SUMMARY
Respiratory syncytial virus (RSV) is the primary cause of bronchiolitis in young children. In general RSV is considered to be a poor inducer of type I (α/β) interferons (IFNs). Measurement of active type I IFN production during infections in vivo is demanding since multiple IFN subtypes with overlapping activities are produced. In contrast, Mx gene expression, which is tightly regulated by type I IFN, is easily determined. We therefore measured Mx expression as a reliable surrogate marker of type I IFN activity during RSV infection in vivo in the cotton rat model. We showed that the expression of Mx genes was dramatically augmented in lungs of infected animals in a dose- and virus strain-dependent manner. The expression of Mx genes in lungs was paralleled by their induction in nose and spleen, although in spleen no simultaneous virus gene expression was detected. Re-infection of RSV-immune animals leads to abortive virus replication in the lungs. Thus, type I IFN and Mx gene expression was triggered in re-infected animals even though virus could not be isolated from their lungs. Furthermore, we demonstrated that immunity to RSV wanes with time. Viral replication and Mx gene expression became more prominent with increasing intervals between primary infection and re-infection. Our results highlight the role of type I IFN in the modulation of the immune response to RSV.
INTRODUCTION
Respiratory syncytial virus (RSV) is a negative strand RNA virus of the paramyxovirus family. It causes acute respiratory tract infections, resulting in substantial morbidity and some mortality in humans, particularly at the extremes of age (Thompson et al., 2003; Welliver, 2003; Falsey et al., 2005). RSV is generally considered to be a poor inducer of type I IFN in comparison to other RNA viruses (Hall et al., 1978; Roberts et al., 1992). Two nonstructural proteins of RSV, NS1 and NS2, are known to suppress IFN production (Bossert et al., 2003; Spann et al., 2004). Surprisingly, however, RSV is found to induce high level expression of IFN-β in cultures of various human respiratory epithelial cells and fibroblasts (Garofalo et al., 1996; Jamaluddin et al., 2001). RSV also induces high levels of IFN-α in different subsets of dendritic cells (DC) (Hornung et al., 2004; Schlender et al., 2005; Guerrero-Plata et al., 2006; Wang et al., 2006).
Type I IFN production plays an important role in limiting RSV- induced pathology during infection (Durbin et al., 2002). Studies on type I IFN production in vivo are complicated by the high number of type I IFN genes involved. IFN-α genes are represented by a multigenic family of intronless genes clustered in a 400 kb region, containing also the only IFN-β gene, on the human chromosome 9 and on a syntenic region of chromosome 4 in the mouse (Diaz et al., 1994). They have been shown to be coordinately induced by viruses in human and mouse cells but to differ in the level of expression of their individual mRNAs (Hiscott et al., 1984). Secreted type I IFNs act through a common receptor which consists of two subunits (IFNAR-1 and IFNAR-2) present in virtually all cells (Smith et al., 2005, Stark et al., 1998). Receptor binding leads to a signaling cascade which results in activation of Interferon–Stimulated Genes (ISGs). The best studied ISGs with antiviral properties are the 2′,5′-oligo-adenylate synthetase (2′, 5′-OAS)/RNAseL, the protein kinase R (PKR), and the Mx genes. In contrast to 2′, 5′-OAS and PKR, Mx expression is stimulated exclusively by IFN-α/β or IFN-λ (Holzinger et al., 2007) and does not respond to other cytokines such as IL-1 or TNF-α (Simon et al., 1991). Accordingly, Mx expression has been shown to be an excellent marker for type I IFN action in clinical settings (Roers et al., 1994; Forster et al., 1996; Halminen et al., 1997).
Here we have established the value of measuring the cotton rat Mx gene mRNA induction for monitoring the production of type I IFNs during RSV infection in vivo. We showed that expression of Mx1 and Mx2 mRNAs was strongly induced in the lungs of infected animals. In most cases a parallel induction of cotton rat IFN-α1 and IFN-β was detected. The capacity to trigger type I IFN and Mx gene expression depended on the virus strain used. In RSV-immune animals, the expression of these genes occurred at early time points after a new virus challenge and was influenced by the time interval between primary infection and re-challenge.
METHODS
Virus preparations and cell cultures
HEp-2 and VERO cells (ATCC CCL-23 and CCL-81 correspondingly) were used to prepare virus stocks. Virus titers for all RSV stocks were determined by plaque assay on HEp-2 cells. Uninfected cell culture supernatants (mock preparations) were used as a control. RSV A/Long (ATCC VR-26) virus stock (3.3×107pfu/ml) was prepared in HEp-2 cells and stabilized with sucrose and fetal calf serum. UV inactivation was done by exposure of the virus stock to UV light until no infectious virus was detected. Primary human RSV isolates A074 (group A), B006 and B007 (group B) were recovered from hospitalized children and provided by the laboratory of Dr. Val Hemming of the Uniformed Services University of the Health Sciences (USUHS, Bethesda, MD). All RSV clinical isolates as well as human RSV strains A2 (ATCC VR-1540) and Long were first amplified by two passages on HEp-2 cells and titrated before preparing IFN-free virus stocks in VERO cells (MOI=0.1) for the experiment in which different virus isolates were used to infect animals.
Animals
An inbred cotton rat (Sigmodon hispidus) colony was maintained at Virion Systems, Inc., Rockville, MD. The cotton rats were housed in sterile conditions and fed a diet of rodent chow and water. The cotton rat colony was maintained free of paramyxoviruses, RSV, and rodent viruses. The animals were 4–6 weeks old and weighed ~100 g at the onset of experiment. All animal experimentation procedures were done following NIH and USDA guidelines and were approved by the Virion Systems Inc. Institutional Animals Care and Use Committee.
Infection
Cotton rats were inoculated intranasally under isoflurane anesthesia with 100 μl of RSV suspension containing the indicated pfu. Uninfected rats and rats inoculated with either uninfected cell culture supernatant (mock) or with UV-inactivated virus suspensions were used as controls. Rats were sacrificed by carbon dioxide inhalation at the indicated intervals thereafter. Tissues (lung, spleen, and turbinates) were dissected and snap frozen in liquid nitrogen.
