Abstract
Background
Early growth monitoring may not identify infants at-risk for later growth faltering because it is difficult for the provider to recognize how large of a negative shift might be problematic.
Aim
The aim of this study was to determine whether a slowing in early weight-for-age could be used to identify children at increased risk of later growth faltering.
Methods
Longitudinal data for infants aged birth to two years were analyzed for 1978 healthy, term infants born between 1999-2001. Logistic regression techniques were used to determine whether a negative change in weight-for-age, across well-child visit intervals, can identify infants at risk for growth faltering.
Results
The period prevalence of underweight was 24%. The odds ratio (OR) for infants with a negative shift in z-scores ≥ -0.85 between four and six months was 2.4 (95% CI 1.5, 3.9) compared to those without this shift, holding birth weight constant. Sensitivity analyses revealed the model was significant when either the 2000 CDC growth charts (p<.0001) or the 2006 WHO growth charts (p<.0001) were used as the reference, although the prevalence of underweight was lower (14.7%) when the 2006 WHO growth charts were the reference.
Conclusion
The findings support the hypothesis that a downward shift in weight-for-age of this magnitude during early infancy when well-child visits are most frequent can be used to identify children at-risk of later poor growth.
Keywords: Early Intervention, Failure-to-thrive, Growth, Infant, Weight, Sensitivity
INTRODUCTION
Growth monitoring is an integral part of routine well-child visits designed to monitor health and development. Yet there are few data to suggest that it is effective in identifying infants and young children early in the course of growth faltering (1-3). Growth often begins to slow in the first 6 months of life in infants later diagnosed with growth faltering (4); this is the same time interval when well-child visits are most frequent, allowing multiple opportunities to identify slowing growth in infants who later become underweight. However, Chen and colleagues (2000) found that in 55% of the charts they reviewed, a growth perturbance was either not identified or not documented (2). In a busy clinical practice, practitioners need a method of identifying those infants whose growth may indicate a need for further probing of feeding interactions.
One reason growth monitoring may be ineffective in identifying growth faltering is the fact that many infants cross percentile channels (both up and down) on the growth chart, making it difficult for the primary care provider to accurately identify those infants whose growth is problematic (5). Identifying percentiles that are shifting away from the mean may be useful as an early prognosticator for growth perturbations. However, a defined or recognized rate of deceleration that is indicative of increased risk of adverse outcomes and that is easily implemented into clinical practice is lacking. A standard of growth velocity for identification of infants who are at risk for later growth faltering may be advantageous.
The goal of the research was to identify whether early deceleration in weight gain could be used to predict subsequent early childhood growth faltering. The authors endeavored to identify a level of slowed weight-gain that is predictive of reaching a weight-for-length ≤ 5th percentile, using information gathered during regularly-scheduled well-child visits between two and six months of age. The age interval of four-to-six months was chosen initially because it reflects the typical timing of well-child visits and there are a number of developmental changes related to feeding skill development and changing nutritional requirements that could influence growth (6, 7). Additionally, there are data that suggest weight gain prior to six months of age is predictive of increased risk of overweight (8-10). The authors tested the hypothesis that a change in weight-for-age in a negative direction of more than -0.85 standard deviation between the four and six month well-child visit was predictive of a child reaching a weight-for-length ratio ≤ the 5th percentile (“underweight”) at some point during the first two years of life. A negative change of more than -0.85 was chosen a priori based upon data published by Mei and colleagues (2004) that suggested approximately 17% of infants demonstrated a negative change in weight-for-age ≥1 standard deviation between birth and six months of age, and this study was examining a shorter time interval. A second hypothesis tested was whether a similar change in the two-to-four month time period would be equally predictive, as we were interested in finding the earliest time interval useful for prognostic purposes. We conducted sensitivity analyses to determine the effect of the growth reference (2006 WHO vs. 2000 CDC) on the robustness of the model.
METHODS
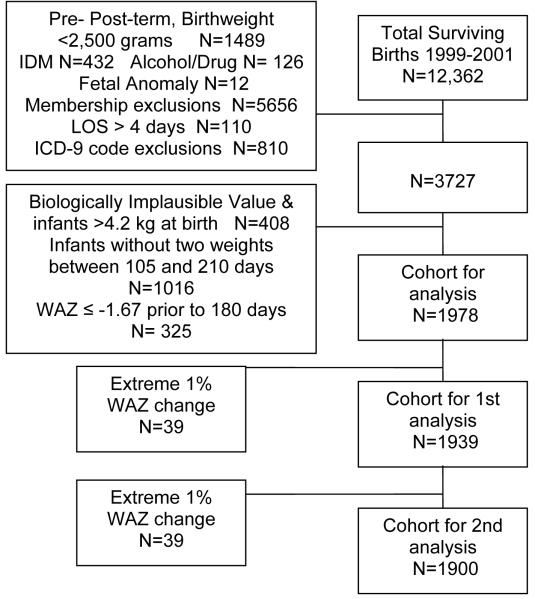
This study was a secondary database analysis of healthy, term infants born in the years 1999-2001, who were served by a private comprehensive health care system for the first two consecutive years of life. Weights, lengths and demographic information were extracted from the electronic medical record database. Inclusion criteria consisted of term gestation (≥37 weeks, ≤ 42 weeks), birthweight >2.5 kg, no known prenatal exposure to alcohol or illicit drugs, and an initial hospital stay of ≤ four days. Infants were excluded if they were born with any known, documented congenital or genetic defects or if there was any documented indication of gestational diabetes in the mother. Additionally, because this was a longitudinal study of growth, infants were excluded if they did not maintain enrollment in the health care system for the first two years of life, with an allowable lapse in membership of ≤ 45 consecutive days. Diagnostic codes used by the primary care provider within the first 4 months of life that may be associated with feeding and/or growth problems (e.g., V55.0, Attention to Tracheostomy) were applied to eliminate potential confounding variables. Infants with a birthweight >4.2 kg were then eliminated from analysis to control for regression to the mean, resulting in a dataset with birthweights >5%tile and <95%tile. Figure 1 details the development of the database.
