Abstract
Purpose
Gallic acid, a natural agent present wide-range of fruits and vegetables, has been of potential interest as anti-cancer agent; herein, we evaluated its efficacy in androgen-independent DU145 and androgen-dependent-22Rv1 human prostate cancer (PCa) cells
Materials and Methods
Cell viability was determined by MTT and apoptosis by Annexin V-PI assays. In vivo anti-cancer efficacy was assessed by DU145 and 22Rv1 xenograft growth in nude mice given normal drinking water or one supplemented with 0.3% or 1% (w/v) gallic acid. PCNA, TUNEL and CD31 immunostaining was performed in tumor tissues for in vivo anti-proliferative, apoptotic and anti-angiogenic effects of gallic acid.
Results
Gallic acid decreased cell viability in a dose-dependent manner in both DU145 and 22Rv1 cells largely via apoptosis induction. In tumor studies, gallic acid feeding inhibited the growth of DU145 and 22Rv1 PCa xenografts in nude mice. Immunohistochemical analysis revealed significant inhibition of tumor cell proliferation, induction of apoptosis, and reduction of microvessel density in tumor xenografts from gallic acid-fed mice as compared to controls in both DU145 and 22Rv1 models
Conclusion
Taken together, our findings show the anti-PCa efficacy of gallic acid providing a rationale for additional studies with this naturally-occurring agent for its efficacy against PCa.
Keywords: Chemoprevention, prostate cancer, gallic acid, apoptosis, cell proliferation
Introduction
Inadequacy in current modalities for the treatment of cancer has fueled the search for new strategies, which are far more effective and have greater patient acceptability. Due to high systemic toxicities associated with current treatment regimens for prostate cancer (PCa), an increasing number of patients are resorting to complementary therapies, which most often involve the usage of natural products; specifically phytochemicals (1). Because of the widespread use of complementary therapies by cancer patients including PCa, it has become necessary to scientifically characterize the agents used as supplements in complimentary medicine and understand their mechanism of action.
Grape seed extract (GSE) is one such plant product, which is consumed as health supplement. Most of its health beneficial effects have been attributed to its strong anti-oxidant capacity due to its high proanthocyanidin content (2). In case of cancer, GSE has been shown to exhibit anti-cancer efficacy against numerous cancer cell lines of different anatomical origin such as lung, breast, colon and prostate (3–5). However, GSE in itself is a mixture of various polyphenols, making it pertinent to isolate and characterize individual components in order to carry out further studies to fully understand its mechanism of action in various health conditions including cancer. Accordingly, we have devoted tremendous effort in recent years to fractionate GSE in to individual components and evaluate their efficacy against PCa (6). In this regard, as reported recently, we have identified gallic acid as one of the most active GSE constituents with significantly strong anti-cancer activity against human PCa DU145 cells in culture (6).
In addition to GSE, gallic acid is widely distributed throughout the plant kingdom where it is either present in free form or more commonly as a constituent of tannins, namely gallotannin (7). Strawberries, pineapples, bananas, lemons, red and white wines, gallnuts, sumac, witch hazel, tea leaves, oak bark and apple-peels are some of the natural products which are rich in gallic acid (8, 9). Regarding its biological activity, gallic acid exerts anti-bacterial, anti-viral, anti-inflammatory and antioxidant effects (9–12); and anti-melanogenic activity via the inhibition of tyrosinase activity (13). It also inhibits high fat diet induced dyslipidaemia, hepatosteatosis and oxidative stress (14) and mutagenic effects of benzidine, a human bladder carcinogen (15). Anti-cancer activity of gallic acid has been reported in various cancer cells such as leukemia, oral tumor and esophageal (16, 17). Regarding its efficacy against PCa, our recent studies have shown both anti-cancer and cancer chemopreventive effects of gallic acid in human PCa DU145 cells in culture and TRAMP model, respectively (18, 19).
The first line of treatment for PCa patients is usually androgen ablation; however, the disease resurfaces over the period of time and presents itself as resistant to hormone ablation therapy (20). Recent clinical studies have also shown that even in androgen ablation therapy resistant PCa, the role of androgen receptor remains critical (21). Based on these facts and that gallic acid was already found effective by us in androgen-independent human PCa DU1454 cells in culture, in the present study, we evaluated in detail the anti-cancer efficacy of gallic acid employing both androgen-responsive and – independent human PCa 22Rv1 and DU145 cell lines in culture and tumor xenografts in athymic male nude mice.
MATERIALS AND METHODS
Chemicals
Gallic acid, Thiazolyl Blue Tetrazolium Bromide (MTT), 3,3′-diaminobenzidine (DAB) and Harris hematoxylin were procured from Sigma-Aldrich Chemical Co. (St. Louis, MO). Vybrant Apoptosis Assay Kit 2 was from Molecular Probes (Eugene, OR). Mouse monoclonal anti-proliferating cell nuclear antigen (PCNA)antibody and biotinylated rabbit anti-mouse antibody were procured from DAKO (Carpinteria, CA). Dead End Colorimetric TUNEL system was procured from Promega (Madison, WI). Goat anti-CD31 polyclonal antibody and biotinylated rabbit anti-goat secondary antibody were procured from Santa Cruz Biotechnology Inc., Santa Cruz, CA.
