Abstract
To gain insight into the mechanical determinants of walking energetics, we investigated the effects of aging and arm swing on the metabolic cost of stabilization. We tested two hypotheses: 1) elderly adults consume more metabolic energy during walking than young adults because they consume more metabolic energy for lateral stabilization, and 2) arm swing reduces the metabolic cost of stabilization during walking in young and elderly adults. To test these hypotheses, we provided external lateral stabilization by applying bilateral forces (10% body weight) to a waist belt via elastic cords while young and elderly subjects walked at 1.3 m/s on a motorized treadmill with arm swing and with no arm swing. We found that the external stabilizer reduced the net rate of metabolic energy consumption to a similar extent in elderly and young subjects. This reduction was greater (6–7%) when subjects walked with no arm swing than when they walked normally (3–4%). When young or elderly subjects eliminated arm swing while walking with no external stabilization, net metabolic power increased by 5–6%. We conclude that the greater metabolic cost of walking in elderly adults is not caused by a greater cost of lateral stabilization. Moreover, arm swing reduces the metabolic cost of walking in both young and elderly adults likely by contributing to stability.
Keywords: biomechanics, aging, arm swing, locomotion, stability, gait
1. Introduction
Elderly adults consume about 15–20% more metabolic energy per kilogram of body mass for walking than young adults (Martin et al., 1992; Waters et al., 1988) but the reasons are not known. One possible cause of the greater metabolic cost of walking in elderly adults is a greater cost of lateral stabilization. Human walking is laterally unstable but implementing active control of step width counteracts this instability (Bauby and Kuo, 2000; Townsend, 1985). However, active control of step width requires that humans perform more mechanical work and consume more metabolic energy (Donelan et al., 2001).
As humans age, their ability to actively control lateral stabilization during walking may decline due to progressive impairment of neuromuscular function (Campbell et al., 1973; Lauretani et al., 2003; Stelmach and Worringham, 1985; Tomlinson and Irving, 1977). Consequently, to maintain lateral stability during walking, elderly adults may adopt stabilization strategies that increase metabolic cost like co-contraction of antagonist muscle pairs (Benjuya et al., 2004). Although co-contraction of antagonist muscle pairs can maintain stability despite reduced sensory information (Benjuya et al., 2004), it exacts a metabolic penalty because agonist muscles must generate more force to overcome antagonist force generation.
Similarly, elderly adults may enhance lateral stability despite reduced sensory input by taking wider steps (Bauby and Kuo, 2000; Collins, 2003) and adjusting step width more frequently (Collins, 2003; Owings and Grabiner, 2004) although this strategy exacts a metabolic penalty (Donelan et al., 2001). Indeed, when an external stabilization device reduces the need for active stabilization during walking, step width decreases 50–70% and metabolic cost decreases 5.7% in young adults (Donelan et al., 2004) and 6.5% in elderly adults (P>0.05, Dean et al., 2007). However, those studies examined the effects of external stabilization on walking without arm swing, and arm swing may help stabilize the body (Elftman, 1939; Hinrichs and Cavanagh, 1981). Thus, it remains unclear whether elderly and young adults have a similar metabolic cost of lateral stabilization during walking with normal arm swing.
Arm swing may help stabilize the body during walking by balancing the angular momentum of the body and reducing the lateral displacement of the center of mass. At intermediate walking speeds, the arms swing in a pendulum-like motion opposite to leg motion and thereby counteract the angular momentum of the legs (Elftman, 1939; Hinrichs and Cavanagh, 1981). Although arm swing is not entirely passive (Jackson et al., 1978), it may reduce the need for more metabolically expensive stabilization techniques like increasing trunk muscle force generation or adjusting step width.
This study tested the hypotheses that: 1) elderly adults consume more metabolic energy during walking than young adults because they have a greater cost of lateral stabilization, and 2) arm swing reduces the metabolic cost of stabilization during walking in elderly and young adults. We began by quantifying the difference in metabolic cost and kinematics of walking at a moderate speed between elderly and young adults. To test our first hypothesis, we examined the effect of an external stabilization device on metabolic cost and kinematics when walking with arm swing and with no arm swing. To test our second hypothesis, we quantified the effect of eliminating arm swing on the metabolic cost and kinematics of walking normally and walking with external stabilization. We predicted that eliminating arm swing would have a smaller effect on metabolic cost when walking with external stabilization because the stabilizer applies a stabilizing twisting torque to the trunk.