Figure 1. Database development.
Database of Weights and Lengths for 3727 Infants Delivered to Study Team
IDM: Infant of a Diabetic Mother
LOS: Length of Stay
WAZ: Weight-for-Age Z-Score
This study was approved by the Colorado Multiple Institutional Review Board and received approvals for waiver of informed consent and HIPAA waiver for authorization (COMIRB 05-1055). This study was also approved by both the Research Review Committee and the Institutional Review Board for the health care system that held the database, with similar waivers (CO-05SPharo-01.)
All statistical analyses were conducted using SAS version 9.1, Windows XP Platform. An alpha level of 0.05 was set for all models. The authors obtained weight-for-age (WAZ), length-for-age (LAZ), and weight-for-length ratio (WLZ) z-scores, stratified by sex using the Epi Info Version 3.3 program based upon the 2000 CDC Growth Charts, and using the Epi Info Version 3.4.3. program based upon the 2006 WHO Growth Charts. Infants with values that were classified as “biologically implausible (coded BIV)” by the CDC software (n=326) were eliminated from the analysis to control for data entry errors, as were infants whose birthweight was > 4.2 kg (n=82). Infants were eliminated from analysis if they did not have at least two weights collected between 105 and 210 days of age (n=1016), because the difference in WAZ standardized percentile scores (z-scores) during this time period was the predictor for the first hypothesis. Infants who had a WAZ ≤ -1.67 (≤ 5th percentile) prior to 180 days of life were eliminated from further analysis to control for infants who already met the case criterion prior to the latest time period used as the predictor (six months of age, n=325.)
The predictor variable was defined as a change in WAZ between the first and the last weights collected within the four-to-six month time period (primary hypothesis) or within the two-to-four month time period (secondary hypothesis.) Weight-for-age was chosen over weight-for-length as the predictor variable because weight is the most accurately collected anthropometric measurement in the infant under one year of age (11, 12). The predictor variable was created by subtracting WAZ1 (the first WAZ collected) from WAZ2 (the last WAZ.) A negative value implies a deceleration in weight-gain velocity; a positive value implies accelerated weight-gain velocity. The predictor criterion was a negative change in the WAZ of more than -0.85. Weights collected from three and one-half to seven months of age were accepted for the change in weight during the four-to-six month time period, and weights collected from one and one-half to five months were accepted for the two-to-four month time period. The outcome variable was the lowest WLZ recorded during the time periods of 7-12, 12-18, or 18-24 months of age. WLZ is preferred as the indicator for underweight by the CDC, the World Health Organization (WHO), and the American Academy of Pediatrics (AAP) (13). Weight-for-length ratio is also the most reflective of a nutritional deficit (underweight); therefore, it can prompt a nutritional intervention. The authors decided to use the lowest WLZ, reflecting the a priori determination that one instance of a WLZ of ≤-1.67 resulted in becoming a case.
For each predictor time period (four-to-six months or two-to-four months), the most extreme values of change in WAZ for the entire cohort (<1%, >99%) were further eliminated to adjust for potential data entry errors, resulting in a total of 1939 infants in the four-to-six month time period and a total of 1900 infants in the two-to-four month time period. Period prevalence was calculated for 7-12, 12-18, and 18-24 months of age, and for the aggregate time period, and compared with published prevalence rates for the United States in the year 2000 (14). Means and standard deviations were calculated for age in months, length in centimeters, and weight in kilograms for each time period of interest. The cohort was then stratified categorically by birth weight (2.5 kg to < 2.75 kg, ≥2.75 kg to < 3.0 kg, ≥3.0 kg to ≤ 4.0 kg (REFERENCE), and >4.0 kg to ≤ 4.2 kg) to assess the influence of birth weight on becoming a case. The reference category was chosen as it included roughly the middle 50% of the birth cohort.
