Abstract
Many viruses have evolved strategies to either evade or hijack host cell immune programs, as a means of promoting their own reproduction. For example, the human cytomegalovirus (HCMV) immediate-early protein vMIA/UL37ex1 inhibits host cell apoptosis, and its expression during infection aids virus replication. Here it is shown that stable expression of vMIA/UL37ex1 reduces cleavage of the innate immune response-proteins MAVS and RIG-I by caspases during apoptosis. Unexpectedly, it is demonstrated that RIG-I, but not MAVS, is degraded during HCMV infection. This process occurs in a non-apoptotic manner, and provides new evidence that HCMV may have evolved a unique strategy to evade RIG-I-mediated immune responses.
Keywords: RIG-I, MAVS, IPS-1, CARDIF, VMIA, UL37X1, CLEAVAGE, APOPTOSIS, CYTOMEGALOVIRUS
1. Introduction
In order to aid their replication, viruses employ a number of methods to evade the immune systems of their hosts [1]. The human cytomegalovirus (HCMV; a β-herpesvirus) targets numerous pathways in the innate and adaptive immune systems, leading to the establishment of persistent infection [2,3]. These include: (1) production of glycoproteins that inhibit expression of major histocompatibility complex (MHC) proteins on the surface of infected cells, thereby reducing their recognition and destruction by CD8+ T lymphocytes; (2) expression of a cytomegalovirus homologue of human IL-10, an anti-inflammatory cytokine, which blocks immune NF-κB signaling; and (3) blocking host-cell apoptosis to reduce the clearance of infected cells [1–4]. To block apoptosis, HCMV encodes two proteins that ultimately inhibit the ability of an infected cell to activate cell-degrading caspases. vICA/UL36 prevents the activation of caspase-8, which is normally associated with the external apoptotic pathway and tumor necrosis factor (TNF) signaling; while vMIA/UL37ex1 prevents the activation of caspase-9, by inhibiting the release of pro-apoptotic factors from mitochondria [5,6].
The RIG-I-like helicase (RLH) pathway of the mammalian innate immune system senses intracellular viral pathogens and initiates a signaling cascade that ends in the production of type-1 interferon (reviewed in [7]). The pathway consists of the apical RNA helicases RIG-I and MDA-5, which sense cytoplasmic viral nucleotides and signal to the mitochondrial antiviral protein MAVS, through a CARD:CARD domain interaction [8,9]. This interaction leads to the activation of signaling kinases such as IRF3/7 and NF-κB, and ultimately in the production of antiviral cytokines [7]. Recent research has shown that MAVS is targeted by both viral proteases and host-cell caspases during infection and apoptosis [10–13], indicating that this protein is an important focal point in several immune pathways.
Here the role of HCMV in the regulation of RLH proteins was investigated. Expression of the anti-apoptotic protein vMIA prevents the degradation of MAVS and RIG-I during apoptosis, by blocking the activation of caspases and subsequent proteolysis. HCMV infection does not appear to modify levels of MAVS; however, RIG-I is first upregulated, then degraded in a non-apoptotic manner, indicating that this protein is targeted by HCMV.
2. Materials and Methods
2.1 Cell culture and reagents
Wild-type and vMIA HeLa cells, HepG2 cells and human foreskin fibroblasts (HFFs) were grown in DMEM supplemented with 10% FBS and penicillin-streptomycin. Cells were treated with staurosporine (Sigma) or zVAD-fmk (Calbiochem) dissolved in DMSO (Sigma), or Poly(I:C) (Sigma), which was reconstituted in ddH20, for the indicated times. Poly(I:C) transfections were carried out with Lipofectamine 2000 (Invitrogen), while plasmids were transfected with Fugene 6 (Roche). YFP-MAVS and FLAG-RIG-I have been described previously [8,14], while mito-dsRed was obtained from Clontech. Antibodies against MAVS (Bethyl Laboratories), FLAG (Stratagene), RIG-I (Abcam), PARP (Biomol), Tim23 (BD Biosciences) and β-actin (Sigma) were obtained commercially, while the HCMV IE 1/2 antibody was a kind gift of Anamaris Colberg-Poley. Secondary antibodies for western analysis (Amersham) and immunofluroesence (Invitrogen) were used according to manufacturers’ instructions. HFF cells were infected with HCMV (AD169) at a MOI of 1.0 for the indicated times.