Analysis of tissue mRNA by reverse transcription-PCR
Total RNA was isolated using a Qiagen RNA isolation kit (cat #75144) and treated with DNAse I (Qiagen) to remove traces of contaminant DNA. Total RNA (1 μg) from each tissue sample was utilized for the preparation of cDNA using oligo dT primers and Super Script II Reverse Transcriptase (Invitrogen, CA). Relative quantities of mRNA for each gene of interest were determined by semiquantitative PCR (Blanco et al., 2002) or by Real-time PCR using iQ™ SYBR Green Supermix (BIO-RAD) and MyiQ™ Single-Color Real-Time PCR Detection System (BIO-RAD). The primers (sense [S], and anti-sense [AS]) and probes ([P]) for each analyzed gene were as follows: IFN-β, 5′-GCAGCATTTCAGAATGTCTAGAGC-3′ [S], 5′-ATCTTAATAAGTCTTCCCATGATGG-3′ [AS}, and 5′-TCCATCTCGTTACGGAGTTTATCC-3′ [P]. Due to differences in the sequences of the F gene for RSV type A and RSV type B, we generated two sets of primers for amplification of the F gene: type A, 5′-CAGACTACTAGAGATTACCAGGGAAT-3 [S], 5′-TGGCTCCTAGAGATGTGATAACGGAGC-3′ [AS]; type B, 5′-CAGATTGTTGGAAATCACCAGAGAAT-3′ [S], 5′-TAGCTCCAAGAGAAGTAATTACTGAGC-3′ [AS]. Both sets amplify the same region of the gene with the same efficiency. The probe for detection of F gene amplicon of both RSV types was the same, 5′-GGTGTAACTACACCTGTAAGCAC-3′ [P]. RSV/NS1 type A, 5′-GCAGCAATTCGTTGAGTATG-3′ [S], 5′-GATCAAATCCAAGTAATTCAG-3′ [AS], 5′-CATTGTGTTTGTGCATGTTATTAC-5′ [P]; type B, 5′ GTGCAATTCACTGAGCATGA-3′ [S], 5′ GAGATCAAGCCCAAGTAAATC-3′ [AS]. RSV/NS2 type A, 5′-GACACAACCCACAATGATA-3′ [S], 5′-CATGGATTGAGATCATACTTG-3′ [AS], 5′-CAACTATGAAATGAAACTATTGCAC-3′ [P]. The primers and probes for other genes assessed in this study have been published previously (Blanco et al., 2002; Pletneva et al., 2006). PCR conditions were as follows: IFN-α, 62 °C for annealing and 30 sec for extension; IFN-β, 55 °C and 30 sec; F gene, 60 °C and 30 sec; NS1 and NS2 genes, 60 °C and 30 sec; β-actin, 65 °C and 30 sec. The optimum number of cycles for each particular gene in lung tissue was determined empirically. Briefly, pilot PCR reactions were performed using positive lung cDNA control samples for each gene and the number of cycles needed to achieve the strongest unsaturated signal was used in subsequent experiments. The number of cycles used for each gene was as follows: IFN-α: 28 cycles; IFN-β: 26 cycles; Mx1 and Mx2: 19 cycles; F: 22 cycles in lungs and turbinates and 26 cycles in the spleen; NS1 and NS2: 19 cycles in lungs and turbinates and 26 cycles in the spleen. In cases where the cycle numbers are different from those mentioned above, correct numbers are indicated in the legend of the figure. β-Actin was included as a housekeeping gene to control for differences in cDNA amount for each treatment during the amplification reaction. Standard Southern blot technique was utilized to analyze mRNA expression for each gene.
Preparation of protein extracts and Western blot analysis
Whole cell extracts were prepared from lung tissue using a lysis buffer containing 20 mM Tris-HCl pH 7.9, 100 mM NaCl, 1% NP-40, 4 mM DTT, 7.5 mM NaF, 2 mM EDTA, 0.5 mM PMSF and protease inhibitors. Cell extracts were subjected to 8% SDS-PAGE, transferred to Immobilon-P membranes (Millipore Corp. Bedford, MA), and probed with the indicated antibodies. Horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibodies and reagents for enhanced chemiluminescence (ECL) detection were obtained from Amersham (Arlington Heights, IL).
Statistical analysis
Results are expressed as mean ± SEM. Statistical significance was calculated by Student’s t test as indicated in the legends.
RESULTS
RSV infection induced Mx proteins in cotton rat lungs
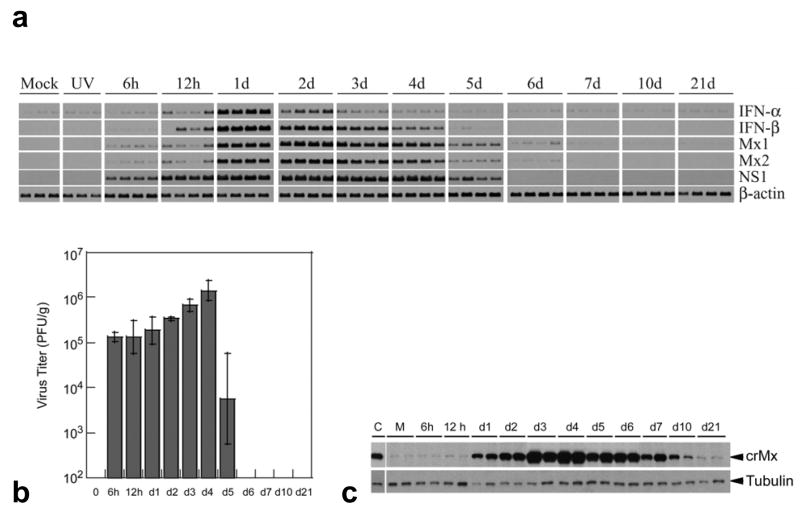
We have previously cloned the cotton rat Mx1 and Mx2 genes and found that they exhibit considerable similarity to the human and mouse genes (Pletneva et al., 2006). Using cotton rat cell lines we further verified that these genes were responsive to type I IFN and not to type II IFN (IFN-γ) or other inflammatory cytokines such as IL-1 and TNF-α (data not shown). Since the expression of these genes accurately reflects the presence of active type I IFN, we have analyzed their expression in vivo during infection with RSV. Groups of cotton rats were infected intranasally with 3 × 106 pfu/100 g body weight; on the indicated days post-infection (p.i.) four animals from each group were sacrificed, and lung samples trisected for determination of viral titers, gene and protein expression. Steady state levels of host mRNA for IFN-α, IFN-β, Mx1 and Mx2 and viral mRNA for NS1 were analyzed by RT-PCR (Fig. 1a). RSV infection induced a rapid increase in the steady state levels of all tested genes. Particularly, the expression of both Mx genes was detected as early as 6 h post-infection (p.i.) whereas IFN-α/β mRNAs were detected at 12 h p.i. Expression of both IFN genes analyzed peaked on day 1–2 p.i. and their levels decreased to almost undetectable by day 7. Both Mx mRNAs showed overlapping patterns of induction peaking on day 2–3 p.i. and decreasing to almost undetectable levels by day 7. Replicating virus was required for activation of mRNA expression of type I IFN and Mx genes since induction of these genes was not detected when animals were inoculated with UV-inactivated virus. In addition, the mRNA expression for most of these genes was undetectable or very low (in the case of IFN-α) in animals inoculated with a mock preparation of the virus and sacrificed at different times post-treatment. The mRNA expression for the host genes paralleled the presence of NS1 mRNA (Fig 1a) and the virus in the lungs (Fig. 1b) and preceded the peak of lung pathology that occurs on day 6 after infection (Prince et al., 1999).