The authors used logistic regression analyses to determine whether a rate of deceleration in WAZ (negative change in z-score) of ≥ -0.85 during the four-to-six month time period was associated with an increased risk of underweight in the first two years of life, after adjusting for birth weight. The covariates of birth weight category, a negative change in z-score of ≥ -0.85, and the interaction term (birth weight category*change in z-score) were entered and tested for significance, using the -2 log likelihood test (15). We then assessed the time period of two-to-four months as a predictor, using the same logistic regression modeling techniques. The 95% confidence intervals for the c-statistics for each of the logistic regression models for the two predictor time periods were then compared to determine whether one time period was a better predictor of growth faltering than the other time period. Odds ratios and 95% confidence intervals, as well as relative risks, are reported.
Post-hoc sensitivity analyses were conducted to determine both the effect of exclusions that limited the final cohort, and the effect of a-priori decisions on the study results. The primary analyses were run using the full cohort (n=3727). Alternate thresholds were tested for the predictor variable (change in WAZ).and the outcome criterion of ≤ 5th percentile in weight-for-length was altered to both a weight-for-length ≤ 3rd percentile, and to weight-for-age percentiles ≤ both the 5th and 3rd percentiles. To assess the influence of errors in length measures, we conducted a sensitivity analysis subtracting 1.3 cm from all of the lengths originally recorded. Lipman and colleagues (2004) found that length measurements collected as part of routine care in pediatric practices were inaccurate by an average of 1.3 cm (16). Additionally, feeding regimen (breastmilk vs. formula and introduction of solid foods) was unavailable in this dataset. Therefore, we used the WHO growth charts in place of the CDC growth charts as reference to calculate WAZ and WLZ scores to determine whether the feeding regimen might influence our findings. The WHO growth charts represent the longitudinal growth of breastfed infants, while the CDC growth charts were developed using cohorts that were primarily formula-fed (17). We compared prevalence rates as well as the efficacy of using a negative change in weight-for-age as a predictor of later growth faltering.
RESULTS
Table 1 describes the maternal and infant demographic characteristics of those infants included in the analysis. To examine the potential bias created by limiting the initial birth cohort, the characteristics for full received cohort (FRC, n=3727) as well as the cohort used for final analyses (FC, n=1978) were compared to the 2000 census data published for the State of Colorado to determine how similar the infants were to those born within the state during the same time period. The infants in the FC were similar in all areas to those excluded (n=1729) from the FRC, with the exception of a greater frequency of first-time pregnancy in the mothers of infants included in the final analysis. The infants in the FC were within two percentage points to the published census data for distribution of sex and delivery method, and within three percentage points for distribution of the age of the mother, parity and number of singleton and twin births. Race/Ethnicity of the mother was not reported for 8.6% of the FC, which may have contributed to the lower than expected number of mother identified as Hispanic (15.9% vs. 27.2% in the census data.)
Table 1.
Demographic Characteristics of Infants Included in the Database Analysis (FC), compared to the Full Received Cohort (FRC) and the census data for the State of Colorado, 2000.
| Characteristic | Final Cohort (n=1978) |
Full Received Cohort (n=3727) |
Colorado 2000* (n=65,429) |
|
|---|---|---|---|---|
| n | (%) | (%) | (%) | |
| Sex: | ||||
| Male | 1036 | 52.4 | 51.8 | 51.2 |
| Female | 942 | 47.6 | 48.2 | 48.8 |
| Race: | ||||
| Asian or Pacific Islander | 105 | 5.3 | 4.6 | 4.2 |
| Non-Hispanic Black | 107 | 5.4 | 5.3 | 4.6 |
| Hispanic | 314 | 15.9 | 16.1 | 27.2 |
| Non-Hispanic White | 1281 | 64.8 | 65.5 | 63.9 |
| Unknown | 171 | 8.6 | 8.5 | 0 |
| Maternal age: | ||||
| Young (<20) | 83 | 4.2 | 4.8 | 7.5 |
| Mid (≥ 20, ≤35) | 1512 | 76.44 | 76.2 | 74.2 |
| Older (>35) | 383 | 19.36 | 18.9 | 18.3 |
| Parity | ||||
| 0 | 864 | 43.7 | 40.0 | 42.1 |
| 1 | 719 | 36.3 | 37.0 | 32.7 |
| 2 | 269 | 13.6 | 16.0 | 15.9 |
| 3 | 93 | 4.7 | 5.0 | 6.1 |
| >3 | 33 | 1.7 | 2.0 | 3.3 |
| Singleton | 1960 | 99.1 | 99.0 | 97.0 |
| Twin | 18 | 0.9 | 1.0 | 3.0 |
| Delivery method: | ||||
| C-section | 339 | 17.1 | 16.8 | 18.3 |
| Vaginal | 1639 | 82.9 | 83.2 | 81.7 |
Table 2 shows the period prevalence of underweight for each time period of interest, and for the entire time period using the 2000 CDC growth chart as a reference. Table 3 presents means and standard deviation for age in months, length in centimeters, and weight in kilograms, stratified by caseness within each time period. The period prevalence of underweight was higher than expected for all time periods when compared with the expected prevalence rate published for the United States (14). Sensitivity analysis revealed that, even when the criterion to become a case was changed to define underweight as a WLZ ratio ≤ -2.0 (≤ 3rd percentile), the prevalence remained elevated (16.1% (p<0.0001)). We conducted an additional sensitivity analysis using the entire cohort (n=3727) to explore the influence of excluded infants on the overall prevalence rate. The prevalence rate of underweight remained elevated in the entire cohort (n=3727) using a WLZ score ≤ -1.67 (21.47%); weight-for-age z-score of ≤ -1.67 was 14.5% for the entire cohort.