2. Methods
2.1. Subjects
We collected kinematic and metabolic data during walking for twelve healthy young adults (six male, six female; age 22.7 ± 3.7 years, mass 64.8 ± 10.4 kg leg length 0.89 ± 0.07, mean ± s.d.) and twelve healthy elderly adults (six male, six female, age 74.5 ± 2.9 years, mass 68.3 ± 13.3 kg, leg length 0.89 ± 0.05, mean ± s.d.). Leg length was defined as the vertical distance from the ground to the greater trochanter (L). All subjects were physically active, had no known orthopedic, neurological, or cardiovascular disease, and gave their written informed consent. The University of Colorado Committee for the Protection of Human Subjects approved this protocol.
2.2. Protocol
Each subject participated in two sessions. In the first session, subjects were given a physical examination, and body composition was measured. During this session, elderly candidates also performed a graded exercise test to determine their cardiovascular health. During the second session at least three days after the first session, we measured standing metabolic rate, familiarized subjects to treadmill walking, and conducted the experimental walking trials. At the start of the second session, we measured metabolic rate as each subject stood quietly for seven minutes. Subsequently, for familiarization, each subject walked on a motorized treadmill (Model 18–60, Quinton Instruments, Seattle, WA, USA) for at least thirty minutes using normal arm swing, no arm swing, and external stabilization and until each subject reported feeling comfortable walking under the imposed condition. This familiarization period exceeded the recommended minimum treadmill habituation time of 10 minutes (Van de Putte et al., 2006; Wall and Charteris, 1981).
For the experimental trials, each subject walked at 1.3 m/s and completed one trial for each of four randomly ordered conditions separated by at least three minutes: 1) normal arm swing without external stabilization, 2) normal arm swing with external stabilization, 3) no arm swing (i.e., arms folded across chest) without external stabilization, and 4) no arm swing with external stabilization. Within the last three minutes of each seven minute trial, we collected data to determine the rate of O2 consumption and CO2 production, step frequency, step width, and the force applied by the external stabilizer.
2.3. External Stabilization Device
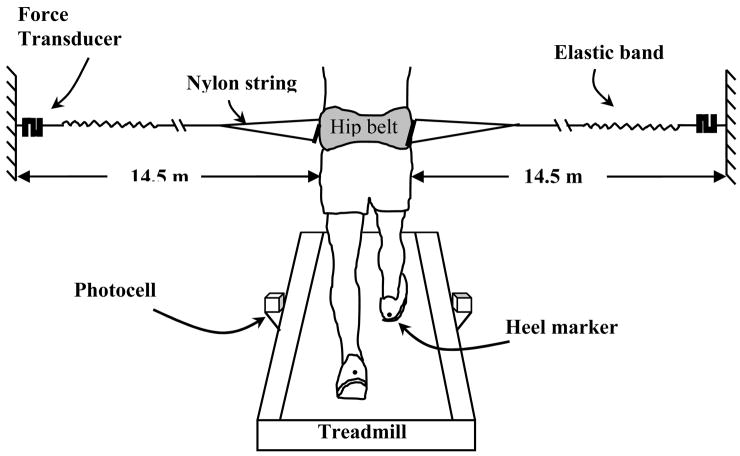
The external stabilization device consisted of two elastic cords that attached to the subject via a customized padded belt that allowed normal arm swing (Fig. 1). The cords pulled in opposite lateral directions and were approximately aligned with the whole body center of mass when the subject faced straight ahead (Donelan et al., 2004). The device provided ‘lateral stabilization’ by resisting lateral motion of the center of mass and ‘twisting stabilization’ by exerting a restoring torque about the trunk’s long axis whenever the trunk was not facing straight ahead.
Figure 1.
Schematic of external lateral stabilizer and step width measurement apparatus. Two lightweight elastic cords attached to a padded belt approximately in alignment with the center of mass when the subject faced straight ahead. Step width was measured by digitizing the medio-lateral positions of heel markers in rear-view video at the instant near mid-stance when the photocell was triggered. Adapted from Donelan et al. (Donelan et al., 2004).
