Abstract
Burkitt lymphoma (BL) is a highly aggressive non-Hodgkin lymphoma (NHL) with endemic, sporadic and immunodeficiency-associated clinical variants composed of monomorphic medium-size B-cells with a high proliferation rate and a translocation involving the C-MYC locus. Classically the immunophenotype of Burkitt lymphoma has been considered to be of germinal center type. In most reports, all cases of BL are reported to be MUM1 negative. MUM1 expression is seen in plasma cells and in a small fraction of B cells located in the light zone of germinal centers corresponding to the final step of intra-germinal center (GC) B-cell differentiation, and in activated T-cells. Therefore, MUM1 expression may denote the final step of intra-GC B-cell differentiation at centrocyte stage, as well as the subsequent steps of B-cell maturation towards plasma cells. Unlike most normal GC B-cells, in which the expression of MUM1 and bcl-6 are mutually exclusive, the tumor cells in approximately 50% of MUM1 positive DLBCL show co-expression of bcl-6, suggesting that the expression of these proteins may be deregulated. In one of the few studies in the literature, 25 BL-cases, including 19 associated with HIV; two of these cases showed occasional MUM1+ cells, less than the 20% cut-off for positivity. We studied 222 cases of well-characterized Burkitt lymphoma with the classic phenotype and C-MYC translocation, and found 90 cases (40.5%) with MUM1 nuclear expression suggesting a late germinal center stage of differentiation.
Keywords: Burkitt lymphoma, MUM1/IRF4, TMA, immunohistochemistry, EBV, transcription factor
Introduction
Burkitt lymphoma (BL) is a highly aggressive non-Hodgkin lymphoma (NHL) with endemic, sporadic and immunodeficiency-associated clinical variants composed of monomorphic medium-size B-cells with a high proliferation rate (1). Morphologically, in addition to classical BL, there are two cytological variants: BL with plasmacytoid differentiation and atypical BL (aBL), the latter showing greater pleomorphism in nuclear size and shape than the classical type. Immunophenotypically all types are similar, expressing CD20, CD10, bcl-6, membranous IgM but not IgD, bcl-2, or terminal deoxynucleotidyl transferase (TdT). The Ki-67 proliferation index (PI) is nearly 100%. Cytogenetically, all cases have a translocation involving the C-MYC gene at 8q24 with the immunoglobulin heavy chain (IGH) gene on 14q32 or less commonly with the kappa light chain locus (IGK) at 2q11 or the lambda light chain locus (IGL) at 22q11 (2).
MUM1/IRF4, multiple myeloma-1/interferon regulatory factor 4 protein, is a lymphocyte-specific member of the interferon regulatory factor family of transcription factors (3), and is encoded by the MUM1/IRF4 gene, which has been identified as a myeloma-associated oncogene activated at the transcriptional level as a result of t(6;14) (p25;q32) (4). MUM1 is induced by antigen receptor-mediated stimuli and plays a crucial role in cell proliferation, differentiation and survival (5). Loss of MUM1 function results in the absence of activated lymphoid cells and Ig-secreting plasma cells. Tsuboi et al (6) demonstrated MUM1 expression in plasma cells and in a small fraction of B cells located in the light zone of germinal centers corresponding to the final step of intra-germinal center (GC) B-cell differentiation, and in activated T-cells. Therefore, MUM1 expression may denote the final step of intra-GC B-cell differentiation at the centrocyte stage, as well as subsequent steps of B-cell maturation towards plasma cells. Inside the GC, the centrocytes are the first cells to express MUM1, and this expression is maintained during post-GC maturation, in contrast to bcl-6 expression, which is observed immediately after the B-cell enters the GC and is maintained only until GC exit. Thus MUM1 is considered to be a histogenetic marker of the late-GC and post- GC B-cell (7, 8), and the morphologic spectrum of positive cells ranges from that of a centrocyte to that of a plasmablast/plasma cell. In a PCR analysis of single MUM1+ cells from GC, Falini et al (9) demonstrated that they contained rearranged Ig heavy chain genes with a varying number of VH somatic mutations, suggesting that MUM1 cells represent surviving centrocytes whose progeny is committed to exit the GC and to differentiate into plasma cells. Unlike most normal GC B-cells, in which the expression of MUM1 and bcl-6 are mutually exclusive, the tumor cells in approximately 50% of MUM1 positive DLBCL show co-expression of bcl-6, suggesting that the expression of these proteins may be deregulated (9, 10). One of the first well-characterized transcription factors found to be involved in regulating cell proliferation and growth was c-myc; its repression is required for normal plasma cell differentiation (11). Deregulation of C-MYC prevents B-cell differentiation and it is an oncogenic hallmark in Burkitt lymphoma (12, 13).
