154–166. doi: 10.1111/j.1471-4159.2006.04170.x. [DOI] [PubMed] [Google Scholar]
- 49.Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 50.Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci. 2006;26:12152–12164. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lisé MF, El-Husseini A. The neuroligin and neurexin families: from structure to function at the synapse. Cell Mol Life Sci. 2006;63:1833–1849. doi: 10.1007/s00018-006-6061-3. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujita E, Kouroku Y, Ozeki S, Tanabe Y, Toyama Y, et al. Oligo-asthenoteratozoospermia in mice lacking RA175/TSLC1/SynCAM/IGSF4A, a cell adhesion molecule in the immunoglobulin superfamily. Mol Cell Biol. 2006;26:718–726. doi: 10.1128/MCB.26.2.718-726.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABAA receptor 6 subunit. J Neurosci. 1996a;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mellor JR, Merlo D, Jones A, Wisden W, Randall AD. Mouse cerebellar granule cell differentiation: electrical activity regulates the GABAA receptor α6 subunit gene. J Neurosci. 1998;18:2822–2833. doi: 10.1523/JNEUROSCI.18-08-02822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mellor JR, Wisden W, Randall AD. Somato-synaptic variation of GABAA receptors in cultured murine cerebellar granule cells: investigation of the role of the alpha6 subunit. Neuropharmacology. 2000;39:1495–1513. doi: 10.1016/s0028-3908(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 56.Dudanova I, Sedej S, Ahmad M, Masius H, Sargsyan V, et al. Important contribution of alpha-neurexins to Ca2+-triggered exocytosis of secretory granules. J Neurosci. 2006;26:10599–10613. doi: 10.1523/JNEUROSCI.1913-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang W, Rohlmann A, Sargsyan V, Aramuni G, Hammer RE, et al. Extracellular domains of alpha-neurexins participate in regulating synaptic transmission by selectively affecting N- and P/Q-type Ca2+ channels. J Neurosci. 2005;25:4330–4342. doi: 10.1523/JNEUROSCI.0497-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, et al. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- 59.Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, et al. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu C, Fu Z, Karavanov I, Yasuda RP, Wolfe BB, et al. NMDA receptor subtypes at autaptic synapses of cerebellar granule neurons. J Neurophysiol. 2006;96:2282–2294. doi: 10.1152/jn.00078.2006. [DOI] [PubMed] [Google Scholar]
- 61.Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- 62.Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Fiszman ML, Barberis A, Lu C, Fu Z, Erdélyi F, Szabó G, Vicini S. NMDA receptors increase the size of GABAergic terminals and enhance GABA release. J Neurosci. 2005;25:2024–2031. doi: 10.1523/JNEUROSCI.4980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, et al. Stargazing regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 65.Losi G, Prybylowski K, Fu Z, Luo JH, Vicini S. Silent synapses in developing cerebellar granule neurons. J Neurophysiol. 2002;87:1263–1270. doi: 10.1152/jn.00633.2001. [DOI] [PubMed] [Google Scholar]
- 66.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]