Each elastic cord was comprised of a 14 m long nylon cord attached in series to 0.5 m of hollow latex rubber tubing. To allow normal arm swing, each cord attached to the front and back of a lightweight hollow fiberglass rod fastened to the waist belt and oriented in the fore-aft direction (Fig. 1). The other end of each elastic cord was attached to a wall mount via a force transducer. We adjusted the wall mount height for each subject so that the cord was parallel to the ground. Given the long cord length, small deviations from its intended orientation did not lead to significant vertical or horizontal (fore-aft) forces on the subject. The elastic cords were stretched so that each cord applied an average force of 10% (SEM 0.7) of body weight. By oscillating a known mass between the two cords and using a 2nd order damped oscillator model (Donelan et al., 2004), we determined that the system had an effective spring constant of approximately 1900 N/m and minimal damping (26 Ns/m). For non-stabilized trials, subjects wore the waist belt but the cords were not attached to it.
2.4. Metabolic power
We measured the rates of oxygen consumption (V̇O2) and carbon dioxide production (V̇CO2) using open-circuit respirometry (Physio-Dyne Instruments, Quogue, NY, USA). We calculated average metabolic rate per kilogram body mass (W/kg) (Brockway, 1987) using the average V̇O2 (ml O2/min) and V̇CO2 (ml CO2/min) for a two minute period during the last three minutes of each trial when V̇O2 had reached steady state. We subtracted the standing metabolic rate from gross metabolic rate for walking and divided by body mass to calculate net metabolic power (W/kg) for walking.
2.5. Kinematics
We determined average step width and its variability from rear view high speed video (200 fields/s, JC Labs, Mountain View, CA, USA) for twenty steps during the last three minutes of each trial. Reflective markers were placed on the heels of the subjects’ shoes (Fig. 1). We digitized (Peak Performance Technologies, Englewood, CO, USA) and filtered marker position using a fourth-order zero-lag Butterworth filter with a cutoff frequency of 6 Hz. We calculated step width as the lateral distance between left and right heel markers at approximately mid-stance for each foot and calculated its standard deviation to represent step width variability. To normalize for body size, we expressed step width relative to leg length.
2.6. Statistics
We used repeated-measures analysis of variance (ANOVA, p<0.05) to determine statistical differences due to age, external stabilization, arm swing, and the interaction of these variables. When appropriate, we applied independent t-tests to determine differences between elderly and young subjects for each condition and paired t-tests to test the effects of external stabilization and arm swing. We used SPSS software for these tests (SPSS Inc., Chicago, IL). All data are reported as mean ± SEM unless otherwise specified.
3. Results
3.1. Effect of age on walking cost and kinematics
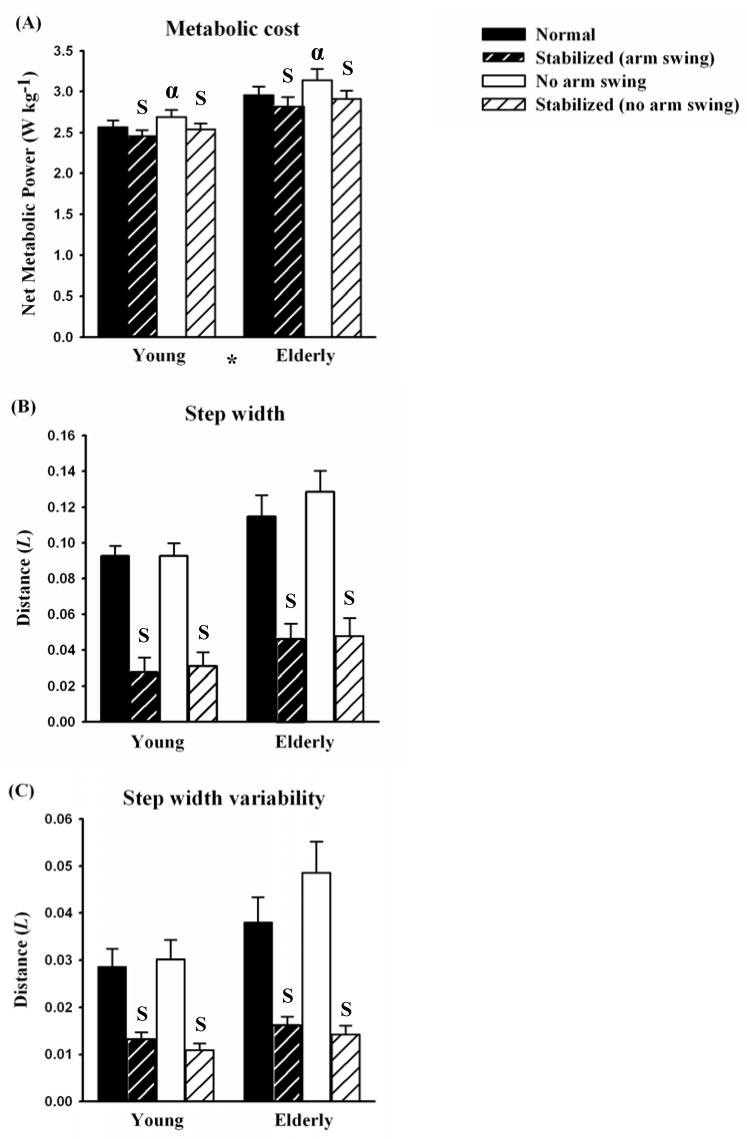
When walking normally (with arm swing) at 1.3 m/s with no external stabilization, elderly subjects consumed metabolic energy faster than young subjects but had similar kinematics (Table 1 and Fig. 2). Elderly subjects consumed metabolic energy 15% faster per kilogram of body mass (W/kg) than young subjects (p=0.005; Fig. 2A). Elderly subjects used a similar step frequency, step width, and step width variability as young subjects (Table 1, Fig. 2B and 2C).