2.2 Western blotting and subcellular fractionation
For general western blot analysis, cells were harvested by scraping in 1× PBS and pelleted by centrifugation at 200 g. Cells were lysed on ice in buffer containing 2 % CHAPS, 300 mM NaCl and 25 mM HEPES-KOH and whole-cell lysates were recovered by centrifugation at 10000 g for 10 min at 4 °C. Protein extracts were boiled for 10 min in reducing NuPage sample buffer then separated by SDS-PAGE (NuPage, Invitrogen). For subcellular fractionation, cells were harvested as above and resuspended in detergent-free sucrose lysis buffer on ice for 1 h. Cells were homogenized by passaging through 25-gauge needles, followed by a spin at 500 g to remove nuclei and unbroken cells. The remaining supernatant was centrifuged at 6000 g to separate heavy membranes and cytosol. 30 µg of each fraction was then separated by SDS-PAGE.
Following electrophoresis, proteins were transferred to nitrocellulose membrane for western analysis. Membranes were then blocked in non-fat milk before being probed with the indicated antibody. Following washes in 1× PBS-T, HRP-conjugated secondary antibodies (Amersham) were added to permit chemiluminescent identification (ECL Plus, Amersham).
2.3 Confocal microscopy and imaging
Cells were imaged using a Zeiss 510 confocal microscope with filters/lasers for YFP (FITC; 514 nm) and Alexa 568/mito-dsRed (Rhodamine; 546 nm). Figures were prepared with minimal processing in Photoshop (Adobe), and all panels within and between figures were subject to the same editing procedures.
3. Results
3.1 vMIA protects MAVS and RIG-I from cleavage during apoptosis
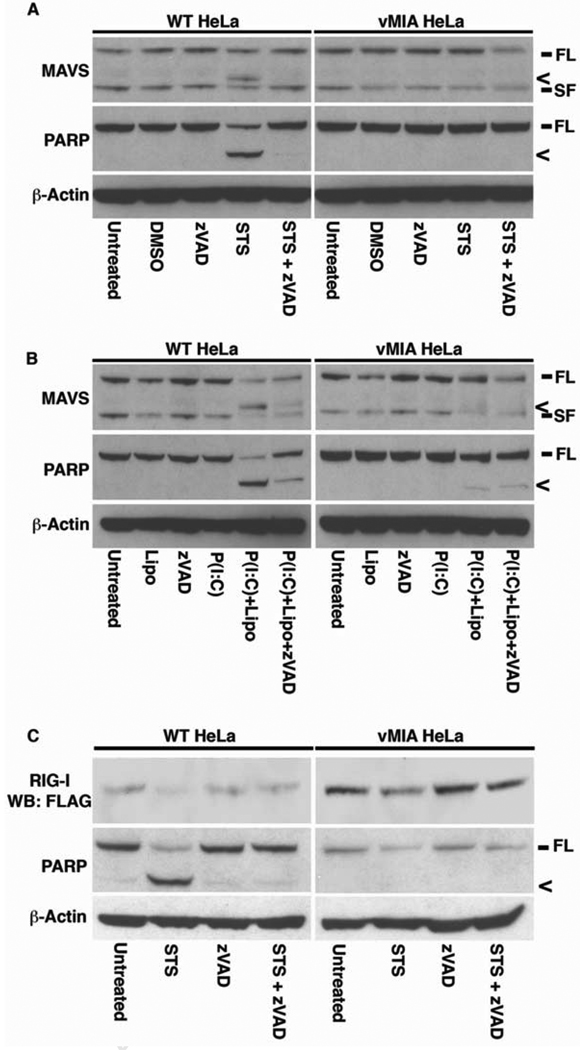
The RLH-pathway proteins MAVS and MDA-5 are targeted for cleavage during apoptosis [12,13,15,16]. As MAVS is localized to mitochondria [9], the question of whether the HCMV anti-apoptotic protein vMIA (which is also mitochondrial) could prevent the degradation of MAVS during cell death, was examined. In wild-type HeLa cells, treatment with the pro-apoptotic kinase inhibitor staurosporine (STS) led to cleavage of MAVS, as indicated by the presence of a faster-migrating portion during western analysis (Fig. 1A, left). This cleavage was mirrored by that shown by the caspase-3 target PARP, a classic indicator of apoptosis (Fig. 1A, left). The degradation of both proteins was abrogated by the addition of the caspase inhibitor zVAD-fmk (Fig. 1A, left), indicating that the cleavage is caspase-dependent, in line with previous reports [12,13]. In contrast, HeLa cells stably-expressing vMIA displayed no cleavage of either MAVS or PARP after STS treatment (Fig. 1A, right), indicating that vMIA blocks MAVS degradation by inhibiting the activation of caspases.
