Abstract
Prostacyclin is a pulmonary vasodilator and is produced by prostacyclin synthase and stimulates adenylate cyclase (AC) via the prostacyclin receptor (IP) to produce cAMP. Forskolin is a direct stimulant of AC. Phosphodiesterase 3 hydrolyzes cAMP and is inhibited by milrinone.
Objective
To characterize the prostacyclin-AC-cAMP pathway in the ovine ductal ligation model of persistent pulmonary hypertension of the newborn (PPHN).
Setting
University-based laboratory animal facility.
Subjects
Lambs delivered to time-dated pregnant ewes.
Interventions
Fifth generation pulmonary arteries (PA) and lung parenchyma were isolated from control fetal lambs (n = 8) and fetal lambs with PPHN induced by antenatal ductal ligation (n = 9). We studied relaxation responses to various agonists (milrinone, forskolin, prostacyclin, and iloprost, a prostacyclin analog) that increase cAMP in PA after half-maximal constriction with norepinephrine and pretreatment with propranolol ± indo-methacin. Lung protein levels of prostacyclin synthase, IP, AC2, and phosphodiesterase 3A were analyzed by Western blot and cAMP by enzyme-linked immunoassay.
Main Results
Milrinone relaxed control and PPHN PA and pretreatment with indomethacin significantly impaired this response. Relaxation to milrinone, prostacyclin, and iloprost were significantly impaired in PA from PPHN lambs. Pretreatment with milrinone markedly enhanced relaxation to prostacyclin and iloprost in PPHN PA, similar to relaxation in control PA. Relaxation to forskolin was similar in control and PPHN PAs indicating normal AC activity. Protein levels of prostacyclin synthase and IP were decreased in PPHN lungs compared with control, but AC2, cAMP, and phosphodiesterase 3A remained unchanged.
Conclusions
Prostacyclin and iloprost are dilators of PAs from PPHN lambs and their effect is enhanced by milrinone. This combination therapy may be an effective strategy in the management of patients with PPHN.
Keywords: pulmonary hypertension, nitric oxide, prostacyclin, newborn, phosphodiesterase, cyclic AMP
Persistent pulmonary hypertension of the newborn (PPHN) is a serious disorder with high morbidity and mortality affecting approximately 1 in 500 neonates (1). Antenatal ligation of the ductus arteriosus results in pulmonary hypertension in newborn lambs with clinical, hemodynamic, and pathologic changes resembling the human syndrome (2). Multiple studies using this model have helped us understand the pathophysiology of PPHN and to establish new management strategies, including inhaled nitric oxide (NO) (3). Although inhaled NO is a standard therapy for PPHN, the beneficial effects are transient or minimal in 40% of infants with PPHN (4, 5). Prostacyclin analogs, by intravenous or inhalation routes have been used in term and preterm infants with PPHN (6 – 8) and found to be effective even in infants not responding to inhaled NO (9, 10). Iloprost is a recently approved analog of prostacyclin approved as a nebulized treatment of pulmonary arterial hypertension in adults (11) and has been used anecdotally in neonates with PPHN (12).
Endogenous prostacyclin is produced by cyclo-oxygenase (COX) and prostacyclin synthase (PGIS) enzymes (Fig. 1). Indomethacin is an inhibitor of COX. Prostacyclin stimulates adenylate cyclase (AC) by a prostacyclin receptor (IP)-coupled mechanism. Approximately ten isoforms of AC are found in the lung, but isoform 2 (AC2) is found predominantly in the pulmonary arterial smooth muscle cells (13). Forskolin is a receptor independent, direct stimulant of AC (14). AC catalyzes the formation of cAMP, and cAMP is hydrolyzed to AMP by at least three phosphodiesterase (PDE) isozymes in the pulmonary vasculature (15, 16), namely PDE1, PDE3, and PDE4. Of all the inhibitors of these enzymes, milrinone, a PDE3 inhibitor, is widely used in intensive care settings as an inotrope and afterload reducing agent. Milrinone has been used to reduce pulmonary hypertension in animal models (17) and human neonates (18) and in infants after cardiac bypass (19, 20). The PDE3 family includes two genes, PDE3A and PDE3B, whose products possess similar kinetic and regulatory properties (21, 22). PDE3A is historically referred to as the cardiovascular PDE3 and is highly expressed in cardiac and vascular myocytes (22–24).
Figure 1.
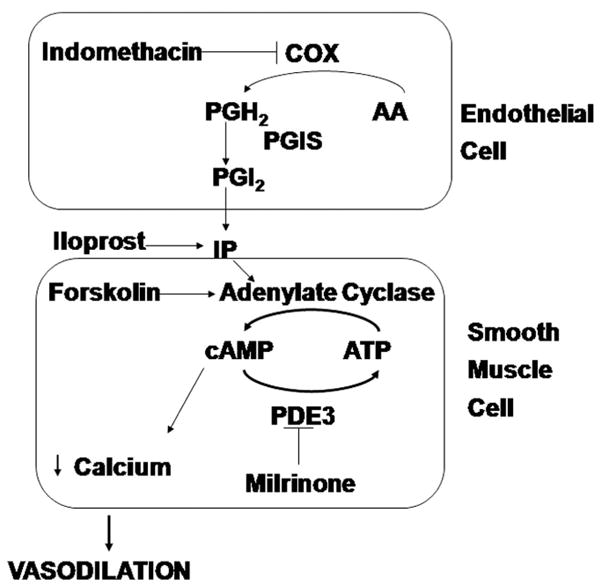
Schematic showing the pathway for synthesis and mode of action of prostacyclin (PGI2). Various agents used in this study are also shown in the figure. COX, cyclo-oxygenase; AA, arachidonic acid; PGH2, prostaglandin H2; PGIS, prostacyclin synthase; IP, prostacyclin receptor; PDE3, phosphodiesterase 3.
