Abstract
HMGI-C belongs to the high-mobility-group-protein (HMG) family of architectural transcription factors and considerable interest has recently been shown in its expression in neoplastic tissues and apparent involvement in tumorigenesis. We could previously demonstrate an expression of HMGI-C mRNA in the peripheral blood of breast cancer patients for the first time. In this prospective study, we evaluated the independent prognostic power of HMGI-C mRNA expression in the peripheral blood of an unselected cohort of 69 patients with metastatic breast cancer using a hemi-nested reverse transcriptase polymerase chain reaction (RT–PCR) followed by sequence analysis of the resulting PCR products. Multivariate analysis was performed using the Cox regression model. HMGI-C mRNA was detected in peripheral blood from 21 out of 69 (30%) patients with metastatic breast cancer. Median survival was 15.9 months in patients expressing HMGI-C, while in the group of patients without HMGI-C expression the median survival had not been reached yet after a median follow-up of 24.7 months and 85.4% were still alive in this group. Disease-specific survival was significantly worse for patients positive for HMGI-C in comparison to those not expressing HMGI-C (P=0.0001). In a multivariate regression analysis, HMGI-C remained an independent prognostic factor for overall survival (P=0.001) besides oestrogen receptor status (P=0.024) and presence of metastases in liver and lungs (P=0.029). HMGI-C expression in the peripheral blood of patients with metastatic breast cancer is a powerful independent indicator for poor overall survival and this is the first study to demonstrate its prognostic relevance in univariate and multivariate analysis.
Keywords: HMGI-C, metastatic breast cancer, independent prognostic factor
Breast cancer is the most common malignancy affecting women. One area of intense breast cancer research has been in assessing prognostic factors of patient outcomes, and several molecular markers have been evaluated in association with established histologic and clinical prognostic parameters of breast cancer (Honkoop et al, 1998; Rizzieri et al, 1999; Sabbatini et al, 2000; Silva et al, 2001).
Considerable interest in HMGI proteins, a subfamily of the high-mobility-group-proteins, has been stimulated by observations that they are involved in the fundamental biological processes of cell proliferation and differentiationQ2 (Zhou and Chada, 1998). They serve as transcription factors and take part in the regulation of chromatin structure and function. HMGI proteins contain DNA binding domains and are involved in the assembly of the correct three-dimensional configuration of protein–DNA complexes (Tallini and Dal Cin, 1999). The HMGI family comprises three proteins: HMGI and HMGI(Y), which result from differential splicing from a single gene, and HMGI-C, which is encoded by a different gene, but shares some structural homologies with the former two (Bustin and Reeves, 1996; Goodwin, 1998; Chau et al, 1999; Tallini and Dal Cin, 1999). HMGI proteins facilitate gene activation through enhanceosome formation on inducible genes during embryonal development and within rapidly dividing cells. The family member HMGI-C was also shown to enhance the activity of the transcription factor NF-κB (Mantovani et al, 1998). The HMGI-C gene is normally almost exclusively expressed during embryonic development and in haematopoietic stem cells in adults (Rogalla et al, 1996). Experiments with knockout mice having both HMGI-C alleles disrupted led to a pygmy phenotype in these mice (Zhou et al, 1995).
An expression of antisense HMGI-C RNA was shown to prevent retrovirally induced neoplastic transformation in rat thyroid cells by Berlingieri et al (1995). These findings and studies on HMGI-C expression in a variety of tumours (Chiappetta et al, 1995; Kazmierczak et al, 1996,1998; Staats et al, 1996; Rogalla et al, 1997,1998; Rommel et al, 1997; Giannini et al, 1999; Klotzbucher et al, 1999) have substantiated the important role of HMGI-C in the control of cell growth, differentiation and tumorigenesis. It could also be detected in the peripheral blood of patients with leukaemia, but no HMGI-C expression could be found in the peripheral blood samples of healthy donors (Rommel et al, 1997; Sezer et al, 2000). We could recently demonstrate for the first time that HMGI-C is expressed in the peripheral blood of breast cancer patients and that this expression is restricted to patients with metastatic disease (Sezer et al, 2000).
The purpose of the present study was to analyse the correlation between HMGI-C expression in the peripheral blood of metastatic breast cancer patients and clinicopathologic characteristics and this is the first study to evaluate the prognostic relevance of HMGI-C expression for survival using univariate and multivariate analysis.