Figure 1. vMIA protects MAVS and RIG-I from capsase-mediated cleavage during apoptosis.
A WT and vMIA HeLa cells were treated with 0.5 µM staurosporine (STS) in the presence or absence of 50 µM zVAD-fmk (zVAD). Untreated and vehicle-only (DMSO) cells were used as controls. After 2 h, cells were harvested and 50 µg of protein extracts were analyzed for MAVS and PARP by western blotting, with β-actin being used as a loading control. B WT and vMIA HeLa cells were treated with 5 µg ml−1 Poly (I:C) for 6 h, either added to the growth medium (P(I:C)), or transfected into cells (P(I:C) + Lipo) using Lipofectamine 2000, in the presence or absence of 50 µM zVAD. 50 µg of protein extracts were analyzed for MAVS, PARP and β-actin as above. C WT and vMIA HeLa cells were transfected for 16 h with FLAG-RIG-I, then treated for 2 h with 0.5 µM STS in the presence or absence of 50 µM zVAD. 50 µg of protein extracts were analyzed for RIG-I (α-FLAG), PARP and β-actin as above. Symbols: FL – full-length protein; SF – short-form of protein, < – cleavage product.
The synthetic dsRNA analogue Poly(I:C) is commonly used to mimic infection by a number of viruses [9,16]. Previous work has shown that Poly(I:C) can activate the 2'-5' oligoadenylate synthetase (OAS) and ribonuclease RNase L pathways, leading to apoptosis [17,18], and that transfection of Poly(I:C) leads to apoptotic MAVS cleavage [12]. Addition of Poly(I:C) to the cell culture medium of WT HeLa cells had no effect on the status of MAVS and PARP after 6 h (Fig. 1B, left). In contrast, transfection of the same concentration of the chemical into the cells induced apoptosis (as evidenced by degradation of PARP) and cleavage of MAVS, which again could be ablated by the addition of zVAD (Fig. 1B, left). As with STS treatment, the cleavage of MAVS and PARP caused by Poly(I:C) transfection was vastly reduced in cells expressing vMIA (Fig. 1B, right), confirming that this protein can negate the effects of multiple apoptotic stimuli to prevent MAVS cleavage.
While previous reports have shown that MDA-5 is cleaved by caspases during apoptosis, there have been no similar studies of the behavior of RIG-I during cell death. To investigate whether RIG-I is also a caspase target, WT and vMIA HeLa cells were transfected overnight with a plasmid encoding FLAG-RIG-I, then treated cells with STS for 2 h. Western analysis of protein extracts showed that in WT cells STS treatment led to a vast reduction in levels of FLAG-RIG-I, in a manner that correlated with PARP cleavage, and that this change could be blocked by incubation with zVAD (Fig. 1C, left). The reduction in FLAG-RIG-I levels was greatly reduced in vMIA-expressing HeLa cells treated with STS relative to WT, consistent with the absence of caspase-mediated PARP cleavage (Fig. 1C, right). These data indicate that RIG-I, in common with the other innate immune system CARD proteins MDA-5 and MAVS, is cleaved during apoptosis.