Fig. 1.
(a) Time course of IFN-α, IFN-β, Mx1, Mx2, and NS1 mRNA expression in lungs of cotton rats infected with the Long strain of RSV, as assessed by RT-PCR analysis. Mock (M), or UV-inactivated (UV) preparations of the virus were used as controls. Three control animals and 4 infected animals per time point were sacrificed. Each lane represents one animal. One result for each mock and UV-inactivated group is included as the representative result for the entire time course. (b) RSV replication in lungs of cotton rats sacrificed at the indicated times p.i. Viral titers were determined by plaque assay and mean viral titers are shown. For day 5 p.i., 2 rats out of 4 did not have detectable virus in the lung. (c) Western blot analysis of the expression of Mx protein(s) at the indicated times p.i. in lungs of infected cotton rats. Each lane represents one animal. Identical Western blots were developed with antibodies against tubulin as a control for protein loading. C indicates MxA protein loaded as a control, M indicates mock infection. Results are representative of 2 independent experiments.
In parallel, lung samples from RSV infected animals were analyzed for the expression of Mx proteins by Western blot using the monoclonal antibody M143 prepared against human MxA that recognizes the N-terminal region of Mx proteins from most species, including mouse, rat, and cotton rat (Flohr et al., 1999; Pletneva et al., 2006) (Fig. 1c). The presence of a band that co-migrated with human MxA protein used as a control was detected as early as 12 h p.i. and was not detected in mock-treated animals. Since cotton rat Mx1 and Mx2 proteins have similar estimated molecular weights (74.7 and 74.9 kDa, respectively), it was not possible to discriminate their individual level of expression during infection in lung samples by this methodology. The peak expression of the cotton rat Mx proteins occurred on day 4, and declined by day 7 when no virus could be isolated from the lungs (Fig. 1b). After day 7 p.i. the amount of Mx protein gradually decreased but was still detectable 21 days p.i.
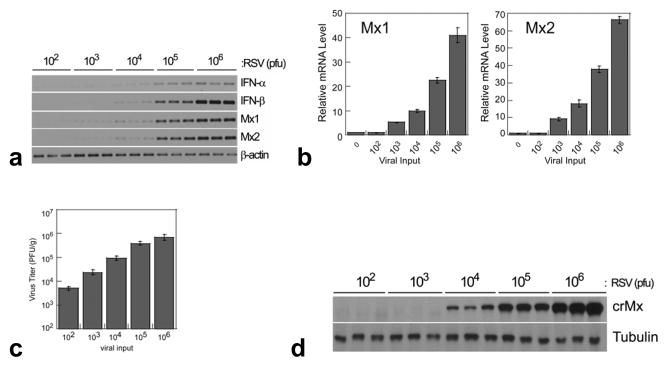
The expression of type I IFN, Mx1 and Mx2 mRNA was evaluated by semiquantitative RT-PCR (Fig. 2a) and qRT-PCR for Mx1 and Mx2 (Fig. 2b) on day 3 (peak Mx mRNA expression) after inoculation of cotton rats with different doses of RSV. IFN-α, IFN-β, Mx1, and Mx2 mRNAs were induced in lungs of infected cotton rats in a dose-dependent manner correlating with the replication of RSV in the lung (Fig. 2c); the smallest inoculum that generated detectable production was 103 pfu/100 g animal (Fig. 2b). The expression of Mx proteins was also tightly correlated with the levels of mRNA (Fig. 2d). Taken together these data demonstrate that RSV induced a strong production of Mx and, therefore, active type I IFN in the lungs of cotton rats and their expression was tightly associated with the viral infection in a dose-dependent manner.
Fig. 2.
(a) Dose response analysis of the Mx1 and Mx2 mRNA levels by semiquantitative RT-PCR during RSV infection (total pfu of RSV Long/100 g as indicated) in lungs of cotton rats was performed on day 3 p.i. (b) qRT-PCR of Mx1 and Mx2 gene expression. (c) RSV replication in lungs of cotton rats after infection with the indicated doses of virus and sacrificed on days 3 p.i. (d) Dose response analysis of the expression of cotton rat Mx proteins in the lung of RSV infected animals on day 3 p.i. Identical Western blots were developed with antibodies against tubulin as a control for protein loading.