MATERIALS AND METHODS
Peripheral blood samples from an unselected cohort of 69 patients with metastatic breast cancer prior to the initiation of a chemotherapy were analysed in the present study. Institutional ethical approval was obtained and informed consent was given by the patients and healthy donors. Blood samples (5 ml) were immediately stabilised with DNA/RNA stabilisation reagent (Roche Diagnostics, formerly Boehringer Mannheim, Germany) after being drawn from the patient and processed according to the manufacturer's instructions.
Reverse transcription–polymerase chain reaction for assessing the expression of HMGI-C mRNA
Briefly, mRNA was obtained using an mRNA kit (mRNA Isolation Kit, Roche Diagnostics, formerly Boehringer Mannheim, Germany), cDNA was synthesised using the adapter primer (AP2) and Superscript II reverse transcriptase (RT) (Life Technologies, Eggenstein, Germany) and HMGI-C expression was determined using a hemi-nested reverse transcription–polymerase chain reaction (RT–PCR), as previously described (Rogalla et al, 1996; Sezer et al, 2000) and confirmed by DNA sequencing (ABI Prism DNA sequencer, Perkin-Elmer).
Statistical analysis
All statistical analyses were performed using SPSS software (Version 10.0, SPSS Inc, Chicago, IL, USA). Possible differences in clinicopathologic characteristics of the patient groups with and without circulating HMGI-C mRNA were analysed using exact χ2- and Mann–Whitney tests. The Kaplan–Meier method was used to estimate survival rates and log-rank tests were applied to compare survival curves between two groups. Cox multivariate regression analysis was used to assess the prognostic significances of the various factors for overall survival, with variables entering the model if changes in minus twice the log likelihood were statistically significant at the 5% significance level. Proportional hazard was tested with the log minus log plot.
Results
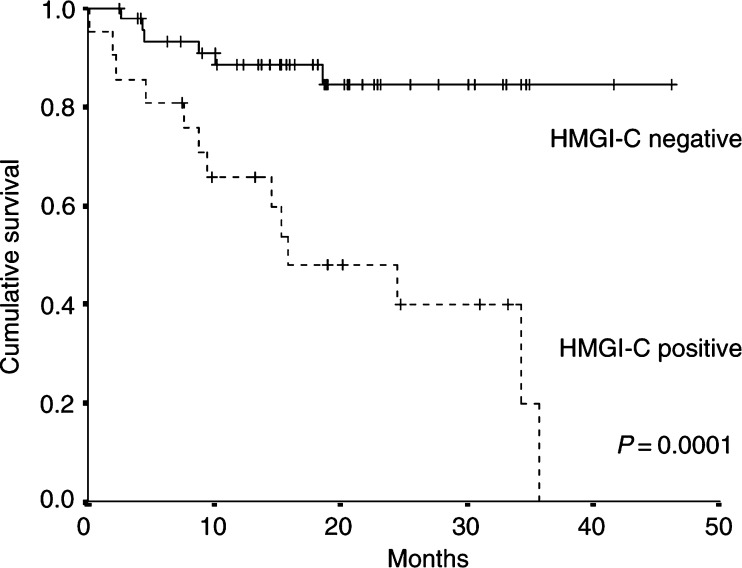
Expression of HMGI-C mRNA in the peripheral blood was detected in 21 (30.4%) of the 69 patients with metastatic breast cancer. Patient characteristics according to HMGI-C expression status are listed in Table 1 . There was no statistically significant difference between the group of patients positive for HMGI-C expression and the group of patients without detectable HMGI-C in terms of age, time from diagnosis to first metastatic event, menopausal status, histologic type, hormone receptor status and sites of metastases. The two groups did not differ with respect to having ever received chemotherapy, having received adjuvant or palliative chemotherapy, treatment with taxanes or anthracyclines, endocrine therapy or radiation therapy. Median length of follow-up was 24.7 months for all patients. Median survival was 15.9 months in patients expressing HMGI-C, 38% were still alive at the last follow-up contact, while in the group of patients without HMGI-C expression the median survival had not been reached yet, and 85.4% were still alive at last follow-up contact (P=0.0001). Kaplan–Meier analysis showed that patients with HMGI-C expression had statistically significant worse overall survival (P=0.0001) (Figure 1). Cox multivariate regression analysis performed after univariate analyses identified HMGI-C to be an independent poor prognostic factor for patient survival (P=0.001). In addition, positive oestrogen receptor status was significant for better overall survival (P=0.024) and presence of metastases in both liver and lung was significant for worse outcome (P=0.029) (Table 2 and Table 3 ).