3.2 vMIA prevents the cellular redistribution of MAVS during apoptosis
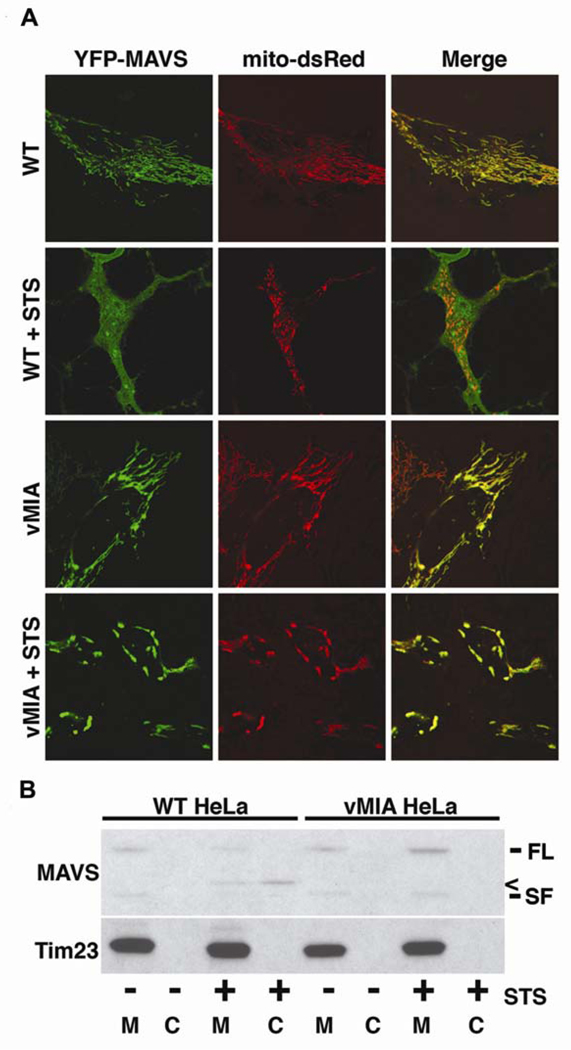
Recently, it was demonstrated that cleavage of MAVS during apoptosis led to a redistribution of the protein. While MAVS is located on the mitochondrial outer membrane in healthy cells, cleavage during apoptosis leads to the majority of the protein being found in the cytosol [12]. Addition of a proteasome inhibitor (MG132) to STS-treated cells blocked this change, indicating that a protein degradation mechanism was behind the alteration [12]. To investigate whether expression of vMIA could prevent the redistribution of MAVS, YFP-MAVS and the mitochondrial marker mito-dsRed were co-expressed in WT and vMIA HeLa cells, then treated with or without STS for 2 h. In WT cells, YFP-MAVS showed a tight co-localization with mito-dsRed when analyzed by confocal microscopy, indicating that the protein is located at the mitochondria (Fig. 2A). In contrast, MAVS in STS-treated WT HeLa cells displayed a diffuse cytoplasmic localization, suggesting that the protein had been cleaved from its C-terminal mitochondrial anchor (Fig. 2A). In both untreated and STS-treated vMIA HeLa cells, YFP-MAVS remained at the mitochondria and displayed co-localization mito-dsRed (Fig. 2A), consistent with the hypothesis that redistribution of MAVS is apoptosis-dependent.
Figure 2. vMIA prevents redistribution of MAVS during apoptosis.
A WT and vMIA HeLa cells were co-transfected for 16 h with YFP-MAVS (green) and the mitochondrial marker mito-dsRed (red), then incubated with or without 0.5 µM STS for 2 h. Cells were then analyzed by confocal microscopy for the localization of YFP-MAVS. B WT and vMIA HeLa cells were treated with or without 0.5 µM STS for 2 h, then cells were harvested and lysed in detergent-free sucrose lysis buffer. Lysates were separated into membrane (M) and cytosolic (C) fractions by differential centrifugation, after which 30 µg of each extract was analyzed for endogenous MAVS by western blot. The mitochondrial inner membrane protein Tim23 was used as a loading control. Symbols: FL – full-length protein; SF – short-form of protein, < – cleavage product.
To ensure that the change in MAVS localization was caused by cleavage of the protein itself, and not the N-terminal YFP tag, the localization of endogenous MAVS was examined in WT and vMIA cells treated with or without STS for 2 h. After treatment, cells were harvested and the protein extracts were separated into heavy membrane (containing mainly mitochondria) and cytosolic (containing soluble and light membrane protein) fractions by differential centrifugation. In untreated WT HeLa cells, MAVS is confined to the heavy membrane fraction (Fig. 2B). However, in STS-treated WT cells, MAVS is greatly reduced in the membrane fraction, while an immunoreactive band corresponding to the cleaved version of MAVS is visible in the cytosol (Fig. 2B). In contrast, MAVS remains exclusively in the membrane fraction in both untreated and STS-treated vMIA HeLa cells, indicating that the endogenous protein remains at the mitochondria, consistent with the data from confocal analysis. In summary, it would appear that vMIA prevents the redistribution of MAVS by inhibiting the activation of caspases, which would normally cleave this protein from the mitochondria during apoptosis.