Induction of Mx genes in vivo was dependent of the RSV strain
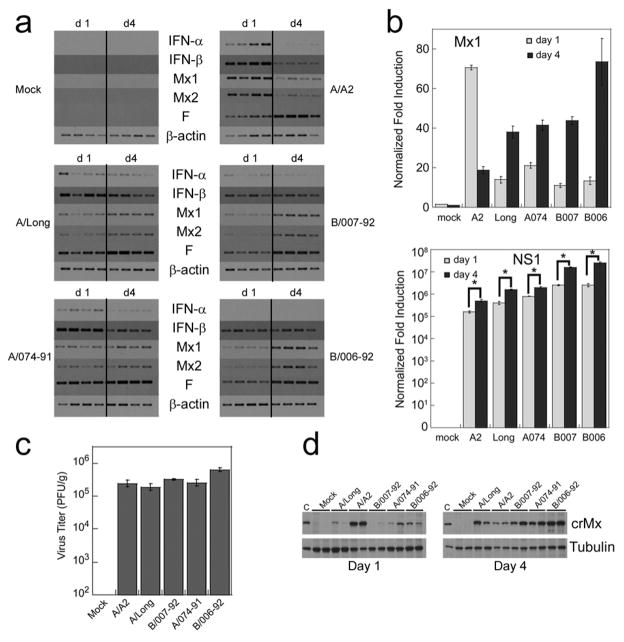
The differential ability of RSV isolates to induce the expression of IFN-α has been recently demonstrated in vitro by using human plasmacytoid dendritic cells (Hornung et al., 2004; Schlender et al., 2005). Since we hypothesize that the expression of Mx genes is directly associated with the total amount of active type I IFN produced in situ, we next determined the extent of type I IFN and Mx gene induction and viral gene expression (F and NS1) in the lungs of animals infected with different RSV isolates of the subtype A, including the prototype strains RSV Long, A2, and the clinical isolate 074-91, and two clinical isolates of the RSV subtype B, B006-92, and B007-92. Animals were infected with equal amounts of each isolate (106 pfu/100 g rat) and sacrificed on days 1 and 4 p.i. All isolates replicated with similar kinetics as evidenced by the levels of expression of the viral F gene (Fig. 3a) or NS1 (Fig 3b, bottom graph), which were higher on day 4 than on day 1, and by the equivalent viral titers in the lung on day 4 (Fig. 3c).
Fig. 3.
Induction of type I IFN, and Mx genes by different RSV isolates. Groups of cotton rats were inoculated with 106 pfu/100 g of each isolate (RSV subtype A: A2, Long and 074-91; RSV subtype B: 007-92 and 006-92) or a mock viral preparation (Mock), and sacrificed on day 1 and 4 p.i. (a) RT-PCR analysis of IFN-α, IFN-β, Mx1, Mx2, and RSV F mRNA levels in the lungs. β-actin is shown as a control for the total amount of RNA used in each reaction. (b) qRT-PCR expression for Mx1 (top graph) and NS1 (botton graph) gene expression on days 1 and 4 p.i. for each strain of RSV. Significant differences were seen in the expression of NS1 when comparing day 1 and day 4 expression of NS1 (*p<0.001). Identical profile as that of Mx1 was obtained for Mx2 (data not shown). (c) RSV replication in lungs of cotton rats on day 4 after infection with the indicated RSV isolate. (d) Western blot analysis showing the expression of cotton rat Mx proteins in lungs of animals infected with the indicated RSV isolates or mock (Mock) and sacrificed at day 1 and day 4 p.i. Western blot for tubulin was included as loading control. Each lane in (A) and (C) represents one animal. The results are representative of two independent experiments.
Importantly, all isolates of the subtype A induced stronger IFN-α/β mRNA expression than the isolates of the subtype B on day 1, whereas the mRNA levels of type I IFN genes induced by each strain were comparable on day 4 (Fig. 3a). In addition, comparison of Mx1 and Mx2 mRNA levels revealed that the A/A2 strain was the strongest activator of these genes on day 1 and also the fastest suppressor of their steady state mRNA levels on day 4 p.i (Fig. 3a and Fig. 3b, top graph), since all the other strains induced peak levels of the Mx1 and Mx2 mRNA on day 4. Importantly, the observations described at the level of Mx mRNA were precisely paralleled by the Mx protein expression analyzed in the same samples by Western blot (Fig. 3d). Samples obtained from animals infected and sacrificed on day 1 showed robust Mx expression when infected with the A/A2 strain of RSV but not with the other strains. In addition, on day 4 p.i. all strains but A/A2 showed high levels of Mx protein expressed, confirming the inhibitory effect on type I IFN of this strain.
These data (1) demonstrate the differential ability of different strains of RSV to induce type I IFN in vivo, (2) confirm previously reported results that showed the capability of the strain A/A2, in contrast to A/Long, to suppress the production of type I IFN, and (3) underscore the utility of measuring Mx mRNA levels as the most accurate indication of total active type I IFN production in vivo, since these differences are not appreciated by only measuring the expression of selected type I IFN forms.
Expression of Mx genes in different tissues upon RSV infection
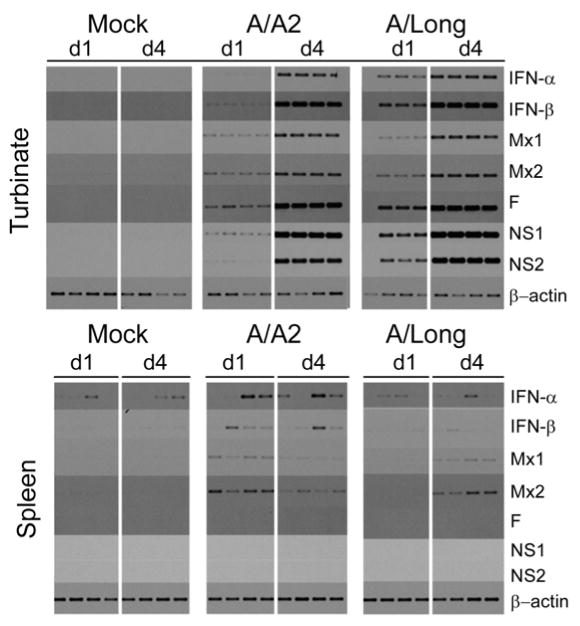
We evaluated the expression of type I IFN and Mx mRNAs in turbinates of infected cotton rats since this is another tissue where sustained RSV replication also takes place. In addition we chose to analyze the expression of the same genes in spleen. In the spleen, among other lymphoid tissues, RSV specific lymphocytes are generated by interacting with antigen presenting cells including plasmacytoid dendritic cells, which migrate from peripheral tissues into the T cell areas and exert their function by producing type I IFN.