Among neoplastic B-cell lymphoproliferations, MUM1 expression is observed in multiple myeloma and lymphoplasmacytic lymphoma, many cases of DLBCL (75%), some cases of chronic lymphocytic leukemia and marginal zone lymphomas, most cases of classical Hodgkin lymphoma, in the setting of the acquired immunodeficiency syndrome and in post-transplant lymphoproliferative disorders. In most reports, there are limited numbers of BL cases included, and all cases of BL are reported to be MUM1 negative (6, 9, 14, 15). Carbone et al (16) studied 19 BL-cases associated with HIV; two of these cases showed occasional MUM1+ cells, less than their 20% cut-off for positivity. Among Burkitt cell lines MUM1 expression has been observed in the Ramos but not in the Daudi line, both lines being bcl-6+ (10). Chuang et al (2) described MUM1 expression in 5 of 28 cases of BL, without specifying the criteria and cut-off for considering a case positive.
In the present study we analyzed the expression pattern of MUM1, bcl-6 and CD138 (syndecan-1) along with the association of EBV in 222 well-characterized cases of BL.
Materials and methods
Case material and clinical data
A total of 222 cases of BL, all showing C-MYC translocation by FISH, were included in the present study. These cases were part of 595 cases with tentative diagnosis of BL obtained retrospectively from the files of Consultoria em Patologia, a large reference consultation service in anatomic pathology located in Botucatu, Sao Paulo State, Brazil between June 1997 and December 2007. The study group cases (222 cases) had the diagnosis of BL confirmed by morphological, immunohistochemical and molecular analysis. Both nodal and extra-nodal BL cases were included. Clinical data including gender, age at diagnosis and anatomic location were obtained from the referring pathologists/oncologists and/or pathology reports. Available hematoxylin and eosin stained slides of each case were reviewed by three of the authors (GG, EMQ, CEB) and representative areas were selected for TMA. A morphologic subclassification of the cases was performed, considering variants included in WHO classification (1).
Tissue microarray construction
Eight tissue microarray (TMA) blocks were constructed, using a tissue arrayer (Beecher Instruments, Sun Prairie, WI, USA). Each individual case was represented by three tumor cores of 0.6 mm that had been taken from the original paraffin blocks. Serial sections of 3μm were cut from the tissue array blocks and used for the immunohistochemical analysis. Proper positive and negative controls cores for each marker were also included in the array block to provide an assessment of the adequacy of the antibodies used in the immunohistochemical study, including tonsil (CD20, CD3, CD10, bcl-2, bcl-6, Ki67, MUM-1, CD138), EBV-positive Hodgkin lymphoma (EBER), and lymphoblastic lymphoma (TdT).
Immunohistochemistry and in situ hybridization
Immunohistochemical studies were performed on each TMA using the Novolink polymer® (Novocastra, Newcastle Upon Tyne, UK) as the detection system, and an epitope-retrieval method was applied as needed for each specific antibody; diaminobenzidine (DAB) was the chromogen. The primary antibodies used were CD20, CD3, CD10, CD138, bcl-6, MUM1, Ki-67, TdT and bcl-2 (Table 1). MUM-1 was considered positive above a 20% cut-off of nuclear staining. Sections from all TMA were examined for the expression of the Epstein-Barr viral RNA by in situ hybridization using the EBER-1 probe, as previously described (17).
Table 1.