3.3 HCMV infection leads to degradation of RIG-I without affecting MAVS
Several viruses, such as Hepatitis A virus (HAV), Hepatitis C virus (HCV) and GB virus B (GBV-B), target MAVS for degradation with self-encoded proteases during infection. The proteolysis removes MAVS from the mitochondrial outer membrane, in a manner that is phenotypically-similar to the cleavage seen during apoptosis. This effectively negates the interferon signaling function of MAVS, aiding viral replication [10,14,19]. As such, the question of whether HCMV infection had the capacity to initiate MAVS cleavage, in a manner similar to these other viruses, was examined.
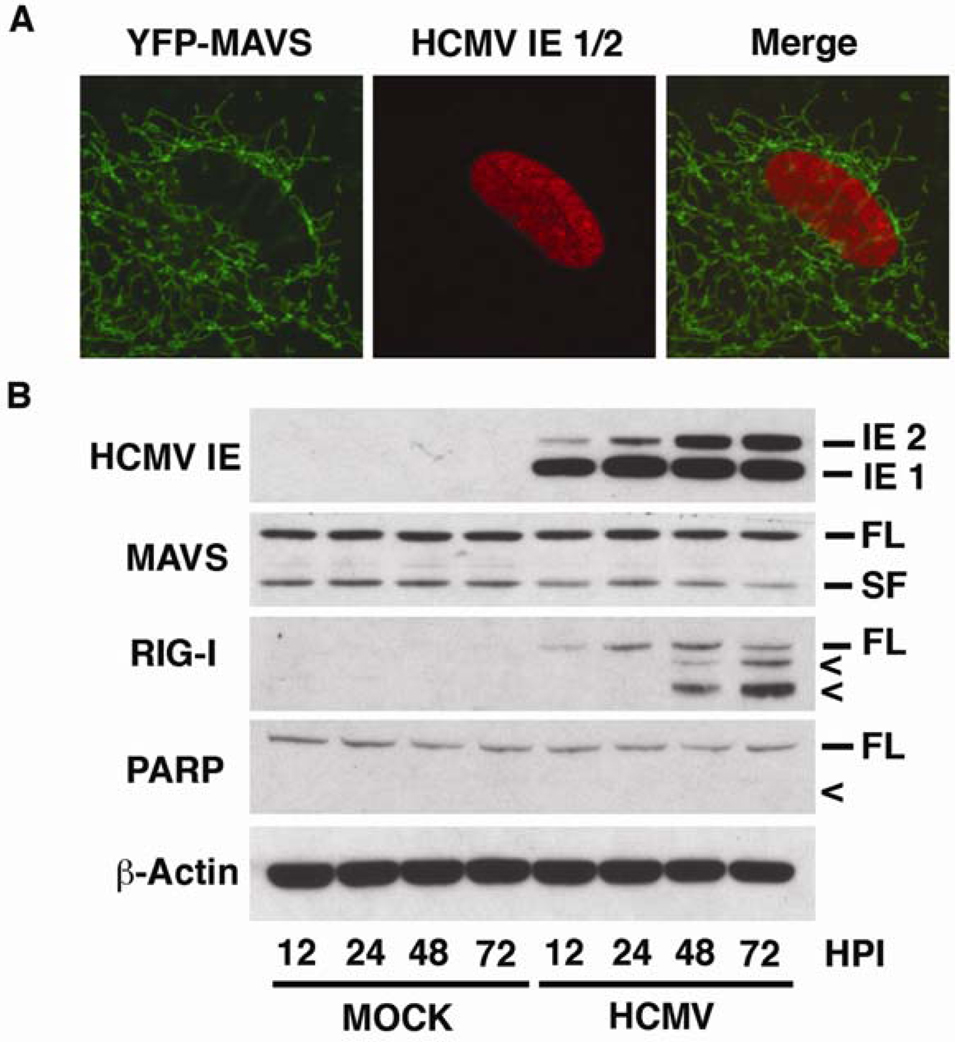
HFF cells grown in chamber slides were transfected with YFP-MAVS overnight, followed by infection with HCMV for 48 h. Cells were then fixed and stained with an antibody against HCMV immediate-early 1 and 2 (HCMV IE 1/2) to identify infected cells. Confocal microscopy analysis demonstrated that in HCMV-positive cells, MAVS maintained an organellar morphology (Fig. 3A). This indicated that the protein had not been cleaved by the virus and that it maintained its wild-type, mitochondrial localization.