Table 1.
Metabolic power consumption and step kinematics (mean and standard errors of means) for young (n = 12) and elderly (n = 12) subjects. Step width and step width variability are normalized to leg length (L).
| Young
|
Elderly
|
Age Effect
|
|||
|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | P | |
| Normal walking | |||||
| Net metabolic power (W kg−1) | 2.57 | 0.09 | 2.96 | 0.11 | 0.005 |
| Step frequency (Hz) | 1.82 | 0.03 | 1.91 | 0.05 | 0.134 |
| Step width (L) | 0.093 | 0.006 | 0.115 | 0.012 | 0.110 |
| Step width variability (L) | 0.029 | 0.004 | 0.038 | 0.005 | 0.083 |
| Stabilized walking (with arm swing) | |||||
| Net metabolic power (W kg−1) | 2.49 | 0.08 | 2.83 | 0.11 | 0.010 |
| Step frequency (Hz) | 1.80 | 0.03 | 1.93 | 0.05 | 0.090 |
| Step width (L) | 0.028 | 0.008 | 0.047 | 0.008 | 0.090 |
| Step width variability (L) | 0.013 | 0.001 | 0.016 | 0.002 | 0.097 |
| Walking with no arm swing | |||||
| Net metabolic power (W kg−1) | 2.69 | 0.08 | 3.14 | 0.14 | 0.006 |
| Step frequency (Hz) | 1.82 | 0.03 | 1.95 | 0.06 | 0.052 |
| Step width (L) | 0.093 | 0.007 | 0.129 | 0.012 | 0.016 |
| Step width variability (L) | 0.030 | 0.004 | 0.049 | 0.007 | 0.014 |
| Stabilized walking (with no arm swing) | |||||
| Net metabolic power (W kg−1) | 2.54 | 0.08 | 2.91 | 0.10 | 0.005 |
| Step frequency (Hz) | 1.80 | 0.03 | 1.93 | 0.05 | 0.056 |
| Step width (L) | 0.031 | 0.008 | 0.048 | 0.010 | 0.200 |
| Step width variability (L) | 0.011 | 0.001 | 0.014 | 0.002 | 0.085 |
Figure 2.
(A) Net metabolic power, (B) step width, and (C) step width variability for young and elderly subjects walking normally (black bars), walking normally while externally stabilized (black with white hatch), walking with no arm swing (white), and walking with no arm swing while externally stabilized (white with black hatch). Values are means for all subjects in each group and error bars represent the standard errors of the means. Elderly subjects walked with greater net metabolic cost (* indicates significant age effect, p < 0.0001 for both arm swing and no arm swing). External stabilization reduced net metabolic power, step width, and step width variability to a similar extent in young and elderly adults (S indicates significant stabilization effect, p < 0.05). Eliminating arm swing increased net metabolic power (α indicates significant arm swing effect, p < 0.05).
3.2. Effects of external stabilization when walking with arm swing
External stabilization during walking decreased the rate of metabolic energy consumption and affected kinematics similarly in young and elderly subjects (Table 1 and Fig. 2). When we applied external stabilization as the subjects walked with normal arm swing, young and elderly subjects consumed 3% and 4% less metabolic energy, respectively (p<0.0001, Fig. 2A). When we applied external stabilization, young and elderly subjects reduced step width by 66% and 76%, respectively (p<0.0001, Fig. 2B), and step width variability by 54% and 57%, respectively (p=0.002, Fig. 2C), compared to normal walking. External stabilization did not affect step frequency in either group (p=0.164; Table 1).