Cell Culture
22Rv1 and DU145 cells were procured from ATCC (Manassas, VA) and were grown in RPMI-1640 medium supplemented with 10% FBS and 100 U/ml each of streptomycin and penicillin. PWR-1E cells (from ATCC) were cultured in Keratinocyte media supplemented with EGF and bovine pituitary extract along with 100 U/ml of streptomycin and penicillin. Cells were maintained under standard culture conditions of 37°C, 95% humidified air and 5% CO2.
Cell Viability Assay
Cell viability was studied by MTT assay. Briefly, cells were plated at a density of 2000 cells per well of 96 wells plate for 24 h, and subsequently treated with varying concentration of gallic acid (0–100 μM) in DMSO for 12–48 h. At the end of treatment times, 20μl of MTT stock solution (5mg/ml) was added to each well, and cells were incubated for additional 4 h. Thereafter, media was carefully aspirated from each well, followed by addition of 200 μl of DMSO, and the color formed was determined by measuring the OD at 570 nm using ELISA plate reader.
Apoptosis Assay
For the quantification of apoptotic death, cells (both 22Rv1 and DU145) were plated in 60 mm culture dishes at a density of 5000 cells per cm2 for overnight, and then treated with either DMSO as vehicle control or various concentrations of gallic acid in DMSO for 24 h. Thereafter, cells were collected and stained with Annexin V and PI using Vybrant Apoptosis Assay Kit 2 (Molecular Probes, Eugene, OR) essentially following the instructions of the manufacturer. Apoptotic death was quantified by Fluorescence-Activated Cell Sorting (FACS) analysis of the stained cells using the core facility of the University of Colorado Cancer Center, Denver, Colorado.
In vivo Tumor Xenograft Study
Athymic (BALB/c, nu/nu) male nude mice (4 weeks old) were obtained from NCI(Frederick, MD) and fed autoclaved AIN76A (Dyets Inc., Bethlehem, PA) chow diet and water ad libitum. For xenograft, ~1 million 22Rv1 cells or ~ 4 million DU145 cells were suspended in 0.1 ml serum-free medium mixed with matrigel (1:1) and subcutaneously injected into right flank of each mouse. The next day (day 1)mice were randomly divided into three groups for each cell line (n = 10) and fed plain drinking water(control), or 0.3% and 1% gallic acid w/v in drinking water. Diet and water consumption as well as animal body weight were monitored regularly throughout the study. Once xenograft started growing, their sizes were measured in two dimensions using digital vernier calipers. Tumor volume was calculated using the formula “0.5236 L1 (L2)2 where L1 and L2 represent the long and short axis of tumor respectively. At the end of the study period, tumors were excised, and were fixed in 10% formalin for immunohistochemical analysis. The completed animal research here adhered to the “Principles of Laboratory Animal Care” and was approved by IACUC.
Immunostaining
For immunostaining, tumor tissues collected from mice in xenograft study were fixed in 10% formalin for 8 to 10 hours at 4°C, followed by dehydration in ethanol, and were then cleared in xylene, and finally embedded in PolyFin. Four-μm serial sections were cut, processed, and immunostained either with monoclonal anti-proliferating cell nuclear antigen (PCNA) antibody (1:250 dilution; Dako, Carpinteria, CA) or anti-goat CD31 antibody (1:100 dilutions; Santa Cruz Biotechnology, Santa Cruz, CA), followed by appropriate biotinylated secondary antibodies, and finally with conjugated horseradish peroxidase streptavidin. The sections were then incubated with DAB working solution for 10 min at room temperature and finally slides were counterstained with diluted Harris hematoxylin and mounted. Terminal deoxynucleotidyltransferase-mediated nick end labeling (TUNEL) staining for apoptotic cells was done as published previously by us (22). All immunohistochemical analyses were done using Zeiss Axioscop 2 microscope (Carl Zeiss, Inc., Jena, Germany).
Statistical Analysis
Data were analyzed using the SigmaStat 2.03 software. The statistical significance of differences between control versus all other gallic acid treated groups was determined by unpaired student’s t-test. Differences were considered significant at p<0.05. Analyses for all immunohistochemical studies were done using Zeiss Axioscop 2 microscope (Carl Zeiss Inc, Jena, Germany). The representative images of immunohistochemical studies were taken by AxioCam MrC5 camera at 400× magnification. The images were further processed by AxioVision software documentation system (Carl Zeiss Inc).