The effects of various agents acting via the cAMP pathway, such as milrinone, prostacyclin, and forskolin on pulmonary arteries (PA) isolated from control fetal and newborn lambs have been reported (25–27). Similarly, the effect of various agonists of NO pathway on PA isolated from control and PPHN lambs has been well studied (28 –30). Abnormalities in the prostacyclin-cAMP pathway in hypoxia-induced pulmonary hypertension in rats have also been described (31, 32). However, the effect of indomethacin, milrinone, forskolin, prostacyclin, and iloprost on PA isolated from a ductal ligation model of PPHN is not known. In the current study, we sought to determine the effect of these agents on PA isolated from control fetal lambs and lambs with PPHN induced by antenatal ductal ligation. We correlated the effects of these agents on tone in isolated PA to protein levels of various enzymes and receptors involved in the synthesis and action of prostacyclin such as PGIS, IP, AC2, PDE3A, and cAMP in the lung.
MATERIALS AND METHODS
The Laboratory Animal Care Committee at the State University of New York at Buffalo approved this study. Fetal ductal ligation was performed via thoracotomy at 125–126 days of gestation (term = 146 days) as previously described (3, 33). Briefly, time-dated pregnant sheep (Swartz family farm, Attica, NY) were anesthetized with an intravenous injection of Pentothal (750 mg) followed by 2% halothane inhalation. A left lateral thoracotomy was performed in the fourth intercostal space of the fetus, and the ductus arteriosus ligated (2). The fetal chest wall was closed and the fetus returned to the uterus. After skin closure, the wound was infiltrated with Marcaine for local analgesia and an intramuscular dose of Buprenorphine, a narcotic analgesic was given to the ewe soon after surgery and repeated 12 hrs later. The ewe was allowed to recover for 9 days.
At 135 days gestation, nine lambs with PPHN and eight controls (usually twins) were delivered by cesarean section and killed by rapid exsanguination by direct cardiac puncture while still under anesthesia. The heart and lungs were removed en bloc from the thorax immediately and placed in Krebs-Ringer solution (in mM: 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25.5 NaHCO3, and 5.6 glucose). Fifth generation intralobar PA with inside diameters of 500 microns were isolated, dissected with care to preserve the integrity of the endothelium, cut into rings ~1–2 mm long and 1–2 mg in weight and studied using standard tissue bath techniques as described previously (34). Rings were suspended in water-jacketed chambers filled with aerated (20% O2– 6% CO2 and remainder N2) Krebs-Ringer solution. A continuous recording of isometric force generation was obtained by tying each vessel ring to a force displacement transducer (model UC2, Statham Instruments, Hato Rey, PR) that was connected to a recorder (Gould Instrument Systems, Valley View, OH). After the arterial rings were mounted, they were allowed to equilibrate for 20 min in the bathing solution. A micrometer was used to stretch the tissues repeatedly in small increments over the following 45 min until resting tone remained stable at a passive tension of 0.8 g for control pulmonary arteries and 1.0 g for pulmonary arteries from PPHN lambs. Preliminary experiments determined that this procedure provided an optimal length for generation of active tone to exogenous norepinephrine. Wet tissue weights were obtained at the end of each experiment, and contraction responses were normalized to tissue weight.
The following pharmacologic agents were used: indomethacin, DL-propranolol, norepinephrine hydrochloride (NE), potassium chloride (KCl) milrinone, prostacyclin, iloprost, and forskolin. Iloprost was obtained from Cayman Chemical Company (Ann Arbor, MI). All other drugs were obtained from Sigma-Aldrich (St. Louis, MO). Indomethacin was dissolved in ethanol. Iloprost methyl acetate was dissolved in dimethyl sulfoxide to a 10−3 M concentration and then diluted in distilled water. Forskolin was dissolved in ethanol. Milrinone was dissolved in dimethyl sulfoxide and then diluted in distilled water. All other drugs were dissolved in distilled water. Ethanol and DMSO at the concentrations used in these experiments did not alter the preexisting tone of the PA.
Isolated PA were pretreated with propranolol (10−6 M) to block β adrenergic receptors. Some arteries that were later relaxed with milrinone alone were pretreated with indomethacin (10−5 M) to prevent the formation of vasoactive prostaglandins and to study the relative contribution of COX to milrinone-induced relaxations (Fig. 1). Pulmonary arteries that were relaxed with prostacyclin or iloprost were not pretreated with indomethacin. The arteries were first contracted with increasing concentrations of NE (10−8–3 × 10−7 M). This concentration of NE provided 40%–50% of contraction force (as expressed as grams of force per grams of tissue weight) generated by 118 mM KCl. Isolated pulmonary arteries relaxed with milrinone alone were treated with a dose of 10−7–10−4 M. Vessels in protocols involving relaxation with prostacyclin and iloprost were treated with milrinone (10−7–3 × 10−6 M) and relaxation responses were measured as a percentage of NE contraction. After observing the relaxation response to the 3 × 10−6 M dose of milrinone, the vessels were constricted with an additional dose of NE (2 × 10−7 M) and once a new plateau contraction was reached, PA were relaxed with increasing concentrations of forskolin (10−8–10−5 M), prostacyclin (10−7–10−4 M), or iloprost (10−10–10−4 M). Other vessels were constricted with NE (10−8–3 × 10−7 M) and relaxed with forskolin, prostacyclin, or iloprost without milrinone pretreatment. The starting tension generated by NE in the protocols with and without milrinone before relaxation by forskolin, prostacyclin, or iloprost were similar (133 ± 12 and 157 ± 41 g/g respectively, p = 0.52 by unpaired t test). Two to three PA rings per protocol were studied from each animal.