Primary antibodies used for the immunohistochemical staining
| Antigen | Clone | Dilution | HIER | Source |
|---|---|---|---|---|
| CD20 | L26 | 1:1200 | MW CB | Dako |
| CD3 | SP7 | 1:200 | S CB | Lab Vision |
| CD10 | 56C6 | 1:100 | S CB | Novocastra |
| CD138 | B-B4 | 1:800 | PC CB | Invitrogen |
| BCL-2 | 124 | 1:400 | MW CB | Dako |
| BCL-6 | PG-B6P | 1:100 | T+ S TRIS | Dako |
| MUM-1/IRF4 | MUM1P | 1:1200 | S CB | Dako |
| Ki-67 | MIB-1 | 1:4800 | PC CB | Dako |
| TdT | Polyclonal | 1:1600 | PC EDTA | Dako |
Dako, Carpinteria, CA, USA; Lab Vision Corporation, Fremont, CA, USA; Novocastra, United Kingdom; Invitrogen, Carlsbad, CA, USA; HIER: Heat-induced epitope retrieval; MW-microwave oven, PC-pressure cooker, S-steamer, T-trypsin and CB-citrate buffer
Fluorescence in situ hybridization (FISH)
Locus-specific interphase FISH for translocations involving the C-MYC locus was performed in each case using a 3μm-thick tissue section of each array block as previously described (2). For the detection of breakpoints (splits) in the C-MYC locus a LSI MYC Dual Color Break-apart Rearrangement probe (Vysis, Abbott AG, USA) was applied. This probe hybridizes to the band region 8q24 and it is a mixture of two separate probes: 1. a 3′ SpectrumGreen that starts approximately 1 Mb 3′of the MYC gene, extends toward the telomere for about 400kb probe and contains the vast majority of breakpoints reported for t(8,22)(q24;q11) and t(2;8)(p11;q24); and 2. a 5′ SpectrumOrange probe that begins upstream of the 5′ end of MYC and extends 260kb toward the centromere. The slides were evaluated using spectrum orange and spectrum green filters (Chroma Technology GmbH, Fuerstenfeldbruck, Germany) on a Zeiss Axio Imager M1 fluorescence microscope (Carl Zeiss AG, Germany) and the assistance of Isis FISH Imaging Software (Metasystems, Altlussheim, Germany). Using an extended focus/tile sampling methodology, tiles with distant unpaired signals (≥ 10 pixels in distance) were considered to be positive and the percentage of tiles containing positive signals was calculated. The threshold for positivity was established from a group of immunophenotypically characterized samples (tonsils), which did not contain the translocation of interest. A positive case was defined when the mean number of positive tiles detected was 3 standard deviations above the mean of this negative control group. This threshold established was 2.19% (the mean of this negative control group was 0.73%).
Results
Of the 222 BL cases, 165 (74%) were male and 57 (26%) female. 134 cases (60%) were children 16 or less years old and 82 cases (37%) were adults. In 6 cases the age was unknown. The median age was 19 years (range 1 to 81). Extranodal BL were 69% of the cases (148 cases), primary lymph node involvement was observed in 31% of the cases (66 cases), in 8 cases it was not possible to determine nodal vs. extranodal presentation. In the pediatric population, primary extranodal disease was observed in 104 cases (78%); in contrast, the adult population had an extranodal presentation in 42 (51%) of the cases.
Morphologic features
In the original material reviewed to select the areas for TMA, 96% of BL showed diffuse architecture and rare cases exhibit a focal nodular pattern (2 cases); most of the cases were classic type (91%). Morphologic variants were distributed as follows: 3% (6 cases) of BL showed plasmacytoid differentiation and 6% (14 cases) showed features of atypical BL.