Table 2.
Period Prevalence of Underweight Stratified by Sex, Time Period
| Number of children with a weight/length ratio during each time interval | Cohort (N=1939) |
Expected Prevalence (5.4%)* |
|
|---|---|---|---|
| N = Case in Time Period | Period Prevalence | z-statistic | |
| 6-12 mo of age (N=1684) | |||
| Male (N=888) | 136 | 15.3 | 13.07 |
| Female (N=796) | 121 | 15.2 | 12.23 |
| 12-18 mo of age (N=1569) | |||
| Male (N=827) | 118 | 14.3 | 11.28 |
| Female (N=742) | 122 | 16.4 | 13.31 |
| 19-24 mo of age (N=1549) | |||
| Male (N=813) | 103 | 12.7 | 9.17 |
| Female (N=736) | 104 | 14.1 | 10.48 |
| Total cases within the 6-24 month interval (N=1939) | |||
| Male (N=1014) | 235 | 23.2 | 25.04 |
| Female (N=925) | 232 | 25.1 | 26.48 |
| Total | 467 | 24.0 | 36.40 |
All time periods p< 0.0001 compared to 5.4% prevalence of underweight nationally, Pediatric Nutrition Surveillance Report, 2002.
Table 3.
Mean age (mos), length (cm), weight (kg) across all time periods of interest, stratified by cases and controls.
| Time Period (mos) | Cases | Controls | ||||
|---|---|---|---|---|---|---|
| Mean age-mos (sd) | Mean length- cm (sd) | Mean weight- kg (sd) | Mean age-mos (sd) | Mean length- cm (sd) | Mean weight- kg (sd) | |
| 6-12 | 6.4 (0.7) | 69.5 (2.5) | 6.9 (0.6) | 6.5 (0.8) | 68.4 (2.8) | 7.9 (0.9) |
| 12-18 | 13.0 (1.5) | 77.5 (3.0) | 8.5 (0.7) | 13.0 (1.5) | 77.0 (3.3) | 9.9 (1.1) |
| 18-24 | 20.2 (2.4) | 84.4 (3.6) | 9.9 (0.8) | 21.1 (2.7) | 84.9 (4.0) | 11.8 (1.4) |
A greater percentage of infants with a birth weight of less than 3.0 kg reached case criterion than did infants with heavier birth weights, with 35% of infants (131/372) with a birthweight < 3.0 kg meeting case criterion compared to 21% of infants (327/1157) weighing ≥3.0 kg to ≤ 4.0 kg at birth, and 10.8% of infants (9/83) with a birthweight >4.0 kg and ≤ 4.2 kg.
Using the -2 log likelihood test, birth weight category entered into the model (p< 0.0001.) A negative change in z-score ≥ -0.85 was also significant (p < 0.0019.) The interaction term (birth weight*change in z-score) was not significant when it was entered into the model along with birth weight and the negative change in z-score, and therefore was dropped from the final model. The odds ratios and the 95% confidence intervals of becoming a case for infants with a negative shift in WAZ score ≥ -0.85 during the four-to-six month age range, stratified by birth weight, are reported in Table 4, along with relative risks. Table 5 presents the sensitivity, specificity, and area under the curve (AUC) from ROC analyses conducted using the predictor cut-off of a negative shift in WAZ score ≥ -0.85 during the four-to-six month age range, for the entire cohort as well as stratified by birth weight.
Table 4.
Relationship of Change in Weight-for-Age and Odds of Becoming a Case, Stratified by Birthweight Category, 4-6 Month Time Period (n=1939)
| Parameter | Odds Ratio | 95% CI | Relative Risk | 95% CI |
|---|---|---|---|---|
| Only Birthweight (kg) category in model* | ||||
| <2.75kg | 2.13 | 1.4, 3.3 | 1.25 | 1.1, 1.5 |
| ≥2.75kg, <3.0kg | 1.85 | 1.4, 2.4 | 1.19 | 1.1, 1.3 |
| ≥3.0kg, ≤4.0kg (Reference) | 1.0 | - | ||
| >4.0kg, ≤4.2kg | 0.43 | 0.2, 0.9 | 0.88 | 0.8, 0.9 |
| Only Negative Change in WAZ score ≥ -0.85 in the model** | 2.17 | 1.3, 3.5 | 1.28 | 1.1, 1.5 |
| Both BW and Change WAZ in model* | ||||
| BW<2.75kg | 2.19 | 1.4, 3.3 | ||
| BW ≥2.75kg, <3.0kg | 1.90 | 1.4, 2.5 | ||
| ≥3.0kg, ≤4.0kg (Reference) | 1.0 | |||
| BW >4.0kg, ≤4.2kg | 0.42 | 0.2, 0.9 | ||
| Negative Change in WAZ score ≥ -0.85 | 2.39 | 1.5, 3.9 |
WAZ = weight-for-age z score
p<0.0001;
p=0.0019
Table 5.