Table 1. Association of HMGI-C expression with clinicopathologic characteristics of patients.
|
HMGI-C expression |
|||
|---|---|---|---|
| Variables | Positive (%) | Negative (%) | P-valuea |
| Age (years) | |||
| Mean±s.d. | 55.6±7.8 | 56.0±13.1 | 0.80 |
| Range | 39.9−71.9 | 32.1−85.1 | |
| Median | 55.3 | 58.4 | |
| Time from diagnosis to first metastatic event (months) | |||
| Mean±s.d. | 27.1±19.8 | 31.0±31.0 | 0.87 |
| Range | 0.0−85.0 | 0.0−120.0 | |
| Median | 23.0 | 25.5 | |
| Menopausal status | 0.18 | ||
| Premenopausal | 0 (0) | 5 (10.4) | |
| Postmenopausal | 19 (90.5) | 38 (79.2) | |
| Unknown | 2 (9.5) | 5 (10.4) | |
| Histology | 1.00 | ||
| Ductal carcinoma | 20 (95.2) | 45 (93.8) | |
| Lobular carcinoma | 1 (4.8) | 3 (6.2) | |
| Oestrogen-receptor status | 0.78 | ||
| Positive | 11 (52.4) | 27 (56.2) | |
| Negative | 8 (38.1) | 16 (33.3) | |
| Unknown | 2 (9.5) | 5 (10.4) | |
| Progesterone-receptor status | 0.79 | ||
| Positive | 8 (38.1) | 19 (39.6) | |
| Negative | 12 (57.1) | 24 (50.0) | |
| Unknown | 1 (4.8) | 5 (10.6) | |
| Site of metastases | |||
| Any visceral | 19 (90.5) | 39 (81.3) | 0.48 |
| Lung | 12 (57.1) | 25 (52.1) | 0.80 |
| Liver | 12 (57.1) | 20 (41.7) | 0.29 |
| Lung and Liver | 5 (23.8) | 8 (16.7) | 0.52 |
| Bone | 14 (66.7) | 21 (42.6) | 0.12 |
| Soft tissues | 5 (23.8) | 13 (27.1) | 1.00 |
| Chemotherapy | |||
| Ever | 16 (76.2) | 29 (60.4) | 0.28 |
| Adjuvant | 12 (57.1) | 20 (41.7) | 0.30 |
| Previous palliative | 7 (33.3) | 10 (20.8) | 0.36 |
| Anthracyclines | 9 (42.9) | 19 (39.6) | 1.00 |
| Taxanes | 7 (33.3) | 10 (20.8) | 0.37 |
| Endocrine therapy | 12 (57.1) | 29 (60.4) | 1.00 |
| Tamoxifen | 11 (52.4) | 26 (54.2) | 1.00 |
| Letrozole | 3 (14.3) | 13 (27.1) | 0.36 |
| Radiation therapy | 12 (57.1) | 22 (45.8) | 0.44 |
p-values for age and time from diagnosis to the first metastatic event were obtained using the Mann–Whitney test; other p-values were derived using the χ2-test.
Figure 1.
Kaplan–Meier curves for disease-specific survival of 69 metastatic breast cancer patients with detectable HMGI-C expression (HMGI-C positive, N=21) vs patients in whom HMGI-C could not be detected (HMGI-C negative, N=48) by RT–PCR in peripheral blood.
Table 2. Prognostic significance of HMGI-C mRNA in peripheral blood and other variables associated with survival as determined by univariate analysis.
| Variables | P-value |
|---|---|
| HMGI-C expression | 0.0001 |
| Oestrogen receptor status | 0.055 |
| Progesterone receptor status | 0.49 |
| Menopausal status | 0.39 |
| Liver and lung metastases | 0.10 |
| Sites of metastases >2 | 0.16 |
| Adjuvant chemotherapy | 0.20 |
Table 3. Multivariate analysis using the stepwise Cox regression model for overall survival, final model.