Total protein was isolated from sheep lung tissue snap frozen in liquid nitrogen using the commercially available PARIS kit (Ambion, Austin, TX) supplemented with a protease inhibitor cocktail (Sigma) and a phosphatase inhibitor cocktail (EMD Biosciences, San Diego, CA). Protein concentration was determined using the Bradford method (35). Total lung protein (40 μg) was separated on a 4%–20% sodium dodecyl sulfate-polyacrylamide gel (Biorad, Hercules, CA), and the protein was then transferred from the gel to a nitrocellulose membrane (Amersham, Arlington Heights, IL). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 (1X TBST) for 1 hr at room temperature. Membranes were then incubated with primary antibody at 4°C overnight at the appropriate dilution in 5% milk in 1X TBST (1:1000 for PGIS [Cayman Chemical, Ann Arbor, MI], 1:500 for IP [Cayman Chemical], 1: 100 for AC2 [Santa Cruz Biotechnology, Santa Cruz, CA], 1:500 for PDE3A (Santa Cruz), 1:2000 for β-actin [Sigma]). After being washed with 1X TBST, the membranes were incubated with the appropriate secondary antibody conjugated to horseradish peroxidase (Pierce, Rockford, IL for anti-mouse and anti-rabbit; Santa Cruz for anti-goat) at a 1:1000 dilution for the anti-mouse antibody (β-actin), a 1:2000 dilution for the anti-rabbit antibody (PGIS, IP, AC2), and a 1:5000 dilution for the anti-goat antibody (PDE3A) in 5% milk + 1X TBST. Membranes were then washed with the appropriate buffer, and the bands visualized via chemiluminescence (Pierce, Rockford, IL), using a Digital Science Image Station (Kodak, Rochester, NY). Expression for PGIS, IP, AC2, and PDE3A was normalized to β-actin expression.
To measure PDE3 hydrolytic activity, lung protein was prepared fresh from snap-frozen tissue as described above. The total lung protein was immediately placed on ice and assayed the same day. The protein was purified over a Centri-Spin 10 column to remove any phosphate contamination (Princeton Separations, Adelphia, NJ), since the assay measurement is dependent on free phosphate. Protein concentration was determined as described above. Total protein (5 μg) was assayed for cAMP hydrolytic activity using a commercially available colorimetric cyclic nucleotide PDE assay kit (Biomol, Plymouth Meeting, PA). The basis for the assay is the cleavage of cAMP by a cyclic nucleotide PDE, such as PDE3, followed by the release of free phosphate by a 5′ nucleotidase from Crotalus atrox venom. The free phosphate is quantified using the Biomol Green reagent (Biomol) in a modified Malachite Green assay (36, 37). The reactions are mixed in a 96-well plate, and reactions are started in a timed-fashion by the addition of the 5′-nucleotidase. Each sample is read in four wells—two without milrinone and two with milrinone (100 μM), to determine PDE3-specific cAMP-hydrolytic activity. The samples are then incubated at 30°C for 30 mins and stopped in a timed-fashion by the addition of the Biomol Green reagent, followed by incubation at room temperature for 20 mins. Results were measured using a Labsystems Multiskan EX automated plate reader at 620 nM wavelength and compared with a standard curve generated with AMP and 5′-nucleotidase. The difference between the picomole cAMP hydrolyzed per milligram total protein per minute without milrinone and the picomole cAMP hydrolyzed per milligram total protein per minute with milrinone represents the PDE3-specific cAMP-hydrolytic activity. Results are shown as the PDE3-specific picomole cAMP hydrolyzed per milligram total protein per minute for each sample.
To measure cAMP levels, sheep lung tissue snap frozen in liquid nitrogen was weighed and homogenized in 10 volumes (milliliter solution per gram of tissue) of 5% trichloroacetic acid (Sigma) in water. Precipitate was removed by centrifugation at 1500 g for 10 mins. The trichloroacetic acid was then extracted using water-saturated ether according to the manufacturer’s protocol (Cayman Chemical). cAMP content of the lung samples was measured by enzyme-linked immunoassay in triplicate, relative to a cAMP standard curve, using a Labsystems Multiskan EX automated plate reader (Thermo Electron, Milford, MA) at 420 nm wavelength. Results are shown as picomole cAMP per milligram frozen lung tissue.
All data are expressed as mean ± SEM, with “n” representing the number of animals studied. Statistical comparisons of the curves were performed with factorial or repeated measures analysis of variance as appropriate. Fisher’s protected least significant difference post-hoc testing was used as needed to compare multiple groups. All statistical analysis for the isolated vessel data were performed with StatView software (Abacus Concepts, Berkley, CA) and all statistical analysis for the Western blot and cAMP data were performed using Prism (GraphPad Software, San Diego, CA). Significance was accepted at p < 0.05.