Sensitivity, Specificity, and Area under the ROC curve by category of birthweight using a negative change in WAZ of ≥ -0.85
| Birthweight category | Sensitivity (95% CI) | Specificity (95% CI) | Area under Curve (AUC) | p-value |
|---|---|---|---|---|
| Aggregate cohort | 0.06 (0.04, 0.09) | 0.97 (0.96, 0.98) | 0.611 | p< .0001 |
| < 3.0 kilograms | 0.02 (0.0, 0.07) | 0.98 (0.96, 1.0) | 0.615 | p= .0004 |
| ≥3.0 kilograms, < 4.2 kilograms | 0.07 (0.05, 0.10) | 0.97 (0.96, 0.98) | 0.619 | p< .0001 |
We then tested the hypothesis that change in WAZ during the time period of four-to-six months was more predictive than the time period of two-to-four months. The negative change in WAZ as a predictor was statistically significant (P=0.0069) during the period of two-to-four months, and therefore the authors compared the c-statistics for the logistic regression models. Because the confidence intervals overlapped (0.607, 95% CI 0.576, 0.637 for the two-to-four month period; 0.610, 95% CI 0.581, 0.639 for the four-to-six month period), the two time periods were not statistically different in their ability to identify infants at increased risk for growth faltering, suggesting that either time period can be used when the 2000 CDC growth charts are the reference.
The sensitivity analyses revealed that this model was robust under a variety of conditions. Using a more conservative definition of underweight (WLZ ≤ -2.0), the model with both birthweight and a negative change in WAZ of more than -0.85 between four and six months of age was still statistically significant (p<.0001). We changed the predictor variable to a negative change in WAZ of more than -0.50 between four and six months of age and the model remained significant (p<.0001). After subtracting 1.3 cm from the length of every measurement and re-calculating z-scores, overall prevalence of a WLZ of ≤-1.67 dropped to 14.7% across the 7-24 month time period, but the model was robust (p<.0001)
The final sensitivity analyses were conducted using the 2006 WHO growth charts in place of the 2000 CDC charts. The overall prevalence of a WLZ ≤ -1.67 dropped to 14.7%, which remained significantly elevated over expected (p<.0001). The model including birthweight and change in WAZ between four and six months of age remained significant (p<.0001), and the odds ratio remained elevated (adjusted OR 3.3). A similar change in WAZ between two and four months of age was not predictive of later underweight when the WHO growth charts were used.
DISCUSSION
This study provides evidence that early deceleration in weight-gain is useful in identifying infants who later become underweight. Infants whose WAZ dropped more than -0.85 standard deviations between either the two-to-four month or four-to-six month intervals were at increased risk of reaching underweight status by 24 months of age compared to those with a more modest change. This study also demonstrated that this drop in weight-for-age (≥ -0.85 sd) is predictive as early as two-to-four months when the 2000 CDC growth charts are the reference, and the sensitivity analyses supported the robust nature of this model. Because of this, a provider may consider probing for additional information from families whose infants are demonstrating early negative shifts in weight-for-age percentiles. In addition, the provider should be mindful of the need for careful assessment of growth measures at subsequent visits and consider an early intervention in order to improve nutrition and growth outcomes. The cut-off level provided in this methodology resulted in a low sensitivity (0.06) and a high specificity (0.97). Providers can feel confident that the extra time spent providing nutritional guidance to the parents of a child whose WAZ shifts to this degree is directed towards those most at-risk of reaching an underweight status. The cut-off level can be set by each provider to best balance the sensitivity and specificity within their own practice. We chose to keep this cut-off given the high specificity of the results, because this was meant to be a screening that assists the provider in identifying those infants for whom it would be helpful to explore nutritional issues even if parental anxiety might be raised. The AUC was greater than 0.5, but it was not very large (0.615 for the infants born <3.0 kg, and 0.619 for infants ≥ 3.0 kilograms at birth). This methodology may be useful in assisting a care provider, when considered along with clinical factors including previous growth history, nutrition, and overall development. Additionally, because change in growth velocity can be automatically calculated in the study system by using the anthropometric indicators already collected as part of routine well-child visits, the resource investment is minimal. Within each population, the provider would need to determine baseline prevalence rates and determine whether a high sensitivity, or high specificity would be preferable.
This study revealed a significantly higher prevalence of underweight than expected (24% of the total cohort.) This was an especially surprising finding given the population studied was adequately insured, one identified barrier to accessing care. There are several possible factors that may have contributed to this high prevalence. We conducted sensitivity analyses to determine the effect of both exclusions and outcome criterion to explore the reasons why the prevalence was elevated. This study used a period prevalence, and allowed a single instance of meeting case criterion to be sufficient. There were repeated opportunities (up to three) to identify underweight.