| Multivariate analysis | |||
|---|---|---|---|
| Variables | Hazard ratio | 95% CI | P-value |
| HMGI-C expression | 6.38 | 2.22–18.34 | 0.001 |
| Estrogen receptor positivity | 0.31 | 0.11–0.86 | 0.024 |
| Liver and lung metastases | 3.46 | 1.13–10.58 | 0.029 |
DISCUSSION
HMGI-C is an architectural transcription factor and plays a key role in important cellular processes such as DNA transcription in rapidly dividing embryonal cells and in neoplastic cells (Tallini and Dal Cin, 1999). It was found to be expressed in a variety of tumour tissues, but not in normal tissue adjacent to the tumour (Rogalla et al, 1996). In tumour tissues, a correlation between HMGI-C expression and grading has been reported, and in a study on HMGI-C expression in breast cancer tissues, HMGI-C was predominantly noted in tumours with high histological grade (Rogalla et al, 1997). Berlingieri et al (1995) demonstrated the appearance of a highly malignant phenotype in relation with HMGI-C expression in differentiated rat thyroid cells transformed with oncogenes. V-mos and v-ras-Ki oncogenes were implied to induce the synthesis of HMGI protein, and the block of this induction by the presence of an HMGI-C antisense construct could be shown to inhibit cell transformation. HMGI-C did not behave like a classical transforming oncogene though, since when transfected in normal thyroid cells, it did not cause their transformation, thus suggesting that its expression is necessary but not sufficient to achieve the transformed phenotype (Berlingieri et al, 1995). Neoplastic transformation was associated with a drastic increase in AP-1 activity, which was blocked by the suppression of HMGI protein synthesis. The absence of AP-1 transcriptional activity induction, directly or indirectly regulated by the HMGI proteins, would inhibit the expression of AP-1-dependent genes such as VEGF, collagenase I and stromelysin, which are required for neoplastic cell transformation (Vallone et al, 1997). It was noted that the high intracellular levels of HMGI proteins in transformed malignant cells are not simply the result of increased cellular proliferation. In fact, nontransformed cells proliferating at approximately the same rate as their transformed counterpart expressed consistently lower HMGI-C or HMGI(Y) (Tallini and Dal Cin, 1999). A recent study by Scala et al (2001) showed though, that in pygmy mice carrying a disrupted HMGI-C gene, induction of thyroid carcinomas with radiation or the E7 papilloma virus oncogene took place with the same frequency as in wildtype mice, therefore indicating that HMGI-C is not necessarily required for in vivo thyroid carcinogenesis (Scala et al, 2001). It was considered that HMGI(Y) proteins, rather than HMGI-C, may be required for thyroid cell transformation.
Similar observations as for HMGI-C concerning its expression in association with grade and stage of tumours were made for HMGI(Y), given its 50% amino-acid sequence homology with HMGI-C (Tallini and Dal Cin, 1999). In a study on colorectal neoplastic tissues a correlation could be found between an increased HMGI(Y) protein expression because of an increase in its mRNA and various clinicopathological parameters, known to be indicative of a poor prognosis (Abe et al, 1999). A significant correlation between HMGI(Y) mRNA expression and tumour grade and stage could be found in another study on prostate cancer (Tamimi et al, 1996). The above data demonstrate that HMGI-C and HMGI(Y) are important elements in tumorigenesis, while a precise definition of their role in tumour initiation and progression is still missing. So far studies on the expression of HMGI-C had focused on tumour tissues and cell lines and only one study was available on HMGI-C expression in the peripheral blood of leukemia patients, until we could recently demonstrate that HMGI-C is expressed in the peripheral blood of a subset of patients with breast cancer. This expression was restricted to patients with metastatic disease.
In the present study, we evaluated the prognostic relevance of HMGI-C expression in the peripheral blood of breast cancer patients with metastatic disease with respect to clinicopathologic parameters. We could show for the first time that HMGI-C expression is highly significant for worse outcome in univariate analysis, and we could furthermore demonstrate in multivariate analysis that circulating HMGI-C mRNA is a powerful independent prognostic indicator for overall survival. The significance of HMGI-C mRNA detection in peripheral blood, even allowing for the relatively small number of patients with metastatic breast cancer studied here, implies that this is a particularly strong prognostic factor. The multivariate analysis also showed that the presence of lung and liver metastases was significantly associated with worse outcome, which is consistent with previous studies, as is the fact that patients who were oestrogen receptor positive had significantly better overall survival (Rizzieri et al, 1999).
Reverse transcription–polymerase chain reaction of peripheral blood for HMGI-C mRNA, identifying those patients with metastatic breast cancer with an unfavourable prognosis, could be of diagnostic importance complementary to current methods for assessment of disease status and prediction of outcome, reflecting distinct biologic features of the disease. So far no expression of HMGI-C in the peripheral blood of healthy controls could be found, which is of advantage, since the specificity of other RT–PCR assays in breast cancer, such as RT–PCR for cytokeratin 19, had to be questioned due to an expression in healthy controls (Bostick et al, 1998; Slade et al, 1999).