3.3. Effect of external stabilization when walking with no arm swing
When walking without arm swing, external stabilization reduced metabolic cost, step width and step width variability similarly in young and elderly adults (Table 1 and Fig. 2). Applying external stabilization to young and elderly subjects walking with no arm swing reduced net metabolic power consumption by 6% and 7%, respectively (p<0.0001; Fig. 2A). Thus, the external stabilizer reduced metabolic power consumption to a greater extent when arm swing was eliminated than with normal arm swing (p=0.040). Moreover, when walking with no arm swing, external stabilization caused young and elderly subjects to take 67% and 63% narrower steps, respectively, (p<0.0001; Fig. 2B) and decrease step width variability by 64% and 71%, respectively (p=0.001; Fig. 2C). Finally, external stabilization did not affect step frequency in either group when walking with no arm swing (p=0.965; Table 1).
3.4. Effects of eliminating arm swing when walking normally
Eliminating arm swing during walking increased the rate of metabolic energy consumption but did not affect kinematics in both young and elderly subjects (Table 1 and Fig. 2). When young and elderly subjects eliminated arm swing, they consumed metabolic energy 5% and 6% faster, respectively, than when they walked normally (p=0.004; Fig. 2A). For both groups, eliminating arm swing did not affect step width (p=0.061), step width variability (p=0.154) (Fig. 2B and 2C), or step frequency (p=0.719; Table 1).
3.5. Effects of eliminating arm swing when walking with external stabilization
When walking with external stabilization, eliminating arm swing did not affect the net metabolic power consumption or kinematics of either young or elderly subjects (Table 1 and Fig. 2). With external stabilization, subjects walking with no arm swing consumed metabolic energy at a similar rate as when they walked with arm swing (p=0.160; Fig. 2A). Thus, it appeared that the stabilizer compensated for the elimination of arm swing. Likewise, eliminating arm swing while in the external stabilizer did not affect step width (p=0.619; Fig. 2B), step width variability (p=0.129; Fig. 2C), or step frequency (p=0.205; Table 1) for either group.
4. Discussion
The results of this study indicate that the cost of lateral stabilization does not explain the elevated metabolic cost of walking in elderly adults compared to young adults and that arm swing reduces the metabolic cost of stabilization during walking in both young and elderly adults. In agreement with past studies (Martin et al., 1992; Mian et al., 2006; Waters et al., 1988), we found that elderly adults consume approximately 15% more metabolic energy per kilogram to walk a meter than young adults. Contrary to our first hypothesis, lateral stabilization contributes a similar fraction of the net metabolic cost of walking in young and elderly adults, and therefore, stabilization cost does not explain why elderly adults consume more metabolic energy for walking than young adults. In agreement with our second hypothesis, eliminating arm swing increases the metabolic cost of walking for both young and elderly adults but does not affect metabolic cost when walking with external stabilization. This finding strongly suggests that arm swing helps stabilize the body in normal walking and thereby reduces the metabolic cost of walking to a similar extent in young and elderly adults.
Although elderly adults consume metabolic energy at a faster rate than young adults during normal walking, we find that they use similar step widths and step width variability as young adults. This finding strongly suggests that the age-related difference in metabolic cost is not due to step width differences. Our observation of similar step width and step width variability in young and elderly subjects walking with normal arm swing agrees with some past research (Gabell and Nayak, 1984; Ortega and Farley, 2007) but disagrees with previous studies showing that elderly adults take wider steps (Collins, 2003) and more variable step widths than young adults (Grabiner et al., 2001; Owings and Grabiner, 2004). The likely reason for these discrepancies is that our study included healthy, physically active elderly adults with no known neurological or orthopedic disorders in order to isolate the intrinsic effects of chronological aging rather than disease effects.