Immunohistochemistry, FISH, and EBV in situ hybridization
All cases had an immunophenotype consistent with BL according to the WHO classification, i.e., CD20+, CD3-, bcl-6+/-, CD10+, Ki-67>95% and bcl-2 negative. In addition, all cases were positive for C-MYC translocation, as detected by the FISH study using the C-MYC breakapart probe. Table 2 summarizes the results of MUM1 expression in relation to all the other parameters evaluated in this study. MUM1 expression was observed in 90 cases (41%), with moderate or strong nuclear positivity in the majority of the neoplastic cells in all MUM1-positive cases (Figure 1); these cases showed a similar frequency in pediatric and adult populations, 43% and 38% respectively. MUM1 positive cases were extranodal in 64 cases (72%), nodal in 23 cases (26%); the anatomical presentation was unknown in 3 cases. MUM1 positive cases represented 45% and 35% of the cases of extranodal and nodal BL, respectively. MUM1 positivity was found in 57% of cases of aBL and 33% of cases of BL with plasmacytoid differentiation. Bcl-6 positivity occurred in 192 cases (87%). Expression of MUM1 was observed in 76 of the bcl-6 positive cases, representing 40% of bcl-6 positive cases and 34% of all the cases. It is worth noting that only 14 cases (6%) showed a MUM1+/bcl-6- phenotype (post-GC phenotype) and 12 cases (5%) showed a double negative “mantle zone cell phenotype”. A GC-phenotype (MUM1- negative/bcl- 6 +) was observed in 116 cases, 52.2% of the cases. In 76 (34%) cases all three markers (CD10, bcl-6, MUM1) were positive.
Table 2.
Distribution of MUM1 positive and negative cases in relation with other parametersanalysed in 222 Burkitt Lymphomas.
| MUM1 status | Nodal | Extranodal | Pediatric | Adult | EBV+ | EBV- | Bcl6+ | Bcl6- | Total |
|---|---|---|---|---|---|---|---|---|---|
| MUM1+ | 23(35) | 64(43)* | 57(43) | 31(38)** | 32(86) | 60(57) | 76(40) | 14(54) | 90 |
| MUM1- | 43(65) | 84(57)* | 77(57) | 51(62)** | 82(72) | 46(43)*** | 116(60) | 12(46)*** | 132 |
| 66(100) | 148(100) | 134(100) | 82(100) | 114(100) | 106(100) | 192(100) | 26(100) | 222 | |
(%) of each column
Three MUM1+-cases and five MUM1-negative cases had no anatomical origin information
Three MUM1 positive-cases and three other MUM1 negative cases had no age information
4 MUM1-negative cases were inconclusive for bc1-6 and two were inconclusive for ISH for EBV
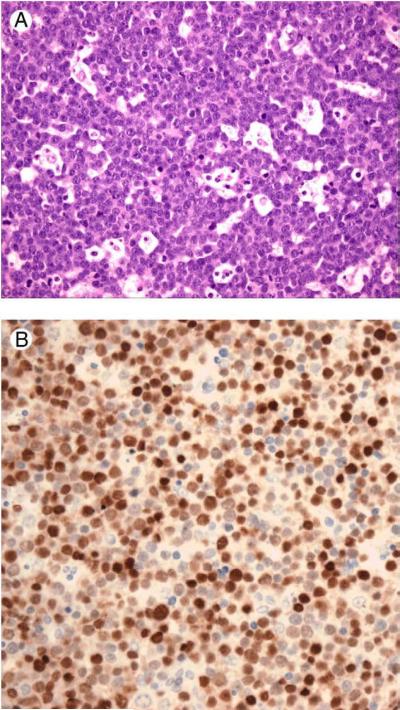
Figure 1.
A. Classical Burkitt lymphoma with a starry sky pattern. Hematoxilin&Eosin, (200X). B. Immunostaining for MUM1 show intense nuclear positivity in large percentage of the cells, (200X).
CD138 positive neoplastic cells were observed only in three cases; none of these cases showed MUM1 expression. ISH for EBV showed 114 positive cases (51%), the majority belonging to the pediatric population, 59%. In the pediatric group there were 77 EBV positive cases, representing 57% of this age group; and in the adult group positivity for EBV was seen in 35 cases, 43% of this age population.
Co-expression of MUM1 and EBV was found in 32 cases (28% of the EBV positive cases). Co-expression of bcl-6, MUM1 and EBV ISH was observed in only 24 patients, six adults and 18 children. Most of the EBV-positive cases belonged to MUM-1-negative/Bcl6+ group, (75 cases, 65% of this phenotypic group). EBV+/MUM-1+ cases were distributed as follows: 24 within the MUM-1+/Bcl-6+ group (late GC-like) and 8 cases in the MUM-1+/Bcl-6 negative (post GC) group, as seen in Table 3. All cases were bcl-2 negative.
Table 3.