Another factor that may have contributed to the elevated prevalence rate is the use of a single criterion (a weight-for-length z-score ≤ -1.67) rather than multiple criteria to define underweight. In a recent study by Olsen and colleagues (2007), 27% of their birth cohort (n=6090) met at least one criterion for growth faltering between 2 and 11 months of life, although there was little concurrence among the seven criteria used in their study (18). The WLZ is recommended by the WHO, the CDC, and the AAP, although the number of children identified as underweight using this criterion may differ from the number that might be identified by a different criterion. Sensitivity analyses revealed that 12.73% of infants had a WAZ ≤ -1.67, which is still significantly elevated compared to expected (P<.0001). Clinically, more than one measure of poor growth is conventionally used to identify growth faltering. However, the study was designed to provide an early indicator to assist the care provider in identifying at-risk infants rather than to provide a diagnosis. In a busy practice, the assistant who typically plots the weight and length on a growth chart can flag those infants whose growth is slowing, so that the care provider can ask additional questions to determine whether further investigation is warranted.
Measurement error in length could also contribute to the elevated prevalence of underweight, as the collection of length data was not standardized and accuracy of the measurements is unknown. Subjects would generally have been measured in the same clinics over the study period; however, an over-estimation of length would increase the number of infants identified as underweight (19). Sensitivity analyses revealed the prevalence of underweight remained elevated when length was reduced by 1.3 cm (14.7%), and the model remained robust (p<.0001).
Additional factors influencing the high prevalence outcome include the possible influence of altitude and feeding regimen, and the influence of the media attention on obesity. There are data that suggest lengths of infants at high altitude are shorter than same-age peers (“stunting”) (20), although there are no data that have directly shown poor weight gain related to altitude. Because this study was conducted with infants born at an altitude > 5000 feet, the bias due to impact of altitude on the primary outcome would have been toward fewer cases. Nevertheless, replication of observations in a similar population born and reared at a lower altitude would be useful.
The authors had no information on the composition of the diet for the infants in the cohort which may have a bearing on the prevalence rate. Data have suggested infants who are exclusively fed breast milk gain weight at a different rate than the national references (21-23). Colorado has a high breastfeeding initiation rate (83.3%-84.8% in the years 1999-2000) although the rate decreases by 6 months of age (39.2-44.4%) (24). This study used the 2000 CDC growth charts because clinically they are common in the United States and were in use during the study period; fewer infants were categorized as underweight when the 2006 WHO growth charts designed for breastfed infants were used as the reference. This finding is similar to that reported in other studies comparing prevalence of underweight across the 2000 CDC and the 2006 WHO growth charts(17). The model including birthweight and change in WAZ between four and six months remained robust using the WHO growth charts (p<.0001).
Finally, while obesity remains a public health concern, thinness may be tolerated at a higher prevalence rate due to heightened concerns about later obesity. Parents may not be concerned about low weight gain because they are inundated with messages about obesity. Studies also have suggested parents demonstrate a poor ability to visually identify either thinness or overweight status in young children (25).
An additional finding was that infants with lower birth weight were at increased risk of underweight than were infants with a heavier birth weight. This finding supports previously published data regarding slower weight-gain in low birth weight infants (26-28). However, the infants in the current study were > 2.5 kg at birth and were term gestation, and no infant had a weight-for-age ≤ -1.67 between birth and 180 days. Infants who are > 2.5 kg are not considered low birth weight, but this study suggests they are still at increased risk of growth faltering, supporting the need for close monitoring of growth for these marginally average-for-gestational age (AGA) infants.
Strengths of this study include the large sample size as well as the fact that longitudinal measurements of growth were available for the first two consecutive years of life. Additionally, the infants in the cohort were selected based upon inclusion criteria designed to focus on healthy term infants. This is in contrast to many studies of growth in ill or hospitalized infants and/or preterm infants (28-31). This population was also born within a three-year period; therefore, temporal changes in clinical and feeding practices were likely minimal. Finally, these infants were all served by a comprehensive, private health care system. While socio-economic information was not available, the cohort represents a population covered under private health care rather than the public health communities often enrolled in studies of infants at-risk for growth faltering (31). Few data have been published on cohorts of privately insured infants, where the assumption is made that access to care is not a barrier.
Weaknesses of this study include possible under-representation of specific ethnic/racial minorities, although the percentage of Caucasian infants in the cohort was similar to the percentage reported during the 2000 U.S. Census for the state of Colorado (64.8% vs. 63.9%). Racial/ethnic background was not reported for 8.6% of the cohort. An additional concern regarding generalization of findings is the fact that the cohort for analyses (n=1978) was significantly smaller than the original birth cohort (n=12,362.) The majority of infants lost to follow-up were lost due to a change in insured status within this HMO (n=5656) or prematurity/low birth weight (n=1489). There may have been an unmeasured or unidentified bias introduced during the data exclusion process. The infants in the final cohort were similar in demographic characteristics to both those infants excluded during the analysis and to those infants born in the state of Colorado during the year 2000. In addition, the lack of developmental and health outcome data limit these findings to describing underweight status. And finally, the lack of information regarding feeding regimen (breast milk or formula, and complementary and weaning foods) is an additional weakness. However, we re-ran the analyses using the WHO growth charts in an attempt to determine the influence of feeding regimen on the model, and found the model to be robust when using the 4 to 6 month time period as a predictor. In future studies, the authors would like to apply this standard of weight-for-age deceleration while collecting weights and lengths by trained research staff, and where data are collected regarding feeding regimen.