RESULTS
Gallic acid selectively reduces the viability of prostate carcinoma cells
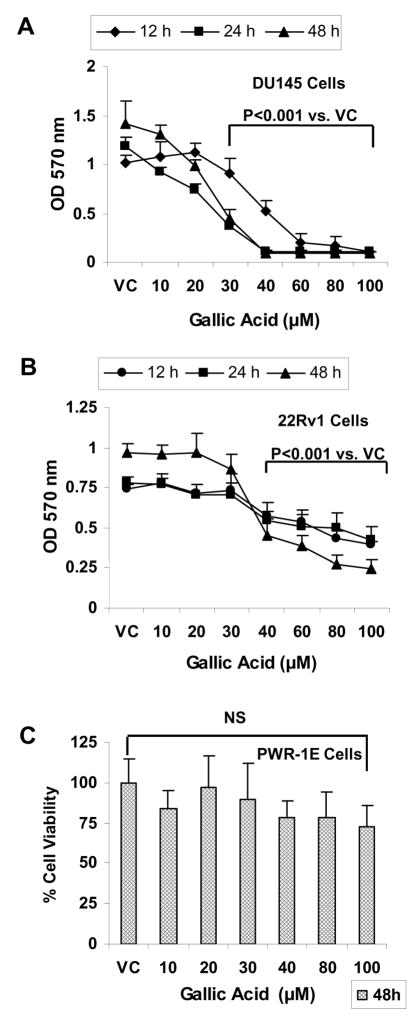
First we studied the effect of gallic acid on the viability of prostate carcinoma (DU145 and 22Rv1) as well as non-neoplastic prostate epithelial (PWR-1E) cells. We observed that treatment of DU145 cells with gallic acid at the concentrations ranging from 10–100 μM for 12–48 h resulted in a concentration-and time- dependent decrease in the viability of cells measured in terms of absorbance of color formed by reduction of MTT dye by live cells. A significant reduction in the viability by 11 to 90% (p<0.001) of these cells as compared to DMSO treated controls was observed at 30–100 μM concentration after 12 h of treatment time. Increase in treatment times to 24 and 48 h further reduced the viability and decrease was evident at even lower concentration of 20 μM (Fig. 1A). In case of 22Rv1 cells, treatment with gallic acid (10–100 μM) decreased the viability of these cells in both time- and dose-dependent manner. The viability of these cells was reduced by 27–47% (p<0.001) after 12 h of treatment time with 40–100 μM concentrations of gallic acid. On increasing the treatment time to 24 and 48 h, the decrease in the viability of cells was 9–47% (p<0.001) and 10–75% (p<0.001) at even lower concentration of 30 μM and above (Fig. 1B). However, when non-neoplastic PWR-1E cells were treated with gallic acid at similar concentration range (10–100 μM) for 48 h, no significant decrease in the viability of the cells was observed (Fig. 1C). From these results, it could be concluded that gallic acid is selectively toxic to prostate carcinoma cells as compared to non-neoplastic prostate epithelial cells.
Fig. 1.
Cell viability effect of gallic acid in prostate carcinoma cell lines and normal prostate epithelial cells in vitro. DU145 cells (A), 22Rv1 cells (B) or PWR-1E cells (C) were seeded in 96 well plates for overnight and were then treated with 0–100 μM gallic acid in DMSO or DMSO alone for 12–48h. At the end of treatment times, cell viability was measured by MTT assay as described under Materials and Methods section. Data indicate mean ± SD, n=3.
Gallic acid induces apoptotic death in prostate carcinoma cells
Since we observed that gallic acid treatment causes significant cytotoxicity in prostate carcinoma cells, we next examined if the cytotoxic effects induced by gallic acid involve induction of apoptosis. Based on the results from MTT assay showing that 100 μM concentration was highly cytotoxic, the apoptosis inducing ability was studied only up to 75 μM of gallic acid concentrations. Flow cytometric analysis of annexin V-PI stained cells revealed that indeed gallic acid causes apoptotic death of prostate carcinoma cells. In case of DU145 cells, gallic acid (25–75 μM) treatment for 24 h resulted in 73–84% (p<0.001) apoptotic death at 50 and 75 μM concentrations as compared to 1.8% observed in DMSO alone treated controls (Fig. 2A). Similarly, in 22Rv1 cells also, a significant increase (52–73%, p<0.001) in apoptotic death was observed at 50 and 75 μM concentration of gallic acid after 24 h of treatment time. In both the cell lines, the lower concentration of 25 μM of gallic acid did not induce significant increase in apoptotic death over the respective DMSO alone treated controls (Fig. 2B).
Fig. 2.
Gallic acid induces apoptotic death in prostate carcinoma cells in vitro. DU145 cells (A) or 22Rv1 cells (B) were plated for overnight at a density of 5000 cells per cm2. Cells were treated with 0–75 μM gallic acid for 24h. At the end of treatment time, adherent and non-adherent cells were collected and stained with annexin V-PI. Stained cells were then analyzed by flow cytometric analysis. Bars indicate mean ± SD, n=3. $; P<0.001 represents statistical significance of differences between control and gallic acid fed groups.