Steady state levels of mRNA for all the genes analyzed in the turbinates were robust after infection with both the A/A2 and A/Long strains of RSV. However, with both viruses greater expression of mRNA for all genes was seen on day 4 than on day 1 p.i. (Fig. 4, top panel). This contrasts to what we have described for the expression of the same genes in the lung, where RSV A/A2 but not RSV A/Long induces stronger Mx expression on day 1.
Fig. 4.
Induction of type I IFN and Mx genes during RSV A/A2 and A/Long infection in nasal turbinates and spleen. Cotton rats were infected i.n. with the indicated isolate of RSV or a mock (Mock) preparation of the virus as control. Animals were sacrificed on day 1 and 4 p.i. and total RNA was extracted from the turbinate and spleen. RT-PCR analysis for turbinate (upper panel) and spleen (lower panel) was performed for the host (IFN-α, IFN-β, Mx1, Mx2) and viral (NS1, NS2, F) genes indicated. A total of 4 animals per group are shown.
In the spleen (Fig 4, bottom panel), analysis of the expression of mRNA for the same cluster of genes revealed a variable expression of type I IFN but a clear pattern of mRNA expression for both Mx genes, with peak mRNA levels on day 1 p.i. for A/A2, and on day 4 p.i. for A/Long - a pattern that correlates to that in the lung. In addition, no viral gene expression was detected, consistent with previous observations that RSV does not replicate outside the respiratory tract (Prince et al., 1978). Our results clearly indicate that upon viral infection different target tissues respond to RSV infection producing different levels type I IFN, most likely due to a differential utilization of innate virus detection mechanism. In addition, we were able to detect virus-specific production of type I IFN in spleen by analyzing the induction of Mx mRNA. To our knowledge, this is the first time that type I IFN production upon RSV infection has been measured in spleen in vivo.
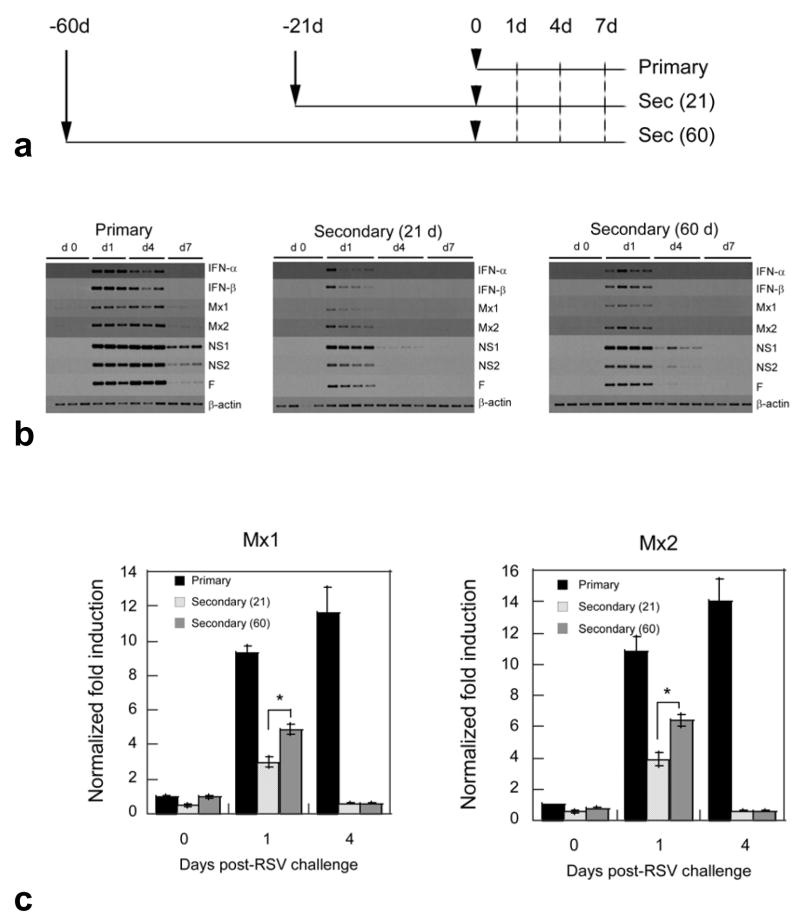
Expression of type I interferon and Mx genes during RSV re-infection
Re-infections with RSV are common. How time length between infection and reinfection reflects on type I IFN induction remains unclear. An analysis of the expression of type I IFN and Mx genes during primary infection and re-infection in cotton rat model was conducted following the indicated time schedule (Fig. 5a). Briefly, animals of the same age were separated in three groups: a primary infection group was inoculated with a mock preparation of the virus on day -60 and infected only once with 106 pfu/100 g of RSV/Long on day 0; a secondary infection 21 (sec (21)) group, was infected twice, firstly on day -21 and secondly on day 0; and another secondary 60 (sec (60)) group, was infected twice, firstly on day -60 and secondly on day 0. Three to 4 animals per group where sacrificed on days 1, 4 and 7 and lung samples collected for determination of viral titers and mRNA analysis. As expected, virus was isolated only from lungs of those animals with the primary infection (data not shown). In addition, these animals showed the strongest mRNA induction for all genes analyzed, and had detectable viral and Mx genes on day 7.
Fig. 5.