Figure 3. HCMV infection leads to degradation of RIG-I without affecting MAVS.
A Human foreskin fibroblast (HFF) cells grown in chamber slides were transfected with YFP-MAVS (green) for 16 h, and then infected with HCMV (AD169) for 48 h. Cells were fixed with paraformaldehyde and probed with an antibody to HCMV immediate-early 1/2 (IE 1/2), followed by staining with Alexa 568 (red). Cells were analyzed by confocal microscopy for MAVS localization in HCMV infected cells. B HFF cells were either mock treated or infected with HCMV (AD169) for 12, 24, 48 or 72 h. Cells were harvested and 50 µg of protein extracts were analyzed by western blotting with antibodies against HCMV IE 1/2, MAVS, RIG-I and PARP. β-actin was used as a loading control. Symbols: FL – full-length protein; SF – short-form of protein, < – cleavage product.
To assess whether this was the case for endogenous MAVS, HFF cells were either mock- or HCMV-infected for 12 – 72 h, after which protein extracts were examined by western blot. Consistent with the confocal data, there was no apparent cleavage of MAVS over the course of the experiment in either the mock or HCMV-infected cells (Fig. 3B). From these data, it is concluded that MAVS does not undergo HCMV-induced cleavage during acute infection.
In HCMV-infected cells, consistent with the immune response elicited by other viruses, there was a rapid up-regulation of RIG-I protein levels that continued for the first 24 h (Fig. 3B). Surprisingly, after 48 h there was a decrease in the expression of full-length RIG-I, coupled with the appearance of two smaller immunoreactive bands that grew in intensity from 48 – 72 h (Fig. 3B). It would appear that these bands are not the result of the same apoptotic cleavage demonstrated above (Fig. 1C), as there is clear expression of immediate-early proteins, which include the anti-apoptotic vMIA (Fig. 3B). Additionally, there is no cleavage of PARP, indicating that there has been no activation of caspases over the course of the experiment (Fig. 3B). From these data, it is concluded that RIG-I is subject to proteolytic degradation following HCMV infection.
4. Discussion
The ability of a virus to evade the immune system of its intended host, to a large extent, controls its success as a pathogen. As such, viruses that attack higher organisms have to produce new tools to disrupt the constantly-evolving immune programs of their targets. The RLH signaling pathway in humans is a prime example of this “tit-for-tat” (equivalent retaliation) process in action, where several members of the pathway are targeted by different viral agents in order to abrogate an immune response. For example, two unrelated viruses - HAV (Picornaviridae) and HCV (Flaviviridae) - both target MAVS for proteolytic cleavage from the mitochondrial membrane, using viral-encoded proteases. This cleavage ablates its signaling function and prevents the production of type-1 interferon [10,19]. MAVS, and the upstream RNA helicase MDA-5, are both degraded by caspases during poliovirus (Picornaviridae) infection, possibly aiding viral survival [13,16].
While these results demonstrate that several viruses have evolved strategies to counter RLH immune signaling, there have been no reports thus far of similar methods to disrupt the prototypic member of the pathway, RIG-I. Data presented here indicates that, in a manner similar to MDA-5 and MAVS, RIG-I is targeted for degradation during the induction of apoptosis (Fig. 1C). As this cleavage is blocked by the caspase-inhibitor zVAD, and expression of vMIA (which blocks caspase activation by preventing the release of pro-apoptotic factors from the mitochondrion; [20]), it would appear that RIG-I is a bone fide target of caspases. As with MDA-5 and MAVS, it remains to be determined whether of not this cleavage provides a benefit to invading viruses in terms of aiding replication [13,16].