RESULTS
Isolated Pulmonary Artery Studies
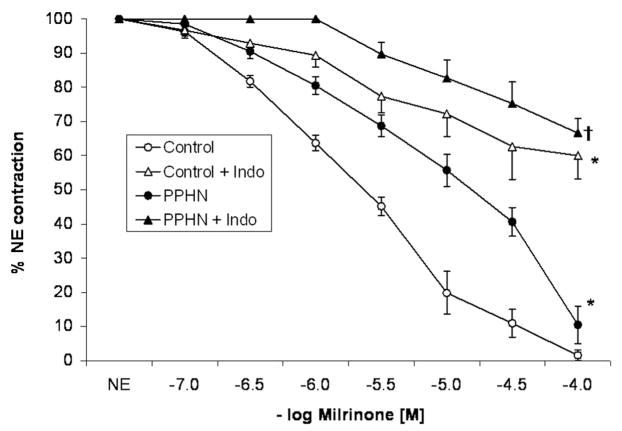
Pulmonary arteries isolated from control fetal lambs and contracted by NE relaxed well to milrinone in a concentration-dependent manner (Fig. 2). Pretreatment with indomethacin significantly decreased the relaxation to milrinone. Relaxations to milrinone were significantly impaired in pulmonary arteries isolated from lambs with PPHN compared with controls. Similar to control PA, pretreatment with indomethacin significantly impaired the relaxation to milrinone in PPHN PA (Fig. 2).
Figure 2.
Cumulative concentration response curve to milrinone in pulmonary arteries isolated from control fetal lambs (control, n = 8) and lambs with persistent pulmonary hypertension of the newborn (PPHN) (n = 9). Some vessels were pretreated with indomethacin (Indo, 10−5 M). Data represents mean ± SEM and “n” refers to number of lambs. (*p < 0.05 compared with control and †p < 0.01 compared with PPHN by analysis of variance repeated measures.)
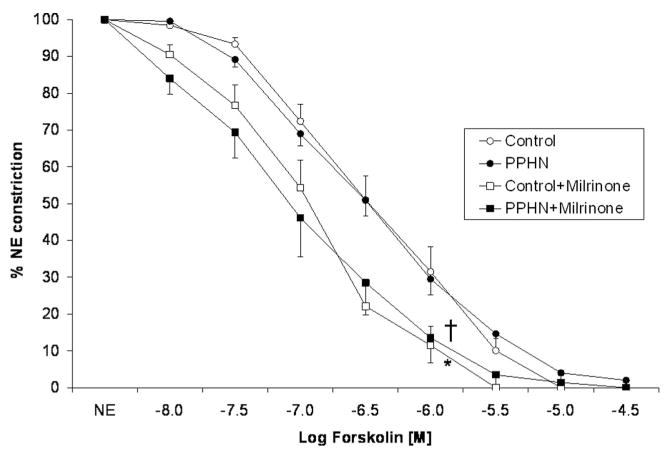
Forskolin, a receptor-independent stimulator of AC completely relaxed control PA and this response was significantly increased by pretreatment with milrinone (Fig. 3). In sharp contrast to relaxations induced by milrinone, forskolin-mediated relaxations were not significantly different between PA isolated from control and PPHN lambs (Fig. 2). Pre-treatment with milrinone significantly enhanced relaxation to forskolin in PA from control and PPHN lambs.
Figure 3.
Cumulative concentration response curve to forskolin in pulmonary arteries isolated from control fetal lambs (control, n = 7) and lambs with persistent pulmonary hypertension of the newborn (PPHN) (n = 7). Some vessels were pretreated with milrinone (10−5.5 M). “n” refers to number of lambs. (*p < 0.01 compared with control and †p < 0.01 compared with persistent pulmonary hypertension of the newborn by analysis of variance repeated measures.)
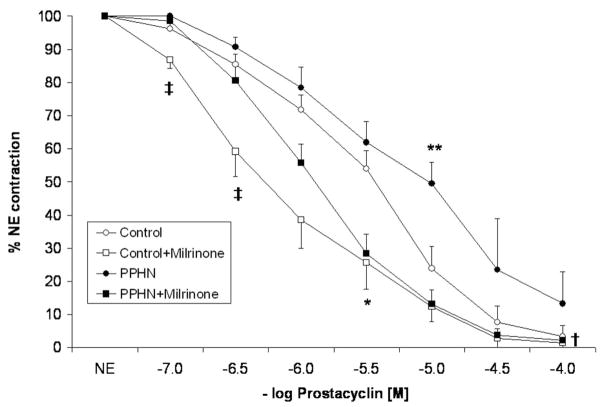
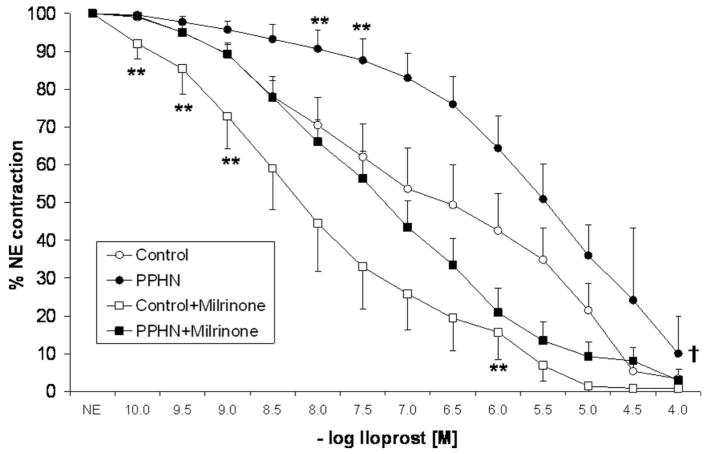
Relaxation to prostacyclin in control PA was enhanced by pretreatment with milrinone (Fig. 4). However, this enhancement was significant at 10−7 M and 3 × 10−7 M concentrations of prostacyclin only. At 10−5 M prostacyclin, control PA relaxed to <20% of the NE-induced contraction either with or without milrinone. The relaxation responses to 10−5 M prostacyclin were significantly impaired in PA from PPHN lambs. At higher doses (10−4 M), there was no significant difference in relaxation to prostacyclin in control and PPHN lambs. However, pretreatment and milrinone significantly enhanced the relaxation response to prostacyclin with relaxation at 10−5 M prostacyclin similar to that of control PA (Fig. 4). Similarly, iloprost relaxed control PA and this response was enhanced by pretreatment with milrinone at 10−10 M–10−9 M and 10−6 M concentrations of iloprost. Similar to prostacyclin, relaxation to iloprost was impaired in PA isolated from PPHN lambs and enhanced after pretreatment with milrinone (Fig. 5).