Induction of type I IFN and Mx genes in the lungs of cotton rats during infection and re-infection with RSV A/Long. (a) Experimental setting for infection (Primary) and re-infections 21 [sec (21)] or 60 [sec (60)] days after the first challenge. Groups of cotton rats were inoculated intranasally with 106 pfu/100 g rat of virus suspension in case of sec(21) and sec(60) groups on day -21 and day -60 correspondently or with an identical volume (100 μl) of a mock preparation in case of Primary group on day -60. Twenty one or 60 days after primary inoculation, 3–4 animals per group were sacrificed for day 0 time-point and the rest of animals in all groups were inoculated with the same dose of virus and sacrificed at the indicated times after second inoculation (b) Semiquantitative RT-PCR analysis for the induction of the host genes IFN-α, IFN-β, Mx1, Mx2 and the viral genes NS1 (24 cycles), NS2 (24 cycles) and F. β-actin is shown as control for total amount of RNA used in each reaction. (c) qRT-PCR of the levels of Mx1 (left) and Mx2 (right) mRNA in the lung of animals infected (primary), and reinfected 21 [secondary (21)] or 60 [secondary (60)] days post-primary infection. β-actin was used as a reference gene. The mean ± SEM for 4 animals per group is shown. Significant differences were seen in the expression of Mx when comparing the indicated groups (*p<0.001).
As previously reported (Prince et al., 1999), secondary infection did not yield any virus amplification, although abortive replication was detected (as indicated by the measurable expression of all viral genes on day 1). Most importantly, the abortive viral gene expression was sufficient for the induction of type I IFN and Mx gene expression (Fig. 5b). Furthermore, after comparison of the two groups of secondary infection [sec (21) and sec (60)] it was evident that expression of type I IFN, Mx and viral genes was stronger in animals reinfected after 60 days of primary challenge than in those reinfected after 21 days. The difference was clear for the transcription of the viral NS1 and NS2 since their mRNA was detected in all rats on day 4 of sec (60) infection whereas it was almost undetectable for the same day in animals of the sec (21) group. Less evident but also significant (as seen by qRT-PCR, Fig. 5c) was the difference in the expression of Mx genes on day 1 post-challenge. All together these data demonstrate that abortive RSV replication, seen during secondary infection in the lung of cotton rats is sufficient to activate type I IFN production. In addition, we demonstrated that both abortive viral gene expression and type I IFN can be influenced by the length of the time period between infections with RSV, suggesting that time between infections is an important factor determining the extent of abortive viral gene expression, interferon production and most likely the outcome of the disease.
DISCUSSION
In humans infected with RSV the detection of type I IFN was sporadic in nasopharyngeal secretions (Hall et al., 1978; McIntosh, 1978; Hall et al., 1981; Isaacs, 1989; Taylor et al., 1989; Nakayama et al., 1993). However, these studies are difficult to interpret considering the nature of the samples analyzed (nasal washes, nasal aspirates, sera), variation of the subject age, timing of sample isolation, and IFN measurement techniques. The fact that all the studies demonstrate the presence of type I IFN in a portion of the nasopharyngeal samples tested suggests that type I IFN plays an important but transient role during RSV infection.
Production of type I IFN in response to RSV infection has been previously studied in other systems. Many different factors seem to determine the production of type I IFN by RSV, including cell types and viral strains (Garofalo et al., 1996; Jamaluddin et al., 2001; Hornung et al., 2004; Schlender et al., 2005; Guerrero-Plata et al., 2006; Wang et al., 2006). However, it is clear from the literature that RSV stimulates type I IFN to a lesser extent than other human respiratory viruses (i.e., influenza, PIV-1, hMPV) (Hall et al., 1978; Chonmaitree et al., 1981, Roberts et al., 1992; Guerrero-Plata et al., 2006).
The in vivo expression of type I IFN and Mx genes in response to influenza infection in the cotton rat has previously been studied (Pletneva et al., 2006) and their antiviral potential tested (Stertz et al., in press). We now show that similarly to influenza, RSV infection activated type I IFN and Mx gene expression in the lung quite early during infection (6–12 h p.i.).
Importantly, in infections with RSV/A/A2 we showed that the peak of type I IFN and Mx mRNA and Mx protein preceded the peak of viral replication (Fig. 3a and b). Recently, induction of type I IFN by RSV was shown to be virus strain-dependent (Schlender et al., 2005). In various types of human cells, including epithelial cell lines (A 549, HEp-2 and 293), and freshly isolated human B cells, T cells, monocytes, and plasmacytoid dendritic cells, only RSV/A/Long strain induces high levels of IFN-α whereas the other isolates tested (including RSV/A/A2) are at least 10 fold less efficient in IFN-α induction. Our results extended those of Schlender et al., by further demonstrating that RSV/A/Long has a lesser ability to counteract cellular mechanisms of type I IFN production (thus producing type I IFN and Mx for a longer period of time) than the RSV/A/A2 strain in vivo.
We demonstrated that RSV infection also induced type I IFN and Mx mRNA expression in tissues other than the lung. First, we performed the analysis of nasal turbinates since RSV robustly replicates in the nose of cotton rats with a peak in viral titers on days 3–5 p.i. (Prince et al., 1978). In contrast to what we described for the expression of type I IFN in the lungs, both RSV/A/A2 and RSV/A/Long followed a similar profile of expression in the nasal turbinates with the strongest expression on day 4 p.i. This implies that during RSV infection there is a differential type I IFN response depending on the target tissue.
In addition to their antiviral effect, type I IFN play pleiotropic roles in immunomodulation, both at the innate and adaptive immune level (Theofilopoulos et al., 2005). Adjuvants currently under development exert part of their effect by inducing local expression of type I IFN through Toll-like receptors (Seya et al., 2006), some of them previously tested in cotton rats (Prince et al., 2003; Boukhvalova et al., 2006). Moreover, IFN-α was previously tested as adjuvant for influenza vaccines and was demonstrated to potentiate the immunity to the virus (Bracci et al., 2006; Tovey et al., 2006). Understanding the mechanisms that determine the tissue specific induction of type I IFN by RSV could become decisive at the time of determining the route of immunization for RSV vaccines.
We analyzed the type I IFN production through Mx mRNA expression in the spleen since this organ mounts a specific immunological response to RSV and no viral replication was detected in this organ (Richter et al., 2005). Interestingly, the pattern of Mx1 and Mx2 mRNA expression in the spleen after infection with RSV/A/Long and RSV/A/A2 reflected exactly the pattern of mRNA expression seen in the lung, with RSV/A/A2 having the strongest induction on day 1 and RSV/A/Long showing stronger expression on day 4 p.i. These results suggest that antigen presenting cells draining from the lung, but not from the turbinate are most likely those secreting type I IFN and activating Mx expression in the spleen.