Gallic acid inhibits DU145 and 22Rv1 xenograft growth in athymic nude mice
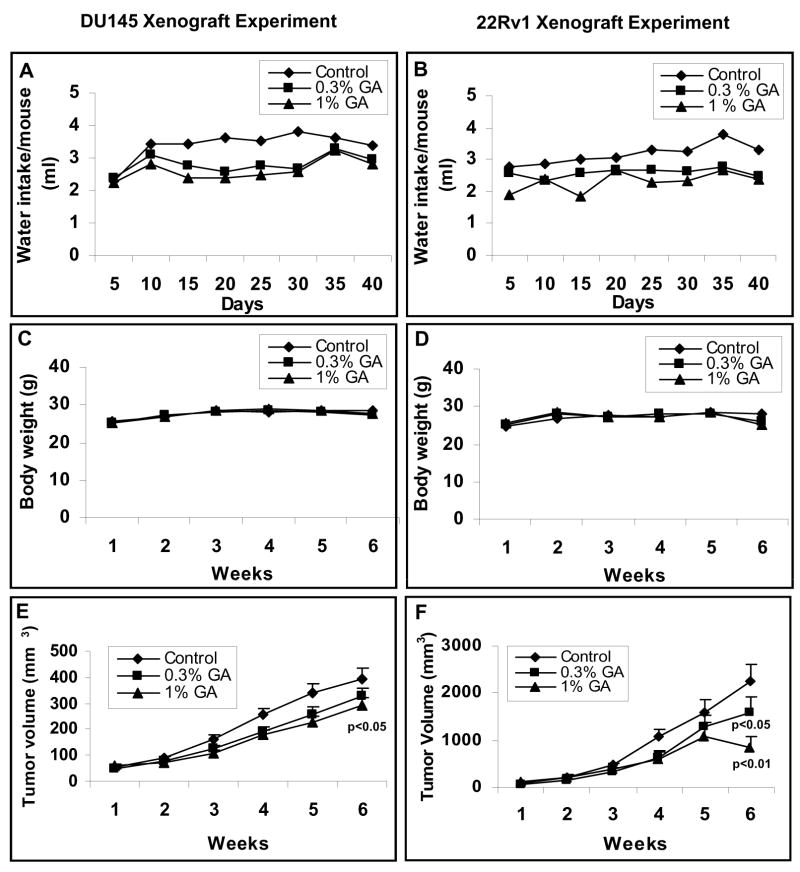
After studying the anticancer efficacy of gallic acid against prostate carcinoma cell lines in vitro, we next examined the in vivo efficacy of gallic acid in prostate tumor (DU145 and 22Rv1) mouse xenograft model. In this study, daily oral administration of gallic acid in drinking water at 0.3% and 1% dose levels (w/v) for six weeks caused inhibition in the growth of both DU145 and 22Rv1 tumor xenografts (Fig. 3). Moreover, in both the studies, there was no apparent sign of toxicity (measured in terms of significant weight loss on gallic acid administration) during the six weeks of experimental period. As shown in Fig 3A–3D, water intake and gain in body weights did not differ significantly among the control and gallic acid-fed groups till the end of these studies. At the end of the experimental period, gallic acid reduced the tumor volume per mouse in DU145 xenograft study from 392 mm3 in control to 324 and 293 mm3 (P<0.05) in 0.3% and 1% (w/v) gallic acid fed group respectively (Fig. 3E). In case of 22Rv1 tumor xenograft, at the end of experimental period of 6 weeks, gallic acid administration through drinking water at same dose levels led to a stronger reduction in average tumor volume per mouse from 2252mm3 in control group to 1589 mm3 (p<0.05) and 848 mm3 (p<0.01) with a time-dependent growth inhibitory effect of gallic acid throughout the study time (Fig. 3F).
Fig. 3.
Gallic acid feeding inhibits the growth of DU145 and 22Rv1 xenograft growth in athymic nude mice. Four million DU145 cells (A–C) or one million 22Rv1 cells (D–F) were mixed in Matrigel and were ectopically implanted on the right flank of each mouse. After 24 hours, mice were fed plain water (control group) or 0.3%, 1% (w/v) dose of gallic acid in water for 5 days/wk for 6 weeks: (A, B) average water consumption per mouse per day (ml), (C, D) mean body weight/mouse (g), and (E, F) average tumor volume (mm3) are plotted as a function of week of gallic acid feeding. Data shown represents mean of eight to nine mice in each group; bars, SE. Statistical significance of difference between control and gallic acid-fed groups was calculated by one-way ANOVA followed by Bonferroni t test.