Distribution of all EBV positive BL cases according to their phenotype.
| Phenotype | GC (MUM1-/BCL6+) | Late-GC (MUM1+/BCL6+) | Post-GC (MUM1+/BCL6-) | Mantle cell-like (MUM1-/BCL6-) |
|---|---|---|---|---|
| No of cases(Total EBV+ =114) | 75 | 24 | 8 | 7 |
| % in each group | 65% | 32% | 57% | 58% |
GC- germinal center.
Discussion
Our BL cases showed a male and pediatric predominance, a high frequency of extranodal presentation, with lymph node involvement more common among adults than among children, in concordance with the literature (1, 18, 19, 20).
From a morphological point of view, most of the cases were classic BL (91%), with the two variants, plasmacytoid and atypical found in 3 and 6% of cases, respectively (18). BL is a B-cell lymphoma that shows clonally rearranged immunoglobulin genes and carries a high load of somatic mutations consistent with a germinal center stage of differentiation, besides the characteristic C-MYC translocation (21, 22).
A clear-cut distinction between BL and other high-grade lymphomas, based on the current WHO classification, may be problematic in some cases. This is particularly well-documented by the low reproducibility rate of 53% for the diagnosis of BL and aBL (22). In gene expression profile studies a genomic molecular definition for BL was described (21, 22), and it was demonstrated that the current pathological criteria for distinguishing BL from DLBCL are often inadequate. In studies of DLBCL and BL subjected to immunophenotyping panels, including markers of GC (CD10, bcl-6) and activated-B-cells (ABC) (bcl-2, CD44, CD138, MUM1) type differentiation, hierarchical clustering has yielded two major groups: one with a high CG/low ABC score, that tends to include the lymphomas morphologically interpreted as BL and a second group with a low GC/high ABC score that includes DLBCL. However there is a continuum of expression of GC-markers and ABC-markers, with no distinct separation of the two groups, suggesting there may be a true biological continuum between BL and DLBCL (23, 24). Similarly, all BL harbor a C-MYC translocation, but 5-15% of DLBCL also may have a C-MYC rearrangement (18, 19, 23).
The MUM1/IRF4 gene encodes a transcription factor thought to play a key role in lymphoid development, since IRF4-deficient mice have a block in peripheral B-cell maturation with absence of GC and plasma cells, associated with a lack of a cytotoxic T-cell response (25). In normal tissues MUM1 protein is detected mainly in plasma cells and in small number of GC-B-cells found in the light zone of GC. The post-germinal phenotype MUM1+/bcl-6-/Ki67- differs from that of most GC cells (MUM1-/bcl-6+/Ki67+) and mantle cells (MUM1-/bcl-6-/Ki67-). In addition MUM1 is expressed in a small percentage of T-cells (9). In lymphoid neoplasms, in accordance with its expression at late stages of B-cell differentiation, and after T-cell activation, MUM1 protein is strongly expressed in lymphoplasmacytic lymphoma, myeloma, DLBCL, primary effusion lymphoma, Hodgkin lymphoma and anaplastic large cell lymphoma (7, 10). Falini and coworkers (10, 26) demonstrated that in normal tissues MUM1 positive GC-cells fail to express bcl-6 protein; but the same authors found that 50% of MUM1-positive DLBCL co-express bcl-6 (23/30).
Bcl-6 protein is a POZ/zinc finger transcription repressor and is required for GC formation and T-helper-2 mediated responses. Bcl-6 and MUM1 proteins have been used as phenotypic markers for the characterization of B-cell maturation and for lymphoma histogenesis (9, 26, 27). Expression of bcl-6 is restricted to GC-B-cells (14), and MUM1 marks the final step of intra-GC differentiation and subsequent early post-GC events. Bcl-6 expression in BL has been observed in 70 to 100% of pediatric cases and 70 to 82% of adult cases (20, 24).