Figure 4.
Cumulative concentration response curves to prostacyclin in pulmonary arteries isolated from control fetal lambs (control, n = 8) and lambs with persistent pulmonary hypertension of the newborn (PPHN) (n = 9). Some vessels were pretreated with milrinone (10−5.5 M). “n” represents number of lambs. (**p < 0.05 compared with control by analysis of variance with Fisher’s protected least significant difference at 10−5 M of prostacyclin, *p < 0.05 compared with control by analysis of variance (ANOVA) repeated measures, †p < 0.005 compared with PPHN by ANOVA repeated measures, ‡p < 0.05 compared to Milrinone + PPHN by ANOVA with Fisher’s protected least significant difference at 10−7 and 10−6.5 M of prostacyclin.)
Figure 5.
Cumulative concentration response curves to iloprost in pulmonary arteries isolated from control fetal lambs (control, n = 7) and lambs with persistent pulmonary hypertension of the newborn (PPHN) (n = 8). Some vessels were pretreated with milrinone (10−5.5 M). “n” represents number of lambs. (**p < 0.05 compared with control by analysis of variance with Fisher’s protected least significant difference at a specific concentration of iloprost, †p < 0.05 compared with PPHN by analysis of variance repeated measures.)
Molecular Analysis of Lung Tissue
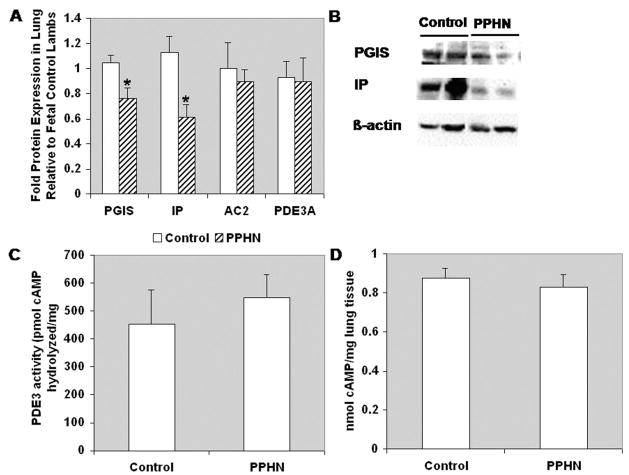
Protein levels PGIS and IP were significantly decreased in PPHN lung tissue compared with control lung tissue (0.76 ± 0.09-fold and 0.62 ± 0.1-fold, respectively, p < 0.05; Fig. 6, A and B). In contrast, protein levels of AC2 and PDE3A were unchanged in the PPHN lung tissue compared with control lung tissue (Fig. 6A). Similarly, baseline PDE3 hydrolytic activity (Fig. 6C) and steadystate cAMP levels (Fig. 6D) were unchanged in PPHN lungs compared with control.
Figure 6.
Prostacyclin synthase (PGIS) and IP receptor are decreased in persistent pulmonary hypertension of the newborn lamb lung relative to control lamb lung. (A) Total protein harvested from frozen lung tissue was subjected to Western blot analysis for PGIS, IP receptor, AC2, and PDE3A (n = 7). Results are normalized for β-actin expression. *p < 0.01 for PGIS and IP receptor in persistent pulmonary hypertension of the newborn vs. Control. (B) Representative Western blots for PGIS and IP receptor. (C) Total protein harvested from frozen lung tissue was assayed for PDE3-specific cAMP hydrolytic activity (n = 7, read in duplicate). Results are shown as picomole cAMP hydrolyzed/microgram lung protein/min. (D) Cyclic nucleotide content was extracted from frozen lung tissue and subjected to cAMP analysis by EIA (n = 7, read in triplicate). Results are shown as nanomole cAMP/mg frozen lung tissue.
DISCUSSION
In this study, we report relaxation responses to various agonists of the cAMP pathway in pulmonary arteries isolated from control lambs and lambs with PPHN induced by antenatal ductal ligation. The recent approval of iloprost (a stable analog of prostacyclin for inhalation/nebulization) by the FDA has sparked further interest in prostacyclin-cAMP pathway in PPHN.