Application of this weight-for-age deceleration standard can assist both the researcher and the clinical provider in identifying infants at-risk for growth faltering as early as the interval between four and six months of age, regardless of the growth chart reference used by the clinician. Identifying at-risk infants is imperative in designing prospective research studies. In the clinical setting, healthcare providers may scrutinize early growth patterns more closely and use this standard to interpret changes in growth percentile channels. Growth monitoring is a screening tool, inexpensive and readily available. In the sites used for this current study, the weights and lengths are plotted automatically using a nutrition program from the CDC, called Epi Info Nutrition. Weight-for-age, length-for-age, and the weight-for-length ratio percentiles and z-scores can be requested easily using this computer program. The change in z-scores can be quickly calculated once the z-scores are obtained using this public domain program. Given the higher than expected prevalence rate of underweight found in this population, the methods described in this study (combined with ROC analyses) should be replicated in additional populations to determine the best cut-off score for identifying early growth faltering in that population.
Early evidence of growth faltering should trigger additional questions and possibly assessment of an infant's health and development, and feeding patterns. With the expense that is incurred by families whose children demonstrate poor growth and feeding habits in their toddler and preschool years, this screening measure is an inexpensive way to begin identifying those infants who may benefit from anticipatory guidance from their primary care provider.
Acknowledgements
Kaiser-Permanente of Colorado provided the data for this study and funded this dissertation project. NIH #5 T32 DK 07658-17 (Krebs, PI) provided funding for manuscript preparation and publication. We would like to acknowledge the support of Kaiser-Permanente, as well as the research and clinical expertise of Susan Pharo, M.D., and Kimberly Bischoff, MSPH, both of whom provided supports from within Kaiser-Permanente to see this project to completion. We would also like to acknowledge Allan Prochazka, M.D., M.Sc. who provided expertise on study design, methodology, data analysis, and had input on the manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: We have no potential conflicts of interest or corporate sponsors to disclose.
References
- 1.Panpanich R, Garner P. Growth monitoring in children. Cochrane Database of Systematic Reviews. 2000(2):CD001443. doi: 10.1002/14651858.CD001443. [DOI] [PubMed] [Google Scholar]
- 2.Chen RS, Shiffman RN. Assessing growth patterns--routine but sometimes overlooked. Clin Pediatr (Phila) 2000 Feb;39(2):97–102. doi: 10.1177/000992280003900204. [DOI] [PubMed] [Google Scholar]
- 3.Batchelor JA. Failure to thrive in young children: Research and practice evaluated. The Children's Society; London: 1999. [Google Scholar]
- 4.Shrimpton R, Victora CG, de Onis M, Lima RC, Blossner M, Clugston G. Worldwide timing of growth faltering: implications for nutritional interventions. Pediatrics. 2001 May;107(5):E75. doi: 10.1542/peds.107.5.e75. [DOI] [PubMed] [Google Scholar]
- 5.Mei Z, Grummer-Strawn LM, Thompson D, Dietz WH. Shifts in percentiles of growth during early childhood: analysis of longitudinal data from the California Child Health and Development Study. Pediatrics. 2004 Jun;113(6):e617–27. doi: 10.1542/peds.113.6.e617. [DOI] [PubMed] [Google Scholar]
- 6.Wolf L, Glass R. Feeding and Swallowing Disorders in Infancy. Therapy Skill Builders; Tucson, AZ: 1992. [Google Scholar]
- 7.Arvedson J. Oral-motor and feeding assessment. In: Arvedson J, Brodsky L, editors. Pediatric swallowing and feeding: assessment and management. Singular Publishing Group, Inc.; San Diego: 1993. pp. 249–92. [Google Scholar]
- 8.Botton J, Heude B, Maccario J, Ducimetiere P, Charles MA. Postnatal weight and height growth velocities at different ages between birth and 5 y and body composition in adolescent boys and girls. Am J Clin Nutr. 2008 Jun;87(6):1760–8. doi: 10.1093/ajcn/87.6.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stettler N, Stallings VA, Troxel AB, Zhao J, Schinnar R, Nelson SE, et al. Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Circulation. 2005 Apr 19;111(15):1897–903. doi: 10.1161/01.CIR.0000161797.67671.A7. [DOI] [PubMed] [Google Scholar]