In our cases of BL we found MUM1 and bcl-6 positivity in 41% and 87%, respectively. In our multi-marker comparison, we found 52% of cases of BL to have a MUM1 negative/bcl-6 positive GC-like phenotype; on the other hand 34% showed a MUM1 positive/bcl-6 positive phenotype, consistent with a late GC phenotype, 6% of the cases showed a MUM1 positive/bcl-6 negative post-GC phenotype and 5.4% showed a double-negative mantle B-cell immunophenotype. The MUM1 positive/bcl-6 positive phenotype observed in 34% of the cases suggests an intermediate pathologic stage of differentiation because this profile that does not correspond to the differentiation immunophenotype of normal B-cells, suggesting that the differentiation process of BL cells is not complete in a proportion of the cells (28) or is in evolution at the time of the biopsy. It is interesting to note that in studies using gene-expression microarray technology, Dave et al (22) and Hummel et al (21) reported a genetic signature for BL that clearly distinguishes this type of tumor from cases of DLBCL; but Hummel et al. found 22% of their cases had an intermediate signature, including cases diagnosed as aBL.
Our study involving 222 cases of BL demonstrates that 41% of the cases express MUM1, with a similar proportion of pediatric and adult patients, and with frequent extranodal presentation. This large series of BL contrasts with the findings described by other groups, many of them stating that BL is a MUM1 negative lymphoma (15); most of these papers refer to less than 5 cases (8, 9, 10, 14). In 19 cases Carbone et al (16) found 2 BL-cases MUM1 + in occasional cells, corresponding to 11% of cases and Chuang et al (2) found 5 of 28 BL with MUM-1 expression, (17.8%).
In other B-cell lymphomas MUM1 is positive in a variable proportion of cases. In follicular lymphoma, the rate of positivity ranges 0% to 37%, being more frequent in high-grade cases. In DLBCL, the rate of positivity is between 50 to 75%, including around 100% in DLBCL associated with HIV, while immunoblastic/plasmablastic myeloma, ALCL and HL have an almost 100% rate of positivity.
It is well established that there is a significant association of BL with EBV with a variable frequency, depending on the clinicopathologic variant. EBV is present in the majority of endemic cases of BL, and nearly 30% of cases of sporadic BL (1, 29). In Brazil a high association of EBV with BL has been demonstrated in tropical areas, especially in the Northeast region (30, 31, 32). With respect to immunoglobulin gene analysis, Bellan et al (33) revealed two distinct cells of origin for EBV positive and EBV negative BL; EBV negative-BL has a lower average mutation frequency, indicating an origin in the early centroblast for sporadic BL, whereas EBV positive-BL has a much higher mutation frequency, corresponding to the late GC-B-cells that have begun the differentiation process into memory B-cells, mostly seen in endemic and AIDS-related BL. One may hypothesize that the higher frequency of MUM-1 positive BL cases may be related with the relatively high frequency of EBV infection found in our population. We found evidence of EBV infection in 51% of the cases, including 60% in the pediatric population and 44% in adults, an intermediate value between EBV frequency reported in well-developed and African countries (34). When the distribution of EBV positive cases was correlated with cell phenotype, the late CG group was less associated with EBV infection in comparison with post-GC B-cell phenotype and GC-B-cell type groups, MUM1 was expressed in only 32 EBV-positive cases, so EBV infection seems not to be the only factor explaining this higher MUM-1 expression.
Acknowledgments
We acknowledge the outstanding service of Consultoria em Patologia staff for skillful technical assistance.