Young and elderly subjects reduce metabolic energy consumption to a similar extent when walking with external stabilization. In both groups, external stabilization reduces metabolic cost by 3–4% when walking with arm swing and 6–7% when walking with no arm swing. Despite the absence of a age-stabilization interaction effect on the rate of metabolic energy consumption (p=0.309), it should be noted that the absolute decrease in metabolic power as a result of external stabilization in elderly walkers was nearly 50% greater than the decrease in young walkers (Table 1). This is related to the fact that the elderly subjects consumed metabolic energy at a higher rate than young adults during normal walking. Although we know of no prior studies that quantified the effect of stabilization on walking with normal arm swing, these results broadly agree with prior studies that have investigated the effect of stabilization on walking with no arm swing. For example, we found that external stabilization causes young adults to consume 5.8% less metabolic energy when walking without arm swing, compared to 5.7% in Donelan et al. (Donelan et al., 2004). Moreover, our results also show external stabilization decreases metabolic cost similarly in young and elderly adults (6–7%) walking without arm swing while Dean et al. (Dean et al., 2007) found that external stabilization reduces cost to a similar extent (5.4–6.5%) as our study but their finding was not significant. This discrepancy is likely due to Dean et al. (2007) using fewer subjects and might also be related to differences in the lateral stabilization devices. Our stabilization device has longer cords (14.5 m compared to 3 m in Dean et al. 2007) which reduces unwanted vertical and fore-aft forces. Moreover, our device had greater stiffness (1900 N/m) than the device used in Dean et al. (Dean et al., 2007) (1200 N/m). Higher cord stiffness improves stabilization (Donelan et al., 2004).
The similar metabolic cost of lateral stabilization in young and elderly adults likely results from similar strategies for controlling lateral stability. This conclusion is also supported by our observations that young and elderly adults reduce step width and step width variability to a similar extent when walking with external stabilization. Thus, external stabilization causes both young and elderly adults to rely less heavily on taking wide steps and frequently adjusting step width to maintain lateral stability. Taking narrower steps has the advantage of reducing the lateral forces that walkers must apply to the ground and thereby reducing the individual limb mechanical work performed to redirect and accelerate the center of mass during step-to-step transitions (Donelan et al., 2001).
Although adjusting step width likely has the greatest effect on the cost of stabilization, arm swing likely reduces the cost of stabilization during walking because it reduces the need for more costly stabilization strategies. When young or elderly adults eliminate arm swing, the rate of metabolic energy consumption increases 5–6% for walking. Because elderly adult consume metabolic energy at a higher rate than young adults during normal walking, the increase in metabolic power (W/kg) as a result of external stabilization was 50% greater than the increase observed in young walkers (Table 1). During normal walking, arm swing compensates for the angular momentum of the swinging legs and thereby helps control the twisting motion of the trunk about its long axis (Collins et al., 2001; Elftman, 1939; Hinrichs and Cavanagh, 1981). When humans eliminate arm swing during walking, they likely use more metabolically expensive mechanisms, such as trunk muscle force generation, to prevent excessive trunk twisting and maintain a straight path. Moreover, without arm swing, the center of mass undergoes larger lateral oscillations (Shibukawa et al., 2001), and consequently, walkers must perform more mechanical work and consume more metabolic energy (Donelan et al., 2001).
External stabilization mechanically compensates for eliminating arm swing and prevents an increase in metabolic cost for young and elderly adults. When young and elderly adults walk in the external stabilizer, metabolic cost is the same regardless of whether they swing their arms. The reason is that the stabilizer resists the twisting motion of the trunk and thereby substitutes for arm swing. When the trunk faces forward, the stabilizer’s cords apply lateral forces that are approximately aligned with the center of mass and thus do not create a twisting torque. However, when the trunk starts to twist about the vertical axis, the external stabilizer forces are no longer aligned with the center of mass and create a restoring torque that tends to twist the trunk back to its forward-facing orientation. Thus, the stabilizer allows people to avoid using more costly active mechanisms for controlling trunk motion when they eliminate arm swing.
The reason why elderly adults consume more metabolic energy during walking than young adults might be that they have a greater cost of generating force to support body weight or use more antagonist muscle co-contraction. First, due to the possible 20–30% reduction in force generated per cross sectional area of muscle (Frontera et al., 1991; Morse et al., 2005) in elderly adults compared to young adults, elderly adults likely have to recruit more motor units and a greater fraction of the total cross sectional area of each muscle during walking. Second, the greater metabolic cost of walking in elderly adults may be caused by greater co-contraction of antagonist muscle pairs than in young adults (Mian et al., 2006). Greater co-contraction of antagonist muscle pairs increases the metabolic cost of walking because each agonist muscle must produce extra force and consume more metabolic energy to offset the opposing force of the antagonist muscles.