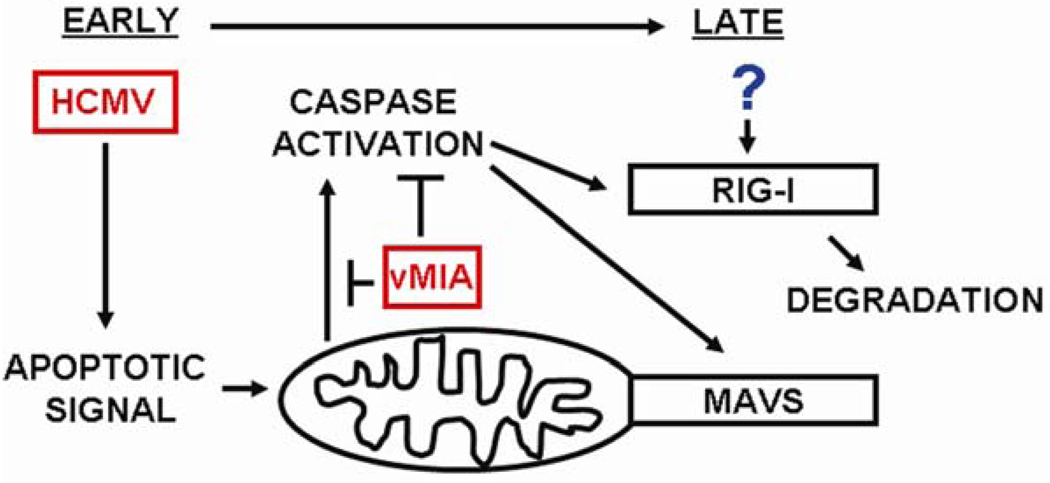
As stated above, expression of the anti-apoptotic HCMV protein vMIA affords protection to MAVS and RIG-I from cleavage during apoptosis (Fig. 1). While preventing apoptosis may be beneficial to HCMV, allowing replication without clearance of infected cells, this may in turn maintain the integrity of the host innate immune system. Specifically, protecting MAVS and RIG-I from cleavage may permit continued interferon signaling through the RLH pathway, possibly negating the benefits accrued through apoptosis prevention. As such, the degradation of RIG-I observed after HCMV infection (Fig. 3B) may provide an answer to this potential conflict. As depicted in the working model of RLH proteins during HCMV infection (Fig. 4), the early expression of vMIA may prevent the initial induction of caspases, protecting the cell from apoptosis and allowing HCMV replication. As the infection progresses, expression of an unidentified protein may target and degrade RIG-I, preventing further immune signaling through this pathway.
Figure 4. Model of the roles of RLH proteins during HCMV infection.
vMIA is expressed early during HCMV infection, protecting host cells from the intrinsic apoptosis pathway, presumably as a mechanism to aid viral replication. However, this protection also prevents the caspase-mediated cleavage of RIG-I and MAVS, leaving this interferon pathway intact. As such, expression of an unknown protein may degrade RIG-I to help abrogate this immune response and aid HCMV persistence.
As a double-stranded DNA (dsDNA) virus, HCMV is perhaps not immediately obvious as a candidate to induce RIG-I degradation, as the RLH pathway is primarily associated with RNA viruses [8]. However, previous studies have shown that dsDNA viruses can be sensed by the RLH pathway, and that RIG-I and MAVS are required for interferon signaling in response to dsDNA in human liver cells [21]. Additionally, expression of HCMV pp65 (UL83) has been shown to block IRF3 signaling, which sits downstream of RIG-I/MAVS in the RLH pathway, showing that the virus attacks interferon induction at multiple related steps [22,23]. Therefore, it may be concluded that the RLH pathway is a legitimate target for HCMV, and that degradation of RIG-I may be part of this process. Future studies will now concentrate on identifying the factor(s) that lead to RIG-I degradation during HCMV infection.