Partially supported by NCI 5R01CA082274, 5R01CA112217 (WH) and the (NCI) AIDS Malignancy Consortium
References
- 1.Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon: 2001. World Health Organization Classification of Tumours. [Google Scholar]
- 2.Chuang S, Ye H, Du MQ, et al. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern. Am J Clin Pathol. 2007;128:558–64. doi: 10.1309/EQJR3D3V0CCQGP04. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen H, Hiscott J, Pitha P. The growing family of interferon regulatory factors. Cytok Growth Factor Rev. 1997;8:293–312. doi: 10.1016/s1359-6101(97)00019-1. [DOI] [PubMed] [Google Scholar]
- 4.Iida S, Rao P, Butler M, et al. Deregulation of MUM1/IRF4 by chromosomal translocation in multiple myeloma. Nat Genet. 1997;17:226–30. doi: 10.1038/ng1097-226. [DOI] [PubMed] [Google Scholar]
- 5.Naresh KN. MUM1 expression dichotomizes follicular lymphoma into predominantly, MUM1-negative low-grade and MUM1-positive high-grade subtypes. Haematologica. 2007;92(2):267–8. doi: 10.3324/haematol.10682. [DOI] [PubMed] [Google Scholar]
- 6.Tsuboi K, Iida S, Inagaki H, et al. MUM1/IRF4 expression as a frequent event in mature lymphoid malignancies. Leukemia. 2000;14(3):449–56. doi: 10.1038/sj.leu.2401696. [DOI] [PubMed] [Google Scholar]
- 7.Gaidano G, Carbone A. MUM1: a step ahead toward the understanding of lymphoma histogenesis. Leukemia. 2000;14(4):563–6. doi: 10.1038/sj.leu.2401748. [DOI] [PubMed] [Google Scholar]
- 8.Carbone A, Gloghini A, Cozzi MR, et al. Expression of MUM1/IRF4 selectively clusters with primary effusion lymphoma among lymphomatous effusions: implications for disease histogenesis and pathogenesis. Br J Haematol. 2000;111(1):247–57. doi: 10.1046/j.1365-2141.2000.02329.x. [DOI] [PubMed] [Google Scholar]
- 9.Falini B, Mason DY. Proteins encoded by genes involved in chromosomal alterations in lymphoma and leukemia: clinical value of their detection by immunocytochemistry. Blood. 2002;99(2):409–426. doi: 10.1182/blood.v99.2.409. [DOI] [PubMed] [Google Scholar]
- 10.Falini B, Fizzotti M, Pucciarini A, et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood. 2000;95(6):2084–92. [PubMed] [Google Scholar]
- 11.Dang CV. C-Myc target genes involved in cell growth, apoptosis and metabolism. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin P, Medeiros LJ. High-grade B-cell lymphoma/leukemia associated with t(14;18) and 8q24/MYC rearrangement: a neoplasm of germinal center immunophenotype with poor prognosis. Haematologica. 2007;92(10):1297–301. doi: 10.3324/haematol.11263. [DOI] [PubMed] [Google Scholar]
- 13.Niller HH, Salamon D, Ilg K, et al. The in vivo binding site for oncoprotein c-Myc in the promoter for Epstein-Barr virus (EBV) encoding RNA (EBER) 1 suggests a specific role for EBV in lymphomagenesis. Med Sci Monit. 2003;9(1):1–9. [PubMed] [Google Scholar]
- 14.Natkunam Y, Warnke RA, Montgomery K, Falini B, van De Rijn M. Analysis of MUM1/IRF4 protein expression using tissue microarrays and immunohistochemistry. Mod Pathol. 2001;14(7):686–94. doi: 10.1038/modpathol.3880373. [DOI] [PubMed] [Google Scholar]
- 15.Ponzoni M, Arrigoni G, Doglioni C. New transcription factors in diagnostic hematopathology. Adv Anat Pathol. 2007;14(1):25–35. doi: 10.1097/PAP.0b013e31802f0495. [DOI] [PubMed] [Google Scholar]
- 16.Carbone A, Gloghini A, Larocca LM, et al. Expression profile of MUM1/IRF4, BCL-6, and CD138/syndecan-1 defines novel histogenetic subsets of human immunodeficiency virus-related lymphomas. Blood. 2001;97(3):744–51. doi: 10.1182/blood.v97.3.744. [DOI] [PubMed] [Google Scholar]
- 17.Bacchi M, Bacchi C, Alvarenga M, Miranda R, Chen Y, Weiss L. Burkitt's lymphoma in Brazil: strong association with Epstein-Barr virus. Mod Pathol. 1996;9:63–7. [PubMed] [Google Scholar]