- 10.Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002 Feb;109(2):194–9. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 11.Corkins MR, Lewis P, Cruse W, Gupta SK, Fitzgerald JF. Accuracy of infant admission lengths. Pediatrics. 2002 June;109(6):1108–11. doi: 10.1542/peds.109.6.1108. [DOI] [PubMed] [Google Scholar]
- 12.Johnson TS, Engstrom JL, Gelhar DK. Intra- and interexaminer reliability of anthropometric measurements of term infants. J Pediatr Gastroenterol Nutr. 1997 May;24(5):497–505. doi: 10.1097/00005176-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 13.WHO An evaluation of infant growth: the use and interpretation of anthropometry in infants. Bulletin of the World Health Organization. 1995;73(2):165–74. [PMC free article] [PubMed] [Google Scholar]
- 14.Polhamus B, Dalenius K, Thompson D, Scanlon KS, Borland E, Smith BA, et al. Pediatric Nutrition Surveillance 2002 Report. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta: 2004. [Google Scholar]
- 15.Hosmer D, Lemeshow S. Applied Logistic Regression. 2nd ed. John Wiley & Sons, Inc.; New York: 2000. [Google Scholar]
- 16.Lipman TH, Hench KD, Benyi T, Delaune J, Gilluly KA, Johnson L, et al. A multicentre randomised controlled trial of an intervention to improve the accuracy of linear growth measurement. Arch Dis Child. 2004 Apr;89(4):342–6. doi: 10.1136/adc.2003.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Onis M, Garza C, Onyango AW, Borghi E. Comparison of the WHO Child Growth Standards and the CDC 2000 Growth Charts. J Nutr. 2007 Jan;137(1):144–8. doi: 10.1093/jn/137.1.144. [DOI] [PubMed] [Google Scholar]
- 18.Olsen EM, Petersen J, Skovgaard AM, Weile B, Jorgensen T, Wright CM. Failure to thrive: the prevalence and concurrence of anthropometric criteria in a general infant population. Arch Dis Child. 2007 Feb;92(2):109–14. doi: 10.1136/adc.2005.080333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rifas-Shiman SL, Rich-Edwards JW, Scanlon KS, Kleinman KP, Gillman MW. Misdiagnosis of overweight and underweight children younger than 2 years of age due to length measurement bias. MedGenMed. 2005;7(4):56. [PMC free article] [PubMed] [Google Scholar]
- 20.de Meer K, Heymans HS, Zijlstra WG. Physical adaptation of children to life at high altitude. Eur J Pediatr. 1995 Apr;154(4):263–72. [PubMed] [Google Scholar]
- 21.Dewey KG. Growth characteristics of breast-fed compared to formula-fed infants. Biol Neonate. 1998;74(2):94–105. doi: 10.1159/000014016. [DOI] [PubMed] [Google Scholar]
- 22.Dewey KG, Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B. Growth of breast-fed and formula-fed infants from 0 to 18 months: the DARLING Study. Pediatrics. 1992 Jun;89(6 Pt 1):1035–41. [PubMed] [Google Scholar]
- 23.Krebs NF, Reidinger CJ, Robertson AD, Hambidge KM. Growth and intakes of energy and zinc in infants fed human milk. J Pediatr. 1994 Jan;124(1):32–9. doi: 10.1016/s0022-3476(94)70251-9. [DOI] [PubMed] [Google Scholar]
- 24. http://abbottnutrition.com/resources/en-US/news_and_media/media_center/BF_Trends_2003.pdf. Mothers Survey, Ross Products Division of Abbott. 2007; Available from: http://abbottnutrition.com/resources/en-US/news_and_media/media_center/BF_Trends_2003.pdf.
- 25.Huang JS, Becerra K, Oda T, Walker E, Xu R, Donohue M, et al. Parental ability to discriminate the weight status of children: results of a survey. Pediatrics. 2007 Jul;120(1):e112–9. doi: 10.1542/peds.2006-2143. [DOI] [PubMed] [Google Scholar]
- 26.Kelleher KJ, Casey PH, Bradley RH, Pope SK, Whiteside L, Barrett KW, et al. Risk factors and outcomes for failure to thrive in low birth weight preterm infants. Pediatrics. 1993 May;91(5):941–8. erratum appears in Pediatrics 1993 Jul;92(1):190. [PubMed] [Google Scholar]
- 27.Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: growth and associated problems in children born at 25 weeks of gestational age or less. Arch Dis Child Fetal Neonatal Ed. 2003 Nov;88(6):F492–500. doi: 10.1136/fn.88.6.F492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Semin Perinatol. 2003 Aug;27(4):302–10. doi: 10.1016/s0146-0005(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 29.Kelleher KJ, Casey PH, Bradley RH, Pope SK, Whiteside L, Barrett KW, et al. Risk factors and outcomes for failure to thrive in low birth weight preterm infants. Pediatrics. 1993 May;91(5):941–8. [PubMed] [Google Scholar]
- 30.Cerro N, Zeunert S, Simmer KN, Daniels LA. Eating behaviour of children 1.5-3.5 years born preterm: parents' perceptions. J Paediatr Child Health. 2002 Feb;38(1):72–8. doi: 10.1046/j.1440-1754.2002.00728.x. [DOI] [PubMed] [Google Scholar]
- 31.Boddy JM, Skuse DH. The process of parenting in failure to thrive. Journal of Child Psychology & Psychiatry & Allied Disciplines. 1994;35(3):401–24. doi: 10.1111/j.1469-7610.1994.tb01731.x. [DOI] [PubMed] [Google Scholar]