RSV re-infections in humans are frequent and their severity in general decreases with each subsequent infection but in some cases is comparable to the first encounter (Henderson et al., 1979). The implications of multiple re-infections on RSV-induced type I IFNs production were not previously evaluated. In the cotton rat model of RSV re-infection it has been demonstrated that abortive replication takes place in immune animals (Boukhvalova et al., 2007). In our study viral gene expression (NS1 is the most sensitive marker of viral persistence) was detected even four days post secondary challenge. Importantly, abortive viral replication was followed by transient induction of type I IFN and Mx gene expression in the lung and this coincided with the pathology previously described during secondary infection in the cotton rat (Prince et al., 1999). More importantly, we showed for the first time that both abortive viral replication and type I IFN production are enhanced when the time between consecutive RSV infections is prolonged, clearly evidencing a decay in the immunity to RSV.
Acknowledgments
This work was funded by NIH grant RO1-AI057575-01 to J. C. G. B. and by Virion Systems Inc. corporate funds. The authors would like to thank Dr. Val Hemming and Sally Hansen for providing RSV clinical isolates, Kevin Yim, Marisa Stock, LyAvia Goodwin for preparation of RSV Long viral stock and RSV titrations, and Lorraine Ward, Charles Smith, and Freddy Rivera for animal care.
References
- Blanco JC, Richardson JY, Darnell ME, Rowzee A, Pletneva L, Porter DD, Prince GA. Cytokine and chemokine gene expression after primary and secondary respiratory syncytial virus infection in cotton rats. J Infect Dis. 2002;185:1780–1785. doi: 10.1086/340823. [DOI] [PubMed] [Google Scholar]
- Bossert B, Marozin S, Conzelmann KK. Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3. J Virol. 2003;77:8661–8668. doi: 10.1128/JVI.77.16.8661-8668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhvalova MS, Prince GA, Blanco JCG. Respiratory Syncytial virus infects and abortively replicates in the lungs in spite of pre-existing immunity. J Virol. 2007;81:9443–9450. doi: 10.1128/JVI.00102-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhvalova MS, Prince GA, Soroush L, Harrigan DC, Vogel SN, Blanco JC. The TLR4 agonist, monophosphoryl lipid A, attenuates the cytokine storm associated with respiratory syncytial virus vaccine-enhanced disease. Vaccine. 2006;24:5027–5035. doi: 10.1016/j.vaccine.2006.03.064. [DOI] [PubMed] [Google Scholar]
- Bracci L, Canini I, Venditti M, Spada M, Puzelli S, Donatelli I, Belardelli F, Proietti E. Type I IFN as a vaccine adjuvant for both systemic and mucosal vaccination against influenza virus. Vaccine. 2006;24(Suppl 2):56–57. doi: 10.1016/j.vaccine.2005.01.121. [DOI] [PubMed] [Google Scholar]
- Chonmaitree T, Roberts NJ, Jr, Douglas RG, Jr, Hall CB, Simons RL. Interferon production by human mononuclear leukocytes: differences between respiratory syncytial virus and influenza viruses. Infect Immun. 1981;32:300–303. doi: 10.1128/iai.32.1.300-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MO, Pomykala HM, Bohlander SK, Maltepe E, Malik K, Brownstein B, Olopade OI. Structure of the human type-I interferon gene cluster determined from a YAC clone contig. Genomics. 1994;22:540–552. doi: 10.1006/geno.1994.1427. [DOI] [PubMed] [Google Scholar]
- Durbin JE, Johnson TR, Durbin RK, Mertz SE, Morotti RA, Peebles RS, Graham BS. The role of IFN in respiratory syncytial virus pathogenesis. J Immunol. 2002;168:2944–2952. doi: 10.4049/jimmunol.168.6.2944. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- Flohr F, Schneider-Schaulies S, Haller O, Kochs G. The central interactive region of human MxA GTPase is involved in GTPase activation and interaction with viral target structures. FEBS Lett. 1999;463:24–28. doi: 10.1016/s0014-5793(99)01598-7. [DOI] [PubMed] [Google Scholar]
- Forster J, Schweizer M, Schumacher RF, Kaufmehl K, Lob S. MxA protein in infants and children with respiratory tract infection. Acta Paediatr. 1996;85:163–167. doi: 10.1111/j.1651-2227.1996.tb13985.x. [DOI] [PubMed] [Google Scholar]
- Garofalo R, Mei F, Espejo R, Ye G, Haeberle H, Baron S, Ogra PL, Reyes VE. Respiratory syncytial virus infection of human respiratory epithelial cells up-regulates class I MHC expression through the induction of IFN-beta and IL-1 alpha. J Immunol. 1996;157:2506–2513. [PubMed] [Google Scholar]
- Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, Garofalo RP. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol. 2006;34:320–329. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Douglas RG, Jr, Simons RL. Interferon production in adults with respiratory syncytial viral infection. Ann Intern Med. 1981;94:53–55. doi: 10.7326/0003-4819-94-1-53. [DOI] [PubMed] [Google Scholar]
- Hall CB, Douglas RG, Jr, Simons RL, Geiman JM. Interferon production in children with respiratory syncytial, influenza, and parainfluenza virus infections. J Pediatr. 1978;93:28–32. doi: 10.1016/s0022-3476(78)80594-0. [DOI] [PubMed] [Google Scholar]
- Halminen M, Ilonen J, Julkunen I, Ruuskanen O, Simell O, Makela MJ. Expression of MxA protein in blood lymphocytes discriminates between viral and bacterial infections in febrile children. Pediatr Res. 1997;41:647–650. doi: 10.1203/00006450-199705000-00008. [DOI] [PubMed] [Google Scholar]