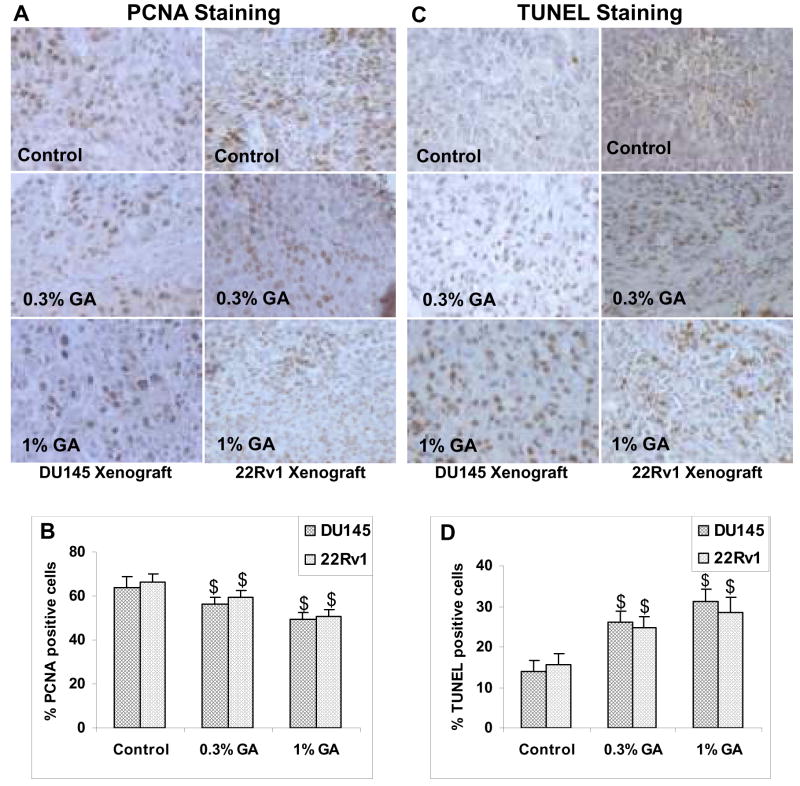
Gallic acid exhibits in vivo anti-proliferative as well as pro-apoptotic effects in xenograft models
Qualitative analysis of PCNA stained tumor sections obtained from DU145 xenograft studies revealed a decrease in PCNA positive tumor cells in gallic acid fed groups as compared to control groups (Fig. 4A; left panel). Further, quantitative analysis of the percentage of the PCNA positive cells in tumor sections from DU145 xenograft study showed 56.3±3.4 and 49.6±2.8% PCNA positive cells in tumor sections from 0.3% and 1% (w/v) gallic acid fed mice as compared to 64±5% in controls accounting for 12–22.5% decrease as compared to control (p< 0.001, Fig. 4B). Similar analysis in 22Rv1 xenograft study also revealed the decreased PCNA stained cells in tumor sections from mice in gallic acid fed groups as compared to those from control animals (Fig. 4A; right panel). It was observed that percentage of PCNA positive cells decreased from 66.5±3.32 % in control group to 59.5±3.15 and 50.7±2.73% (10–24% decrease as compared to control, p<0.001) in 0.3% and 1% (w/v) gallic acid fed groups, respectively (Fig. 4B).
Fig. 4.
In vivo anti-proliferative effects of gallic acid feeding on DU145 and 22Rv1 tumor xenograft in athymic nude mice. At the end of six weeks of xenograft studies, tumors were excised and processed for immunohistochemical staining for proliferation cell nuclear antigen (PCNA), and terminal deoxynucleotidyltransferase-mediated nick end labeling (TUNEL). The representative pictures were taken at 400X magnification of microscopic field from each group (Fig. 4A and 4C; left side vertical panels for DU145 xenograft study, and right side vertical panels for 22Rv1 study, respectively). Bar diagrams represent quantitative analysis (mean counts ± SD) of PCNA positive cells (Fig. 4B) and TUNEL positive cells (Fig. 4D) in various groups for both DU145 and 22Rv1 xenograft studies. $; P<0.001 represents statistical significance of differences between control and gallic acid fed groups.
Next, we examined the pro-apoptotic effects of gallic acid under in vivo conditions. For this, the TUNEL staining was performed on tumor sections from control and gallic acid fed groups from both DU145 and 22Rv1xenograft studies (Fig. 4C). We found that percentage of TUNEL positive cells significantly increased from 13.9±2.6% in tumor sections from control group to 26±2.8 and 31.3±2.8% (1.9 and 2.25 folds increase, p<0.001) in 0.3 and 1% gallic acid fed groups in DU145 tumor xenograft study (Fig. 4D). In case of 22Rv1 tumor xenograft study similar results were observed wherein, percentage of TUNEL positive cells increased significantly from 15.6±2.6 in control group to 24.7±2.9 (1.6 folds increase, p<0.001) in 0.3% (w/v) gallic acid fed group, and to 28.8±3.9% (1.8 folds increase, p<0.001) in 1% (w/v) gallic acid fed group (Fig. 4D). Taken together, these findings suggest that gallic acid possesses in vivo efficacy in inhibiting the growth of prostate tumors by decreasing proliferation and promoting the apoptosis.