Acknowledgements
The author thanks Richard Youle for support and equipment, Chad Williamson for help and advice with viral experiments, Wade Gibson for helpful discussions and both Victor Goldmacher and Kui Li for materials. This research was supported by the intramural research program of the National Institute of Neurological Disorders and Stroke, National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu. Rev. Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 2.Mocarski ES. Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 2002;10:332–339. doi: 10.1016/s0966-842x(02)02393-4. [DOI] [PubMed] [Google Scholar]
- 3.Crough T, Khanna R. Immunobiology of Human Cytomegalovirus: from Bench to Bedside. Clin. Microbiol Rev. 2009;22:76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotenko SV, Saccani S, Izotova LS, Mirochnitchenko OV, Pestka S. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10) Proc. Natl. Acad. Sci. USA. 2000;97:1695–1700. doi: 10.1073/pnas.97.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldmacher VS, Bartle LM, Skaletskaya A, Dionne CA, Kedersha NL, Vater CA, Han JW, Lutz RJ, Watanabe S, Cahir McFarland ED, Kieff ED, Mocarski ES, Chittenden T. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. USA. 1999;96:12536–12541. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skaletskaya A, Bartle LM, Chittenden T, McCormick AL, Mocarski ES, Goldmacher VS. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. USA. 2001;98:7829–7834. doi: 10.1073/pnas.141108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott I. Mitochondrial factors in the regulation of innate immunity. Microb. Infect. 2009 doi: 10.1016/j.micinf.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 9.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 11.Li K, Foy E, Ferreon JC, Nakamura M, Ferreon ACM, Ikeda M, Ray SC, Gale M, Lemon SM. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. USA. 2005;102:2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott I, Norris KL. The mitochondrial antiviral signaling protein, MAVS, is cleaved during apoptosis. Biochem. Biophys. Res. Commun. 2008;375:101–106. doi: 10.1016/j.bbrc.2008.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebsamen M, Meylan E, Curran J, Tschopp J. The antiviral adaptor proteins Cardif and Trif are processed and inactivated by caspases. Cell Death Differ. 2008;15:1804–1811. doi: 10.1038/cdd.2008.119. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Benureau Y, Rijnbrand R, Yi J, Wang T, Warter L, Lanford RE, Weinman SA, Lemon SM, Martin A, Li K. GB virus B disrupts RIG-I signaling by NS3/4A-mediated cleavage of the adaptor protein MAVS. J. Virol. 2007;81:964–976. doi: 10.1128/JVI.02076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovacsovics M, Martinon F, Micheau O, Bodmer JL, Hofmann K, Tschopp J. Overexpression of Helicard, a CARD-Containing Helicase Cleaved during Apoptosis, Accelerates DNA Degradation. Curr. Biol. 2002;12:838–843. doi: 10.1016/s0960-9822(02)00842-4. [DOI] [PubMed] [Google Scholar]
- 16.Barral PM, Morrison JM, Drahos J, Gupta P, Sarkar D, Fisher PB, Racaniello VR. MDA-5 is cleaved in poliovirus-infected cells. J. Virol. 2007;81:3677–3684. doi: 10.1128/JVI.01360-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castelli JC, Hassel BA, Maran A, Paranjape J, Hewitt JA, Li XL, Hsu YT, Silverman RH, Youle RJ. The role of 20–50 oligoadenylate-activated ribonuclease L in apoptosis. Cell. Death Differ. 1998;5:313–320. doi: 10.1038/sj.cdd.4400352. [DOI] [PubMed] [Google Scholar]
- 18.Domingo-Gil E, Esteban M. Role of mitochondria in apoptosis induced by the 2-5A system and mechanisms involved. Apoptosis. 2006;11:725–738. doi: 10.1007/s10495-006-5541-0. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Liang Y, Qu L, Chen Z, Yi M, Li K, Lemon SM. Disruption of innate immunity due to mitochondrial targeting of a picornaviral protease precursor. Proc. Natl. Acad. Sci. USA. 2007;104:7253–7258. doi: 10.1073/pnas.0611506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnoult D, Bartle LM, Skaletskaya A, Poncet D, Zamzami N, Park PU, Sharpe J, Youle RJ, Goldmacher VS. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. USA. 2004;101:7988–7993. doi: 10.1073/pnas.0401897101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng G, Zhong J, Chung J, Chisari FV. Double-stranded DNA and double-stranded RNA induce a common antiviral signaling pathway in human cells. Proc. Natl. Acad. Sci. USA. 2007;104:9035–9040. doi: 10.1073/pnas.0703285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abate DA, Watanabe S, Mocarski ES. Major Human Cytomegalovirus Structural Protein pp65 (ppUL83) Prevents Interferon Response Factor 3 Activation in the Interferon Response. J. Virol. 2004;78:10995–11006. doi: 10.1128/JVI.78.20.10995-11006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoneyama M, Fujita T. Function of RIG-I-like Receptors in Antiviral Innate Immunity. J. Biol. Chem. 2007;282:15315–15318. doi: 10.1074/jbc.R700007200. [DOI] [PubMed] [Google Scholar]