- 18.Ferry JA. Burkitt's lymphoma: clinicopathologic features and differential diagnosis. Oncologist. 2006;11(4):375–83. doi: 10.1634/theoncologist.11-4-375. [DOI] [PubMed] [Google Scholar]
- 19.McClure RF, Remstein ED, Macon WR, et al. Adult B-cell lymphomas with Burkitt-like morphology are phenotypically and genotypically heterogeneous with aggressive clinical behavior. Am J Surg Pathol. 2005;29(12):1652–60. doi: 10.1097/01.pas.0000180442.87022.08. [DOI] [PubMed] [Google Scholar]
- 20.Harris NL, Horning SJ. Burkitt's lymphoma-the message from microarrays. N Engl J Med. 2006;354(23):2495–8. doi: 10.1056/NEJMe068075. [DOI] [PubMed] [Google Scholar]
- 21.Hummel M, Bentink S, Berger H, Klapper W, et al. A biologic definition of Burkitt's lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354(23):2419–30. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 22.Dave SS, Fu K, Wright GW, et al. Lymphoma/Leukemia Molecular Profiling Project. Molecular diagnosis of Burkitt's lymphoma. N Engl J Med. 2006;354(23):2431–42. doi: 10.1056/NEJMoa055759. [DOI] [PubMed] [Google Scholar]
- 23.Frost M, Newell J, Lones M, Tripp S, Cairo M, Perkins S. Comparative immunohistochemical analysis of pediatric Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Clin Pathol. 2004;121:384–92. doi: 10.1309/8WYN-VUTG-V9RP-HUQH. [DOI] [PubMed] [Google Scholar]
- 24.Gormley RP, Madan R, Dulau AE, et al. Germinal center and activated B-cell profiles separate Burkitt lymphoma and diffuse large B-cell lymphoma in AIDS and non-AIDS cases. Am J Clin Pathol. 2005;124(5):790–8. doi: 10.1309/7CEA-WV0D-NLLU-WQTF. [DOI] [PubMed] [Google Scholar]
- 25.Mittrücker HW, Matsuyama T, Grossman A, et al. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science. 1997;275(5299):540–3. doi: 10.1126/science.275.5299.540. [DOI] [PubMed] [Google Scholar]
- 26.Carbone A, Gloghini A, Aldinucci D, Gattei V, Dalla-Favera R, Gaidano G. Expression pattern of MUM1/IRF4 in the spectrum of pathology of Hodgkin's disease. Br J Haematol. 2002;117(2):366–72. doi: 10.1046/j.1365-2141.2002.03456.x. [DOI] [PubMed] [Google Scholar]
- 27.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 28.Valsami S, Pappa V, Rontogianni D, et al. A clinicopathological study of B-cell differentiation markers and transcription factors in classical Hodgkin's lymphoma: a potential prognostic role of MUM1/IRF4. Haematologica. 2007;92(10):1343–50. doi: 10.3324/haematol.11523. [DOI] [PubMed] [Google Scholar]
- 29.Cogliatti SB, Novak U, Henz S, Schmid U, Möller P, Barth TF. Swiss Group for Clinical Cancer Research (SAKK). Diagnosis of Burkitt lymphoma in due time: a practical approach. Br J Haematol. 2006;134(3):294–301. doi: 10.1111/j.1365-2141.2006.06194.x. [DOI] [PubMed] [Google Scholar]
- 30.Araujo I, Bittencourt AL, Barbosa HS, et al. The high frequency of EBV infection in pediatric Hodgkin lymphoma is related to the classical type in Bahia, Brazil. Virchows Arch. 2006;449(3):315–19. doi: 10.1007/s00428-006-0244-z. [DOI] [PubMed] [Google Scholar]
- 31.Araujo I, Foss HD, Bittencourt A, et al. Expression of Epstein-Barr virus-gene products in Burkitt's lymphoma in Northeast Brazil. Blood. 1996;87(12):5279–86. [PubMed] [Google Scholar]
- 32.Sandlund JT, Fonseca T, Leimig T, et al. Predominance and characteristics of Burkitt lymphoma among children with non-Hodgkin lymphoma in northeastern Brazil. Leukemia. 1997;11(5):743–46. doi: 10.1038/sj.leu.2400609. [DOI] [PubMed] [Google Scholar]
- 33.Bellan C, Lazzi S, Hummel M, et al. Immunoglobulin gene analysis reveled 2 distinct cells of origin for EBV-positive and EBV-negative Burkitt lymphoma. Blood. 2005;106:1031–36. doi: 10.1182/blood-2005-01-0168. [DOI] [PubMed] [Google Scholar]
- 34.Queiroga EM, Gualco G, Weiss LM, et al. Burkitt Lymphoma in Brazil is characterized by geographically distinct clinico-pathological features. Am J Clin Pathol. 2008 doi: 10.1309/AJCP64YOHAWLUMPK. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]