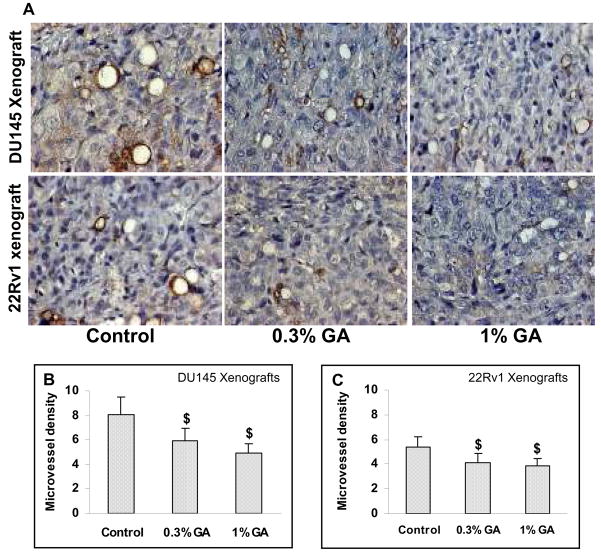
Gallic acid exhibits in vivo anti-angiogenic effects in xenograft model
To examine if gallic acid induced inhibition in growth of tumors in vivo is accompanied by anti-angiogenic effects, CD31 immunohistochemical staining was performed to quantify microvessel density in tumors harvested from both DU145 and 22Rv1 xenograft studies. The immunohistochemical staining for CD31 showed significantly more positive staining in control group of tumors from both the studies but comparatively lesser number of positive cells in the tumors from the gallic acid fed animals (Fig. 5A). The quantitative analysis of CD31 staining in control tumors showed 8.07±1.42 CD31 positive cells as compared to 5.97±1.08 (26% decrease, p<0.001) and 4.93±0.76 (39% decrease, p<0.001) CD31 positive cells in 0.3% and 1% doses of gallic acid treated tumors in DU145 xenograft study, respectively (Fig. 5B). Similarly, in case of 22Rv1 xenograft study, number of CD31 positive cells were reduced significantly from 5.42±0.30 in control tumors to 4.13±0.74 and 3.84±0.64 (24% and 29% decrease, p<0.001) in 0.3% and 1% (w/v) gallic acid treated groups, respectively (Fig. 5C). These results suggest that tumor growth suppression by gallic acid may partly be attributed to its anti-angiogenic effects.
Fig. 5.
Gallic acid exhibits in vivo anti-angiogenic effects in DU145 (Top panel) and 22Rv1 (Bottom panel) tumor xenograft. At the end of six weeks of xenograft studies, tumors were excised and processed for immunohistochemical staining for CD31, a marker to measure microvessel density, and representative pictures were taken at 400× magnification of microscopic field from each group (Fig. 5A). Bar diagrams represent quantitative analysis (mean counts ± SD) of CD31 positive cells in various groups for both DU145 (Fig. 5B) and 22Rv1(Fig. 5C) xenograft studies. $; P<0.001 represents statistical significance of differences between control and gallic acid fed groups.
DISCUSSION
Findings from various epidemiological studies looking at geographical variation in incidence of cancer, as well as studies conducted in transmigrated population reveal that diet has a major influence in overall risk of cancer. Numerous studies have later revealed that increased consumption of fruits and vegetables is associated with decreased incidence of cancer (23, 24). These findings further fueled the interest in examining the potential role of various chemical constituents of fruits and vegetables, and plants in general, in control or prevention of cancer. Not only these epidemiological studies, even the widespread use of various plants and their extracts in traditional medicine throughout the world shifted the focus of research in exploring the beneficial effects of various plant constituents especially phytochemicals in various human health conditions including cancer. Currently, numerous reports in literature show that many phytochemicals exhibit anti-cancer efficacy in various in vitro and in vivo models of cancer (25). We have also extensively studied the anti-cancer efficacy of grape seed extract against prostate cancer (26–29). In our previous studies, we found that grape seed extract exhibited anticancer efficacy against androgen independent prostate carcinoma DU145 cells both in vitro (26) and in vivo (27). We observed that grape seed extract decreased the viability of these cells by induction of intrinsic pathway of apoptosis, whereas under in vivo conditions, it inhibited the growth of advanced human prostate tumors, promoted apoptosis in the tumor, and exerted anti-angiogenic activity. We also observed that grape seed extract treatment led to up-regulation of circulating levels of IGFBP-3, a biomarker related to prostate cancer risk, growth and prognosis. Based on the findings of these studies, and to have further detailed insight into the mechanism for its anticancer efficacy, we next fractionated the grape seed extract into individual chemical constituents. During the screening of these constituents, we found that gallic acid had significant anti-cancer efficacy against DU145 cells, and thus might be the major contributory factor to the grape seed extract biological activity (6, 16). Based on our previous observations, in the present study, we extended our work to evaluate the anti-cancer efficacy of gallic acid against both androgen-dependent 22Rv1 and androgen-independent DU145 cells under both in vitro and in vivo conditions. In concurrence with the results of previous studies, gallic acid decreased the viability of DU145 cells and induced apoptotic death. Additionally, similar effects were observed in 22Rv1 cells. However, contrary to the effects observed in these cells, gallic acid did not affect the viability of non-neoplastic PWR-1E cells. From these observations, it is clear that gallic acid is selectively toxic to cancer cells. A similar observation has been reported previously, where gallic acid selectively induced death in cancer cells (17). Further analysis into cytotoxic effects of gallic acid in DU145 and 22Rv1 revealed that the decrease in the viability of these cell lines was due to induction of apoptosis.