The additional information gained by RT–PCR for HMGI-C may help to improve treatment strategies in allowing for a selection of breast cancer patients with metastatic disease with a worse prognosis. Further studies are needed to evaluate tailored therapy options for this risk group of patients with a particularly poor prognosis.
References
- Abe N, Watanabe T, Sugiyama M, Uchimura H, Chiapetta G, Fusco A, Atomi Y (1999) Determination of high mobility group I(Y) expression levels in colorectal neoplasias: a potential diagnostic marker. Cancer Res 59: 1169–1174 [PubMed] [Google Scholar]
- Berlingieri MT, Manfioletti G, Santoro M, Bandiera A, Visconti R, Giancotti V, Fusco A (1995) Inhibition of HMGI-C protein synthesis suppresses retrovirally induced neoplastic transformation of rat thyroid cells. Mol Cell Biol 15: 1545–1553 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bostick PJ, Chatterjee S, Chi DD, Huynh KT, Giuliano AE, Cote R, Hoon DS (1998) Limitations of specific reverse-transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol 16: 2632–2640 [DOI] [PubMed] [Google Scholar]
- Bustin M, Reeves R (1996) High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. Prog Nucl Acid Res Mol Biol 54: 35–100 [DOI] [PubMed] [Google Scholar]
- Chau K, Arlotta P, Patel UA, Crane-Robinson C, Manfioletti G, Ono SJ (1999) A novel downstream positive regulatory element mediating transcription of the human high-mobility-group-protein (HMGI-C) gene. FEBS Lett 457: 429–436 [DOI] [PubMed] [Google Scholar]
- Chiappetta G, Bandiera A, Berlingieri MT, Visconti R, Manfioletti G, Battista S, Martinez-Tello FJ, Santoro M, Giancotti V, Fusco A (1995) The expression of the high mobility group HMGI(Y) proteins correlates with the malignant phenotype of human thyroid neoplasias. Oncogene 10: 1307–1314 [PubMed] [Google Scholar]
- Giannini G, Di Marcotullio L, Ristori E, Zani M, Crescenzi M, Scarpa S, Piaggio G, Vacca A, Peverali FA, Diana F, Screpanti I, Frati L, Gulino A (1999) HMGI(Y) and HMGI-. Cancer Res 59: 2484–2492 [PubMed] [Google Scholar]
- Goodwin G (1998) Molecules in focus –the high mobility group protein HMGI-C. Int J Biochem Cell Biol 30: 761–766 [DOI] [PubMed] [Google Scholar]
- Honkoop AH, van Diest PJ, de Jong JS, Linn SC, Giaccone G, Hoekman K, Wagstaff J, Pinedo HM (1998) Prognostic role of clinical, pathological and biological characteristics in patients with locally advanced breast cancer. Br J Cancer 77: 621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak B, Bullerdiek J, Pham KH, Bartnitzke S, Wiesner H (1998) Intron 3 of HMGI-C is the most frequent target of chromosomal aberrations in human tumors and has been conserved basically for at least 30 million years. Cancer Genet Cytogenet 103: 175–177 [DOI] [PubMed] [Google Scholar]
- Kazmierczak B, Rosigkeit J, Wanschura S, Meyer-Bolte K, Van de Ven WJ, Kayser K, Krieghoff B, Kastendiek H, Bartnitzke S, Bullerdiek J (1996) HMGI-C rearrangements as the molecular basis for the majority of pulmonary chondroid hamartomas: a survey of 30 tumors. Oncogene 12: 515–521 [PubMed] [Google Scholar]
- Klotzbucher M, Wasserfall A, Fuhrmann U (1999) Misexpression of wild-type and truncated isoforms of the high mobility group I proteins HMGI-C and HMGI(Y) in uterine leiomyomas. Am J Pathol 155: 1535–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani F, Covaceuszach S, Rustighi A, Sgarra R, Heath C, Goodwin GH, Manfioletti G (1998) NF-kappaB mediated activation is enhanced by the architectural factor HMGI-C. Nucleic Acids Res 26: 1433–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzieri DA, Vredenburgh JJ, Jones R, Ross M, Shpall EJ, Hussein A, Broadwater G, Berry D, Petros WP, Gilbert C, Affronti ML, Coniglio D, Rubin P, Elkordy M, Long GD, Chao NJ, Peters WP (1999) Prognostic and predictive factors for patients with metastatic breast cancer undergoing aggressive induction therapy followed by high-dose chemotherapy with autologous stem-cell support. J Clin Oncol 17: 3064–3074 [DOI] [PubMed] [Google Scholar]