Milrinone is a PDE3 inhibitor and relaxations to milrinone appear to be dependent on endogenous production of cAMP in the PA. In support of these findings, inhibition of COX by indomethacin significantly reduced relaxations to milrinone in control pulmonary artery (Fig. 2). Similarly, pretreatment with indomethacin results in a significant decrease in basal release of prostacyclin from pulmonary arteries isolated from newborn lambs (25). Furthermore, basal cAMP production in fetal ovine pulmonary arteries is fully attenuated by indomethacin (27). These results indicate that prostaglandins generated by COX are the predominant source of basal cAMP in these vessels.
Forskolin, prostacyclin, and iloprost relaxed pulmonary arteries isolated from control lambs. Pretreatment with milrinone enhanced these relaxation responses (Figs. 3–5). The bath concentration of milrinone (10−5.5 M or approximately 630 ng/mL) was based on target serum levels (180 –300 ng/mL) obtained from pharmacokinetic studies in preterm neonates (38). Similar enhanced responses with milrinone have been reported in newborn ovine pulmonary arteries with prostaglandin E2 and forskolin and are associated with an increase in intracellular content of cAMP (26).
Steady-state lung cAMP levels are unchanged in PPHN lambs, but PA isolated from PPHN lambs had a significantly reduced relaxation to milrinone (Fig. 2), indicating decreased endogenous production of cAMP from upstream sources. Decreased endogenous cAMP production can be secondary to reduced levels of PGIS or IP receptor or AC in PPHN. We have shown that protein levels of PGIS and IP are decreased in PPHN lungs, whereas AC levels remain unchanged. Similarly, a decrease in lung PGIS expression has been described in adult patients with primary and secondary pulmonary hypertension (39).
Relaxation to 10−5 M prostacyclin was significantly impaired in PA isolated from PPHN lambs (Fig. 3). This could be secondary to reduced levels of prostacyclin IP receptor, reduced activity of AC or increased hydrolysis of cAMP by PDEs. We have shown that the lung IP receptor levels are decreased in PPHN (Fig. 6A), whereas PDE3 hydrolytic activity is unchanged in PPHN lambs. Furthermore, normal relaxation responses to forskolin in PA isolated from lambs with PPHN (Fig. 3) indicate that AC activity is not altered in this model of PPHN. However, pulmonary arteries isolated from rats with hypoxia-induced pulmonary hypertension relaxed poorly to forskolin (32). A decreased prostacyclin stimulated AC activity with normal basal AC activity also has been described in hypoxia-induced pulmonary hypertension in rats (40). Forskolin stimulated AC activity was also not altered in this model (31). Prostaglandin receptor binding characteristics were not quantified in this model as radioligands available at that time had low affinity for vascular prostaglandin receptors (40). We speculate that this difference in the response to forskolin is secondary to changes in AC activity between various models of pulmonary hypertension (hypoxia induced- vs. ductal ligation-induced pulmonary hypertension).
Pretreatment of PA from PPHN lambs with milrinone significantly enhanced the relaxation to prostacyclin (Fig. 4) and iloprost (Fig. 5). The relaxation to 10−5 M–10−3 M of prostacyclin in the presence of milrinone in PPHN was similar to PA isolated from control lambs either with or without milrinone (Fig. 4). We speculate that the ability of higher doses of prostacyclin and iloprost in the presence of milrinone pretreatment to relax PPHN pulmonary arteries similar to control arteries is due to the ability of those higher doses to overcome the upstream defect in cAMP production, specifically due to decreased levels of PGIS and IP.
We acknowledge several drawbacks in this study. The tissue baths were bubbled with 20% oxygen and 6% carbon dioxide mixture. This results in a partial pressure of oxygen of 90 –100 mm Hg in the tissue bath. This level is higher than the pulmonary arterial PO2 in a fetal lamb or a neonatal lamb with PPHN. However, the smooth muscle layer of isolated pulmonary arteries from PPHN lambs is thick and is oxygenated purely by diffusion in the tissue bath. Lack of perfusion of tunica media by vasa vasorum in an in vitro arterial ring can cause hypoxia in the vascular smooth muscle if tissue bath oxygen tensions are very low. The protein levels of PGIS, IP, AC2, and PDE3A, as well as PDE3 activity and cAMP levels were estimated in whole lung parenchyma and not in pulmonary arteries. There may be other nonvascular sources of these enzymes in lung parenchyma, and it may be inappropriate to correlate responses in the isolated pulmonary arteries to lung enzyme/receptor levels.
CONCLUSIONS
In summary, in the ovine ductal ligation model of PPHN, decreased relaxation responses of pulmonary arteries to milrinone and prostacyclin/iloprost are probably secondary to reduced activity of PGIS enzyme and IP receptor. Forskolin-induced relaxation is not altered suggesting normal AC activity. Pretreatment with milrinone results in significant enhancement of prostacyclin-induced relaxations in pulmonary arteries isolated from PPHN lambs. Similar enhancement of NO activity by PDE 5 inhibitor, sildenafil has been reported in neonatal porcine PA (41). We speculate that a combination of intravenous milrinone and inhaled prostacyclin (intratracheal or by nebulization), as suggested by some preliminary animal experiments in the same model of PPHN (17), may be an effective approach to treating neonates with PPHN.
Acknowledgments
Supported, in part, by HL#54705 (to RHS), K08 HL086715 (to KNF) and the Department of Pediatrics, University at Buffalo.