We next assessed whether gallic acid exerts similar effects under in vivo conditions employing xenograft model of prostate cancer. In our study, we found that gallic acid inhibited the growth of both DU145 and 22Rv1 tumor xenograft in nude mice. The extent of growth inhibition by gallic acid was more in case of 22Rv1 tumor xenografts as compared to DU145 tumor xenografts. This effect was in contrast to growth inhibitory effects observed under in vitro condition where DU145 cells were more sensitive to gallic acid than 22Rv1 cells. This may be due to the differences in the growth kinetics of DU145 and 22Rv1 cells under culture conditions versus tumor xenograft in nude mice and the fact that gallic acid might be more effectively in targeting the rapidly proliferating cells versus slow growing cells. In support of this observation, our results clearly showed that in vivo conditions were less conducive to DU145 growth than in vitro conditions where they grew relatively faster; a reverse scenario existed for 22Rv1 cells. We next investigated the mechanism of tumor suppressive effects of gallic acid. Immunohistochemical analysis of tumors harvested from this study revealed that gallic acid inhibited the growth of these tumors as evident by reduced reactivity of PCNA in gallic acid fed animals for both DU145 and 22Rv1 xenograft studies. In addition, there was also increased number of TUNEL positive cells, a marker for apoptosis induction in gallic acid fed groups. Another important aspect of tumor growth and metastasis to distant sites is angiogenesis. In our study, we found that tumors harvested from gallic acid treated groups from both DU145 and 22Rv1 study had significantly less staining for CD31, a marker for tumor angiogenesis as compared to tumors from plain water fed groups. These findings are in accordance with the report in literature where gallic acid was found to be one of the active ingredients of Rubus leaf extract responsible for its anti-angiogenic activity (30). These in vivo findings are also consistent with results of our in vitro studies where we observed that gallic acid inhibited the growth of these cell lines, and induced apoptotic cell death. Taken together, the findings of present studies demonstrate the potential anti-cancer efficacy of gallic acid against androgen-dependent and androgen-independent prostate cancer cells in culture and xenograft animal model. However, additional studies to understand other molecular mechanisms which are responsible for its anti-cancer efficacy are warranted to claim gallic acid as potent anti-cancer agent against prostate cancer.
Acknowledgments
This study was supported by the National Cancer Institute, NIH RO1 grantCA091883.
Abbreviations
- MTT
Thiazolyl Blue Tetrazolium Bromide
- DAB
3,3′-diaminobenzidine
- PCNA
proliferating cell nuclear antigen
- TUNEL
terminal deoxynucleotidyl transferase-mediated UTP nick end labeling
References
- 1.Bemis DL, Capodice JL, Costello JeE, Vorys GC, Katz AE, Buttyan R. The use of herbal and over-the-counter dietary supplements for the prevention of prostate cancer. Curr Urol Rep. 2006;7:166–174. doi: 10.1007/s11934-006-0017-x. [DOI] [PubMed] [Google Scholar]
- 2.Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray SD, Kuszynski CA, Joshi SS, Pruess HG. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology. 2000;148:187–197. doi: 10.1016/s0300-483x(00)00210-9. [DOI] [PubMed] [Google Scholar]
- 3.Ye X, Krohn RL, Liu W, Joshi SS, Kuszynski CA, McGinn TR, Bagchi M, Preuss HG, Stohs SJ, Bagchi D. The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultured human cancer cells. Mol Cell Biochem. 1999;196:99–108. [PubMed] [Google Scholar]
- 4.Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res. 2006;12:6194–6202. doi: 10.1158/1078-0432.CCR-06-1465. [DOI] [PubMed] [Google Scholar]
- 5.Durak I, Cetin R, Devrim E, Erguder IB. Effects of black grape extract on activities of DNA turn-over enzymes in cancerous and non cancerous human colon tissues. Life Sci. 2005;76:2995–3000. doi: 10.1016/j.lfs.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Veluri R, Singh RP, Liu Z, Thompson JA, Agarwal R, Agarwal C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2006;27:1445–1453. doi: 10.1093/carcin/bgi347. [DOI] [PubMed] [Google Scholar]
- 7.Niemetz R, Gross GG. Enzymology of gallotannin and ellagitannin biosynthesis. Phytochemistry. 2005;66:2001–2011. doi: 10.1016/j.phytochem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Chu YF, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- 10.Kang MS, Oh JS, Kang IC, Hong SJ, Choi CH. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J Microbiol. 2008;46:744–750. doi: 10.1007/s12275-008-0235-7. [DOI] [PubMed] [Google Scholar]