Our conclusions are subject to several experimental limitations. First, our protocol required subjects to walk at a single speed of 1.3 m/s. Although some studies have shown elderly adults have a slower preferred walking speed than young adults, we chose this speed based on evidence that healthy young and elderly adults prefer walking speeds at or near the intermediate speed of 1.3 m/s (Blanke and Hageman, 1989; Gabell and Nayak, 1984; Oberg et al., 1993; Ortega and Farley, 2007; Ostrosky et al., 1994). Nonetheless, because gait parameters such a lateral trunk acceleration and step width may change with speed in elderly adults (Helbostad and Moe-Nilssen, 2003), it would be beneficial for a future study to investigate the cost of lateral stabilization in young and elderly adults at different walking speeds. Another potential limitation of the study is that subjects walked with only a single external stabilization force of 10% body weight. However, preliminary tests indicate the effect of stabilization on metabolic cost does not change when using different moderate levels of stabilization forces (10–25% body weight; p= 0.806). This is likely related to the fact that the stiffness and damping characteristics of the stabilizer were comparable across this range of stabilizing force.
In conclusion, our findings show that the elevated metabolic cost of walking in elderly adults does not result from a greater cost of laterally stabilization, and that arm swing plays a stabilizing role that reduces the metabolic cost of walking. The metabolic cost of lateral stabilization is similar for young and elderly adults likely because they use similar stabilization strategies and therefore perform a similar amount of limb mechanical work (Ortega and Farley, 2007). Arm swing reduces walking metabolic cost similarly in young and elderly adults likely because it controls trunk twisting during walking. Future studies should explore alternative explanations for the elevated metabolic cost of walking in elderly adults such as whether elderly adults have a greater metabolic cost of generating muscle force or more co-contraction of antagonist muscle pairs during walking than young adults.
Acknowledgments
This study was supported by National Institutes of Health grants AG 00279 and M01 #RR00051. The authors thank Dr. Max Donelan of Simon Fraser University and the members of the Locomotion Laboratory including Josh Fearn for their help with this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bauby CE, Kuo AD. Active control of lateral balance in human walking. Journal of Biomechanics. 2000;33:1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Benjuya N, Melzer I, Kaplanski J. Aging-induced shifts from a reliance on sensory input to muscle cocontraction during balanced standing. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2004;59:166–171. doi: 10.1093/gerona/59.2.m166. [DOI] [PubMed] [Google Scholar]
- Blanke DJ, Hageman PA. Comparison of gait of young men and elderly men. Physical Therapy. 1989;69:144–148. doi: 10.1093/ptj/69.2.144. [DOI] [PubMed] [Google Scholar]
- Brockway JM. Derivation of formulae used to calculate energy expenditure in man. Human Nutrition: Clinical Nutrition. 1987;41:463–471. [PubMed] [Google Scholar]
- Campbell MJ, McComas AJ, Petito F. Physiological changes in ageing muscles. Journal of Neurology, Neurosurgery, And Psychiatry. 1973;36:174–182. doi: 10.1136/jnnp.36.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SH, Bauby CE, Kuo AD. Control of balance during walking in young and elderly adults. Proceedings of the 27th Annual Meeting of the American Society of Biomechanics; Toledo, Ohio. 2003. [Google Scholar]
- Collins SH, Wisse M, Ruina A. A three-dimensional passive-dynamic walking robot with two legs and knees. The International Journal of Robotics Research. 2001;20:607–615. [Google Scholar]
- Dean JC, Alexander NB, Kuo AD. The effect of lateral stabilization on walking in young and old adults. IEEE Transactions on Biomedical Engineering. 2007;54:1919–1926. doi: 10.1109/TBME.2007.901031. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Kram R, Kuo AD. Mechanical and metabolic determinants of the preferred step width in human walking. Proceedings of the Royal Society of London [Biolological Sciences] 2001;268:1985–1992. doi: 10.1098/rspb.2001.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan JM, Shipman DW, Kram R, Kuo ADAD. Mechanical and metabolic requirements for active lateral stabilization in human walking. Journal of Biomechanics. 2004;37:827–835. doi: 10.1016/j.jbiomech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Elftman H. The function of arms in walking. Human Biology. 1939;11:529–535. [Google Scholar]
- Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. Journal of Applied Physiology. 1991;71:644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- Gabell A, Nayak USL. The effect of age on variability in gait. Journals of Gerontology. 1984;39:662–666. doi: 10.1093/geronj/39.6.662. [DOI] [PubMed] [Google Scholar]
- Grabiner PC, Biswas ST, Grabiner MD. Age-related changes in spatial and temporal gait variables. Archives of Physical Medicine & Rehabilitation. 2001;82:31–35. doi: 10.1053/apmr.2001.18219. [DOI] [PubMed] [Google Scholar]
- Helbostad JL, Moe-Nilssen R. The effect of gait speed on lateral balance control during walking in healthy elderly. Gait & Posture. 2003;18:27–36. doi: 10.1016/s0966-6362(02)00197-2. [DOI] [PubMed] [Google Scholar]
- Hinrichs RN, Cavanagh P. Upper Extremity function in treadmill walking. Annual meeting of the American College of Sports Medicine; Miami, FL. 1981. [Google Scholar]
- Jackson KM, Joseph J, Wyard SJ. A mathematical model of arm swing during human locomotion. Journal of Biomechanics. 1978;11:277–289. doi: 10.1016/0021-9290(78)90061-1. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. Journal of Applied Physiology. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- Martin PE, Rothstein DE, Larish DD. Effects of age and physical activity status on the speed-aerobic demand relationship of walking. Journal of Applied Physiology. 1992;73:200–206. doi: 10.1152/jappl.1992.73.1.200. [DOI] [PubMed] [Google Scholar]
- Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiologica Scandinavica. 2006;186:127–139. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Morse CI, Thom JM, Reeves ND, Birch KM, Narici MV. In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. Journal of Applied Physiology. 2005;99:1050–1055. doi: 10.1152/japplphysiol.01186.2004. [DOI] [PubMed] [Google Scholar]
- Oberg T, Karsznia A, Oberg K. Basic gait parameters: reference data for normal subjects, 10–79 years of age. Journal of Rehabilitation Research & Development. 1993;30:210–223. [PubMed] [Google Scholar]
- Ortega JD, Farley CT. Individual limb work does not explain the greater metabolic cost of walking in elderly adults. Journal of Applied Physiology. 2007;102:2266–2273. doi: 10.1152/japplphysiol.00583.2006. [DOI] [PubMed] [Google Scholar]
- Ostrosky KM, VanSwearingen JM, Burdett RG, Gee Z. A comparison of gait characteristics in young and old subjects. Physical Therapy. 1994;74:637–644. doi: 10.1093/ptj/74.7.637. discussion 644–636. [DOI] [PubMed] [Google Scholar]
- Owings TM, Grabiner MD. Variability of step kinematics in young and older adults. Gait & Posture. 2004;20:26–29. doi: 10.1016/S0966-6362(03)00088-2. [DOI] [PubMed] [Google Scholar]
- Shibukawa M, Sugitani K, Hong R, Kasamatsu K, Suzuki S, Ninomija SP. The relationship between arm movement and walking stability in bipedal walking. Proceedings of the 23rd Annual Engineering in Medicine and Biology International Conference; Istanbul, Turkey. 2001. [Google Scholar]
- Stelmach GE, Worringham CJ. Sensorimotor deficits related to postural stability. Implications for falling in the elderly. Clinics in Geriatric Medicine. 1985;1:679–694. [PubMed] [Google Scholar]
- Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. Journal of the Neurological Sciences. 1977;34:213–219. doi: 10.1016/0022-510x(77)90069-7. [DOI] [PubMed] [Google Scholar]
- Townsend MA. Biped gait stabilization via foot placement. Journal of Biomechanics. 1985;18:21–38. doi: 10.1016/0021-9290(85)90042-9. [DOI] [PubMed] [Google Scholar]
- Van de Putte M, Hagemeister N, St-Onge N, Parent G, de Guise JA. Habituation to treadmill walking. Biomedical Materials & Engineering. 2006;16:43–52. [PubMed] [Google Scholar]
- Wall JC, Charteris J. A kinematic study of long-term habituation to treadmill walking. Ergonomics. 1981;24:531–542. doi: 10.1080/00140138108924874. [DOI] [PubMed] [Google Scholar]
- Waters RL, Lunsford BR, Perry J, Byrd R. Energy-speed relationship of walking: standard tables. Journal of Orthopedic Research. 1988;6:215–222. doi: 10.1002/jor.1100060208. [DOI] [PubMed] [Google Scholar]