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Walsh-Sukys MC, Tyson JE, Wright LL, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: Practice variation and outcomes. Pediatrics. 2000;105(1 Pt 1):14 –20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- 2.Morin FC., III Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res. 1989;25:245–250. doi: 10.1203/00006450-198903000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Zayek M, Cleveland D, Morin FC., III Treatment of persistent pulmonary hypertension in the newborn lamb by inhaled nitric oxide. J Pediatr. 1993;122(5 Pt 1):743–750. doi: 10.1016/s0022-3476(06)80020-x. [DOI] [PubMed] [Google Scholar]
- 4.Roberts JD, Jr, Fineman JR, Morin FC, III, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med. 1997;336:605– 610. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- 5.Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med. 1997;336:597– 604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 6.De Jaegere AP, van den Anker JN. Endotracheal instillation of prostacyclin in preterm infants with persistent pulmonary hypertension. Eur Respir J. 1998;12:932–934. doi: 10.1183/09031936.98.12040932. [DOI] [PubMed] [Google Scholar]
- 7.Soditt V, Aring C, Groneck P. Improvement of oxygenation induced by aerosolized prostacyclin in a preterm infant with persistent pulmonary hypertension of the newborn. Intensive Care Med. 1997;23:1275–1278. doi: 10.1007/s001340050498. [DOI] [PubMed] [Google Scholar]
- 8.Kaapa P, Koivisto M, Ylikorkala O, et al. Prostacyclin in the treatment of neonatal pulmonary hypertension. J Pediatr. 1985;107:951–953. doi: 10.1016/s0022-3476(85)80200-6. [DOI] [PubMed] [Google Scholar]
- 9.Kelly LK, Porta NF, Goodman DM, et al. Inhaled prostacyclin for term infants with persistent pulmonary hypertension refractory to inhaled nitric oxide. J Pediatr. 2002;141:830 – 832. doi: 10.1067/mpd.2002.129849. [DOI] [PubMed] [Google Scholar]
- 10.Golzand E, Bar-Oz B, Arad I. Intravenous prostacyclin in the treatment of persistent pulmonary hypertension of the newborn refractory to inhaled nitric oxide. Isr Med Assoc J. 2005;7:408 – 409. [PubMed] [Google Scholar]
- 11.Baker SE, Hockman RH. Inhaled iloprost in pulmonary arterial hypertension. Ann Pharmacother. 2005;39:1265–1274. doi: 10.1345/aph.1E575. [DOI] [PubMed] [Google Scholar]
- 12.Concheiro Guisan A, Sousa Rouco C, Suarez Traba B, et al. Inhaled iloprost: A therapeutic alternative for persistent pulmonary hypertension of the newborn. An Pediatr (Barc) 2005;63:175–176. doi: 10.1157/13077463. [DOI] [PubMed] [Google Scholar]
- 13.Jourdan KB, Mason NA, Long L, et al. Characterization of adenylyl cyclase isoforms in rat peripheral pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1359 –L1369. doi: 10.1152/ajplung.2001.280.6.L1359. [DOI] [PubMed] [Google Scholar]
- 14.Lincoln TM, Fisher-Simpson V. A comparison of the effects of forskolin and nitroprusside on cyclic nucleotides and relaxation in the rat aorta. Eur J Pharmacol. 1984;101:17–27. doi: 10.1016/0014-2999(84)90026-8. [DOI] [PubMed] [Google Scholar]
- 15.Rabe KF, Tenor H, Dent G, et al. Identification of PDE isozymes in human pulmonary artery and effect of selective PDE inhibitors. Am J Physiol. 1994;266:L536 –L543. doi: 10.1152/ajplung.1994.266.5.L536. [DOI] [PubMed] [Google Scholar]
- 16.Dent G, Magnussen H, Rabe KF. Cyclic nucleotide phosphodiesterases in the human lung. Lung. 1994;172:129 –146. doi: 10.1007/BF00175942. [DOI] [PubMed] [Google Scholar]
- 17.Rashid N, Morin FC, III, Swartz DD, et al. Effects of prostacyclin and milrinone on pulmonary hemodynamics in newborn lambs with persistent pulmonary hypertension induced by ductal ligation. Pediatr Res. 2006;60:624 – 629. doi: 10.1203/01.pdr.0000242343.84510.81. [DOI] [PubMed] [Google Scholar]
- 18.Bassler D, Choong K, McNamara P, et al. Neonatal persistent pulmonary hypertension treated with milrinone: Four case reports. Biol Neonate. 2005;89:1–5. doi: 10.1159/000088192. [DOI] [PubMed] [Google Scholar]
- 19.Danhaive O, Margossian R, Geva T, et al. Pulmonary hypertension and right ventricular dysfunction in growth-restricted, extremely low birth weight neonates. J Perinatol. 2005;25:495– 499. doi: 10.1038/sj.jp.7211299. [DOI] [PubMed] [Google Scholar]
- 20.Khazin V, Kaufman Y, Zabeeda D, et al. Milrinone and nitric oxide: Combined effect on pulmonary artery pressures after cardiopulmonary bypass in children. J Cardiothorac Vasc Anesth. 2004;18:156 –159. doi: 10.1053/j.jvca.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Degerman E, Belfrage P, Manganiello VC. Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3) J Biol Chem. 1997;272:6823– 6826. doi: 10.1074/jbc.272.11.6823. [DOI] [PubMed] [Google Scholar]