- 11.Kratz JM, Andrighetti-Frohner CR, Leal PC, Nunes RJ, Yunes RA, Trybala E, Bergstorm T, Barardi CR, Simoes CM. Evaluation of anti-HSV-2 activity of gallic acid and pentyl gallate. Biol Pharm Bull. 2008;31:903–907. doi: 10.1248/bpb.31.903. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Jun CD, Suk K, Choi BJ, Lim H, Park S, Lee SH, Shin HY, Kim DK, Shin TY. Gallic acid inhibits histamine release and pro-inflammatory cytokine productionin mast cells. Toxicol Sci. 2006;91:123–131. doi: 10.1093/toxsci/kfj063. [DOI] [PubMed] [Google Scholar]
- 13.Kim YJ. Antimelanogenic and antioxidant properties of gallic acid. Biol Pharm Bull. 2007;30:1052–1055. doi: 10.1248/bpb.30.1052. [DOI] [PubMed] [Google Scholar]
- 14.Hsu CL, Yen GC. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br J Nutr. 2007;3:1–9. doi: 10.1017/S000711450774686X. [DOI] [PubMed] [Google Scholar]
- 15.Makena PS, Chung KT. Effects of various plant polyphenols on bladder carcinogen benzidine-induced mutagenicity. Food Chem Toxicol. 2007;45:1899–1909. doi: 10.1016/j.fct.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Inoue M, Sakaguchi N, Isuzugawa K, Tani H, Ogihara Y. Role of reactive oxygen species in gallic acid-induced apoptosis. Biol Pharm Bull. 2000;23:1153–1157. doi: 10.1248/bpb.23.1153. [DOI] [PubMed] [Google Scholar]
- 17.Faried A, Kurnia D, Faried LS, Usman N, Miyazaki T, Kato H, Kuwano H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int J Oncol. 2007;30:605–613. doi: 10.3892/ijo.30.3.605. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal C, Tyagi A, Agarwal R. Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol Cancer Ther. 2006;5:3294–3302. doi: 10.1158/1535-7163.MCT-06-0483. [DOI] [PubMed] [Google Scholar]
- 19.Raina K, Rajamanickam S, Deep G, Singh M, Agarwal R, Agarwal C. Chemopreventive effects of oral gallic acid feeding on tumor growth and progression in TRAMP mice. Mol Cancer Ther. 2008;7:1258–1267. doi: 10.1158/1535-7163.MCT-07-2220. [DOI] [PubMed] [Google Scholar]
- 20.Rubin MA. Targeted therapy of cancer: new roles for pathologists--prostate cancer. Mod Pathol. 2008;21:S44–55. doi: 10.1038/modpathol.2008.11. [DOI] [PubMed] [Google Scholar]
- 21.Huang H, Tindall DJ. The role of the androgen receptor in prostate cancer. Crit Rev Eukaryot Gene Expr. 2002;12:193–207. doi: 10.1615/critreveukaryotgeneexpr.v12.i3.30. [DOI] [PubMed] [Google Scholar]
- 22.Gu M, Dhanalakshmi S, Mohan S, Singh RP, Agarwal R. Silibinin inhibits ultraviolet B radiation-induced mitogenic and survival signaling, and associated biological responses in SKH-1 mouse skin. Carcinogenesis. 2005;26:1404–1413. doi: 10.1093/carcin/bgi096. [DOI] [PubMed] [Google Scholar]
- 23.Pavia M, Pileggi C, Nobile CG, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. Am J Clin Nutr. 2006;83:1126–1134. doi: 10.1093/ajcn/83.5.1126. [DOI] [PubMed] [Google Scholar]
- 24.Larsson SC, Bergkvist L, Wolk A. Fruit and vegetable consumption and incidence of gastric cancer: a prospective study. Cancer Epidemiol Biomarkers Prev. 2006;15:1998–2001. doi: 10.1158/1055-9965.EPI-06-0402. [DOI] [PubMed] [Google Scholar]
- 25.Nishino H, Satomi Y, Tokuda H, Masuda M. Cancer control by phytochemicals. Curr Pharm Des. 2007;13:3394–3399. [PubMed] [Google Scholar]
- 26.Agarwal C, Singh RP, Agarwal R. Grape seed extract induces apoptotic death of human prostate carcinoma DU145 cells via caspases activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis. 2002;23:1869–1876. doi: 10.1093/carcin/23.11.1869. [DOI] [PubMed] [Google Scholar]
- 27.Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004;108:733–740. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 28.Raina K, Singh RP, Agarwal R, Agarwal C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res. 2007;67:5976–5982. doi: 10.1158/0008-5472.CAN-07-0295. [DOI] [PubMed] [Google Scholar]
- 29.Kaur M, Agarwal R, Agarwal C. Grape seed extract induces anoikis and caspase-mediated apoptosis in human prostate carcinoma LNCaP cells: possible role of ataxia telangiectasia mutated-p53 activation. Mol Cancer Ther. 2006;5:1265–1274. doi: 10.1158/1535-7163.MCT-06-0014. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Schwimer J, Liu D, Lewis J, Greenway FL, York DA, Woltering EA. Gallic acid is partially responsible for the antiangiogenic activities of Rubus leaf extract. Phytother Res. 2006;20:806–813. doi: 10.1002/ptr.1966. [DOI] [PubMed] [Google Scholar]