- Rogalla P, Drechsler K, Frey G, Hennig Y, Helmke B, Bonk U, Bullerdiek J (1996) HMGI-C expression patterns in human tissues –implications for the genesis of frequent mesenchymal tumors. Am J Pathol 149: 775–779 [PMC free article] [PubMed] [Google Scholar]
- Rogalla P, Drechsler K, Kazmierczak B, Rippe V, Bonk U, Bullerdiek J (1997) Expression of HMGI-C, a member of the high mobility group family, in a subset of breast cancers: relationship to histologic grade. Mol Carcinogen 19: 153–156 [DOI] [PubMed] [Google Scholar]
- Rogalla P, Drechsler K, Schröder-Babo W, Eberhardt K, Bullerdiek J (1998) HMGI-C expression patterns in non-small lung cancer and surrounding tissue. Anticancer Res 18: 3327–3330 [PubMed] [Google Scholar]
- Rommel B, Rogalla P, Jox A, Kalle CV, Kazmierczak B, Wolf J, Bullerdiek J (1997) HMGI-C, a member of the high mobility group family of proteins, is expressed in hematopoietic stem cells and in leukemic cells. Leuk Lymphoma 26: 603–607 [DOI] [PubMed] [Google Scholar]
- Sabbatini R, Federico M, Morselli M, Depenni R, Cagossi K, Luppi M, Torelli G, Silingardi V (2000) Detection of circulating tumor cells by reverse transcriptase polymerase chain reaction of maspin in patients with breast cancer undergoing conventional-dose chemotherapy. J Clin Oncol 18: 1914–1920 [DOI] [PubMed] [Google Scholar]
- Scala S, Portella G, Vitagliano D, Ledent C, Chiappetta G, Giancotti V, Dumont J, Fusco A (2001) HMGI-C gene expression is not required for in vivo thyroid cell transformation. Carcinogenesis 22: 251–256 [DOI] [PubMed] [Google Scholar]
- Sezer O, Langelotz C, Blohmer JU, Schmid P, Akrivakis K, Possinger K (2000) Detection of HMGI-C in the peripheral blood of breast cancer patients. Eur J Cancer 36: 1944–1948 [DOI] [PubMed] [Google Scholar]
- Silva JM, Dominguez G, Silva J, Garcia JM, Sanchez A, Rodriguez O, Provencio M, Espana P, Bonilla F (2001) Detection of epithelial messenger RNA in the plasma of breast cancer patients is associated with poor prognosis tumor characteristics. Clin Cancer Res 7: 2821–2825 [PubMed] [Google Scholar]
- Slade MJ, Smith BM, Sinnett HD, Cross NC, Coombes RC (1999) Quantitative polymerase chain reaction for the detection of micrometastases in patients with breast cancer. J Clin Oncol 17: 870–879 [DOI] [PubMed] [Google Scholar]
- Staats B, Bonk U, Wanschura S, Hanisch P, Schoenmakers EF, Van de Ven WJ, Bartnitzke S, Bullerdiek J (1996) A fibroadenoma with a t(4;12) (q27;q15) affecting the HMGI-C gene, a member of the high mobility group protein gene family. Breast Cancer Res Treat 38: 299–303 [DOI] [PubMed] [Google Scholar]
- Tallini G, Dal Cin P (1999) HMGI(Y) and HMGI-C dysregulation: a common occurrence in human tumors. Adv Anat Pathol 6: 237–246 [PubMed] [Google Scholar]
- Tamimi Y, van der Poel HG, Karthaus HFM, Debruyne FM, Schalken JA (1996) A retrospective study of high mobility group protein I(Y) as progression marker for prostate cancer determined by in situ hybridization. Brit J Cancer 74: 573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallone D, Battista S, Pierantoni GM, Fedele M, Casalino L, Santoro M, Viglietto G, Fusco A, Verde P (1997) Neoplastic transformation of rat thyroid cells requires the JunB and Fra1 gene induction which is dependent on the HMGI-C products. EMBO J 17: 5310–5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Benson KF, Ashar HR, Chada K (1995) Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor HMGI-C. Nature 376: 771–774 [DOI] [PubMed] [Google Scholar]
- Zhou X, Chada K (1998) HMGI family proteins: architectural transcription factors in mammalian development and cancer. Keio J Med 47: 73–77 [DOI] [PubMed] [Google Scholar]