- 22.Palmer D, Maurice DH. Dual expression and differential regulation of phosphodiesterase 3A and phosphodiesterase 3B in human vascular smooth muscle: Implications for phosphodiesterase 3 inhibition in human cardiovascular tissues. Mol Pharmacol. 2000;58:247–252. doi: 10.1124/mol.58.2.247. [DOI] [PubMed] [Google Scholar]
- 23.Smith CJ, Krall J, Manganiello VC, et al. Cytosolic and sarcoplasmic reticulum-associated low Km, cGMP-inhibited cAMP phosphodiesterase in mammalian myocardium. Biochem Biophys Res Commun. 1993;190:516 –521. doi: 10.1006/bbrc.1993.1078. [DOI] [PubMed] [Google Scholar]
- 24.Rascon A, Lindgren S, Stavenow L, et al. Purification and properties of the cGMP-inhibited cAMP phosphodiesterase from bovine aortic smooth muscle. Biochim Biophys Acta. 1992;1134:149 –156. doi: 10.1016/0167-4889(92)90038-d. [DOI] [PubMed] [Google Scholar]
- 25.Gao Y, Zhou H, Ibe BO, et al. Prostaglandins E2 and I2 cause greater relaxations in pulmonary veins than in arteries of newborn lambs. J Appl Physiol. 1996;81:2534 –2539. doi: 10.1152/jappl.1996.81.6.2534. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Tolsa JF, Shen H, et al. Effect of selective phosphodiesterase inhibitors on response of ovine pulmonary arteries to prostaglandin E2. J Appl Physiol. 1998;84:13–18. doi: 10.1152/jappl.1998.84.1.13. [DOI] [PubMed] [Google Scholar]
- 27.Shaul PW, Farrar MA, Magness RR. Prostacyclin synthesis and stimulation of cyclic AMP production in ovine fetal vasculature: Heterogeneity in pulmonary and systemic arteries. Dev Pharmacol Ther. 1992;18:89 –99. [PubMed] [Google Scholar]
- 28.Steinhorn RH, Morin FC, III, Gugino SF, et al. Developmental differences in endothelium-dependent responses in isolated ovine pulmonary arteries and veins. Am J Physiol. 1993;264(6 Pt 2):H2162–H2167. doi: 10.1152/ajpheart.1993.264.6.H2162. [DOI] [PubMed] [Google Scholar]
- 29.Steinhorn RH, Russell JA, Morin FC., III Disruption of cGMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am J Physiol. 1995;268(4 Pt 2):H1483–H1489. doi: 10.1152/ajpheart.1995.268.4.H1483. [DOI] [PubMed] [Google Scholar]
- 30.Konduri GG, Ou J, Shi Y, et al. Decreased association of HSP90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2003;285:H204 –H211. doi: 10.1152/ajpheart.00837.2002. [DOI] [PubMed] [Google Scholar]
- 31.Shaul PW, Muntz KH, DeBeltz D, et al. Effects of prolonged hypoxia on adenylate cyclase activity and beta-adrenergic receptors in pulmonary and systemic arteries of the rat. Circ Res. 1990;66:1526 –1534. doi: 10.1161/01.res.66.6.1526. [DOI] [PubMed] [Google Scholar]
- 32.Wagner RS, Smith CJ, Taylor AM, et al. Phosphodiesterase inhibition improves agonist-induced relaxation of hypertensive pulmonary arteries. J Pharmacol Exp Ther. 1997;282:1650 –1657. [PubMed] [Google Scholar]
- 33.Wild LM, Nickerson PA, Morin FC., III Ligating the ductus arteriosus before birth remodels the pulmonary vasculature of the lamb. Pediatr Res. 1989;25:251–257. doi: 10.1203/00006450-198903000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Steinhorn RH, Morin FC, III, Gugino SF, et al. Developmental differences in endothelium-dependent responses in isolated ovine pulmonary arteries and veins. Am J Physiol. 1993;264(6 Pt 2):H2162–H2167. doi: 10.1152/ajpheart.1993.264.6.H2162. [DOI] [PubMed] [Google Scholar]
- 35.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 36.Beers SA, Malloy EA, Wu W, et al. Nitroaryl-hydroxymethylphosphonic acids as inhibitors of CD45. Bioorg Med Chem. 1997;5:2203–2211. doi: 10.1016/s0968-0896(97)00174-0. [DOI] [PubMed] [Google Scholar]
- 37.Harder KW, Owen P, Wong LK, et al. Characterization and kinetic analysis of the intra-cellular domain of human protein tyrosine phosphatase beta (HPTP beta) using synthetic phosphopeptides. Biochem J. 1994;298(Pt 2):395– 401. doi: 10.1042/bj2980395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paradisis M, Jiang X, McLachlan AJ, et al. Population pharmacokinetics and dosing regimen design of milrinone in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2007;92:F204 –F209. doi: 10.1136/adc.2005.092817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 40.Shaul PW, Kinane B, Farrar MA, et al. Prostacyclin production and mediation of adenylate cyclase activity in the pulmonary artery. Alterations after prolonged hypoxia in the rat. J Clin Invest. 1991;88:447– 455. doi: 10.1172/JCI115324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreno L, Losada B, Cogolludo A, et al. Postnatal maturation of phosphodiesterase 5 (PDE5) in piglet pulmonary arteries: Activity, expression, effects of PDE5 inhibitors, and role of the nitric oxide/cyclic GMP pathway. Pediatr Res. 2004;56:563–570. doi: 10.1203/01.PDR.0000139412.58594.D0. [DOI] [PubMed] [Google Scholar]