- Henderson FW, Collier AM, Clyde WA, Jr, Denny FW. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- Hiscott J, Cantell K, Weissmann C. Differential expression of human interferon genes. Nucleic Acids Res. 1984;12:3727–3746. doi: 10.1093/nar/12.9.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger D, Jorns C, Stertz S, Boisson-Dupuis S, Thimme R, Weidmann M, Casanova JL, Haller O, Kochs G. Induction of MxA gene expression by influenza A virus requires type I or Type III interferon signaling. J Virol. 2007 doi: 10.1128/JVI.00546–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Schlender J, Guenthner-Biller M, Rothenfusser S, Endres S, Conzelmann KK, Hartmann G. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J Immunol. 2004;173:5935–5943. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- Isaacs D. Production of interferon in respiratory syncytial virus bronchiolitis. Arch Dis Child. 1989;64:92–95. doi: 10.1136/adc.64.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamaluddin M, Wang S, Garofalo RP, Elliott T, Casola A, Baron S, Brasier AR. IFN-beta mediates coordinate expression of antigen-processing genes in RSV-infected pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L248–257. doi: 10.1152/ajplung.2001.280.2.L248. [DOI] [PubMed] [Google Scholar]
- McIntosh K. Interferon in nasal secretions from infants with viral respiratory tract infections. J Pediatr. 1978;93:33–36. doi: 10.1016/s0022-3476(78)80595-2. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Sonoda S, Urano T, Sasaki K, Maehara N, Makino S. Detection of alpha-interferon in nasopharyngeal secretions and sera in children infected with respiratory syncytial virus. Pediatr Infect Dis J. 1993;12:925–929. doi: 10.1097/00006454-199311000-00007. [DOI] [PubMed] [Google Scholar]
- Ottolini MG, Blanco JCG, Eichelberger MC, Porter DD, Pletneva L, Richardson JY, Prince GA. The cotton rat provides a useful small-animal model for the study of influenza virus pathogenesis. J Gen Virol. 2005;86:2823–2830. doi: 10.1099/vir.0.81145-0. [DOI] [PubMed] [Google Scholar]
- Pletneva LM, Haller O, Porter DD, Prince GA, Blanco JC. Interferon-inducible Mx gene expression in cotton rats: cloning, characterization, and expression during influenza viral infection. J Interferon Cytokine Res. 2006;26:914–921. doi: 10.1089/jir.2006.26.914. [DOI] [PubMed] [Google Scholar]
- Prince GA, Jenson AB, Horswood RL, Camargo E, Chanock RM. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol. 1978;93:771–791. [PMC free article] [PubMed] [Google Scholar]
- Prince GA, Prieels JP, Slaoui M, Porter DD. Pulmonary lesions in primary respiratory syncytial virus infection, reinfection, and vaccine-enhanced disease in the cotton rat (Sigmodon hispidus) Lab Invest. 1999;79:1385–1392. [PubMed] [Google Scholar]
- Prince GA, Mond JJ, Porter DD, Yim KC, Lan SJ, Klinman DM. Immunoprotective activity and safety of a respiratory syncytial virus vaccine: mucosal delivery of fusion glycoprotein with a CpG oligodeoxynucleotide adjuvant. J Virol. 2003;77:13156–13160. doi: 10.1128/JVI.77.24.13156-13160.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter BW, Onuska JM, Niewiesk S, Prince GA, Eichelberger MC. Antigen-dependent proliferation and cytokine induction in respiratory syncytial virus-infected cotton rats reflect the presence of effector-memory T cells. Virology. 2005;337:102–110. doi: 10.1016/j.virol.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Roberts NJ, Jr, Hiscott J, Signs DJ. The limited role of the human interferon system response to respiratory syncytial virus challenge: analysis and comparison to influenza virus challenge. Microb Pathog. 1992;12:409–414. doi: 10.1016/0882-4010(92)90003-7. [DOI] [PubMed] [Google Scholar]
- Roers A, Hochkeppel HK, Horisberger MA, Hovanessian A, Haller O. MxA gene expression after live virus vaccination: a sensitive marker for endogenous type I interferon. J Infect Dis. 1994;169:807–813. doi: 10.1093/infdis/169.4.807. [DOI] [PubMed] [Google Scholar]
- Schlender J, Hornung V, Finke S, Gunthner-Biller M, Marozin S, Brzozka K, Moghim S, Endres S, Hartmann G, Conzelmann KK. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J Virol. 2005;79:5507–5515. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T, Akazawa T, Tsujita T, Matsumoto M. Role of Toll-like receptors in adjuvant-augmented immune therapies. Evid Based Complement Alternat Med. 2006;3:31–38. doi: 10.1093/ecam/nek010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A, Fah J, Haller O, Staeheli P. Interferon-regulated Mx genes are not responsive to interleukin-1, tumor necrosis factor, and other cytokines. J Virol. 1991;65:968–971. doi: 10.1128/jvi.65.2.968-971.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PL, Lombardi G, Foster GR. Type I interferons and the innate immune response-more than just antiviral cytokines. Mol Immunol. 2005;42:869–877. doi: 10.1016/j.molimm.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Spann KM, Tran KC, Chi B, Rabin RL, Collins PL. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J Virol. 2004;78:4363–4369. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Stertz S, Dittmann J, Blanco JCG, Pletneva LM, Haller O, Kochs G. The antiviral potential of interferon-induced cotton rat Mx proteins against orthomyxo (influenza)-, rhabdo- and bunyaviruses. J Interferon Cytokine Res. 2007 doi: 10.1089/jir.2006.0176. In press. [DOI] [PubMed] [Google Scholar]
- Taylor CE, Webb MS, Milner AD, Milner PD, Morgan LA, Scott R, Stokes GM, Swarbrick AS, Toms GL. Interferon alfa, infectious virus, and virus antigen secretion in respiratory syncytial virus infections of graded severity. Arch Dis Child. 1989;64:1656–1660. doi: 10.1136/adc.64.12.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- Tovey MG, Lallemand C, Meritet JF, Maury C. Adjuvant activity of interferon alpha: mechanism(s) of action. Vaccine. 2006;24(Suppl 2):46–47. doi: 10.1016/j.vaccine.2005.01.117. [DOI] [PubMed] [Google Scholar]
- Wang H, Peters N, Schwarze J. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J Immunol. 2006;177:6263–6270. doi: 10.4049/jimmunol.177.9.6263. [DOI] [PubMed] [Google Scholar]
- Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr. 2003;143:S112–117. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]